Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anticoagulation is indicated for thromboembolic prophylaxis in patients with atrial fibrillation (AF) who meet established criteria. For a long time, warfarin was the sole drug for this indication. This agent was identified by Karl Link and colleagues in 1940 when they determined that coumarin or sweet clover could be metabolized by fungi into the anticoagulant dicoumaral. In 1950 Askey and Cherry published their landmark study demonstrating the utility of warfarin for the reduction of stroke risk in patients with AF. In their discussion, the authors recognized that “it is probable that a safer drug will be available eventually.” It took 60 years for an alternative to warfarin to be approved for AF-related thromboembolic prophylaxis. These direct acting oral anticoagulants (DOACs) have become widely adopted in clinical practice and now are recommended over warfarin in most patients for thromboembolic prophylaxis in AF. The correct use of anticoagulants for AF thromboembolic prophylaxis requires a thorough understanding of the optimal choice of agent and dosing in specific circumstances.
Warfarin was named after the Wisconsin Alumni Research Foundation (WARF). It inhibits the vitamin K–dependent synthesis of the clotting factors II, VII, IX and X, as well as protein C, protein S, and protein Z. Warfarin’s half-life ranges between 20 and 60 hours, and the time to therapeutic international normalized ratio (INR) is 5 days on average. The daily dosage varies between 0.5 and 20 mg. Loading dosages of warfarin may accelerate the achievement of a therapeutic INR but risk the rare occurrence of acquired protein C depletion and skin necrosis (1:10,000).
An abundance of evidence supports the ability of warfarin to affect a 65% reduction in the risk for stroke in patients with AF. The therapeutic benefit is achieved with INRs greater than 1.8 with a target range of 2 to 3. In fact, the time in therapeutic range (TTR) must be at least 60% to 70% to realize the thromboembolic risk reduction expected with warfarin, which is equivalent to that seen with DOACs. , Many factors influence the ease and stability of anticoagulation with warfarin, most notably medication and dietary interactions ( Table 119.1 ). In addition, variants in the CYP2C9 and VKORC1 genes, which are present in up to 25% of the population, are associated with marked instability in INRs. Efforts to develop genotype-based personalized protocols for warfarin initiation and maintenance have proven somewhat successful but have yet to be shown to be practical or cost effective. , Warfarin is generally best managed through standardized algorithms. Despite the stated difficulties with warfarin usage, patients who have minimal difficulty reaching and maintaining a stable therapeutic INR can use this medication as a durable strategy.
| Drug | Metabolism | Interactions That Increase Drug Level | Interactions That Decrease Drug Level |
|---|---|---|---|
| Warfarin | CYP2C9, CYP3A4, CYP1A2 oxidation | CYP inhibitors: amiodarone, dronedarone, quinidine, propafenone, diltiazem, verapamil, telmisartan, statins (atorvastatin, rosuvastatin, simvastatin), lovastatin, macrolides, metronidazole, isoniazid, quinolones, azole-antifungals, HIV protease inhibitors, NSAIDs, grapefruit juice | CYP inducers: rifampin, carbamazepine, phenytoin, phenobarbital Protein binding displacers: losartan, valsartan, ibuprofen, amlodipine, quinidine GI absorption: PPIs, cimetidine Vitamin K-rich foods: spinach, kale, avocado |
| Dabigatran | P-gp substrate glucuronidation | P-gp inhibitors: amiodarone, verapamil, dronedarone, quinidine, ticagrelor, ketoconazole, macrolides, cyclosporine A/tacrolimus, ketoconazole | CYP/P-gp inducers: rifampin, carbamazepine, phenytoin, phenobarbital GI absorption: PPIs |
| Rivaroxaban | P-gp substrate CYP3A4, CYP3A5, CYP2J2 oxidation | CYP inhibitors: amiodarone, diltiazem, dronedarone, quinidine, verapamil, macrolides, HIV protease inhibitors, azole-antifungals, cyclosporine A/tacrolimus | CYP/P-gp inducers: rifampin, carbamazepine, phenytoin, phenobarbital |
| Apixaban | P-gp substrate CYP3A4, CYP3A5, CYP 2J2 CYP1A2, CYP2C8, CYP2C9, CYP2C19 oxidation BCRP substrate | CYP inhibitors: diltiazem, HIV protease inhibitors, azole-antifungals | CYP/P-gp inducers: rifampin, carbamazepine, phenytoin, phenobarbital |
| Edoxaban | P-gp substrate CYP3A4 oxidation (minimal) | P-gp inhibitors: amiodarone, dronedarone, quinidine, verapamil, macrolides, cyclosporine A/tacrolimus, HIV protease inhibitors | CYP/P-gp inducers: rifampin, carbamazepine, phenytoin, phenobarbital |
Warfarin is associated with a risk for teratogenicity in the first trimester of pregnancy but thereafter is considered a relatively safe form of anticoagulation. The presence of a mechanical heart valve is one circumstance in which warfarin is the preferred agent because of superior efficacy and may be used even in the first trimester if the dose is kept below 5 mg/day. For AF requiring anticoagulation, particularly in the case of mitral stenosis, the recently updated European guidelines recommend using low molecular weight heparin in the first and third trimesters and either warfarin or low molecular weight heparin during the second trimester. Warfarin is not excreted into breast milk and can be used with lactation.
