Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although the treatment and healing of wounds are some of the oldest subjects discussed in the medical literature, and although there have been numerous advances in understanding the steps involved in wound healing, the exact mechanisms underlying wound healing remain unclear.
Attempts to restore mechanical integrity, to repair barriers to fluid loss and infection, and to reestablish normal blood and lymphatic flow patterns are termed wound repair . During wound repair, flawless reconstruction is sacrificed in order to speed up the return to function. In contrast, regeneration, which is the goal of wound healing, is the perfect restoration of the preexisting tissue architecture without scar formation; regeneration is achievable only during embryonic development, in lower organisms, or in certain tissues such as bone and liver.
All wounds undergo the same basic steps of repair. Acute wounds proceed in an orderly and timely reparative process to achieve sustained restoration of structure and function. A chronic wound stalls during a sustained inflammatory phase and fails to heal.
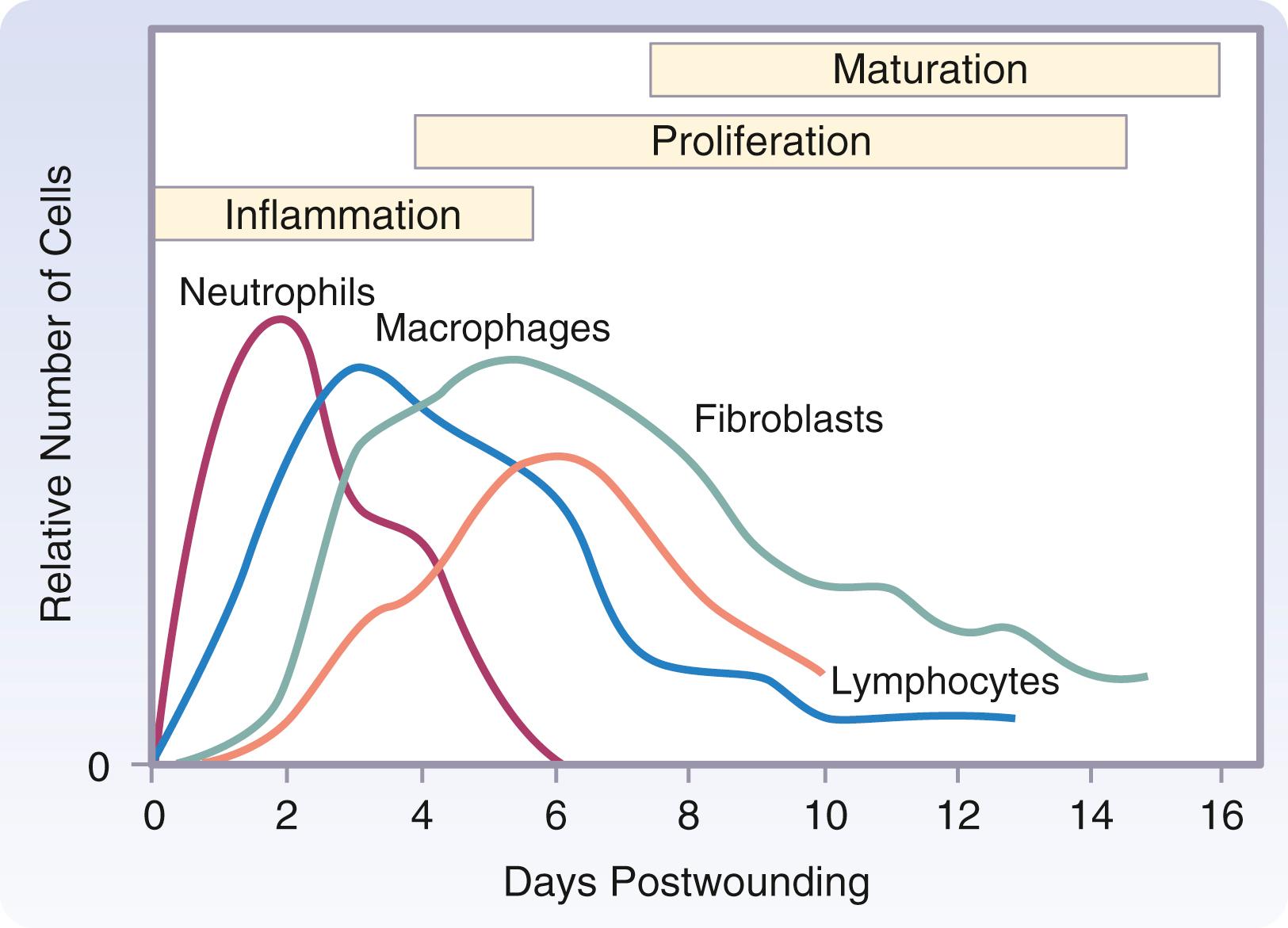
The three phases of wound healing are inflammation, proliferation, and maturation. In a large wound such as a pressure sore, the eschar or fibrinous exudate reflects the inflammatory phase, the granulation tissue is part of the proliferative phase, and the contracting or advancing edge is part of the maturational phase. All three phases may occur simultaneously, and the phases may overlap with their individual processes ( Fig. 6.1 ).
