Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Development of the functioning heart is a complex process that is essential for survival of the embryo. The mechanisms responsible for cardiovascular morphogenesis have been studied for centuries by classical embryologists and more recently by molecular developmental biologists. Major progress in the understanding of cardiac specification and morphogenesis has been made from the study of cardiovascular development in a number of invertebrate and vertebrate model systems, from Drosophila to mouse. The conservation of many aspects of heart development across phylogeny has provided the basis for our increased understanding of the underlying principles of cardiac development and how congenital defects of the heart arise when development goes awry. Congenital heart malformations have long been recognized to be the result of developmental abnormalities affecting cardiac morphogenesis. Developmental defects can occur at any stage of heart development, resulting in phenotypes that vary widely, from severe morphologic abnormalities resulting in early embryonic lethality to mild defects having little physiologic impact. Because of the rapid rate of information entering the field of cardiac embryology and the scope of this chapter, detailed genetic descriptions of specific morphogenetic events are not discussed. Instead, examples are given to illustrate the current paradigms in the field. For the purposes of this book, greater detail is paid to developmental abnormalities of the heart that result in congenital heart defects in humans.
Early in embryonic development, after gastrulation at approximately day 17 to day 19 in human development, cells in the lateral mesoderm adopt a cardiogenic fate. The commitment of progenitor cells in the anterior left and right lateral plate mesoderm to the cardiogenic fate relies on inductive signals from the adjacent endoderm. Specifically, the bone morphogenetic proteins (BMPs) expressed in the endoderm adjacent to the cardiogenic precursors play a role in cardiac induction and act with other signaling factors, including those from the fibroblast growth factor (FGF) family, hedgehog, Wnt ligands, and members of the transforming growth factor (TGF) superfamily. In vertebrates, the heart-forming region is bounded by repressive signals in addition to inductive signals. Repressive signals include members of the Wnt family of secreted molecules. In the cardiogenic region, Wnt antagonists oppose the repressive signals, allowing cells to commit to a cardiogenic fate.
Several lines of evidence suggest that cardiogenic precursors in the lateral plate mesoderm contain prepatterned information regarding their ultimate positional identity and cell fate ( Fig. 104-1 A ). Lineage analysis has demonstrated that cells in the caudal cardiogenic mesoderm contribute to the atria, whereas cells in the rostral cardiogenic mesoderm contribute to the ventricles. Explant experiments also demonstrate phenotypic differences between rostral and caudal precardiac mesoderm. However, cell transplant experiments also demonstrate that rostral-caudal fates of cardiogenic precursors remain plastic and require instructive cues from outside the cardiogenic region. Taken together, these results suggest that regional positional information is prepatterned in cardiogenic precursors before the development of the linear heart tube but that these positional identities appear not to be fixed until later. Several recent studies suggest that cell-type specification information, in addition to positional information, already resides in the field of cardiac progenitors. For example, precursors of both myocardial and endocardial cells can be found in the cardiogenic mesoderm, but no bipotential precursor has been identified.
The definitive heart and associated vascular structures receive contributions not only from the precardiac mesoderm that forms the cardiac crescent but also from two additional populations of cells. An anterior heart–forming field has been identified that lies adjacent to the crescent on its medial aspect, and that contributes to anterior structures of the outflow tract as well as to the myocardial mass of the ventricles. In addition, the neural crest, a population of multipotential migratory cells, contributes to the outflow tract of the heart and is specifically required for outflow tract septation.
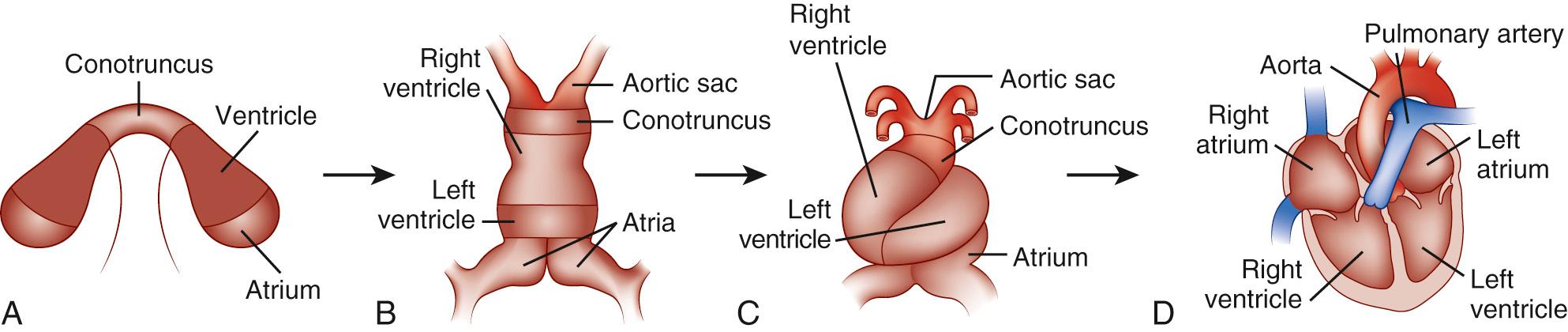
Bilaterally symmetrical cardiogenic primordia are formed from the lateral fields of mesoderm in the early embryo. The convergence and fusion of the bilateral cardiac primordia form the primitive cardiac tube in the ventral midline (see Fig. 104-1 B ). The anterior margins of the cardiogenic mesoderm fuse first to form the cardiac crescent at approximately day 23 of human development.