The risk for bleeding with warfarin is directly related to the INR. In general, significant bleeding, most notably CNS bleeding, occurs with INRs greater than 5. The anticoagulant effects of warfarin can be acutely reversed with prothrombin complex concentrate (PCC) and fresh frozen plasma. For major bleeding, vitamin K should also be given to normalize the INR.
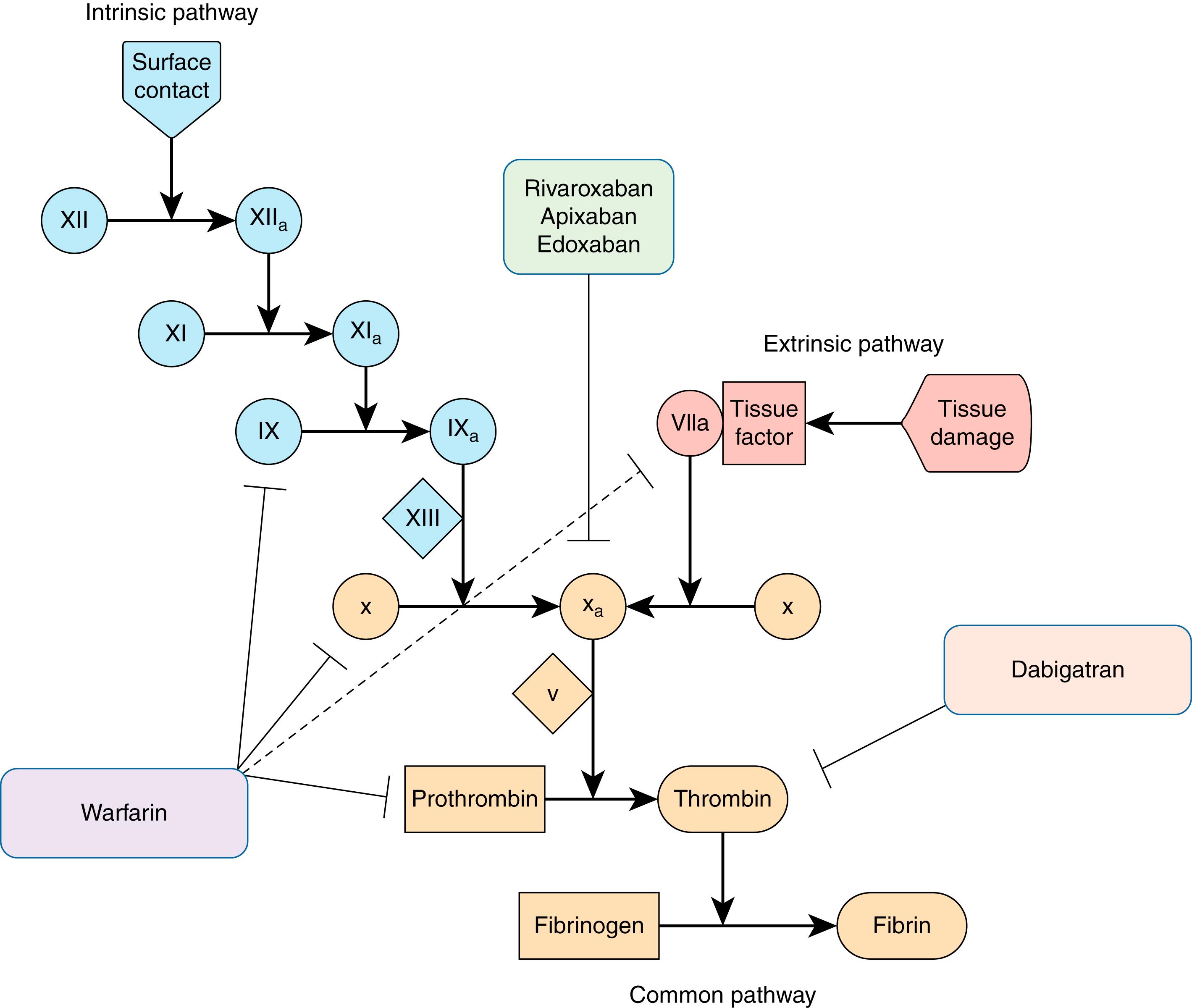
The DOACs inhibit specific enzymes in the coagulation cascade and are composed of two classes of drugs: direct thrombin (factor IIa) inhibitors and factor X inhibitors ( Fig. 119.1 ). All four US Food and Drug Administration (FDA) approved agents have been tested in large clinical trials evaluating the noninferiority of the tested DOAC compared with warfarin for thromboembolic prophylaxis and significant bleeding. Most importantly, each DOAC was associated with a significantly lower risk for intracranial bleeding than warfarin ( Table 119.2 ). There is little published experience with the DOACs in pregnancy or lactation, and so they are avoided in these situations.
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Mechanism | Competitively inhibits Factor II (thrombin) | Competitively inhibits Factor Xa | Competitively inhibits Factor Xa | Competitively inhibits Factor Xa |
| Dosing in AF | CrCl >30 mL/min: 150 mg BID CrCl 15–30 mL/min: 75 mg BID CrCl 15–50 mL/min with P-gp inhibitors: 75 mg BID |
CrCl >50 mL/min: 20 mg QD with largest daily meal CrCl 15–50 mL/min: 15 mg QD with largest daily meal |
5 mg BID If ≥2 of Cr ≥1.5 mg/dL, age ≥80 y, weight ≤60 kg: 2.5 mg BID |
CrCl >50 mL/min: 60 mg QD CrCl 15–50 mL/min: 30 mg QD Avoid if CrCl >95 mL/min because of increased excretion |
| Half-life | 12–17 h Elderly: 14–17 h |
5–9 h Elderly: 11–13 h |
