Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
hematoxylin and eosin
hepatic microcirculatory subunit
keratin ∗
∗ Although the prefix CK is widely used in surgical pathology to designate human cytokeratins, consensus nomenclature recommends the replacement of “cytokeratin” with “keratin” and the prefix “CK” with “K.” (Schweizer J, Bowden PE, Coulombe PA, et al. New consensus nomenclature for mammalian keratins. J Cell Biol . 2006;174:169–174).
nicotinamide adenine dinucleotide phosphate
It would not be misleading to state that the microscopic structure of the liver as visualized in a routine hematoxylin and eosin (H&E)–stained slide is irritatingly bland when compared with its complex and diverse functions. In all truthfulness, when one examines such a section, one is peering down at a sea of monotonous hepatocytes interspersed at reassuring intervals by portal tracts and central veins ( Fig. 1.1 ). Specifically, one fails to notice, even with a healthy dose of imagination, well-demarcated hexagonal lobules, primary or secondary lobules, berry-shaped acini or pyramidal portal units, all so prolifically described in literature ( Fig. 1.2 ). However, it is evident from experimental studies and the zonal distribution of disease processes that there is a definite pattern of functional and metabolic organization to the hepatic parenchyma. Therefore an understanding of our diverse attempts to organize the liver parenchyma into neat functional units is often rewarded by a better insight into liver biopsy material obtained for a variety of indications ( Box 1.1 ).
Elevation of liver tests
Jaundice of unknown cause
Ascites and portal hypertension
Abnormal tests of iron homeostasis
Grading and staging of chronic hepatitis
To facilitate therapeutic decisions
To evaluate therapeutic efficacy
Diagnosis, staging, and grading of fatty liver disease
Quantitation of tissue copper or iron
Enzyme or metabolite analysis for diagnosis of metabolic diseases
Microbiologic cultures for identification of specific infectious agent
Diagnosis and characterization of tumors
Evaluation of liver allograft following transplantation
Although the concept of the hexagonal lobule began with the earliest morphologists such as Malpighi, detailed microscopic characterization was first provided by Kiernan in 1833 who described a roughly hexagonal structure centered around the hepatic vein with portal tracts at its corners. This hexagonal lobule is demarcated by fibrous septa in certain species such as the pig ( Fig. 1.3 ). Although no such septa exist in the human liver, the concept of the classic lobule (see Fig. 1.2A ) as a structural unit is consistent with portocentral gradients, which exist for numerous metabolic pathways and their encoding genes. Similarly, blood flows along this gradient from portal tracts to central veins, whereas bile and lymph flow in the reverse direction.

Other early investigators, particularly Brissaud, Sabourin, and Mall, described the portal or biliary lobule, emphasizing portal tracts and bile flow as the pivotal points around which hepatic architecture is organized. Furthermore, Brissaud and Sabourin have been credited with describing septa that link adjacent central veins, thus demarcating portal lobules in the seal liver. Arey, who clarified that the notion of septa demarcating portal lobules arose from a misinterpretation of Brissaud and Sabourin’s original descriptions in French, could not corroborate the presence of these septa by a study of livers from approximately 25 species.
