Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Inborn errors of metabolism (IEM) caused by defective processing of complex molecules comprise a specific subgroup of genetic disorders. Since these disorders are clinically heterogeneous, their diagnosis requires a high index of clinical suspicion combined with targeted laboratory testing.
This chapter focuses on IEM arising from defective metabolism of “complex molecules.” This IEM group is largely caused by defects in biological processes within intracellular organelles such as lysosomes and peroxisomes. Lysosomes are responsible for the breakdown of complex molecules such as glycosaminoglycans and sphingolipids. Deficiencies in enzymes required for the metabolism of these molecules result in intralysosomal substrate accumulation. Peroxisomes perform a large number of important functions within the cell, including the metabolism of complex molecules such as very long chain fatty acids. Peroxisomal disorders result from defects in their synthesis or in one of their essential catalytic functions. A large and growing number of IEM are caused by congenital defects of protein glycosylation. As such, this chapter describes the clinical and laboratory aspects of the three major types of IEM related to complex molecules: lysosomal storage disorders (LSDs), peroxisomal disorders (PDs), and congenital disorders of glycosylation (CDGs). Although these disorders are individually rare, they are considerably more prevalent as a collective and represent some of the most rapidly expanding IEM groups. The biochemical genetics laboratory plays a key role in the detection of LSDs, PDs, and CDGs. As with many other IEM, early recognition and treatment of these disorders are often associated with improved clinical outcomes. This is particularly true for LSDs, where new treatments such as enzyme replacement therapy have led to their inclusion in public newborn screening programs. Analytical methods used for the identification of these disorders are usually reserved for subspecialty biochemical genetics laboratories. For diagnosis of LSDs, enzyme activity measurement is used in combination with molecular testing. Biochemical screening for PDs requires the measurement of accumulated substrates, such as very long chain fatty acids, using techniques such as gas and liquid chromatography combined with mass spectrometry. Finally, for CDG identification, sophisticated approaches using high-resolution mass spectrometry in combination with molecular testing is often necessary. The diagnosis of IEM of complex molecules increasingly requires integration and correlation of biochemical data with clinical and molecular findings.
First described in 1955 by Christian de Duve, the lysosome is known for its role in the degradation and recycling of proteins, complex carbohydrates, lipids, nucleic acids, sulfates, and phosphates. This is now recognized as an outdated and simplistic view of lysosomal function. The lysosome is, in fact, not the endpoint in the endocytic pathway but rather a central dynamic organelle, serving an important functional role in cell homeostasis, and in particular, the regulation of inflammation and autoimmunity. Present in all nucleated eukaryotic cells, lysosomes are maintained in an acidic state at around pH 4.6 to 5.0 and contain approximately 60 hydrolytic enzymes and various membrane proteins. A deficiency of one particular enzyme within the degradative pathway will lead to the progressive accumulation of a substrate within the lysosome and ultimately to a storage disorder.
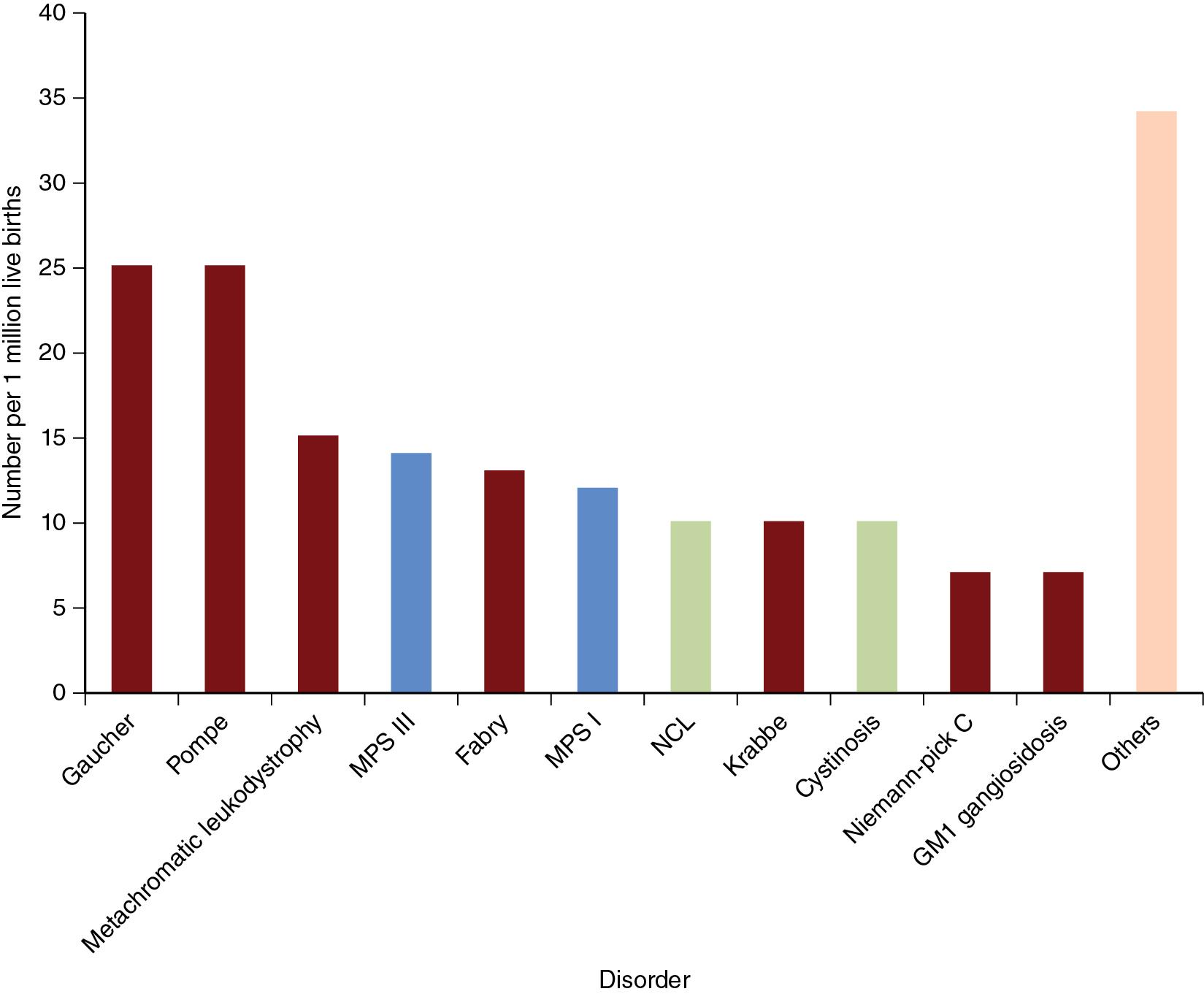
The lysosomal storage disorders (LSDs) comprise a heterogeneous group of approximately 50 conditions caused by defects in lysosomal metabolism. In the majority of cases, LSDs result from mutations in genes encoding intralysosomal catabolic enzymes responsible for the degradation of macromolecules. LSDs may also result from defects in lysosome biogenesis, lysosomal enzyme activators, and membrane transporters. Disorders caused by dysfunction of vesicular traffic or transport within the endosome-lysosome system may also result in problems with lysosomal storage. Regardless of etiology, decreased or absent enzyme activity results in the progressive accumulation of substrates and intermediates within the lysosomal degradative pathway, leading to cellular, tissue, and eventual organ destruction. The clinical phenotype is largely determined by the organ affected, with dysfunction often occurring in the central nervous system (CNS), liver, lungs, eyes, bone, muscle (including cardiac muscle), nervous, and reticuloendothelial systems. Most LSDs are characterized by an autosomal recessive mode of inheritance. However, some LSDs can be X-linked (e.g., Fabry disease [FD]) or autosomal dominant (e.g., sialuria). Although LSDs are individually rare, their cumulative incidence is considerably greater and may be as high as 1:2000 to 1:5000 live births. ,
LSDs have traditionally been classified according to the accumulated substrate. As such, the so-called “classical” sub-groups of LSDs comprise the sphingolipidoses, mucopolysaccharidoses (MPS), mucolipidoses (ML) and oligosaccharidoses. However, this classification does not take into consideration an increasing number of disorders characterized by defects in protein synthesis, trafficking, or membrane-associated proteins. Consequently, the traditional approach of classifying these disorders is increasingly considered obsolete in favor of a more appropriate classification based on functionality. However, due to its current universal acceptance, the traditional classification is discussed in this chapter.
