Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
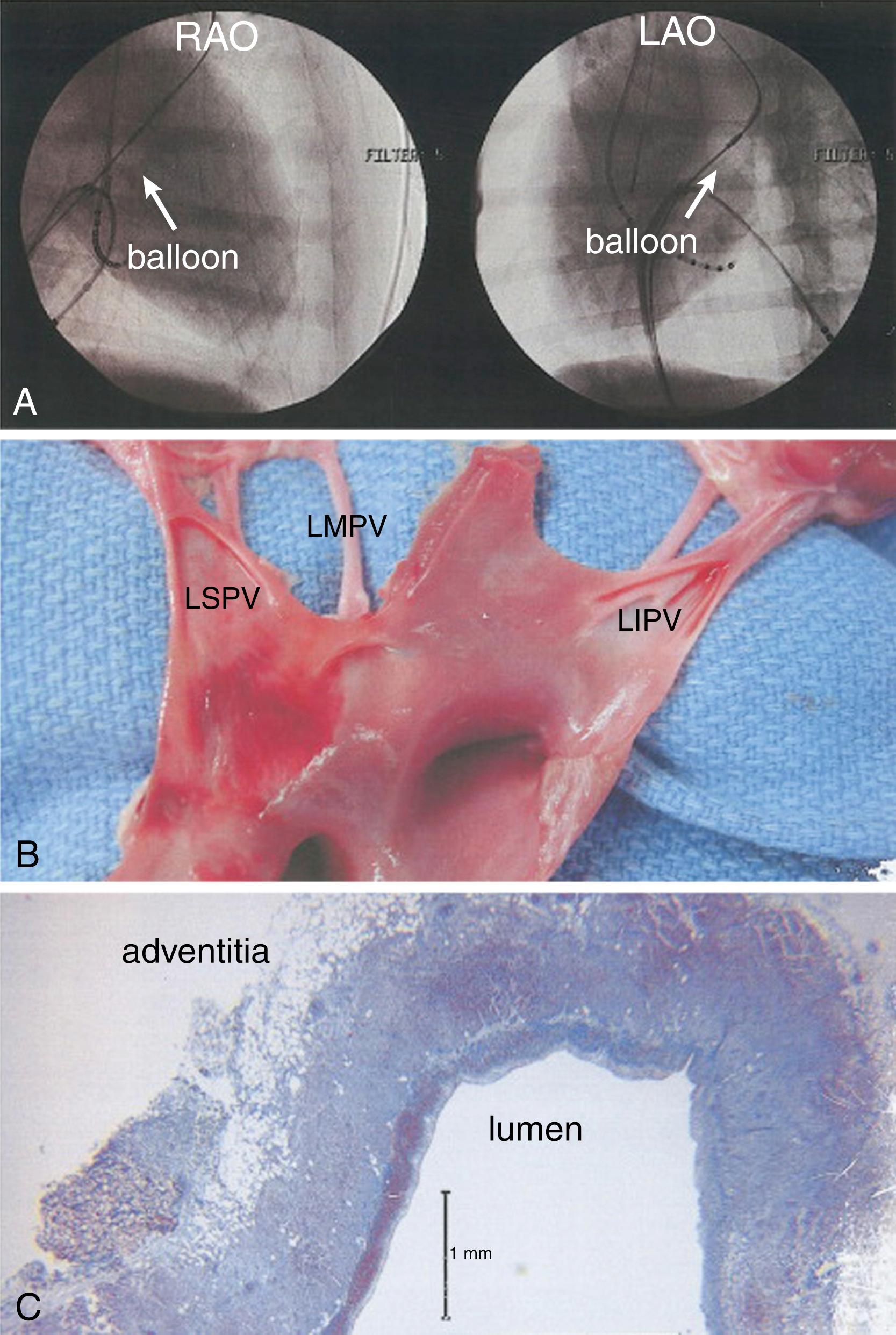
Cryoablation is the removal of heat that results in destruction of tissue. Cryotherapy was invented to treat cancers in the mid 19th century. Cryoablation causes tissue damage by direct and indirect mechanisms: the direct mechanism involves direct injury to the cells, and the indirect mechanisms make the cellular microenvironment hostile to cell viability. There are several ways in which these biocidal mechanisms work, and they are dependent on the minimum cooling temperature, time to minimum cooling, cooling rate, and thaw rate. During cooling, ice crystals that develop in the extracellular space (–15°C) sequester free water, and the resulting hypotonicity leads to withdrawal of intracellular water from the extracellular space culminating in cell dehydration. With rapid cooling, however, there is not enough time for intracellular dehydration, and ice crystals form inside the cell and lead to damage to organelles and interruption of the cell membrane with pore formation (–40°C). The cooling process can also lead to denaturation of proteins in the cell, which can be a reversible process. Mitochondrial damage from cryotherapy can trigger the cysteine-aspartate protease (caspase) system that can lead to programmed cell death and apoptosis. During the thawing process, melting extracellular ice leads to reduced extracellular tonicity and causes water entry into cells, resulting in cellular edema. In addition, ice crystals in the blood vessel wall can also lead to platelet migration and a resultant inflammatory cascade. The resultant lesion is homogeneous and free from endocardial thrombus ( Fig. 131.1 ). Cryotherapy offers unique advantages over radiofrequency (RF) ablation (RFA), including the ability to produce a reversible lesion based on the titration of temperature before achieving lethal temperatures, offering utility for cryomapping. Generally, –20°C to –40°C is the requisite lethal temperature to alter human cellular properties. In murine models, the cell apoptosis rate increases for up to 8 hours after ablation with a second peak of necrosis occurring at 4 days.