Fusion of the bilateral cardiac primordia then occurs in a rostral to caudal direction, with sequential addition of precardiac mesoderm at the caudal end of the developing heart tube. The result is the formation of the primitive linear heart tube. The linear heart tube, already containing distinct myocardial and endocardial layers, initiates contractions at approximately 4 weeks of fetal development in humans. Morphologic and molecular polarity along the heart tube (the anteroposterior axis) exists, with distinct regions from anterior to posterior of the aortic sac, conotruncus, right ventricle, left ventricle, and atria (see Fig. 104-1 B ).
The tubular heart undergoes a morphogenetic process of rightward looping in all vertebrates. During this process, the linear heart tube is converted into an S-shaped heart with a complex, three-dimensional structure and asymmetry about the anterior-posterior, left-right, and dorsal-ventral axes (see Fig. 104-1 C ). Rightward looping is the visible start to the morphogenetic process that results in an asymmetrical, multichambered heart. The specificity of rightward looping is essential for the appropriate orientation of the developing cardiac chambers with one another, with the endocardial cushions that will contribute to chamber septation, and with inflow and outflow vascular connections.
Rightward looping of the heart is the first visible indication of left-right asymmetry in the developing vertebrate body plan. The consistent directionality of this process suggests a highly conserved molecular control mechanism of left-right patterning in the vertebrate embryo. This work demonstrates that significant molecular left-right differences are present in the embryo long before the visible morphogenetic event of rightward heart looping.
Early in embryonic development, during establishment of the lateral plate mesoderm, the Hensen node appears to control the genesis of left-right differences in gene expression despite its midline position. Beating cilia that project from the node undergo a counterclockwise vortex-like movement that creates a leftward flow of fluid across the Hensen node. The unidirectional ciliary movement appears to be established by the inherent molecular chirality of the ciliary protein machinery and its molecular motor on the node. The flow across the node may mediate the movement of secreted molecules across the node in a leftward direction. Such molecules could then function as left-sided determinants on the left side of the node. Alternatively, evenly distributed mechanosensory cilia may be specifically activated on the left side of the node by the fluid flow across the node. The resultant signal transduction downstream of the activated mechanosensory cilia in left-sided cells could then initiate a left-sided molecular cascade.
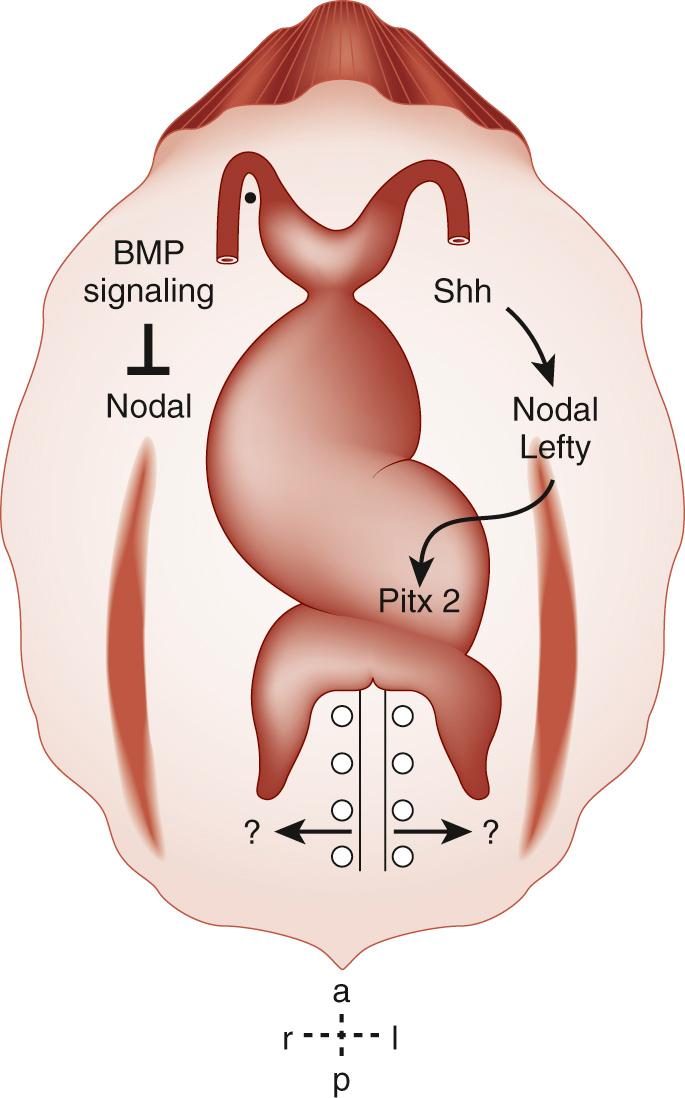
A molecular cascade for left-right determination has been identified, beginning with asymmetrical expression of the morphogen sonic hedgehog (Shh) on the left side of the node ( Fig. 104-2 ). Sonic hedgehog is able to induce left-sided perinodal expression of the left-sided determinant Nodal, a member of the transforming growth factor-β (TGF-β) signaling family. The molecular ground state of laterality in fact appears to be right-sidedness, with the expression of the Nodal normally repressed by BMP signaling. Shh induces the left-sided expression of Caronte, a secreted molecule, which overcomes BMP-mediated repression of Nodal in the lateral plate mesoderm. Thus, left-sided Shh results in the expression of Nodal locally and at a distance, and left-sided positional information is transferred to the left lateral plate mesoderm. One result of left-sided Nodal expression is the left-sided induction of Pitx2, a homeodomain-containing protein, throughout the left lateral plate mesoderm (see Fig. 104-2 ). Pitx2 expression is maintained during subsequent organogenesis on the left side of the developing heart tube, gut, and lungs. This cascade thereby transmits left-sided positional information from the Hensen node to the visceral organs.