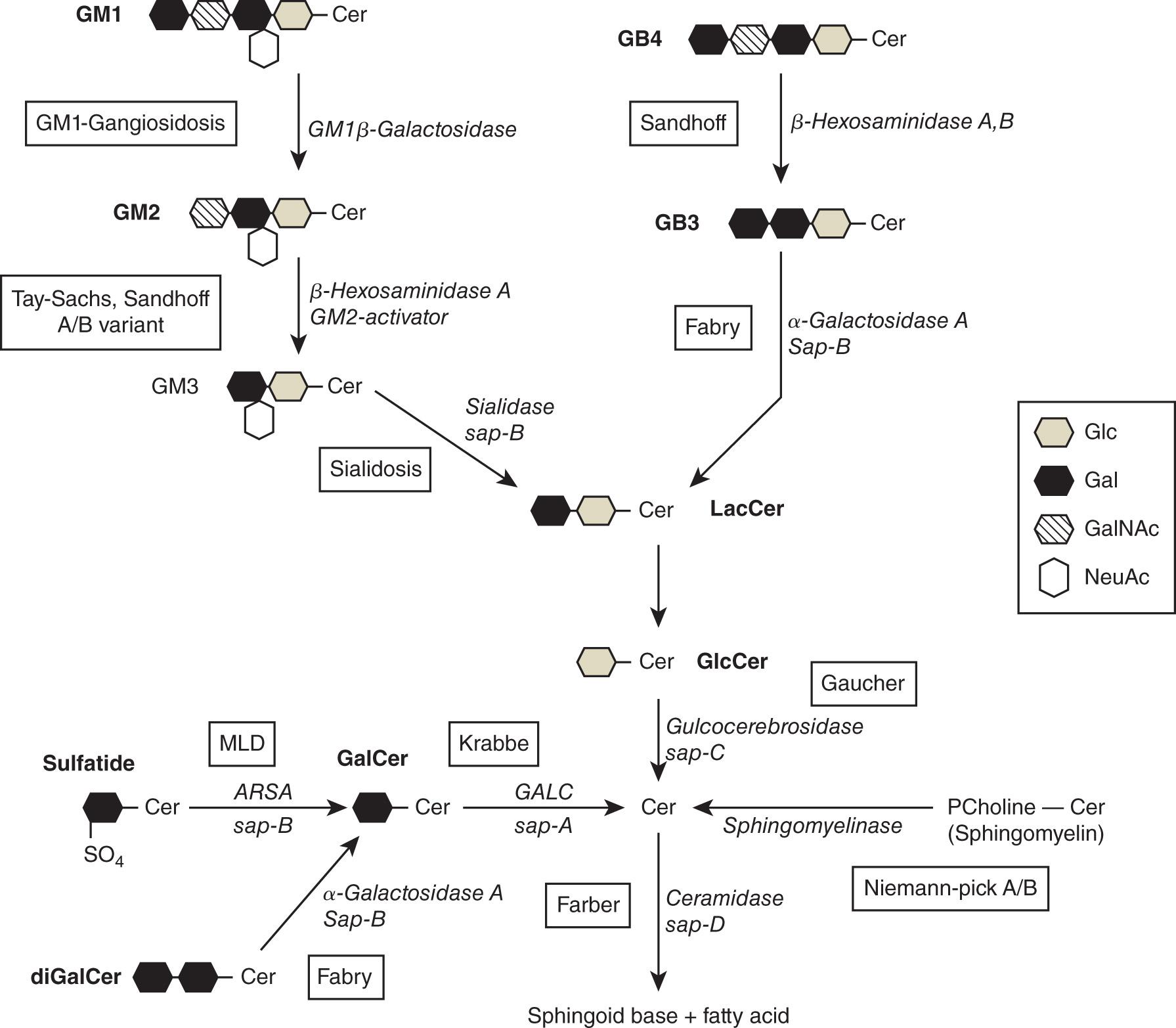
The sphingolipidoses are the most common subgroup of LSDs, accounting for over 50% of all cases ( Fig. 61.1 ). Sphingolipids are derivatives of ceramide and are comprised of variable long chain sphingosines attached to variable long-chain fatty acids. They are hydrolyzed in a stepwise fashion by specific acid hydrolyses, some of which require activator proteins, such as the saposins ( Fig. 61.2 ). Since sphingolipids are highly abundant in neuronal membranes, the majority of the sphingolipidoses have neurologic manifestations. A summary of clinical phenotypes is shown in Table 61.1 . The majority of sphingolipid disorders affect both somatic organs and the CNS, although a small number of conditions have solely non-neurologic manifestations. Somatic features include organomegaly, hypersplenism, anemia, dysmorphic features, and bone disease. The onset of symptoms is variable and may be evident at birth, infancy, childhood, or into adulthood. Although the vast majority of cases are detected in infancy or childhood, an increasing number of older patients are being identified with these disorders. The laboratory investigation of sphingolipid disorders primarily involves enzyme activity measurement. Assays are available for measurement of single enzymes specific for a particular disorder or increasingly are available in multiplex form for panels of enzymes covering multiple disorders. Molecular testing is also used for both identification and confirmatory purposes. In recent years, a number of new treatments have emerged for many sphingolipidoses. This has led to the inclusion of some sphingolipid disorders in public newborn screening (NBS) programs in several countries.