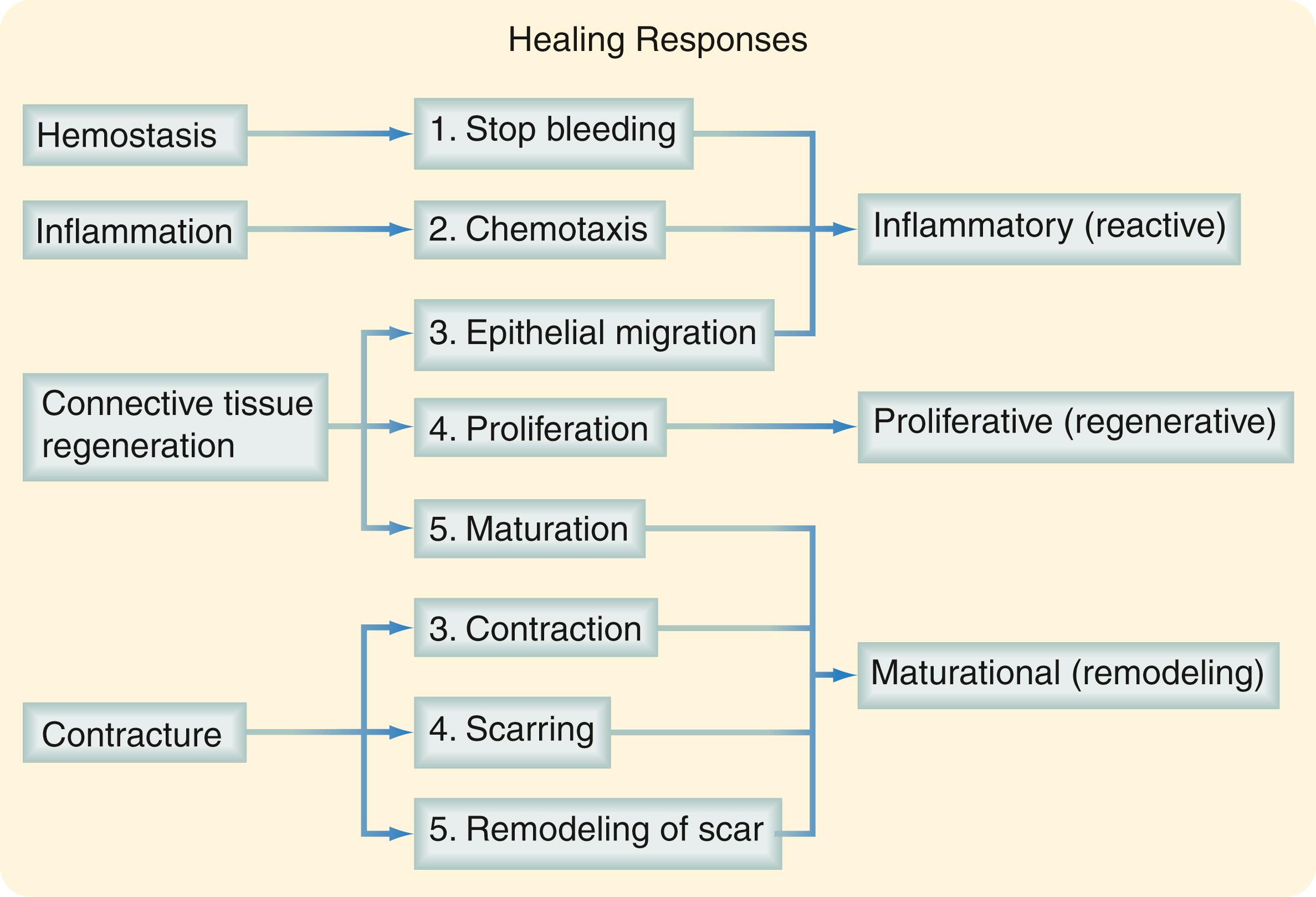
During the immediate reaction of the tissue to injury, hemostasis occurs quickly and is rapidly followed by inflammation. This phase represents an attempt to limit damage by stopping bleeding; sealing the wound surface; and removing necrotic tissue, foreign debris, and bacteria. The inflammatory phase is characterized by increased vascular permeability, migration of cells into the wound by chemotaxis, secretion of cytokines and growth factors into the wound, and activation of the migrating cells.