The study of blood flow by injecting dyed gelatin solutions into the portal vein led Rappaport to a differing perspective of hepatic microarchitecture. Rappaport observed that blood did not flow directly inward from portal tracts to central veins, but rather flowed first laterally through a thin vessel that extended halfway toward the neighboring portal tract before flowing inward from this vessel into several adjacent hexagonal lobules. Rappaport thus observed that the basic unit of the liver is not the hexagonal lobule, or indeed the portal lobule, but the berry-shaped acinus pivoted around this axial vessel, the portal venule, which arises from the smallest, terminal branch of the portal vein. The parenchyma around the portal venule can be arbitrarily divided into three concentric zones: zone 1 is the closest and receives the maximum amount of oxygen and nutrients, whereas zone 3 is the farthest (see Fig. 1.2A ). The acinar concept, which is based on perfusion of the liver parenchyma, explains pathologic lesions that have their basis in parenchymal perfusion, such as perivenular localization of ischemic lesions. The shape of the acinar unit also suggests that zone 3 is star-shaped (see Fig. 1.2B ). On the other hand, by staining for enzymes expressed in periportal hepatocytes (carbamoyl phosphate synthetase and glucose-6-phosphatase) and those expressed in perivenular hepatocytes (glutamine synthetase and NADPH-cytochrome c), Lamers demonstrated that the perivenular area, corresponding to Rappaport acinar zone 3, is discrete and circular, whereas the periportal areas, corresponding to Rappaport acinar zone 1, are irregular in outline and roughly star-shaped (see Fig. 1.2C ). This study showed that three dimensionally and in the context of expression of certain enzymes, the periportal “domain” (around portal tracts) follows the branching pattern of the portal veins and envelopes the discrete and circular perivenular “domain” (around central veins), which follows the branching pattern of the hepatic veins.
The role of the smallest branch of the portal vein, the portal venule, in lobular architecture was also highlighted by Matsumoto, who called this vessel a vascular septum ; the word “septum” was used not to denote a fibrous band but a boundary that demarcates the hexagonal lobule. Each side of the hexagonal lobule is made up of two vascular septa arising from adjacent portal tracts (see Fig. 1.2A and D ). Inlet venules arise from the terminal portal vein and portal venule to end in sinusoids. Sinusoids arising from the terminal portal vein and the proximal portion of the portal venule (ie, near the portal tracts, therefore portal sinusoids) spread transversely before turning inward and heading radially toward the central vein, whereas sinusoids that arise from the distal portion of the portal venule (ie, from the vascular septum, therefore, septal sinusoids) travel radially straight toward the central vein. Metabolic gradients have been described along portal and septal sinusoids, albeit in the rat liver.
The hunt for the functional unit of the liver (the smallest structural unit that can independently subserve all known functions of an organ) led Ekataksin to study and describe the choleohepaton or hepatic microcirculatory subunit (HMS). This structure is even less obvious in surgical pathology material than the lobule or acinus. The HMS is a pyramidal unit with its base at the perimeter of the classic lobule and its apex at the central vein that consists of approximately 19 sinusoids fed by a single inlet venule and drained by a single canal of Hering. The HMS can be highlighted by injection of a fluorescent dye that, after filling up the sinusoids of the pyramidal unit through the inlet venule, accumulates in hepatic plates and then appears a little later as a “chicken wire” pattern of bile canaliculi draining the same area.
Notwithstanding the differing perspectives of the hepatic functional unit in various studies over the course of centuries, a few crucial facts emerge:
As with the seven blind men examining an elephant, each study and each concept of hepatic microarchitecture highlights a specific functional, morphologic, or structural aspect of the liver. Rather than being contradictory or exclusive to each other, every concept complements the others and contributes toward a comprehensive whole.
The arborization patterns of the hepatic and portal veins relative to each other are fundamentally important in establishing the parenchymal architecture of the liver. Matsumoto’s painstakingly detailed reconstruction of hepatic angioarchitecture demonstrated that the hepatic venous system follows the branching order of the portal system right up to the first-order branches of the distributing portal veins. Thereafter, the first-order branches of the distributing portal veins give rise to eleven second-order branches, whereas the hepatic veins do not follow suit, thus resulting in the positioning of six portal veins around one central vein.
Metabolic zonation is dynamic, and the functional zones are not anatomically rigid or limited by fibrous septa. Their boundaries shift depending on feeding cycles and functional needs, and are regulated by local nutritional factors, digestive hormones, and oxygen levels.