| Disorder | OMIM | Inheritance | Affected Gene(s) | Deficient Protein | Estimated Prevalence | Major Clinical Features |
|---|---|---|---|---|---|---|
| Sphingolipdoses and Lipidoses | ||||||
| Gaucher disease Type I | 230800 | AR | GBA | β-Glucocerebrosidase |
|
|
| Gaucher disease Types II and III | 230900/231000 | AR | GBA | β-Glucocerebrosidase |
|
|
| Fabry disease | 301500 | X-linked | GLA | α-Galactosidase A |
|
|
| GM1-gangiosidosis | 230500 | AR | GLB1 | Acid β-galactosidase |
|
|
| GM2-gangiosidosis (Tay Sachs) | 272800 | AR | HEXA | Hexosaminidase A |
|
|
| GM2-gangiosidosis (Sandhoff) | 268800 | AR | HEXA/B | Hexosaminidase A/B |
|
|
| Niemann-Pick type A | 257200 | AR | SMPD1 | Sphingomyelinase |
|
|
| Niemann-Pick type B | 617616 | AR | SMPD1 | Sphingomyelinase |
|
|
| Niemann-Pick type C | 257220 | AR | NPC1/2 | NPC1/NPC2 |
|
|
| Metachromatic leukodystrophy | 250100 | AR | ARSA | Arylsulfatase A |
|
|
| Krabbe disease | 245200 | AR | GALC | Galactocerebrosidase |
|
|
| Farber disease | 228000 | AR | ASAH1 | Acid ceramidase |
|
Painful/swollen joints, developmental delay, hypotonia, nodules, osteopenia. |
| LAL deficiency (Wolman) | 278000 | AR | LIPA | Acid lipase |
|
|
| LAL deficiency (CESD) | 278000 | AR | LIPA | Acid lipase |
|
|
| Neuronal ceroid lipofucinosis (Batten) | 204200 | AR | CLN | Battenin |
|
|
| Glycogen Storage Disease | ||||||
| Pompe disease | 232300 | AR | GAA | α-Glucosidase |
|
|
| Mucopolysaccharidoses | ||||||
| MPS 1 (Hurler and Hurler-Scheie) | 607014 | AR | IDUA | α-L-Iduronidase A |
|
|
| MPS II (Hunter) | 309900 | X-linked | IDS | Iduronate-2-sulfatase |
|
|
| MPS III (Sanfilippo) | 252920 | AR | GNS, HGSNAT NAGLU SGSH | Glucosamine- N -sulfatase |
|
|
| MPS IV (Morquio) | 253000 | AR | GALNS GLB1 | N -acetylgalactosamine-6-sulfatase |
|
|
| MPS VI (Maroteaux-Lamy) | 253200 | AR | ARSB | N -acetylgalactosamine-4-sulfatase |
|
|
| MPS VII (Sly syndrome) | 253220 | AR | GUSB | β-Glucuronidase |
|
|
| MPS IX (Natowicz) | 601492 | AR | HYAL1 | Hyaluronidase |
|
|
| Mucolipidoses | ||||||
| Mucolipidosis I (sialidosis) | 256550 | AR | NAGA | N -acetylneuraminidase |
|
|
| Mucolipidosis II (I-cell disease) | 252500 | AR | GNPTAB | N -acetylglucosamine-1-phosphotransferase |
|
|
| Mucolipidosis III (pseudo-Hurler) | 252600 | AR | GNPTAB | N -acetylglucosamine-1-phosphotransferase |
|
|
| Mucolipidosis IV | 252650 | AR | MCOLN1 | Mucolipin |
|
|
| Oligosaccharidoses | ||||||
| α-Mannosidosis | 248500 | AR | MAN2B1 | α-Mannosidase |
|
|
| ß-Mannosidosis | 248510 | AR | MANBA | ß-Mannosidase |
|
|
| Fucosidosis | 230000 | AR | FUCA1 | α-L-Fucosidase |
|
|
| Galactosialidosis | 256540 | AR | CTSA | Neuraminidase/ß-galactosidase |
|
|
| Schindler disease | 609241 | AR | NAGA | α- N -acetylgalactosaminidase |
|
|
| Aspartylglucos-aminuria | 208400 | AR | AGA | Aspartylglucosaminidase |
|
|
| Pycnodysostosis | 265800 | AR | CTSK | Calthepsin K |
|
|
| Defects of Lysosomal Transport | ||||||
| Cystinosis | 219800 | AR | CTNS | Cystinosin |
|
|
| Sialic acid storage disorder (Salla) | 269920 | AR | SLC17A5 | Sialin |
|
|
Gaucher disease (GD) is the most common LSD worldwide with an estimated prevalence of around 1 in 40,000 live births. Its frequency is highest within individuals of Ashkenazi Jewish descent at around 1 in 450 live births. GD is caused by deficiency of β-glucocerebrosidase (GBA), leading to intralysosomal accumulation of its substrate, glucosylceramide, which primarily affects the macrophage/monocyte system. In GD, the bone marrow and reticuloendothelial organs become infiltrated with lipid-laden “foam” or Gaucher cells. Patients develop massive liver and spleen enlargement, pancytopenia, and severe skeletal disease resulting in bone pain and fractures. Three types of GD have been described based on clinical presentation and the natural history of the disease. GD, non-neuronopathic type (GD type 1) presents from infancy to late adulthood and is characterized by painless hepatosplenomegaly leading to abdominal distension with anemia and thrombocytopenia. The organomegaly is often profound with the spleen typically much more enlarged than the liver. Bone disease manifests as painful crises, osteoporosis, and fractures with vascular necrosis and deformities like kyphosis. GD type 2 is associated with more severe neurologic disease and tends to present in infancy. GD type 3 is characterized by a later onset and a more chronic disease progression with a milder neurologic component. Additionally, the visceral and bone marrow disease associated with type 1 disease is also observed in type 3 disease and is often more severe in the latter. Ocular manifestations such as fast eye movement, strabismus, and bulbar palsy are also associated with both GD types 2 and 3. The laboratory diagnosis of GD involves the measurement of GBA activity in a variety of sample types, including fibroblasts, leukocytes, and dried blood spots (DBS; laboratory methods are discussed later in this chapter). The exogenous administration of purified enzyme to replace a deficient enzyme was first described for GD over 40 years ago. Several versions of recombinant enzyme replacement therapy (ERT) are now approved for the treatment of non-neuronopathic GD type I ( Table 61.2 ). ERT involves the intravenous administration of recombinant human enzyme that is taken up by cells and delivered to the lysosome, where it can compensate for the underlying enzyme deficiency. ERT has proven effective in treating the visceral complications of GD, such as the reduction of hepatosplenomegaly. Substrate reduction therapy (SRT) is also used for the treatment of GD. SRT involves the use of small molecular weight compounds that inhibit biosynthesis of enzyme substrates, thus achieving balance between substrate production and utilization. In contrast to ERT, SRT has the potential to cross the blood-brain barrier and treat CNS disease in LSDs. The route of administration for SRT is also advantageous as these compounds may be given orally, and unlike ERT, SRT does not elicit immune responses. Small molecules have been developed and approved for use in the treatment of neuronopathic GD type 1. Miglustat, approved in 2006, is an iminosugar that inhibits the production of glucosylceramide and other sphingolipids. This compound has shown modest effectiveness in the treatment of GD type 1.