Initial efforts used thermoelectric cooling (Peltier effect; cooling by passage of current through dissimilar metal junctions), but these were quickly superseded by gas cooling, which offered superior thermal efficiency. Current cryoenergy devices are argon- and nitrous oxide–based and can deliver up to 50 W to 85 W of cooling power. Theoretically nitrous oxide– and argon-based devices can reach minimal temperatures of –89°C and –189°C, respectively. Based on Joule-Thomson physics in which gas expansion leads to cooling, the commercial platforms yield nearly equivalent cooling power at the tip with temperatures of –80°C less than 1 mm from the probe surface owing to limited heat extraction capacity. To overcome the limitations of the current gas delivery systems, there has been interest in the development of an ideal liquid cryogen medium; however, the absorption of tissue heat causes a phase transition from liquid to gas. The gas can block the flow of the liquid cryogen into the return duct and cause vapor lock. Near critical nitrogen temperature technology uses liquid nitrogen, which is always maintained close to its critical point (defined as the temperature and pressure where the density of the gas is similar to that of the liquid state). With a system of pressurized nitrogen near its critical point, there is no distinct phase transition between liquid and gas. The nitrogen retains the viscosity of gas, permitting easy transit, allowing for smaller catheter designs, and maintaining fluid density for effective rapid cooling. In addition, the development of a new super critical nitrogen-based delivery system that enables rapid delivery of ultracold temperatures could obviate the requirement for 3- to 5-minute freezing applications.
Most of the preclinical data has been performed in the oncology space where this technology was developed. Preclinical studies on canine models demonstrated that the lesions created by focal cryothermal ablation have a smaller surface area compared with RFA, likely from discrete catheter contact and absence of sliding contact during ablation because of cryoadhesion. The lesion depth is similar. Histologically the lesions have a dense, clearly demarcated region of homogenous fibrosis. The collagen and fibroblasts, however, are resistant to hypothermia, which leads to lesions with preserved ultrastructural integrity. This may lead to less arrhythmogenicity. Minimal endothelial disruption is also noted, which may decrease the risk for thrombosis.
Preclinical data from cardiac canine models with linear lesions created during normothermic bypass with a liquid carbon dioxide cryoprobe found satisfactory epicardial and intramural lesion creation with no inducible ventricular arrhythmias. The authors observed that multiple cryolinear lesions should be overlapping by 2.5 mm to prevent discontinuity in lesions. Serial magnetic resonance imaging (MRI) was performed in a canine study to assess acute and chronic lesion maturation and edema formation after RFA and cryoablation. Ventricular ablation lesions were created in 11 canines and serially imaged over 2 weeks for T2-weighted and late gadolinium characteristics. Cryoablation had lower tissue edema for similar lesion size compared with RFA. Edema resolution was noted in 1 to 2 weeks. The acute to chronic late gadolinium enhancement (LGE) ratio was 2.7 for cryoablation energy, similar to RF energy delivery. Longer freeze times correlated with larger lesion size and edema. The preclinical studies for the cryoballoon conducted in eight canine models demonstrated lesions with well-demarcated borders with inflammatory infiltrate, necrotic myocardial cells, mild degrees of fibrosis, and discrete subendothelial proliferation. Similar to focal cryoapplications, the endothelial surface was noted to be intact with no thrombus formation. Four of the eight dogs had phrenic nerve injury.