Blood vessel injury results in intense local arteriolar and capillary vasoconstriction followed by vasodilatation and increased vascular permeability ( Fig. 6.2 ). Erythrocytes and platelets adhere to the damaged capillary endothelium, resulting in plugging of capillaries and leading to cessation of hemorrhage. Platelet adhesion to the endothelium is primarily mediated through the interaction between high-affinity glycoprotein receptors and the integrin receptor GPIIb-IIIa (α IIb β 3 ). Platelets also express other integrin receptors that mediate direct binding to collagen (α 2 β 1 ) and laminin (α 6 β 1 ) or indirect binding to subendothelial matrix-bound fibronectin (α 5 β 1 ), vitronectin (α v β 3 ), and other ligands. Platelet activation occurs by binding to exposed type IV and type V collagen from the damaged endothelium, resulting in platelet aggregation. The initial contact between platelets and collagen requires von Willebrand factor VIII, a heterodimeric protein synthesized by megakaryocytes and endothelial cells.
Platelet binding results in conformational changes in platelets that trigger intracellular signal transduction pathways that lead to platelet activation and the release of biologically active proteins. Platelets release factors from two different sources: alpha granules and dense bodies. Platelet alpha granules are storage organelles that contain platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), insulin-like growth factor 1 (IGF-1), fibronectin, fibrinogen, thrombospondin, and von Willebrand factor. The dense bodies contain vasoactive amines, such as serotonin, that cause vasodilatation and increased vascular permeability. Mast cells adherent to the endothelial surface release histamine and serotonin, resulting in increased permeability of endothelial cells and causing leakage of plasma from the intravascular space to the extracellular compartment.
The platelets become activated, and the membrane phospholipids bind factor V, which allows interaction with factor X. Membrane-bound prothrombinase activity is generated and potentiates thrombin production exponentially. The thrombin itself activates platelets and catalyzes the conversion of fibrinogen to fibrin. The fibrin strands trap red blood cells to form the clot and seal the wound. The resulting lattice framework is the scaffold for endothelial cells, inflammatory cells, and fibroblasts to repair the damaged vessel.
Thromboxane A2 and prostaglandin F2α, formed from the degradation of cell membranes in the arachidonic acid cascade, also assist in platelet aggregation and vasoconstriction. Although these activities serve to limit the amount of injury, they can also cause localized ischemia, resulting in further damage to cell membranes and the release of more prostaglandin F2α and thromboxane A2.
Chemokines stimulate the migration of different cell types, particularly inflammatory cells, into the wound and are active participants in the regulation of the different phases of wound healing. The CXC, CC, and C ligand families bind to G protein–coupled surface receptors called CXC receptors and CC receptors.
Macrophage chemoattractant protein (MCP-1 or CCL2) is induced in keratinocytes after injury. It is a potent chemoattractant for monocytes/macrophages, T lymphocytes, and mast cells. Expression of this chemokine is sustained in chronic wounds and results in the prolonged presence of polymorphonuclear cells (PMNs) and macrophages, leading to the prolonged inflammatory response. Chemokine (C-X-C motif) ligand 1 (CXCL1; previously called GRO-α) is a potent PMN chemotactic regulator and is increased in acute wounds. It is also involved in reepithelialization. Interleukin-8 (IL-8; also known as CXCL8) expression is increased in acute and chronic wounds. It is involved in reepithelialization and induces the leukocyte expression of matrix metalloproteinases (MMPs), which stimulates remodeling. It is also a strong chemoattractant for PMNs and participates in inflammation. Relatively low levels of IL-8 are found in fetal wounds and may be why fetal wounds have decreased inflammation and heal without scars. Expression of the keratinocyte-produced CXCL10 is elevated in acute wounds and chronic inflammatory conditions in response to interferon-γ (IFN-γ). It impairs wound healing by increasing inflammation and recruiting lymphocytes to the wound. It also inhibits proliferation by decreasing reepithelialization and angiogenesis and preventing fibroblast migration. Stromal cell–derived factor-1 (SDF-1, also known as CXCL12) is expressed by endothelial cells, myofibroblasts, and keratinocytes and is involved in inflammation by recruiting lymphocytes to the wound and promoting angiogenesis. It is a potent chemoattractant for endothelial cells and bone marrow progenitors from the circulation to peripheral tissues. It also enhances keratinocyte proliferation, resulting in reepithelialization.
The release of histamine and serotonin leads to vascular permeability of the capillary bed. Complement factors such as C5a and leukotriene B4 promote neutrophil adherence and chemoattraction. In the presence of thrombin, endothelial cells exposed to leukotriene C4 and D4 release platelet-aggregating factor, which further enhances neutrophil adhesion. Monocytes and endothelial cells produce the inflammatory mediators IL-1 and tumor necrosis factor-α (TNF-α), and these mediators further promote endothelial-neutrophil adherence. Increased capillary permeability and chemotactic factors facilitate diapedesis of neutrophils into the inflammatory site. As the neutrophils begin their migration, they release the contents of their lysosomes and enzymes such as elastase and other proteases into the extracellular matrix (ECM), which further facilitates neutrophil migration. The combination of intense vasodilatation and increased vascular permeability leads to clinical findings of inflammation, rubor (redness), tumor (swelling), calor (heat), and dolor (pain). Local tissue swelling is further promoted by the deposition of fibrin, a protein end product of coagulation that becomes entrapped in lymphatic vessels.