| Disorder | Therapy | Class | Company | Status |
|---|---|---|---|---|
| Gaucher disease (Type 1) | Imiglucerase (Cerezyme) Velaglucerase alfa (VPRIV) Taliglucerase alfa (Elelyso) Miglustat (Zavesca) Eliglustat tartrate (Cerdelga) |
ERT ERT ERT SRT SRT |
Sanofi Genzyme Shire Protalix-Pfizer Actelion Sanofi Genzyme |
Approved 1991 (US), 1997 (EU) Approved 2010 a Approved 2002 Approved 2002 (EU), 2003 (US) Approved 2014 (US), 2015 (EU) |
| Fabry disease | Agalsidase beta (Fabrazyme) Agalsidase alpha (Replagal) |
ERT ERT |
Sanofi Genzyme Shire |
Approved 2001 (EU) and 2003 (US) Approved 2001 b |
| Pompe disease | Alglucosidase alfa (Myozyme) Alglucosidase alfa (Lumizyme) |
ERT ERT |
Sanofi Genzyme Sanofi Genzyme |
Approved 2006 Approved 2010 a |
| MPS I | Laronidase (Aldurazyme) | ERT | BioMarin-Genzyme | Approved 2003 |
| MPS II | Idursulfase (Elaprase) | ERT | Shire | Approved 2006 (US), 2007 (EU) |
| MPS IIIb | Recombinant human alpha-Nacetylglucosaminidase HGT-SNB-088 |
ERT ERT |
Synageva Shire |
ODD 2013 Phase III 2014 |
| MPS IIIa | Recombinant human heparan N -sulfatase (HGT-SAN-067) |
ERT | Shire | Phase II |
| MPS IVa | Vimizim (Elosulfase alfa) | ERT | BioMarin | Approved 2014 |
| MPS VI | Galsulfase (Naglazyme) | ERT | BioMarin | Approved 2005 (US), 2006 (EU) |
| MPS VII | Vestronidase alfa (Mepsevii) | ERT | Ultragenyx | Approved 2017 |
| Metachromatic leukodystrophy | Recombinant human Arylsulfatase A (SHP611) | ERT | Shire | Phase II |
| Niemann-Pick type B | Recombinant human acid sphingomyelinase (Olipudase alpha) | ERT | Genzyme | Phase II/III |
| Niemann-Pick type C | Miglustat (Zavesca) | SRT | Actelion | Approved 2006 (US), 2009 (EU) |
| LAL deficiency | Recombinant human lysosomal acid lipase | ERT | Synageva | Phase III |
| GM2 gangliosidosis | Miglustat (Zavesca) | SRT | Actelion | Phase III |
| CLN2 (TPP1 deficiency) | Brineura | ERT | Biomarin | Approved 2017 |
Fabry disease (FD) is caused by deficiency of α-galactosidase A (GLA) due to pathogenic variants in the GLA gene. Enzyme deficiency leads to progressive accumulation of its substrate globotriaosylceramide (Gb3) in several cell types, including endothelial, renal, cardiac, and dorsal root ganglion neuronal cells (see Fig 61.2 ). An X-linked disorder with an estimated prevalence of between 1 in 40,000 to 117,000 live births, FD exhibits a highly variable expressivity, and the natural history of the disease is often difficult to predict. FD is categorized into “classical” and “nonclassical” forms depending on the age of onset and presenting features. In the classical form, patients are usually hemizygous males with no residual enzyme activity. Childhood presentation is typical, with males being affected more severely and earlier than females. Acroparesthesia, cutaneous angiokeratoma, and hypohidrosis are often the first signs of the disorder. In later presentations, hypertrophic cardiomyopathy, renal failure, cerebrovascular events (strokes), skeletal abnormalities (osteopenia and osteoporosis), and ocular manifestations (corneal opacities) are also typical. Patients with “nonclassical” disease have higher levels of residual GLA activity. These patients follow a milder disease course and typically present in the fourth to sixth decades with clinical manifestations confined to a single organ system, usually the heart (hypertrophic cardiomyopathy or myocardial infarction) and/or the kidneys (proteinuria). Measurement of GLA activity in fibroblasts, leukocytes, or DBS is the most useful laboratory investigation for affected males. However, molecular analysis of the GLA gene is required for identification of heterozygote females (laboratory methods are discussed later in this chapter). Although the treatment of FD with ERT has been established since 2001 (see Table 61.2 ), minimal outcome data are available to truly assess its long-term effectiveness, and many patients receiving therapy develop complications of the disease.
The Niemann-Pick (NP) diseases are characterized by accumulation of sphingomyelin, causing widespread neurologic dysfunction. They are sub-classified into three types (types A, B, and C) based on the organs affected and age of onset. NP types A and B are neurovisceral lipid storage diseases caused by mutations in the acid sphingomyelinase gene ( SMPD1 ) mapped to chromosome 11p15.4. Deficiency of acid sphingomyelinase (ASM) results in accumulation of sphingomyelin in cell membranes and myelin sheaths. Type A patients typically have infantile disease onset with neurologic symptoms, including hypotonia and seizures. About 50% of patients develop cherry-red retinal spots. Type A patients rarely survive beyond two years of age. NP type B usually presents in childhood with hepatosplenomegaly followed by a slow, progressive deterioration that may involve infiltration of the lungs. Prognosis is more favorable in type B disease, with many patients surviving into adulthood. NP type C is caused by mutations in the NPC1 gene locus mapped to chromosome 18q11.2. In contrast to types A and B, there is only a modest accumulation of sphingomyelin in NP type C. Presentation is usually in childhood, characterized by neonatal jaundice. There is only moderate visceromegaly, and foam cells may be present in the bone marrow. There is often cognitive impairment, extrapyramidal dysfunction with dystonia, choreoathetosis, and vertical supranuclear palsy. Laboratory investigation of NP types A and B involves measurement of ASM activity in leukocytes, fibroblasts, or DBS. The traditional approach for diagnosing type C disease involves the demonstration of reduced ability to esterify cholesterol in cultured fibroblast cells following loading with low-density lipoprotein (LDL) cholesterol. Affected patients are identified by the accumulation of unesterified cholesterol through Filipin staining. However, this approach has been largely superseded by assessment of plasma oxysterols combined with molecular analysis of the NPC1 and NPC2 genes. Measurement of cholestane-3β, 5α, 6β-triol (C-triol), and 7-ketocholesterol (7-KC) in plasma is now recommended as a sensitive front-line screening test for NP type C. Therapeutic options for the NP diseases are limited. To date, no specific treatments are available for NP types A and B, although clinical trials of SRT are currently being conducted (see Table 61.2 ). However, the use of Miglustat for the treatment of NP type C has been approved in the United States since 2006.