A small clinical study has added to the preclinical data elucidating the pathophysiology of cryoablation. The IVUS (IntraVascular UltraSound) Cryo study evaluated the tissue characteristics using intravenous ultrasound technique before and after cryoballoon ablation in a small series of atrial fibrillation (AF) patients. The pulmonary veins were noted to have intravein edema, but the overall caliber was increased. This may result from either the low temperature paralyzing the pulmonary vein wall or acute elevation of pulmonary venous pressure during occlusion of the ablated vein. Tissue characteristics suggested that cryoablation affected areas up to 3 to 4 cm proximal to the application site, possibly from the low temperatures transmitted through cooled or frozen blood.
Cryoablation has been approved for management of arrhythmias and has a class I recommendation for use for AF ablation. The role of cryoablation in non-AF cases is generally limited to supraventricular tachycardias (SVTs), notably atrioventricular nodal reentrant tachycardia (AVNRT) in children and is guided by patient characteristics and operator preferences. A recent European survey analyzed cryoablation practices in 49 centers from 18 countries. Six percent of centers used linear cryocatheters for SVT ablations, and the major deterrent was higher recurrence rate. Up to 90% of centers reported using cryoballoon for AF and cited simplicity, low procedural complication rate, safety, and low recurrence rate as benefits identified by high volume operators. The major limitations of cryoballoon AF ablation were difficulty dealing with deviant pulmonary vein anatomies, a relatively high use of fluoroscopy and contrast, challenges with ablation of concomitant atrial flutter requiring employment of a second ablation system during the case, and inconsistent long-term outcomes in ablation of patients with persistent AF. In contrast to AF ablation, only 2% to 6% of centers reported use of cryoablation for SVT and fewer than 10% of the centers reported using cryoablation for ventricular tachycardia (VT). This study reflects the contemporary practice that cryoablation has gained significant use for AF ablation with the single-shot approach.
There have been several trials establishing the efficacy of cryoablation in AF and comparing it with antiarrhythmic drugs (AADs) and RF modalities. Of four early nonrandomized studies of pulmonary vein isolation for the treatment of AF comparing cryoballoon ablation with RFA, all four reported similarly successful pulmonary vein isolation with one study reporting better long-term freedom from AF with cryotherapy. The North American Arctic Front (STOP-AF) randomized control trial demonstrated approximately 70% efficacy of cryoablation compared with 7% for the AAD approach. The FREEZE AF trial randomized 332 patients to cryoablation or RFA. This study concluded that the higher safety profile in the RFA group was driven by more phrenic nerve injuries in the cryoablation group.
The Fire and Ice Trial randomized 762 patients to first-generation cryoablation versus RFA. The acute procedural success was 97.8% and 98.9% in the two groups, respectively. Cryoablation was associated with longer fluoroscopy times (17 vs. 22 minutes), shorter procedural times (124 vs. 141 minutes), and shorter left atrial dwell times (92 vs. 109 minutes). Cryoablation was noninferior to RFA for the primary endpoint, which was the time to event-free survival after the 90-day blanking period. Phrenic nerve injury rates for the cryoablation group were only 2.7%, much lower than the 13.5% reported in the STOP AF trial. Permanent phrenic nerve injury, lasting 12 months or longer, occurred in 0.3% of patients. , Groin complications were more frequent in the RFA group. There have been several efforts to reduce phrenic injury with monitoring of electromyographic diaphragmatic or compound motor action potentials. ,
A French-based, multicenter prospective survey including 860 patients reported lower intercenter and interoperator variability for cryoablation procedures compared with RF for AF ablation. This result was likely driven by the simple one-shot approach and quick learning curve for cryoablation technology. This contrasts with point-to-point RFA lesion application, where there is significant variation in the power and time delivery by different operators and in different anatomic regions.
In a meta-analysis that included 10 prospective studies with over 100 patients who had no prior AF ablation, cryoballoon and RFA for pulmonary vein isolation had similar clinical safety efficacy but shorter procedural times for cases with the second-generation cryoballoon technology. There was a higher risk for phrenic palsy and a lower risk for cardiac tamponade with cryoballoon technology. Other concerns, such as pulmonary vein stenosis from distal balloon application or hemoptysis/bronchial injury stemming from the close proximity of the pulmonary vein ostium and the bronchi, have been reported.