The actual shape of the unit described, irrespective of the one being studied, varies by its physical location within the liver and its relationship to neighboring units. Variations in size and shape have been shown for the hexagonal lobule as well as the HMS, each of which appears more flattened under the capsule than in the deeper liver. Teutsch, through three-dimensional reconstruction studies, has demonstrated the modular architecture of the liver units that mold to each other like Lego blocks, thus conforming to the shape of their neighbors and at the periphery, to the overall shape of the organ.
These observations cast doubt on whether a structurally well-defined functional unit, as embodied by the quintessential nephron in the kidney, exists in the liver. One may safely conclude that the lack of a morphologically distinct unit is consistent with the lack of a distinct functional unit, the shape of which depends on the metabolic and physiologic function being subserved. The microarchitecture of the liver thus allows for flexibility of function as a hexagon, an acinus, and a portal unit or an enzymatic zone, with each unit being subservient to a precise metabolic function. Therefore, an apparently simple configuration belies a sophisticated microarchitecture that facilitates versatility of function with economy of form. In this, the liver is unique.
Furthermore, a modular architecture allows the units to conform to each other, to traversing large portal tracts or hepatic veins, as well as to the overall shape of the organ, so that there is no tail or trunk unceremoniously sticking out, as in the analogous elephant alluded to earlier. Finally, it appears that the branching pattern of the hepatic vein relative to that of the portal vein sets the basic framework for the overall microarchitecture.
Liver architecture is best appreciated on low power or scanning magnification. Preservation of normal architecture is indicated by the presence of portal tracts and central veins at regular intervals within the hepatic parenchyma (see Fig. 1.1 ) ( eSlide 1.1 ). The perivenular areas are further recognized by the presence of hepatic plates that radiate outward from the central veins ( Fig. 1.4 ); hepatic plates appear more compact in the periportal regions. Zonally distributed pigments serve as additional landmarks in the assessment of architecture; thus, lipofuscin, which is present in perivenular hepatocytes, indicates a centrilobular location (see Fig. 1.4 ), whereas iron accumulation, which begins in hepatocytes around the portal tracts, indicates a periportal location. The presence of lipofuscin at regular intervals attests to the maintenance of normal architecture and aids in the identification of small or tangentially cut central veins, which may not be otherwise visible on an H&E stain.

Trichrome and reticulin stains greatly assist in the assessment of architecture ( Table 1.1 ). Trichrome stain highlights portal tracts and central veins that may not be easily visualized on an H&E stain, either because they have not been sectioned en face or are too small to be easily recognizable (see Fig. 1.4 and eSlide 1.2 ). Similarly, the trichrome stain highlights portal tracts that lack bile ducts, the absence of which may render portal tracts less prominent. The reticulin stain, a silver impregnation technique that stains collagen III (reticulin) fibers in the Disse space, is most useful for assessing hepatic plate architecture ( Fig. 1.5 ; see also Fig. 1.4B and E ) and trabecular thickness, which are seen to advantage with this stain ( eSlide 1.3 ). Thus condensation of reticulin fibers highlights areas of hepatocyte loss, whereas thickened plates highlight areas of regeneration. Markedly thickened plates on a reticulin stain aid in the diagnosis of hepatocellular carcinoma and its distinction from nonmalignant lesions, in which the plates are no more than two or three cells thick. Nodularity on a reticulin stain in the absence of fibrosis indicates nodular regenerative hyperplasia. Patients present with portal hypertension, raising clinical suspicion of cirrhosis; however, bridging fibrosis septa are not seen on trichrome stain. Although reticulin fibers appear black on the silver impregnation stain, collagen I, which constitutes fibrous bands, appears brown (see Fig. 1.5A ).