Metachromatic leukodystrophy (MLD) is a devastating neurologic condition caused by deficiency of arylsulfatase A (ARSA), a lysosomal enzyme responsible for the hydrolysis of sulfatides (see Fig. 61.2 ). Sulfatides are expressed at high abundance in the CNS and are the most abundant sphingolipids present in myelin. In MLD, ARSA deficiency results in the accumulation of sulfatides in nervous tissue, causing progressive demyelination and encephalopathy. MLD is broadly classified into three types based on the onset of symptoms and rate of disease progression. The late infantile form of MLD is the most severe; patients present with delays in motor development and hypotonia, followed by pyramidal signs characterized by increasing spasticity. A juvenile form of MLD is characterized by later onset of the above symptoms, which may be accompanied by behavioral problems and peripheral neuropathies. Patients with the adult form typically present with a wide range of nonspecific neurologic symptoms that include gradual impairment of intellectual function and behavioral problems. The natural progression is generally slower than with the late infantile and juvenile forms of MLD. Laboratory diagnosis of MLD involves measurement of ARSA activity in leukocytes, DBS, or cultured fibroblasts. However, interpretation of ARSA enzyme activity data is complicated by the relatively high frequency of the pseudodeficiency allele, present at a carrier frequency of 15 to 20%. Consequently, homozygotes (carrying pseudodeficiency alleles) and compound heterozygotes (carrying a pseudodeficiency allele together with a pathogenic variant) with no clinical evidence of disease are often detected with decreased ARSA activity. Therefore it is recommended that additional testing such as measurement of sulfatides excretion in urine and molecular analysis are used in cases with milder or atypical clinical phenotypes. For several years, hematopoietic stem cell transplant (HSCT) has been successfully used to treat MLD using cord blood as a stem cell source. HSCT is based on providing a population of healthy donor cells with the capacity to produce the missing enzyme in recipient tissues. There is some evidence to suggest that early treatment of mildly affected MLD patients may slow the progression of cognitive decline, but there is no evidence that it is curative. The use of recombinant human ARSA as a form of ERT has also been proposed for MLD and is currently in the trial phase (see Table 61.2 ).
Krabbe disease (KD) is a devastating neurodegenerative disorder caused by decreased activity of lysosomal β-galactocerebrosidase (GALC), resulting in decreased degradation of galactosylceramide and its accumulation in myelin. Approximately 95% of cases are early-onset, whereby presentation occurs in the first 6 months of life and leads to death by 2 years of age. Patients are initially irritable with limb stiffness and exhibit hypersensitivity to their environment. As the disease progresses, patients become unresponsive, blind, and markedly reduced movement. The later-onset forms are characterized by irritability, progressive psychomotor regression, reduced movement, ataxia, and loss of vision. An adult-onset form of KD has a milder phenotype and generally exhibits a slower rate of progression with a normal life span. However, the natural course is unpredictable and while some individuals remain stable, others decline into a vegetative state with death following in a short time. Laboratory investigation of KD involves measurement of GALC activity in leukocytes, fibroblasts, or DBS. Affected, symptomatic KD patients typically have extremely low (<5% normal) GALC enzyme activity. However, individuals with borderline low activities may carry pseudodeficiency alleles. In such cases, GALC gene mutation analysis is usually required. HSCT has been used to treat KD with variable success and is only potentially effective as an infantile, presymptomatic, intervention. In 2007, KD was the first LSD to be included in a NBS program in the United States in New York State.
Sphingolipidoses are the most common subgroup of lysosomal storage disorders.
Gaucher disease is the most common sphingolipid disorder with highest prevalence within the Ashkenazi Jewish population.
Sphingolipidoses are caused by specific enzyme deficiencies in the breakdown of sphingolipids within lysosomes, leading to substrate accumulation.
All sphingolipid disorders undergo autosomal recessive inheritance with the exception of Fabry disease, which is X-linked.
Although the clinical phenotypes between disorders vary significantly, the majority have neurologic disease due to the high abundance of sphingolipids in neuronal membranes.
Laboratory diagnosis of sphingolipidoses is primarily based on enzyme activity measurement and molecular testing.
Newborn screening is only performed for a small number of sphingolipid disorders (e.g., Krabbe disease).
Therapeutics such as enzyme replacement therapy, substrate reduction therapy, and hematopoietic stem cell transplant are available for several sphingolipidoses.
Early identification and treatment are associated with alleviation of symptoms and improved clinical outcomes for some sphingolipid disorders.
The MPS are a heterogeneous group of LSDs that result from defects in the lysosomal degradation of glycosaminoglycans (GAGs). GAGs are composed of long, highly sulfated sugar chains organized into short, repeating units that are degraded in a stepwise fashion by acid hydrolases ( Fig. 61.3 ). MPS are chronic, progressive disorders that affect the tissues where substrate accumulation occurs. Since GAGs are essential components of connective tissue, the hallmark of MPS is skeletal abnormalities. Patients may experience a range of symptoms from mild joint pain and restriction of movement to a severe, multi-organ phenotype associated with significant morbidity and mortality involving bone dysplasia, intellectual impairment, dysmorphic features, enlarged visceral organs, and fetal hydrops. The traditional approach to the laboratory investigation of MPS begins with analysis of urinary GAGs as a first-line screening test. Measurement of specific enzyme activity in leukocytes or DBS remains the most useful second-line investigation. However, the role of molecular testing for identification of MPS is increasing with the widespread availability of multigene panels covering multiple MPS. Although therapeutic options for MPS have been traditionally based on HSCT, ERT is available for an expanding number of disorders (see Table 61.2 ).