There have been two major technological advances that confound interpretation of the aforementioned studies. These are the advent of contact force–sensing RF technology and the introduction of the second-generation cryoablation balloon, which produces a more uniform region of cryoapplication. A retrospective study evaluating the second-generation cryoablation catheter in 452 patients reported a less than 1% need for redo ablation procedures and 87% procedural success at 12 months. A subsequent meta-analysis of 917 patients undergoing cryoablation with the second-generation cryoballoon catheter from 11 studies showed 99.7% acute success for pulmonary vein isolation, with approximately 68% patients being free of clinical recurrence at a mean follow-up of 16 months. Heterogeneity in clinical studies stems from variable definitions of high-volume center for enrollment, and of success, which is a moving target, contingent on the monitoring modality employed.
Presently the major limitations of cryoablation include lengthy time periods to deliver single lesions (up to 5 minutes) with the occasional requirement to create multiple overlapping lesions, concern for collateral injury with transmurality, higher recurrence rates for SVT, and limited experience in use for ventricular arrhythmias (VAs). The development of catheter platforms using supercritical nitrogen for ultra-rapid heating, which can deliver approximately 120W of cooling power and could potentially create a more durable lesion that is delivered more rapidly, are underway.
Electroporation is the process whereby a high-amplitude electrical field applied across a plasma cell membrane results in increased permeability by means of pore formation. , Electron microscopy (EM) studies have confirmed that the mechanism of permeability is a function of induced pores within the lipid bilayer of the cell membrane. , Pulsed field ablation (PFA) is an irreversible form of electroporation. Myocardial cells have a greater sensitivity (400 V/cm) to PFA compared with the esophagus, blood vessels, and nerves, although the exact mechanisms for tissue selectivity are unclear. Cardiac PFA offers the promise of nonthermal, cardiac tissue–selective ablation, which is rapid and can circumvent complications ensuing from coagulative necrosis.
The optimal waveform for cardiac ablation using PFA has not been elucidated in regard to safety and efficacy. The current delivery system parameter ranges reported are 500 to 3000 V/cm voltage delivered, 1 to 100 pulses delivered, over a wavelength of microseconds, with a frequency range of 1 to 5 Hz. Pulses may be delivered in a monophasic or biphasic format, bipolar or unipolar to an intracardiac site and skin dispersive electrode, and the waveforms can be altered to allow for voltage decay. Depending on the strength of the electric field across the cell membrane and its delivery mode, this may result in no effect, reversible pore formation of the membrane, or irreversible pore formation. The upward titration of the voltage delivered, pulse duration, and the number of pulses delivered have generally resulted in greater tissue destruction. ,
Multiple proprietary PFA systems are presently in development. One of the current systems used in preclinical studies involves a 13 Fr steerable sheath with a 12 Fr over-the-wire PFA ablation catheter with five splines, each containing four electrodes. When fully deployed, the diameter of the distal end is 31 mm. The danger of arcing is obviated with pulse strength, pulse duration, electrode spacing, and design. The PFA waveforms for microsecond scale pulses range from 800 to 1800 V for monophasic and biphasic waveforms, respectively. Another system under active clinical investigation is the nine-electrode circular array pulmonary vein ablation catheter, which can work with a duty-cycled RF generator or a custom-made research generator. , The generator delivers unipolar and bipolar RF energy to the nine electrodes to create contiguous lesion sets ( Fig. 131.2 ). The research pulse generator can deliver high-voltage biphasic pulse trains where electrodes 1, 3, 5, 7, 9 are assigned one polarity, and the electrodes 2, 4, 6, 8 assume the opposite polarity.