| Stain | Diagnostic Use |
|---|---|
| Trichrome | Staging of fibrosis
Highlights the portal tracts and central veins, thus facilitating assessment of architecture |
| Reticulin | Demonstrates hepatic trabecular architecture
Facilitates differentiation of hepatocellular carcinoma from nonmalignant nodules (see Chapter 31 ) |
| Iron (Prussian blue reaction) | Demonstrates presence, extent, and cellular localization (hepatocytes versus sinusoidal lining cells) of hemosiderin deposition Highlights iron containing macrophages |
| Periodic acid–Schiff | Demonstrates presence of storage cells in Gaucher disease and Niemann-Pick disease Demonstrates single or clusters of macrophages as markers of previous hepatocellular damage Demonstrates presence of alpha-1 antitrypsin globules |
| Rhodamine | Demonstrates copper deposition in Wilson disease and chronic biliary diseases |
| Orcein | Demonstrates presence of hepatitis B surface antigen Demonstrates copper deposition in Wilson disease and chronic biliary diseases Demonstrates presence of elastic fibers |
| Victoria blue | Demonstrates presence of hepatitis B surface antigen Demonstrates copper deposition in Wilson disease and chronic biliary diseases Demonstrates presence of elastic fibers |
| Sirius red | Enhances natural birefringence of collagen under polarized light; useful for digitized morphometric analysis of fibrosis |
| Aniline blue | Exclusively stains collagen for digitized morphometric analysis of fibrosis |
| Oil red O | Demonstrates presence of fat in nonfixed tissue |

Long tracts of benign-appearing hepatic parenchyma without regularly appearing portal tracts and central veins raise the suspicion of a hepatocellular adenoma or a well-differentiated hepatocellular carcinoma. Both lesions contain haphazardly distributed arterioles; in addition, hepatocellular adenomas show areas of sinusoidal dilatation and/or hemorrhage ( Fig. 1.6 and Table 1.2 ). High-grade dysplastic nodules and hepatocellular carcinomas that are not well-differentiated also lack portal tracts but are easier to recognize because the hepatocytes do not appear benign, raising suspicion for a dysplastic or malignant lesion. Expanses of hepatic parenchyma that have lost the normal central vein–to–portal tract arrangement but contain irregularly scattered portal tracts may represent a large regenerative nodule in a cirrhotic liver (see Fig. 1.6 and eSlide 1.4 ). The accompanying clinical information in such cases usually mentions the presence of cirrhosis and a dominant nodule that is being biopsied to rule out a carcinoma. Alternatively, the history may be merely one of cirrhosis, and the needle may have inadvertently sampled a large regenerative nodule. The reticulin pattern in these instances shows irregular thickening of hepatic plates. The presence of portal tracts is thus a reassuring sign of a benign lesion. Sometimes, however, portal tracts may not be easily recognizable even when present; this occurs particularly when the portal tracts lack their most prominent component, the bile ducts. In these instances, portal tracts are highlighted by the trichrome stain. Furthermore, in diseases that cause loss of bile ducts, immunohistochemistry for the biliary keratins K7 and K19 may highlight the presence of duct remnants. In addition, membranous positivity of periportal hepatocytes for K7 (biliary metaplasia of hepatocytes), indicating chronic cholestasis, may be present (see Fig. 1.6 ).