Evidence suggests that the migration of PMNs requires sequential adhesive and deadhesive interactions between β 1 and β 2 integrins and ECM components. Integrin molecules are a family of cell surface receptors that are closely coupled with the cell’s cytoskeleton. These molecules interact with components of the ECM, such as fibronectin, to provide adhesion and to transduce signals to the interior of the cell.
Integrins are crucial for cell motility and are required in inflammation and normal wound healing as well as in embryonic development and tumor metastases. After extravasation, PMNs, attracted by chemotaxins, migrate through the ECM via transient interactions between integrin receptors and their ligands. Four phases of integrin-mediated cell motility have been described: adhesion, spreading, contractility or traction, and retraction. Activation of specific integrins through ligand binding has been shown to increase cell adhesion and activate reorganization of the cell’s actin cytoskeleton. Spreading is characterized by the development of lamellipodia and filopodia. Traction at the leading edge of the cell develops through binding of integrin followed by translocation of the cell over the adherent segment of the plasma membrane. The integrin is shifted to the rear of the cell and releases its substrate, permitting cell advancement. Regulation of integrin function by adhesive substrates offers a mechanism for local control of migrant cells. Within the assembled framework of the ECM, binding sites for integrins have been identified on collagen, laminin, and fibronectin.
The chemotactic agent mediates the PMN response through signal transduction as the chemotaxin binds to receptors on the cell surface. Bacterial products such as N -formyl-methionyl-leucyl-phenylalanine bind to induce cyclic adenosine monophosphate (AMP), but if there is maximal receptor occupancy, superoxide is produced at peak rates. Neutrophils also possess receptors for immunoglobulin G (IgG; Fc receptor) and the complement proteins C3b and C3bi. As the complement cascade is released and bacteria are opsonized, binding of these proteins to cell receptors on neutrophils allows recognition by the neutrophils and phagocytosis of the bacteria. When neutrophils are stimulated, they express more CR1 and CR3 receptors, permitting more efficient binding and phagocytosis of these bacteria.
Functional activation occurs after migration of PMNs into the wound site, which may induce new cell surface antigen expression, increased cytotoxicity, or enhanced production and release of cytokines. These activated neutrophils scavenge for necrotic debris, foreign material, and bacteria and generate free oxygen radicals with electrons donated by the reduced form of nicotinamide adenine dinucleotide phosphate. The electrons are transported across the membrane into lysosomes, where superoxide anion (O 2 − ) is formed. Superoxide dismutase catalyzes the formation of hydrogen peroxide (H 2 O 2 ), which is then degraded by myeloperoxidase in the azurophilic granules of neutrophils. This interaction oxidizes halides with the formation of byproducts such as hypochlorous acid. The iron-catalyzed reaction between H 2 O 2 and O 2 − forms hydroxyl radicals (OH⋅). This potent-free radical is bactericidal as well as toxic to neutrophils and surrounding viable tissues.
Migration of PMNs stops after several days or when wound contamination has been controlled. Individual PMNs survive no longer than 24 hours and are replaced predominantly by mononuclear cells. Continuing wound contamination or secondary infection causes complement system activation that provides a steady supply of chemotactic factors and a sustained influx of PMNs into the wound. A prolonged inflammatory phase delays wound healing, destroys normal tissue, and results in abscess formation and possibly systemic infection. PMNs are not essential for wound healing because their phagocytosis and antimicrobial role can be taken over by macrophages. Sterile incisions heal normally without the presence of PMNs.
The macrophage is the one cell that is truly crucial to wound healing by orchestrating the release of cytokines and stimulating many subsequent processes in wound healing ( Fig. 6.3 ). Tissue macrophages are derived from chemotaxis of migrating monocytes and appear within 24 to 48 hours of injury. When neutrophils start to disappear, macrophages appear and induce PMN apoptosis. Monocyte chemotactic factors include bacterial products, complement degradation products (C5a), thrombin, fibronectin, collagen, TGF-β, and PDGF-BB. Monocyte chemotaxis occurs as a result of the interaction of integrin receptors on the monocyte surface with ECM fibrin and fibronectin. The β integrin receptor also transduces the signal to initiate macrophage phagocytic activity. Activated integrin expression mediates monocyte transformation into wound macrophages. Transformation results in increased phagocytic activity and selective expression of cytokines and signal transduction elements by messenger RNA (mRNA), including the early growth response (EGR) genes, EGR2 and c- fos . Macrophages have specific receptors for IgG, C3b (CR1 and CR3), and fibronectin (integrin receptors) that permit surface recognition and phagocytosis of opsonized pathogens.