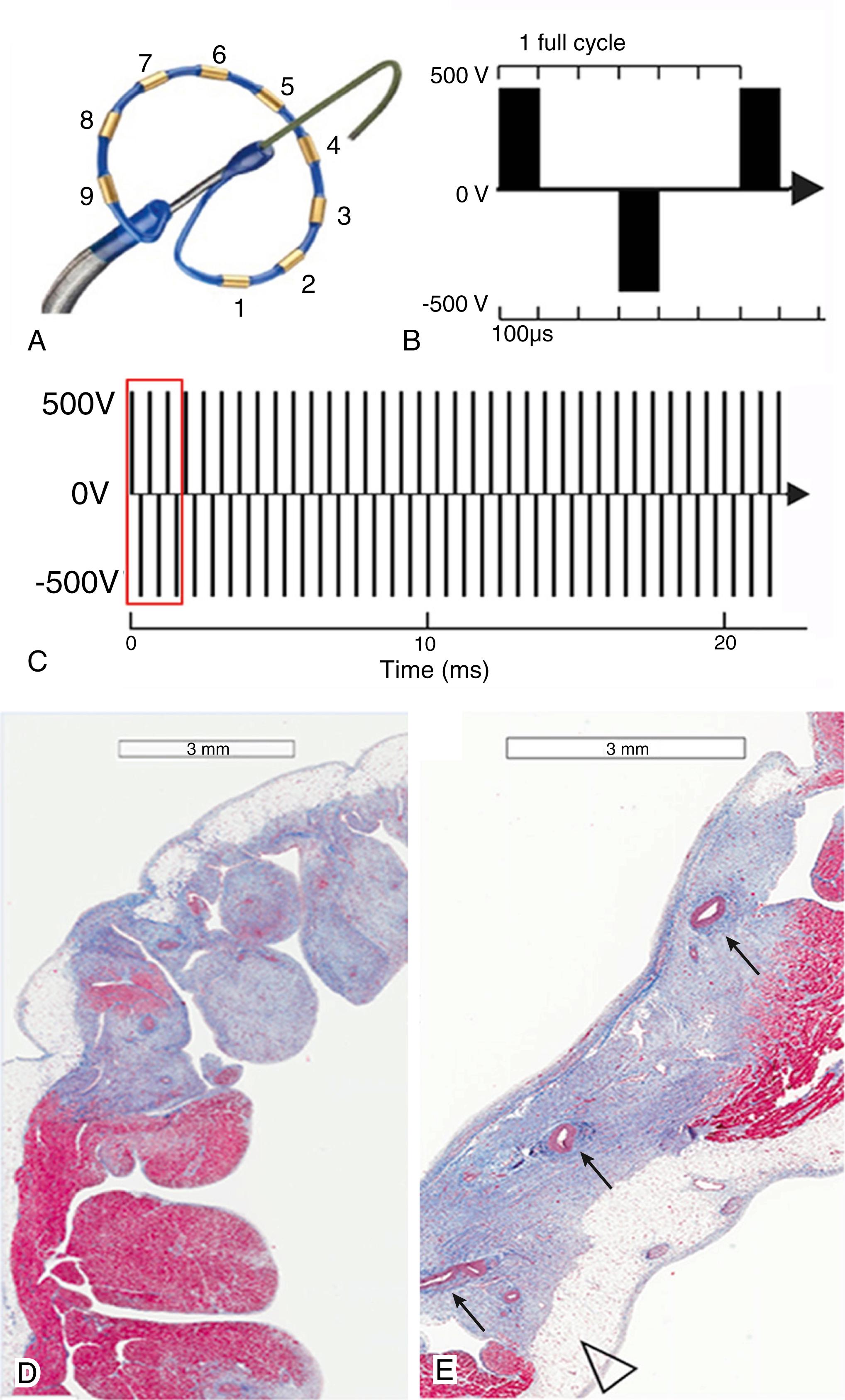
Preclinical studies using rapid freezing EM of human red blood cells revealed three stages of pore formation: initially, there was development of volcano-shaped membrane opening 3 ms after the electrical pulse. These pores rapidly expanded to 20 to 120 nm at 20 ms and beyond that, shrinking and resealing was noted. Direct-current (DC) shock catheter ablation was performed in 1982 and was the first foray into electroporation on the cardiac front, but was later abandoned for RFA because of poor controllability, hydrolysis with dielectric breakdown, and electrical arcing, and then barotrauma from rapid gas globe expansion. Nevertheless, current PFA configurations prevent hydrolysis by limiting power and using individual pulses of very short duration. Both RFA and PFA are effective at creating lesions; however, the gross and histologic examination reveals marked differences between the two modalities.
Gross examination of PFA lesions from experimental animals has demonstrated organized homogenous fibrosis with sharp demarcation of ablated myocardium. Compared with nearby unaffected tissue, consistent findings of the PFA lesions were the elimination of cardiomyocytes but sparing of the structural extracellular matrix, arterioles, nerves, and the presence of remnant fibrosis or fibroblasts. No endothelial thrombus was noted. The center of the PFA lesions was noted to be thinner than the unablated myocardium, putatively from more organized and layered collagenous deposition. An immediate effect of PFA has been to disrupt the sarcolemmal membrane, which can result in conduction block in cardiac tissue and electrical isolation of tissues, such as pulmonary veins, or disruption and inactivation of pathologic automatic or triggered tissue. ,
The first preclinical feasibility of PFA for pulmonary veins was done in a chronic swine model with delivery of 200J shocks between a circular multielectrode catheter and return patch. The authors reported attenuation of pulmonary vein electrograms (EGMs) and lesions as deep as 3.5 mm. Another study compared PFA with phased RFA with a nine-electrode circular mapping catheter to create lesions in the pulmonary vein ostium and right and left atrial appendages. The PFA lesions were devoid of an inflammatory signature with no evidence of epicardial fat inflammation or intralesional remodeling in contrast to the RFA lesions. Both lesion sets had no thrombus.