The molecular hierarchy of left-right positional information establishes global left-right differences in the vertebrate body plan. Defects in the proper establishment of left-right differences of the visceral organs are generally known as heterotaxy syndrome (derived from the Greek meaning “other arrangement”) or atrial isomerism. Often, the clinical phenotype reveals a predominance of one type of sidedness. In simple terms, asplenia syndrome can be thought of as demonstrating a bilateral right-sidedness and polysplenia syndrome can be thought of as demonstrating bilateral left-sidedness. The term situs ambiguus is used if the sidedness cannot be determined. Not surprisingly, mutations in the genes known to have a function in the normal establishment of left-right difference can result in the heterotaxy syndromes, both in animal models and in human patients.
Establishment of left-right asymmetry about the Hensen node has been demonstrated to require the inversus viscerum (iv) gene, encoding left-right dynein, the force-generating component in the cilia responsible for the vortical flow across the node. Mutation in iv, or in other genes that contribute components to the functional ciliary motor, causes immotile cilia syndrome and a failure to generate the initial left-right asymmetry in the Hensen node. Absence of asymmetry about the node results in randomized expression of left-sided determinants Nodal and Pitx2 in the lateral plate mesoderm. The phenotypic result of the failure to generate the appropriate vector for laterality information is randomization of left- and right-sidedness in the visceral organs.
Mutations in single genes can therefore result in both polysplenia and asplenia syndromes, previously thought to be distinct entities, and therefore have distinct causes. The finding that expression of left-sided determinants Nodal and Pitx2 are randomized in these cases helps to explain the variability of defects seen in the heterotaxy syndromes. For example, Pitx2 expression can be normal, presumably resulting in situs solitus, or reversed, presumably resulting in situs inversus (the left-right mirror image specification of the visceral organs). However, it can also be bilateral, absent, or a range of relative left-right levels in between. This range is similar to the wide range of situs abnormalities of the visceral organs observed in this model and in human patients with situs ambiguous. It also may help explain why previous efforts to categorize the specific morphology of heart defects in cases with asplenia and polysplenia have proved so difficult.
The importance of ciliary function in sidedness determination has led to investigations of a mouse model with primary ciliary dyskinesia via a mutated dynein gene (Dnahc5), which showed a 40% incidence of heterotaxy in the homozygous mutant embryos. Translational research subsequently confirmed ciliary dysfunction in 42% of congenital heart disease (CHD) patients with heterotaxy syndrome, which may contribute to respiratory problems in addition to their heart disease.
There is remarkable conservation, between animal models as disparate as Drosophila and vertebrates, of the early transcriptional hierarchy that specifies cardiogenic fate. The tinman gene, encoding a homeodomain-containing transcription factor, is required for formation of the Drosophila dorsal vessel (the dorsal vessel in the fly is analogous to the heart in vertebrates). Tinman directly activates transcription of several genes, including pannier, a GATA transcription factor, and myocyte enhancer factor-2 (MEF-2), a MAD-box–containing transcription factor. MEF-2 and pannier then contribute to the downstream transcriptional activation of cardiomyocyte structural genes.