| Long Tracts of Benign Parenchyma Without Regularly Occurring Portal Tracts | |
| Hepatocellular adenoma | Contains scattered arterioles; areas of sinusoidal dilatation; reticulin stain shows trabeculae that are two or three cells thick |
| Large regenerative nodule | May contain irregularly distributed portal tracts (also see eSlide 1.4 ) |
| Loss of bile ducts, making portal tracts difficult to recognize | Trichrome stain highlights portal tracts; immunohistochemistry for K7 may show duct remnants or membranous staining of periportal hepatocytes |
| Fragmentation of Biopsy Material | |
| Related to procedure | Normal reticulin pattern (also see eSlide 1.5A–C ) |
| Caused by parenchymal fibrosis | Abnormal reticulin pattern showing irregularly distributed thickened trabeculae and nodularity (also see eSlide 1.5D–F ) |
| Subcapsular Tissue | |
| May show fibrous septa and nodularity | Do not assess fibrosis on subcapsular tissue because it may not be representative of deeper parenchyma |
| Overdiagnosis of Fibrosis | |
| Large portal tracts | Contain large branches and tributaries of portal vein, hepatic artery, and bile duct |
| Branching portal tracts | Portal tract constituents run parallel to branching portal tract |
| Longitudinally cut portal tracts | Portal tract constituents run parallel to direction of portal tract (also see eSlide 1.8 ) |
| Areas of confluent necrosis | Reticulin stain shows collapse (also see eSlide 1.14 ) |
Fragmentation of a biopsy specimen may result from the procedure itself or reflect the presence of fibrosis ( eSlide 1.5 ); the incidence caused by procedures has decreased significantly with the increasing use of cutting rather than suction biopsy needles. In current practice, procedural fragmentation is most often the result of obtaining biopsies through the transjugular approach. A fragmented biopsy presents unique challenges in interpretation of architecture and estimation of fibrosis. Although fragments with smooth, rounded edges may indicate the presence of fibrous septa and those with straight, frayed edges may point to mechanical fragmentation, these features are not always reliable indicators. A reticulin stain, on the other hand, greatly aids interpretation by facilitating assessment of the hepatic plate architecture, which is abnormal when fragmentation is the result of parenchymal fibrosis ( Fig. 1.7 and Table 1.2 ).

The relatively new technique of endoscopic ultrasound–guided transgastric liver biopsies produces specimens that may be markedly fragmented and therefore particularly difficult to assess for fibrosis and stage of disease. These specimens are obtained by highly flexible suction needles rather than cutting needles and often include significant amounts of blood clot ( Fig 1.8 and eSlide 1.6 ).

The immediate 2 to 3 mm of subcapsular parenchyma is not ideal for evaluation of fibrosis because bridging fibrous septa and nodularity are normally seen in this location; these are not representative of the rest of the liver and give the false impression of fibrosis ( Fig. 1.9 and eSlide 1.7 ). Petrelli and Scheuer studied biopsies from the inferior edge of the liver in 72 patients ranging in age from 3 months to 93 years, and they found no variation in the subcapsular and deeper parenchyma in 33 patients. However, in 21 cases, there was crowding of central veins and portal tracts, and slender septa were seen emanating from portal tracts in some of these cases. In the remaining 18 cases, thin septa were seen bridging portal tracts with central veins and isolating islands of parenchyma; these patients ranged in age from 15 to 93 years. A needle biopsy, which often includes deeper tissue, is therefore preferable to a wedge biopsy for estimation of fibrosis.

Portal tracts represent cross-sections or tangential sections of a branching system of connective tissue that carries the hepatic arterial and portal venous vessels from the hilum of the liver to the periphery and, in reverse direction, bile ducts from the peripheral hepatic parenchyma to the hilum. As the connective tissue branches and spans outward, the sizes of the portal tracts and their constituent structures decrease, accompanied by a corresponding decrease in the amount of fibrous tissue.
The branching tree analogy is relevant to routine diagnostic pathology because it helps explain the variation in size and shape of portal tracts encountered in surgical pathology specimens; this variation reflects the part of the branching tree being sectioned (eg, large versus small tracts, branching points) and the plane of section (cross section versus tangential versus longitudinal). Thus, whereas terminal portal tracts usually have a round or oval profile, larger portal tracts may be triangular or stellate in shape ( Fig. 1.10 ). In addition, the latter contain more fibrous tissue, which should not be mistaken for pathologic fibrosis, an error that can be avoided by correlating the amount of fibrous tissue with the size of the included structures; large portal tracts have large-caliber vessels and may be expected to contain more fibrous tissue. Sectioned at their branching points or cut longitudinally, portal tracts may mimic fibrous septa; this pitfall can be avoided by noting the longitudinal profiles of the portal tract constituents in these “septa” (see Fig. 1.10 and eSlide 1.8 ).