Tissue specificity of PFA with ablation of myocardium and protection of pulmonary veins, phrenic nerves, the esophagus, and arteries is striking in preclinical studies. The impact of monophasic and biphasic PFA and RFA on phrenic nerve injury with right pulmonary vein and superior vena cava (SVC) isolation was tested in a chronic swine model. Microbubble formation was noted on intracardiac echocardiography with PFA application. For delivery of monophasic energy, paralytics were administered to obviate skeletal muscle capture, which was not an issue for the biphasic delivery module. Monophasic PFA resulted in reversible EGM changes, which have not been observed in clinical studies. There was nerve sparing noted in the PFA group and not in the RF group. In addition, there was evidence of pulmonary vein narrowing and incomplete SVC isolation in the RFA group, unlike the PFA group. Biphasic PFA was noted to be more durable than monophasic PFA and RF application. A protocol assessing the risk for pulmonary vein stenosis by serial computed tomography (CT) angiography showed negligible changes after PFA, whereas significant stenoses were observed with RFA delivered in a similar fashion. When the esophagus is directly ablated with PFA, there typically are no visible lesions, or there is minor damage limited to the muscular layer with sparing of the epithelial and lamina muscularis mucosa. Direct ablation of the porcine esophagus with a linear suction electrode showed no histologic changes 2 months after ablation. Coronary arteries coursing through the middle of PFA myocardial lesions appear to be completely spared. The exact mechanism explaining why cardiac tissues are more sensitive to electric fields than other tissues is not completely understood but may relate to cell size, orientation, membrane characteristics, and sensitivity to nonspecific cation entry. ,
A study assessed the impact of PFA on ventricular tissue in a swine model and noted chronic lesion dimensions were 6.5 mm deep and 22.6 mm wide. There was no evidence of pericarditis or VAs noted during application. In an attempt to eliminate triggers for serious VAs, selective PFA of Purkinje fibers in Langendorff-perfused canine hearts was attempted. This approach yielded a reduction in local EGM amplitude and a significant increase in the lower limit of vulnerability with resultant decrease in VF inducibility. Despite successful ablation of the Purkinje fiber targets, the myocardium was spared.
The first in human studies for proof of concept of PFA for pulmonary vein isolation to treat AF was conducted in 22 patients, with 15 patients undergoing an endocardial approach and 7 undergoing an epicardial approach. Using ablation outputs ranging from 900 to 2500 V, acute isolation was achieved in all but one patient. The average energy applications were 78 J and 1146 J, and the catheter dwell times were 19 and 50 minutes for the endocardial and epicardial groups, respectively. PFA applications were delivered with atrial and ventricular pacing to ensure PFA was not delivered during the vulnerable period of ventricular repolarization. The feasibility, safety, and efficacy of the same system was then reported from two single-center pilot protocols in which 81 patients with AF underwent pulmonary vein isolation with PFA with remapping at a mean 84-day follow-up. The system and waveform were modified over the course of the trials, resulting in a rate of complete pulmonary vein isolation transitioning from 18% initially to 100% at the end of the series. One case of pericardial tamponade was reported. The mean catheter dwell time was 32 minutes, and the mean procedure time was 94 minutes (substantially lower than the times reported in the Fire and Ice Trial). This study highlighted the importance of the PFA waveform optimization in successful application of this technology, and the importance of dose ranging and waveform optimization in preclinical testing.
To date, no studies have been published assessing the reversible effects of PFA on the myocardium or the time course of recovery of normal electrical function. In addition, the current delivery systems do not permit the creation of linear or point lesions. Future development will optimize this energy source for ablation of many targets, including focal sites of VT origin, reentrant VT substrate modification, and cardiac (and extracardiac) ablation of ganglionic plexi. Fluoroscopy still remains an integral part of the procedure workflow but will likely be replaced with nonfluoroscopic imaging as has occurred with RFA. Long-term safety is unknown, although the safety of PFA in its applications in the oncology space over the last decade offers a modicum of reassurance.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here