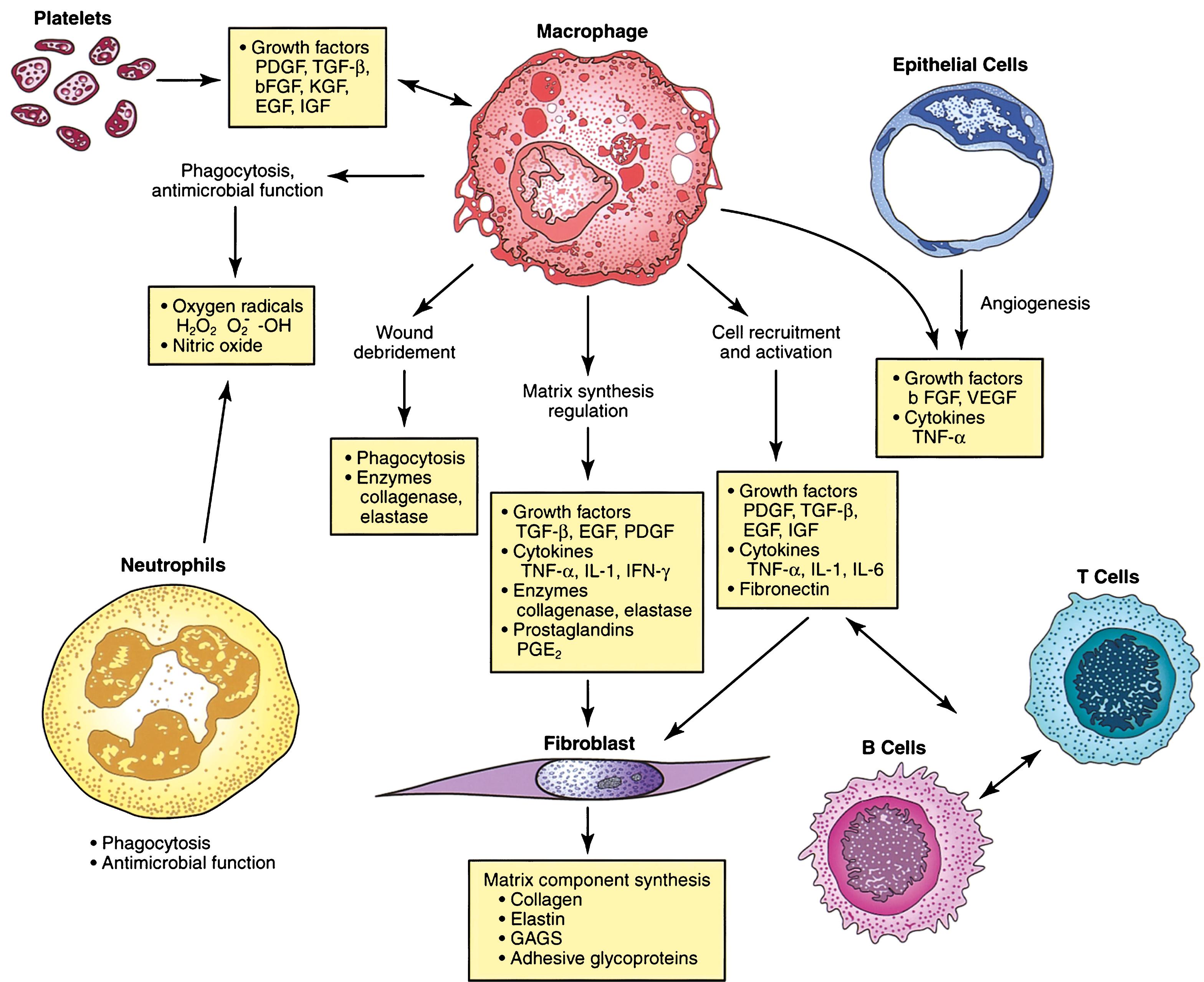
Bacterial debris, such as lipopolysaccharide, activates monocytes to release free radicals and cytokines that mediate angiogenesis and fibroplasia. The presence of IL-2 increases free radical release and enhances bactericidal activity. The activity of the free radicals is potentiated by IL-2. Free radicals generate bacterial debris, which further potentiates the activation of monocytes. Activated wound macrophages also produce nitric oxide (NO), a substance that has been demonstrated to have many functions other than antimicrobial properties.
As the monocyte or macrophage is activated, phospholipase is induced, cell membrane phospholipids are enzymatically degraded, and thromboxane A2 and prostaglandin F2α are released. The macrophage also releases leukotrienes B4 and C4 and 15-hydroxyeicosatetraenoic acid and 5-hydroxyeicosatetraenoic acid. Leukotriene B4 is a potent chemotaxin for neutrophils and increases their adherence to endothelial cells.
Wound macrophages release proteinases, including MMPs (MMP-1, MMP-2, MMP-3, and MMP-9), which degrade the ECM and are crucial for removing foreign material, promoting cell movement through tissue spaces and regulating ECM turnover. This activity is dependent on the cyclic AMP pathway and can be blocked by nonsteroidal antiinflammatory drugs or glucocorticoid drugs. Colchicine and retinoic acid appear to decrease collagenase production as well.