A portal tract contains branches of the hepatic artery and portal vein as well as the tributaries of the bile duct. Although the smallest, terminal portal tracts cut in cross section may show one profile of each as in the classically described portal triad , the number of profiles in larger tracts varies, depending on what the size of the portal tract is, whether they are cut in cross-section or in tangential section, and whether they contain a branching point (see Fig. 1.10 ). That not all portal tracts are triads was apparent in a study of 16 normal biopsy specimens from adult patients, which found more than one profile of a bile duct or hepatic artery in many portal tracts. Interestingly, there were almost as many portal dyads as portal triads, with most dyads missing a portal vein and a minority that lacked a bile duct or a hepatic artery.
In addition to the three components mentioned earlier, portal tracts contain lymphatics that collect lymph formed in the Disse space ( Fig. 1.11 ), which flows toward the portal tracts in the direction of bile flow and against that of blood. Portal lymphatics are not discernible on a routine H&E stain but can be highlighted by immunohistochemical stains for markers expressed on lymphatic endothelium such as D2-40 ( Fig. 1.12 ). Portal tracts also contain nerve fibers that can be visualized on an H&E stain in large, but not small, portal tracts, even though the latter possess neural innervation.
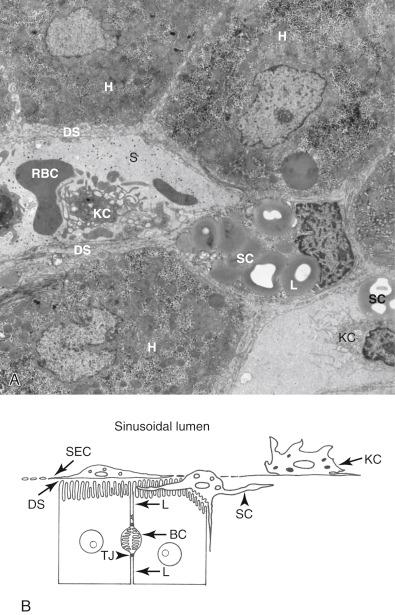

The intrahepatic course of the biliary tree consists of 7 to 10 orders of cholangiographically visible bile ducts. The smallest bile ducts, the interlobular bile ducts, which measure less than 100 μm in diameter, are not visualized radiographically. They are lined by cuboidal cholangiocytes, which rest on a delicate basement membrane and contain central, round, evenly spaced nuclei ( Fig. 1.13 ). Interlobular bile ducts drain bile produced by hepatocytes, which reaches them through an intralobular network of bile canaliculi which drain into the canals of Hering that in turn drain into bile ductules (discussed later). Interlobular bile ducts drain into septal ducts, which measure more than 100 μm in diameter, and, which in turn, drain into segmental ducts, which measure 400 to 800 μm in diameter.

Cholangiocytes lining the larger bile ducts are columnar, with basally situated round-to-oval nuclei that appear even and regularly spaced. Increasing duct size is accompanied by thicker walls, which correspond histologically to increasing amounts of fibrous tissue ( Fig. 1.14 ). When sampled in a biopsy, this robust collar of fibrous tissue around medium and large bile ducts may be mistaken for the “onion skinning” seen in sclerosing cholangitis. This pitfall can be avoided by noting the large size of the ducts and lack of accompanying epithelial damage or basement membrane thickening that invariably precedes advanced fibrosis in sclerosing cholangitis. In addition, in the early stages of primary sclerosing cholangitis, the concentric onion-skinning fibrosis is accompanied by edema and mild inflammatory infiltrate (see Fig. 1.14 ).

The large ducts at the hilum are associated with peribiliary glands and, less commonly, with exocrine pancreatic tissue, which may be sampled on biopsy material. Biliary epithelium marks immunohistochemically for keratins K7 and K19.