12 h | 10–14 h |
| Bio-availability | 3%–7% | 66% without food, 80%–100% with food | 50% | 62% |
| Effect of H2-blocker/PPI | Decreases 12%–30% | No effect | No effect | No effect |
| Prodrug | Yes | No | No | No |
| Time to onset | 1 h (2 h with food) | 2–4 h | 3–4 h | 1–2 h |
| Clearance (renal/nonrenal) | 80%/20% | 35%/65% | 27%/73% | 50%/50% |
| Protein bound | 35% | 95% | 87% | 55% |
| Dialyzability | 50%–60% (partially dialyzable) | Minimal | 14% (partially dialyzable) | Minimal |
| Liver metabolism (CYP450- involvement) | No | Yes (18%) | Yes (25%) | Minimal (<4%) |
| Nonbleeding side effects | Dyspepsia in 5%–10% | Minimal | Minimal | Minimal |
| Monitoring to detect presence | aPTT, ECT, TT | PT, aPTT, anti–Factor Xa activity | PT, aPTT, anti–Factor Xa activity | PT, aPTT, anti–Factor Xa activity |
Dabigatran is a direct thrombin inhibitor. Dabigatran etexilate is a prodrug. Its absorption is mediated by the P-glycoprotein (gp) transporter, and metabolism occurs via esterase-mediated hydrolysis in gastrointestinal (GI) enterocytes. There is no reliance on the cytochrome (CY) P450 system. Drug absorption requires an acidic environment, and therefore the drug preparation includes tartaric acid. The latter is likely responsible for the dyspepsia experienced by some patients. The serum half-life is 12 to 17 hours, and excretion is up to 80% renal ( Table 119.3 ). The approved doses in the United States are 150 mg twice daily for patients with relatively preserved renal function and 75 mg twice daily for patients with a creatinine clearance (CrCl) of 15 to 29 mL/min. A dose of 110 mg twice daily is available and is used outside of the United States in patients older than 75 years. Dabigatran is the most dialyzable of the DOACs.