Macrophages secrete numerous cytokines and growth factors ( Tables 6.1 and 6.2 ). IL-1, a proinflammatory cytokine, is an acute-phase response cytokine. This endogenous pyrogen causes lymphocyte activation and stimulation of the hypothalamus, inducing the febrile response. It also directly affects hemostasis by inducing the release of vasodilators and stimulating coagulation. Its effect is further amplified as endothelial cells produce it in the presence of TNF-α and endotoxin. IL-1 has numerous effects, such as enhancement of collagenase production, stimulation of cartilage degradation and bone reabsorption, activation of neutrophils, regulation of adhesion molecules, and promotion of chemotaxis. It stimulates other cells to secrete proinflammatory cytokines. Its effects extend into the proliferative phase during which it increases fibroblast and keratinocyte growth and collagen synthesis. Studies have demonstrated increased levels of IL-1 in chronic nonhealing wounds, suggesting its role in the pathogenesis of poor wound healing. The early beneficial responses of IL-1 in wound healing appear to be maladaptive if elevated levels last beyond the first week after injury.
| Cytokine | Cell Source | Function | Type of wound | |
|---|---|---|---|---|
| Acute | Chronic | |||
| Proinflammatory Cytokines | ||||
| TNF-α | PMNs, macrophages | Inflammation, reepithelialization, PMN margination and cytotoxicity, with or without collagen synthesis; provides metabolic substrate | Increased levels | Increased levels |
| IL-1 | PMNs, monocytes, macrophages, keratinocytes | Inflammation, reepithelialization, fibroblast and keratinocyte chemotaxis, collagen synthesis | Increased levels | Increased levels |
| IL-2 | T lymphocytes | Increases fibroblast infiltration and metabolism | ||
| IL-6 | PMNs, macrophages, fibroblasts | Inflammation, reepithelialization, fibroblast proliferation, hepatic acute phase protein synthesis | Increased levels | Increased levels |
| IL-8 | Macrophages, fibroblasts | Inflammation, macrophage and PMN chemotaxis; reepithelialization, keratinocyte maturation and proliferation | Increased levels | Increased levels |
| IFN-γ | T lymphocytes, macrophages | Activates macrophages and PMNs, retards collagen synthesis and cross-linking, stimulates collagenase activity | ||
| Antiinflammatory Cytokines | ||||
| IL-4 | T lymphocytes, basophils, mast cells | Inhibition of TNF-α, IL-1, IL-6 production; fibroblast proliferation, collagen synthesis | ||
| IL-10 | T lymphocytes, macrophages, keratinocytes | Inhibition of TNF-α, IL-1, IL-6 production, inhibition of macrophage and PMN activation | ||
| Growth Factor | Cell Source | Function | Type of Wound | |
|---|---|---|---|---|
| Acute | Chronic | |||
| PDGF | Platelets, macrophages, endothelial cells, keratinocytes, fibroblasts | Inflammation; granulation tissue formation; reepithelialization; matrix formation and remodeling; chemotactic for PMNs, macrophages, fibroblasts, and smooth muscle cells; activates PMNs, macrophages, and fibroblasts; mitogenic for fibroblasts and endothelial cells; stimulates production of MMPs, fibronectin, and HA; stimulates angiogenesis and wound contraction | Increased levels | Decreased levels |
| TGF-β (including isoforms β 1 , β 2 , and β 3 ) | Platelets, T lymphocytes, macrophages, endothelial cells, keratinocytes, fibroblasts | Inflammation; granulation tissue formation; reepithelialization; matrix formation and remodeling; chemotactic for PMNs, macrophages, lymphocytes, and fibroblasts; stimulates TIMP synthesis, keratinocyte migration, angiogenesis, and fibroplasia; inhibits production of MMPs and keratinocyte proliferation; induces TGF-β production | Increased levels | Decreased levels |
| EGF | Platelets, macrophages, fibroblasts | Mitogenic for keratinocytes and fibroblasts; stimulates keratinocyte migration | Increased levels | Decreased levels |
| FGF-1 and FGF-2 family | Macrophages, mast cells, T lymphocytes, endothelial cells, fibroblasts, keratinocytes, smooth muscle cells, chondrocytes | Granulation tissue formation; reepithelialization; matrix formation and remodeling; chemotactic for fibroblasts; mitogenic for fibroblasts and keratinocytes; stimulates keratinocyte migration; angiogenesis; wound contraction and matrix deposition | Increased levels | Decreased levels |
| KGF (also called FGF-7) | Fibroblasts, keratinocytes, smooth muscle cells, chondrocytes, endothelial cells, mast cells | Stimulate proliferation and migration of keratinocytes, increase transcription of factors involved in detoxification of ROS; potent mitogen for vascular endothelial cells; upregulates VEGF; stimulates endothelial cell production of UPA | Increased levels | Decreased levels |
| VEGF | Keratinocytes, platelets, PMNs, macrophages, endothelial cells, smooth muscle cells, fibroblasts | Granulation tissue formation; increases vasopermeability; mitogenic for endothelial cells | Increased levels | Decreased levels |
| TGF-α | Macrophages, T lymphocytes, keratinocytes, platelets, fibroblasts, lymphocytes | Reepithelialization; increase keratinocyte migration and proliferation | ||
| IGF-1 | Macrophages, fibroblasts | Stimulates elastin production and collagen synthesis, fibroblast proliferation | ||
Microbial byproducts induce macrophages to release TNF. TNF-α is crucial in initiating the response to injury or bacteria. It upregulates cell surface adhesion molecules that promote the interaction of immune cells and endothelium. TNF-α is detected in a wound within 12 hours and peaks after 72 hours. Its effects include hemostasis, increased vascular permeability, and enhanced endothelial proliferation. Similar to IL-1, TNF-α induces fever, increased collagenase production, resorption of cartilage and bone, and release of PDGF as well as the production of more IL-1. However, excessive production of TNF-α has been associated with multisystem organ failure and increased morbidity and mortality in inflammatory disease states, partly through its effects on activating macrophages and neutrophils. Studies have noted elevated levels of TNF-α in nonhealing versus healing chronic venous ulcers. As in the case of IL-1, TNF-α appears to be essential in the early inflammatory response required for wound healing, but local and systemic persistence of this cytokine may lead to impaired wound maturation.