Hepatic artery branches accompany bile ducts in almost all (96%) portal tracts and approximate the bile ducts in size (see Fig. 1.10A and D ); the ratio of bile duct size to hepatic artery size is approximately 0.8. This close association between artery and duct reflects the crucial and almost exclusive role of the hepatic artery in perfusion of the biliary tree. Hepatic arterial branches form a rich peribiliary vascular plexus consisting of an inner and outer layer around the large ducts, reducing to a single layer as the ducts decrease in size. The smallest tributaries of the biliary tree are supplied by a few capillaries wrapping around and lying in grooves on the surface of the bile duct.
Because the majority of hepatic arteries are accompanied by bile ducts, the finding of hepatic arteries unaccompanied by bile ducts in portal tracts (“isolated” or “unpaired” hepatic arteries) should raise suspicion of diseases characterized by bile duct loss (see Chapter 28 ). Bile ducts may appear appreciably smaller than their accompanying arteries because of epithelial atrophy preceding eventual loss. Although there is some thickening of hepatic arterioles with age and with hypertension, these changes are not as marked or as frequent as elsewhere in the body. Hepatic arteries are often involved in systemic amyloidosis, in which they are thickened by the deposition of amorphous eosinophilic material characteristic of amyloid.
The intrahepatic portal venous system consists of two functionally distinct parts: the conducting system and the distributing system. As the names suggest, the former carries or conducts blood to the far reaches of the hepatic parenchyma, whereas the latter ensures even distribution of blood to individual hepatocytes through the vast sinusoidal network. The most terminal branch of the distributing system (portal venule) corresponds to the vessel around which the acinus described by Rappaport is organized and to the “vascular septum” described by Matsumoto. This vessel, which completely lacks connective tissue and has a sinusoidal appearance, is not discernible on a routine H&E stain.
Portal veins are the largest structures in the portal tracts, with wide lumina lined by a single layer of flattened endothelial cells (see Fig. 1.10A and D ). The larger branches have thin walls consisting of a narrow rim of fibrous connective tissue, whereas the smaller ones are lined by a single layer of endothelial cells. Portal veins may be absent or obliterated (obliterative portal venopathy), giving rise to noncirrhotic portal hypertension; the extrahepatic portal vein is not infrequently thrombosed in cirrhotic patients. Phlebitis of the portal veins is a common manifestation of immunoglobulin G4 (IgG4) disease.
Three main hepatic veins and several smaller veins drain the liver. The latter are surgically significant because they may be of considerable size. The right hepatic vein drains the right lobe of the liver, and after a short extrahepatic course, empties into the anterior surface of the inferior vena cava. The middle hepatic vein drains the middle portion of the left lobe and a variable portion of the right lobe, whereas the left hepatic vein drains the left lobe; these veins often join together after a short extrahepatic course to form a common venous channel that drains into the anterior aspect of the inferior vena cava. Less frequently, they may drain separately into the inferior vena cava. The caudate lobe drains directly into the inferior vena cava, occasionally into its posterior aspect.
The central veins, the smallest tributaries of the hepatic venous system, appear as round-to-oval thin-walled channels lined by a single layer of flattened endothelial cells that may hardly be discernible on an H&E stain (see Fig. 1.4 ). Rows of hepatocytes radiating outward and the presence of intracellular lipofuscin in perivenular hepatocytes facilitate identification of the central veins. Depending on the plane of section, sinusoids may be seen opening into the central veins. Larger tributaries such as the sublobular veins are often invested by an easily visible wall of fibrous tissue, which can be further highlighted with a trichrome stain ( Fig. 1.15 ). The hepatic venules are obliterated in sinusoidal obstruction syndrome/veno-occlusive diseases and, along with the sinusoids, may be dilated in conditions causing obstruction to venous outflow such as congestive cardiac failure or Budd-Chiari syndrome. Perivenular fibrosis is a common feature of alcoholic and nonalcoholic steatohepatitis.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here