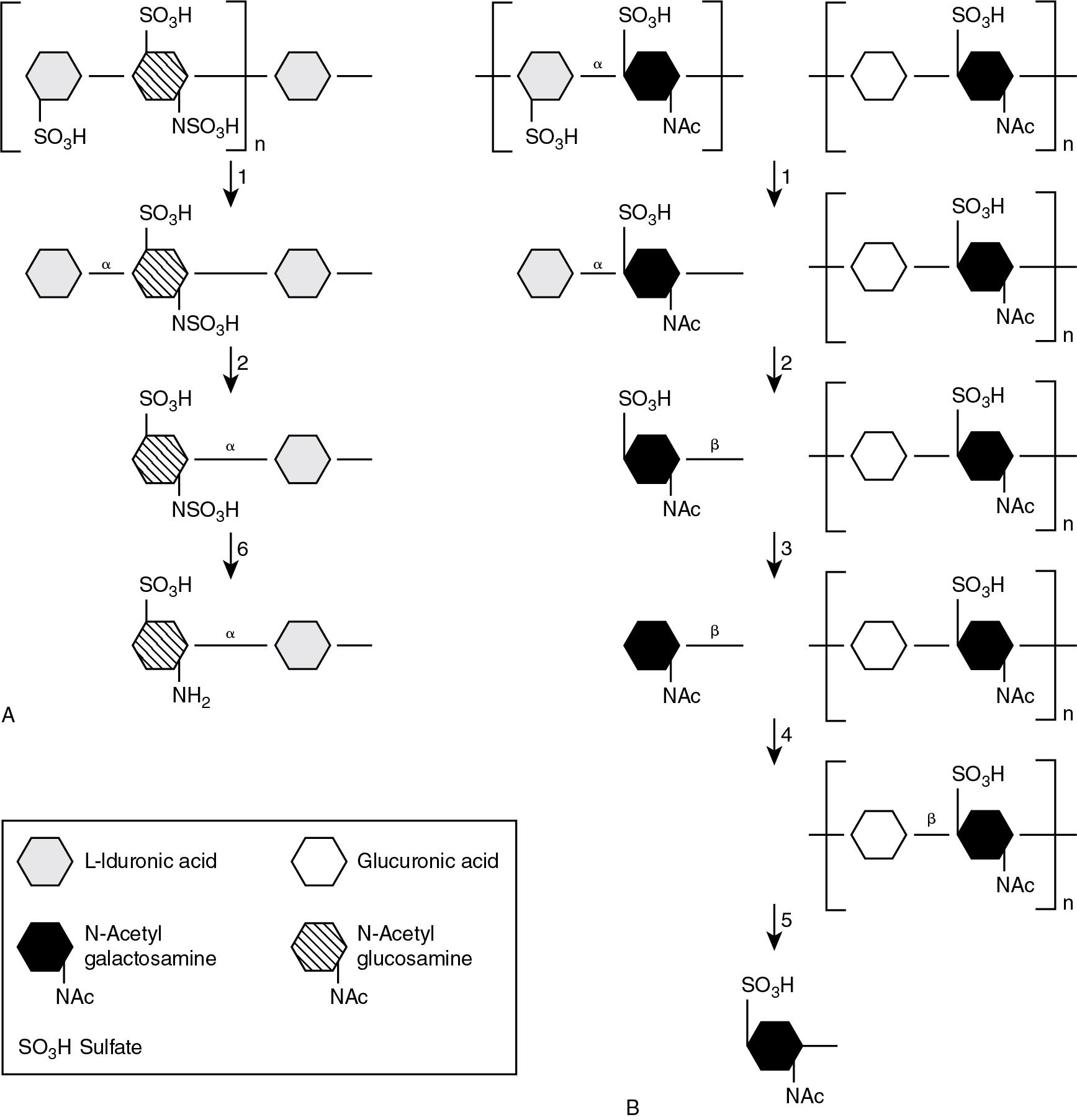
MPS type I (MPS I) is caused by deficiency of α-L-iduronidase A (IDUA), leading to lysosomal accumulation of both dermatan and heparan sulfates (see Fig. 61.3 ). MPS 1 is a disease spectrum disorder, ranging from a severe early-onset form characterized by skeletal disease and neurologic involvement (Hurler syndrome) through an intermediate form (Hurler/Scheie syndrome) to a mild, chronic form without CNS involvement (Scheie syndrome). The severe Hurler syndrome presents in early infancy with dysmorphic coarse facial features and severe skeletal deformities (known as dysostosis multiplex). CNS disease (mental retardation), hepatosplenomegaly, enlarged tongue, corneal clouding, and cardiac disease are invariably present, and death occurs within the first decade. Scheie syndrome patients usually present as teenagers with joint contractures, hepatomegaly, cardiac abnormalities, and corneal clouding. CNS involvement is absent, and the outlook is generally favorable. The intermediate Hurler/Scheie form typically presents in early childhood with variable skeletal and visceral manifestations without cognitive involvement. The outlook for Hurler/Scheie syndrome is not as favorable as the Scheie form, and patients usually do not survive beyond the second decade of life. As previously mentioned, demonstration of increased excretion of dermatan and heparan sulfates by GAG analysis is used to identify MPS I patients. Positive GAG screens are typically followed up by measurement of IDUA activity in leukocytes or DBS. Consensus guidelines recommend HSCT as first-line treatment for Hurler syndrome patients under the age of 2.5 years who have not developed severe neurologic manifestations. Since HSCT has the ability to deliver enzyme to the neurons, it represents the most widely adopted approach in the treatment of severe MPS I (Hurler syndrome) and has improved neurologic outcomes and survival compared with untreated patients. ERT with recombinant iduronidase (Laronidase) has been licensed for the treatment of non-CNS manifestations of MPS I since 2003 (see Table 61.2 ). The general consensus about ERT in MPS I is that, while effective in improving visceral complications, it is relatively ineffective in resolving the brain and skeletal manifestations. In 2016, MPS I was added to the Recommended Uniform Screening Panel (RUSP) in the United States for inclusion in NBS programs.
MPS type II (MPS II, Hunter syndrome) is caused by deficiency of iduronate-2-sulfatase resulting in impaired metabolism of dermatan and heparan sulfates (see Fig. 61.3 ). Since MPS II is an X-linked recessive disorder, young males are almost exclusively affected. MPS II exhibits a chronic, progressive disease phenotype similar to MPS I, although corneal clouding does not feature in the former. The presence of ivory-colored skin lesions known as “pebbling of skin” may also feature and is unique to MPS II. MPS type III (MPS III, Sanfilippo Syndrome) is a group of clinically indistinguishable disorders consisting of four MPS III subtypes (a through d) caused by deficiencies in one of four enzymes involved in the catabolism of heparan sulfate. MPS III is characterized by variable clinical severity, which is often complicated by a milder phenotype compared with other MPS. Patients typically present after 2 years of age with evidence of progressive CNS degeneration (developmental delay and regression), giving rise to musculoskeletal complications (joint stiffness, contractures, scoliosis, hip dysplasia), hearing loss, retinopathy, respiratory tract infections, and cardiac disease. Similar to other types of MPS, analysis of urine GAGs provides a useful first-line screen for MPS types II/III, followed by specific enzyme activity measurement and molecular analysis. Treatment is available for MPS II in the form of HSCT, which has been shown to improve visceral manifestations but has proven inconsistent in treating neurologic and skeletal disease. The use of recombinant idursulfase is approved as a form of ERT to treat the somatic disease manifestations in MPS II patients. Despite several potential therapeutics in development or trials, there are currently no approved therapies for MPS III.