IL-6, which is produced by monocytes and macrophages, is involved in stem cell growth, activation of B cells and T cells, and regulation of the synthesis of hepatic acute-phase proteins. Within acute wounds, IL-6 is also secreted by PMNs and fibroblasts; an increase in IL-6 parallels the increase in the PMN count locally. IL-6 is detectable within 12 hours of experimental wounding and may persist at high concentrations for longer than 1 week. It also works synergistically with IL-1, TNF-α, and endotoxins. It is a potent stimulator of fibroblast proliferation and is decreased in aging fibroblasts and fetal wounds.
IL-8 is secreted primarily by macrophages and fibroblasts in the acute wound with peak expression within the first 24 hours. Its major effects have already been discussed and include increased PMN and monocyte chemotaxis, PMN degranulation, and expression of endothelial cell adhesion molecules.
IFN-γ, another proinflammatory cytokine, is secreted by T lymphocytes and macrophages. Its major effects are macrophage and PMN activation and increased cytotoxicity. It has also been shown to reduce local wound contraction and aid in tissue remodeling. IFN-γ has been used in the treatment of hypertrophic and keloid scars, possibly by its effect in slowing collagen production and cross-linking, whereas collagenase (MMP-1) production increases. Experimentally, it has been shown to impair reepithelialization and wound strength in a dose-dependent manner when applied locally or systemically. These findings suggest that administration of IFN-γ may improve scar hypertrophy by decreasing the strength of the wound.
Macrophages also release growth factors that stimulate fibroblast, endothelial cell, and keratinocyte proliferation and are important in the proliferative phase (see Table 6.2 ). Macrophage-secreted PDGF stimulates collagen and proteoglycan synthesis. PDGF exists as three isomers—PDGF-AA, PDGF-AB, and PDGF-BB. The PDGF-BB isomer is the only growth factor preparation approved by the U.S. Food and Drug Administration and is the most widely studied clinically.
TGF-α and TGF-β are released by activated monocytes. TGF-α stimulates epidermal growth and angiogenesis. TGF-β itself stimulates monocytes to express other peptides, such as TGF-α, IL-1, and PDGF. TGF-β, which is also released by platelets and fibroblasts within wounds, exists as at least three isomers—β 1 , β 2 , and β 3 —and its effects include fibroblast migration and maturation and ECM synthesis. TGF-β 1 has been shown to play an important role in collagen metabolism and healing of gastrointestinal injuries and anastomoses. In experimental models, TGF-β 1 accelerates wound healing in normal, steroid-impaired, and irradiated animals.
TGF-β is the most potent stimulant of fibroplasia, and its strong mitogenic effects have been implicated in the fibrogenesis seen in disease states such as scleroderma and interstitial pulmonary fibrosis. Enhanced expression of TGF-β 1 mRNA is found in keloid and hypertrophic scars. In contrast, fetal wounds have been demonstrated to have a paucity of TGF-β, suggesting that the scarless repair seen in utero occurs because of low or absent amounts of TGF-β. Studies of the three isomers have suggested that although TGF-β 1 and TGF-β 2 play an important role in tissue fibrosis and postinjury scarring, TGF-β 3 may limit scarring. As the concentration of TGF-β increases in the inflammatory site, fibroblasts are directly stimulated to produce collagen and fibronectin, leading to the proliferative phase.
Wound macrophages exhibit different functional phenotypes—M1 (classically activated) and M2 (alternatively activated)—that are at the extremes of a continuum of macrophage function. Lipopolysaccharide and IFN-γ stimulate the differentiation into M1 macrophages that release TNF-α, NO, and IL-6. These mediators are responsible for host defense but at the expense of significant collateral tissue damage. M2 macrophages are activated by IL-4 and IL-13; suppress inflammatory reactions and adaptive immune responses; and play an important role in wound healing, angiogenesis, and defense against parasitic infections. However, despite their beneficial functions, M2 macrophages can also be involved in different diseases, such as allergy, asthma, and fibrosis, which is the result of a helper T-cell (Th2) response predominated by IL-4 or IL-10. Both phenotypes are important when correctly balanced during the different phases of wound healing. In the inflammatory phase, greater M1 macrophage activity is required for macrophage debris scavenging and invading pathogen destruction. In the proliferative phase, M2 macrophages predominate. The balance between M1 and M2 macrophages is likely disturbed during abnormal wound-healing responses.