| Drug | Pivotal Trial for AF | Exclusion Criteria | No. of Patients | Warfarin TTR | Follow-up (y) | CHADS 2 Mean Score | Efficacy Outcomes | Safety |
|---|---|---|---|---|---|---|---|---|
| Dabigatran (150 mg BID or 110 mg BID) | RE-LY (NCT00262600, 2009) | Reversible AF, CrCl <30 mL/min, recent stroke, high bleeding risk condition, severe valve disorder, anticoagulated for another condition, planned AF ablation, liver disease, or pregnancy | 18,113 | 64% | 2 | 2.1 | High dose: dabigatran superior to warfarin for stroke + systemic embolism | Both doses: dabigatran had lower rates of major bleeding, intracranial bleeding, life-threatening bleeding, and major or minor bleeding |
| Low dose: dabigatran noninferior to warfarin for stroke + systemic embolism | GI bleeding higher in high-dose dabigatran group | |||||||
| Rivaroxaban (20 mg QD; 15 mg QD if CrCl 30–49 mL/min) | ROCKET-AF (NCT00403767, 2011) | Reversible AF, CrCl <30 mL/min, recent stroke/TIA, high bleeding risk condition, moderate or severe mitral stenosis or mechanical prosthesis, ASA >100 mg QD or DAPT, planned cardioversion | 14,246 | 55% | 1.9 | 3.5 | Rivaroxaban noninferior to warfarin for stroke + systemic embolism | Rivaroxaban had similar rates of major bleeding and clinically relevant nonmajor bleeding as warfarin |
| Rivaroxaban had lower rates of fatal bleeding, bleeding at a critical site, and intracranial bleeding | ||||||||
| GI bleeding was higher in the rivaroxaban group | ||||||||
| Apixaban (5 mg BID; 2.5 mg BID if ≥2 of age ≥80 y, Cr ≥1.5, or weight ≤60 kg) | ARISTOTLE (NCT00412984, 2012) | Reversible AF, CrCl <25 mL/min or Cr >2.5 mg/dL, recent stroke, high bleeding risk condition, anticoagulated for another condition, significant mitral stenosis or presence of any prosthetic valve, ASA >165 mg QD or DAPT, planned AF ablation | 18,201 | 62% | 1.8 | 2.1 | Apixaban superior to warfarin for stroke + systemic embolism | Apixaban had lower rates of major bleeding, intracranial bleeding, and any bleeding compared with warfarin |
| Rate of all-cause death lower with apixaban compared with warfarin | Apixaban had similar rates of GI bleeding as warfarin | |||||||
| Edoxoban (60 mg QD or 30 mg QD; dose halved if CrCl 30–50 mL/min, weight <60 kg, or certain drugs ) | ENGAGE AF- TIMI 48 (NCT00781391, 2013) | Reversible AF, CrCl <30 mL/min, DAPT, recent ACS or stroke, high bleeding risk condition, moderate or severe mitral stenosis or mechanical prosthesis, anticoagulated for another condition | 21,105 | 68% | 2.8 | 2.8 | High dose: edoxaban superior to warfarin for stroke + systemic embolism | Both doses: edoxaban with lower rates of major bleeding, life threatening bleeding, intracranial bleeding and major + clinically relevant nonmajor bleeding |
| Low dose: edoxaban noninferior to warfarin for stroke + systemic embolism | GI bleeding higher in edoxaban groups |
Dabigatran was compared with warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study (see Table 119.2 ). In this trial of 18,133 patients with AF, dabigatran was noninferior to warfarin for the endpoint of prevention of thromboembolic and hemorrhagic complications. In fact, the 150 mg dose of dabigatran was superior to warfarin for the prevention of ischemic stroke.
Rivaroxaban is a factor Xa inhibitor. The P-gp transporter is involved in intestinal absorption, and concomitant food intake increases bioavailability by roughly 15%. The serum half-life is 5 to 9 hours, and 35% of the excretion occurs via the renal system. The dose is 20 mg once daily in patients with a CrCl of greater than 50 mL/kg. The dose is reduced to 15 mg once daily for a CrCl of 15 to 50 mL/kg. Rivaroxaban is not dialyzable.
Rivaroxaban was compared with warfarin for thromboembolic prophylaxis in AF in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). This study differed from the evaluation of the other DOACs in that the studied population had a higher CHADS score (average 3.5), with nearly half of the ROCKET AF population having had prior stroke or transient ischemic attack (TIA; see Table 119.2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here