MPS type IV (MPS IV, Morquio syndrome) is caused by deficiency in one of the two enzymes required for the degradation of keratan sulfate. Hence, there are two sub-types, depending on the enzyme deficiency; morquio A (deficiency of N -acetylgalactosamine-6-sulfatase) and morquio B (deficiency of β-galactosidase). Since keratin sulfate is a key constituent of cartilage and cornea, MPS IV presents in early infancy with skeletal deformities (short-trunk dwarfism and coarse facial features) and corneal clouding. In contrast to other MPS, intelligence is normal in MPS IV patients. The detection of keratin sulfate in urine by GAG analysis is unique to MPS IV and forms a specific screen for the disorder. MPS type VI (MPS VI, Maroteaux-Lamy syndrome) is caused by deficiency of arylsulfatase B (ARSB) within the dermatan sulfate degradative pathway (see Fig. 61.3 ). The clinical features are similar to MPS I, characterized by dysostosis multiplex, hepatosplenomegaly, and corneal clouding. However, in contrast to MPS I, patients with MPS VI have normal intelligence. MPS type VII (MPS VII, Sly syndrome) is a rare disorder caused by deficiency of β-glucuronidase required for the degradation of dermatan, heparan, and chondroitin sulfates. The clinical phenotype varies considerably between patients with both severe early-onset and mild late-onset forms described. The presence of hydrops fetalis is a common form of presentation in MPS VII. With regards to therapeutic options for these disorders, ERT is available for MPS VI (approved in 2005), MPS IVa (approved in 2014), and more recently, for MPS VII (approved in 2017).
Initially described in 1967 by Leroy and Demars, the ML represent a small subgroup of LSDs that are closely related to the MPS with regards to clinical phenotype. Notably, urine GAGs are usually normal or only mildly increased in ML patients. Laboratory investigation involves assay of oligosaccharides in urine. Impaired breakdown of glycoproteins in ML results in excretion of partially degraded free oligosaccharides (FOS), which forms a useful first-line screening test. To date, four types of ML have been described, for which there are currently no specific therapies available, and management is essentially supportive and symptomatic.
Mucolipidoses type I (ML 1) is caused by pathogenic variants in the NEU1 gene. The gene defect leads to deficiency of sialidase (also known as neuraminidase 1), the enzyme responsible for removal of sialic acid from glycoproteins, resulting in substrate accumulation. For this reason, ML 1 is commonly referred to as sialidosis . Patients with ML 1 usually present either at birth or within the first year of life with coarse facial features, macroglossia, excessive swelling, hypotonia, hepatosplenomegaly, and skeletal malformations. Intellectual impairment and recurrent infections are also common. The condition is progressively severe, and the majority of patients die before 1 year. A mild form of the disorder has also been described with no dysmorphic features, gait abnormalities, myoclonus and the presence of macular “cherry-red spot,” with onset in the second decade. Laboratory investigation of ML 1 involves screening urine for the presence of sialic acid bound oligosaccharides (known as sialyloligosaccharides) followed by NEUI gene sequencing for confirmation.
ML types II (ML II) and III (ML III) are caused by variants in the GNPTAB and GNPTAG genes, respectively. Although both disorders result from deficiency of the enzyme, N -acetylglucosamine-L-phosphotransferase (GlcNAc-1-phosphotransferase), they likely represent opposite ends of a spectrum of disease severity. In contrast to the vast majority of LSDs which are due to single enzyme defects, ML II/III are caused by deficiency of GlcNAc-1-phosphotransferase, which impairs the production of mannose-6-phosphate, a key marker for lysosomal targeting and transportation of newly synthesized glycoproteins (specifically digestive enzymes) within the Golgi. As a result, digestive enzymes are inappropriately secreted from the cell leading to substrate accumulation within the lysosome, forming “inclusion bodies.” Consequently, ML II was originally referred to as inclusion-cell (I-cell) disease. ML II is a severe disorder that closely resembles the clinical phenotype of MPS I. Patients typically exhibit coarse facial features, skeletal disease, short-trunk dwarfism, restricted joint movement, organomegaly, corneal clouding, respiratory tract infections, and valvulopathies. Most patients with ML II die within the first decade as a result of congestive heart failure or recurrent respiratory tract infections. ML III represents a milder disorder associated with later clinical presentation, likely attributed to some residual GlcNAc-1-phosphotransferase activity in these patients. ML III patients also exhibit coarse facial features, skeletal abnormalities, short stature, and corneal clouding but are usually of normal intelligence. Additionally, life expectancy is much longer with many patients surviving until the fourth or fifth decade. As with other ML, partially degraded FOS are excreted in ML types II and III, which may be detected by urine oligosaccharide analysis. Mildly increased GAG excretion, specifically keratin sulfate, has also been described for GNPTAB -related disorders.
Mucolipidosis type IV (ML IV) is caused by variants in the MCOLN1 gene required for the production of mucolipin-1, resulting in either deficiency or production of a nonfunctional protein. Mucolipin-1 is located within the lysosomal and endosomal membranes and is necessary for trafficking between these compartments, impairment of which leads to lysosomal accumulation of lipids. Mucolipin-1 appears to be important for the development and maintenance of the brain and retina. As such, the clinical phenotype involves motor delay, abnormal gait, clouding of the cornea of the eye, and severely reduced vision. Speech is also often severely impaired. Additionally, some ML IV patients are only visually impaired and experience no brain component. Two mutations in the MCOLN1 gene account for the vast majority of ML IV cases in people with Ashkenazi Jewish ancestry. Although analysis of cultured fibroblasts for the presence of granular inclusions has been used to diagnose ML IV, molecular analysis of the MCOLN1 gene is increasingly used.
The mucopolysaccharidoses represent a subgroup of lysosomal storage disorders (LSDs) caused by impaired breakdown of glycosaminoglycans (GAGs) due to enzyme deficiencies.
MPS III is the most prevalent type of MPS in all populations.
All mucopolysaccharidoses undergo autosomal recessive inheritance with the exception of MPS II (Hunter syndrome), which is X-linked.
All types of MPS are associated with skeletal disease since GAGs are found at high abundance in connective tissue.
Analysis of GAGs in urine is a useful first-line screening test for mucopolysaccharidoses.
Newborn screening is currently performed for MPS I in some public screening programs.