Nkx2.5 is the vertebrate ortholog of the Drosophila tinman gene. Nkx2.5 is expressed early in the cardiogenic mesoderm, in part a response to induction of the heart-forming region by BMPs from the endoderm. Like tinman in Drosophila, the Nkx2.5 transcription factor plays a role in cardiogenic differentiation in vertebrates. Nkx2.5 physically interacts with vertebrate GATA factors, and these transcription factors mutually activate one another's promoters. This establishes a positive molecular feedback loop that appears to strengthen the choice of cardiomyocyte fate. As in Drosophila, the GATA and MEF-2 gene families have been demonstrated to play a role in the activation of a cardiomyocyte differentiation program throughout the myocardium. In vertebrates, three GATA family members, GATA-4, -5, and -6, demonstrate expression in cardiac lineages. GATA factors are required for transcriptional control of several cardiac muscle structural genes. GATA family members act in cooperation with transcription factors from other families, including Nkx2.5 and MEF-2, to regulate the expression of target genes. There are four MEF-2 family members in vertebrates. As in Drosophila, these genes appear to directly activate structural genes of myocyte differentiation. For example, mice lacking the gene for one family member, mef2a, die with cardiovascular abnormalities at the looping stage and fail to express a group of muscle-specific structural genes.
Nkx2.5 has roles in cardiogenic differentiation and in pattern formation of the vertebrate heart. The multifaceted nature of its action perhaps results from its promiscuous interactions with transcription factors of other classes. Aside from the GATA class of transcription factors, Nkx2.5 is able to physically interact with the T-box transcription factor Tbx5. The Tbx5 gene is responsible for the human autosomal dominant disorder Holt-Oram syndrome. Cardiac manifestations of the loss of one copy of Tbx5 or Nkx2.5 overlap and include atrial septal defects and conduction system abnormalities. Nkx2 .5 and Tbx5 have been shown to act synergistically on the promoters of genes with high levels of expression in the atria and the conduction system, including atrial natriuretic factor and connexin 40.
Concomitant with the activation of a cardiomyocyte differentiation program is the development of regional differences between the chambers of the heart. As early as the tubular heart stage, the cardiac primordia demonstrate a segmental appearance. Morphologically, five primordial segments can be identified from posterior to anterior: sinus venosus or atrium, atrioventricular canal, left ventricle, right ventricle, and outflow tract (see Fig. 104-1 B ). These morphologically distinct regions must be indicative of distinct transcriptional domains in the developing heart primordia. In fact, a growing number of transcription factors have been identified that demonstrate chamber-specific patterns of transcription.
The chamber-specific expression patterns of the Iroquois family member Irx4, a homeobox-encoding gene, suggest a role in ventricular versus atrial chamber specification. Irx4 is expressed specifically in the developing ventricular chambers of the heart and is excluded from the developing atria. Irx4 has been demonstrated to play an important transcriptional role in the selection of ventricular fate. Loss of Irx4 function in the ventricles results in aberrant activation of atrial gene expression, whereas ectopic expression of Irx4 in atrial chambers results in the aberrant activation of ventricular gene expression. Further evidence for the separate genetic control of atrial and ventricular fate is demonstrated by the zebrafish mutants lonely atrium and pandora, in which the ventricular chamber fails to form but the atrial chamber appears morphologically intact.
The beta helix-loop-helix factors HAND1 (eHAND) and HAND2 (dHAND) have been demonstrated to play a role in determining molecular differences between the ventricles. The chamber-specific expression of HAND1 and HAND2 suggested that these genes may play a role in right-versus-left ventricular chamber specification. Although both HAND1 and HAND2 are initially coexpressed throughout the precardiac mesoderm, HAND2 expression becomes restricted to the right ventricular precursors during cardiac looping, whereas HAND1 becomes restricted to left ventricular and conotruncal precursors. Functional analysis of hand2 has demonstrated a role in chamber formation and may provide a molecular candidate for a severe form of human coronary heart disease. Mice homozygous for a null hand2 allele appear to lack a morphologic right ventricle. The phenotype of the heart in this animal model is similar to human right ventricular hypoplasia, establishing hand2 as a candidate gene for this type of human CHD.
Irx4 and the hand genes suggest that the specification of chamber-specific modularity lies at least in part in the transcriptional localization of single genes. However, the notion that chamber identity as a whole is specified as a result of a small number of chamber-specific modules of gene expression is simplistic. The degree to which regulation of cardiac gene expression is a highly modular process has only begun to be understood. The analysis of the promoters of single cardiac-specific genes has demonstrated that a very large number of small promoter elements are required to recapitulate normal expression patterns. The degree of modularity is so high that some elements drive expression in regions not previously thought to be molecularly distinct from their neighbors. These findings suggest that complex combinatorial networks of transcription factors are required for the molecular regionalization of information in the developing heart.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here