Hematopoietic stem cell transplantation and enzyme replacement therapy represent therapeutic options for MPS.
The mucolipidoses are a small subgroup of LSDs that share many of the clinical features of mucopolysaccharidoses.
Analysis of free oligosaccharides in urine is a useful screening test for mucolipidoses.
The oligosaccharidoses are a subgroup of LSDs caused by deficiencies in lysosomal hydrolases required for degradation of complex oligosaccharide molecules ( Fig. 61.4 ). Also known as glycoprotein storage disorders, oligosaccharidoses are characterized by accumulation of incompletely degraded oligosaccharides in cells and tissues, together with oligosaccharide excretion in urine. Similar to the MPS and ML disorders, the use of separation techniques such as analysis of FOS in urine offers a useful screening assay for oligosaccharidoses (methods are discussed later in this chapter). Although these disorders closely resemble the MPS from a clinical perspective, screening urine for GAGs is not particularly informative. Management of the oligosaccharidoses mainly involves supportive symptomatic treatment, although ERT has been used in some European countries for a small number of disorders.
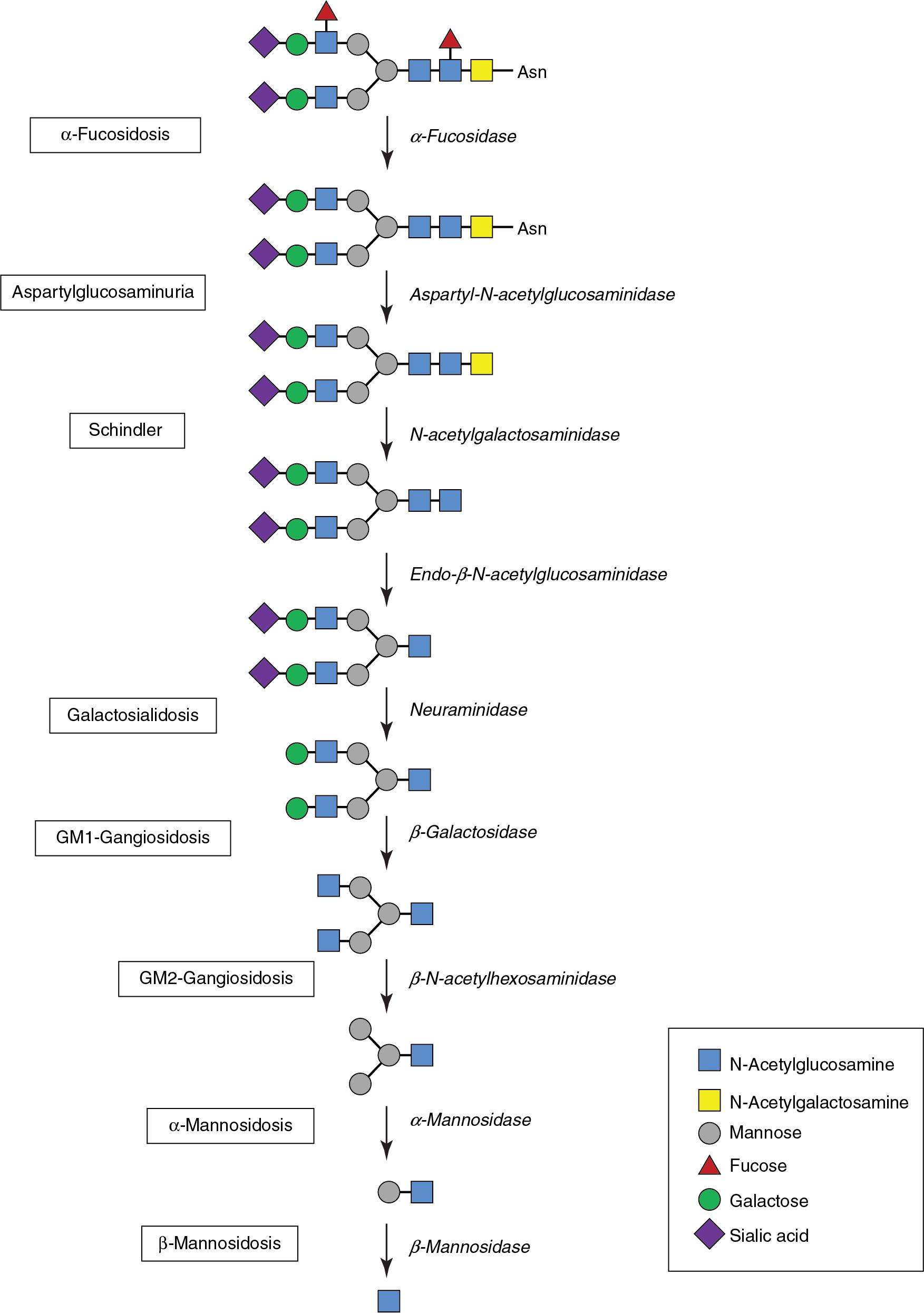
Pathogenic variants in the MAN2B1 gene result in α-mannosidosis, a highly variable disorder with a wide spectrum of clinical phenotypes. Affected patients are often normal at birth and present in early childhood with delayed psychomotor development, coarse facial features, skeletal dysplasia, hearing impairment, hepatosplenomegaly, and immune deficiency. β-mannosidase deficiency is caused by deficiency of β-mannosidase due to homozygous or compound heterozygous variants in MANBA . This disorder is also highly variable, with a wide spectrum of phenotypes ranging from mental retardation, coarse features, skeletal disease, and hearing loss in the most severely affected patients to comparatively mild disease.
Homozygous or compound heterozygous variants in FUCA1 give rise to fucosidosis, a severe neurologic disorder caused by α-fucosidase deficiency. Although the clinical course is variable, patients often develop neurodegenerative decline characterized by epilepsy, dysostosis, and the presence of angiokeratoma.
Galactosialidosis is caused by a combined deficiency of lysosomal neuraminidase (sialidase) and β-galactosidase secondary to a defect in cathepsin A. Under normal conditions, cathepsin A is associated with neuraminidase and β-galactosidase in a complex, which offers a stabilizing or protective role to the latter enzymes, preventing their premature proteolytic processing and degradation. Galactosialidosis may present at various ages and is often characterized by clinical features shared with other LSDs, including hydrops fetalis, intellectual impairment, coarse facies, cherry-red spots, skeletal dysplasia, valvulopathies, and cardiomegaly.
Caused by deficiency of α- N -acetylgalactosaminidase (NAGA), Schindler disease is an extremely rare disorder of lysosomal metabolism with several distinct phenotypes of variable severity. The infantile-onset form is characterized by neuroaxonal dystrophy, whereas later-onset forms are associated with mild intellectual impairment and angiokeratoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here