Several studies have demonstrated the importance of macrophages in wound healing by macrophage depletion. Macrophage depletion delays wound infiltration by fibroblasts and decreased wound fibrosis. Newborn animals that lacked macrophages, mast cells, and functional neutrophils as a result of defective myelopoiesis healed without scarring at the same speed as wild-type animals if their wounds were protected by antibiotic coverage, suggesting that inflammatory cells are not essential for wound closure. However, several models of specific inducible macrophage depletion based on genetically modified mice resulted in a detrimental effect of preinjury depletion of macrophages. Mice depleted before injury typically showed a defect in reepithelialization, granulation tissue formation, angiogenesis, wound cytokine production, and myofibroblast-associated wound contraction. Macrophage depletion 9 days after injury did not result in any morphologic or biologic differences between control and treatment mice, suggesting that macrophages may not be required at later stages of wound healing.
Significant numbers of T lymphocytes appear by day 5 after injury and peak on day 7. B lymphocytes appear to be principally involved in downregulating healing as the wound closes. Lymphocytes stimulate fibroblasts with cytokines (IL-2 and fibroblast-activating factor). Lymphocytes also secrete inhibitory cytokines (TGF-β, TNF-α, and IFN-γ). Antigen-presenting macrophages present bacterial “debris” or enzymatically degraded host proteins to lymphocytes, stimulating lymphocyte proliferation and cytokine release. T cells produce IFN-γ, which stimulates the macrophage to release TNF-α and IL-1. IFN-γ decreases prostaglandin synthesis, enhancing the effect of inflammatory mediators, suppressing collagen synthesis, and inhibiting macrophage exodus. IFN-γ appears to be an important mediator of chronic nonhealing wounds, and its presence suggests that T lymphocytes are primarily involved in chronic wound healing.
Drugs that suppress T-lymphocyte function and proliferation (steroids, cyclosporine, and tacrolimus) result in impaired wound healing in experimental wound models, possibly through decreased NO synthesis. In vivo lymphocyte depletion suggests the existence of an incompletely characterized T-cell lymphocyte population that is neither CD4+ nor CD8+ that seems to be responsible for the promotion of wound healing.
As the acute responses of hemostasis and inflammation begin to resolve, the scaffolding is laid for repair of the wound through angiogenesis, fibroplasia, and epithelialization. This stage is characterized by the formation of granulation tissue, which consists of a capillary bed; fibroblasts; macrophages; and a loose arrangement of collagen, fibronectin, and hyaluronic acid. Numerous studies have used growth factors to modify granulation tissue, particularly fibroplasia. Adenoviral transfer, topical application, and subcutaneous injection of PDGF, TGF-β, keratinocyte growth factor (KGF), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF) have been shown to increase the proliferation of granulation tissue.
Angiogenesis is the process of new blood vessel formation and is necessary to support a healing wound environment. After injury, activated endothelial cells degrade the basement membrane of postcapillary venules, allowing the migration of cells through this gap. Division of these migrating endothelial cells results in tubule or lumen formation. Eventually, deposition of the basement membrane occurs and results in capillary maturation.
After injury, the endothelium is exposed to numerous soluble factors and comes in contact with adhering blood cells. These interactions result in upregulation of the expression of cell surface adhesion molecules, such as vascular cell surface adhesion molecule-1. Matrix-degrading enzymes, such as plasmin and the metalloproteinases, are released and activated and degrade the endothelial basement membrane. Fragmentation of the basement membrane allows migration of endothelial cells into the wound, promoted by fibroblast growth factor (FGF), PDGF, and TGF-β. Injured endothelial cells express adhesion molecules, such as the integrin α v β 3 , which facilitates attachment to fibrin, fibronectin, and fibrinogen and facilitates endothelial cell migration along the provisional matrix scaffold. Platelet endothelial cell adhesion molecule-1 (PECAM-1), also found on endothelial cells, modulates their interaction with each other as they migrate into the wound.
Capillary tube formation is a complex process that involves cell-cell and cell-matrix interactions, modulated by adhesion molecules on endothelial cell surfaces. PECAM-1 has been observed to mediate cell-cell contact, whereas β 1 integrin receptors may aid in stabilizing these contacts and forming tight junctions between endothelial cells. Some of the new capillaries differentiate into arterioles and venules, whereas others undergo involution and apoptosis with subsequent ingestion by macrophages. Regulation of endothelial apoptosis is not well understood.
Angiogenesis appears to be stimulated and manipulated by various cytokines predominantly produced by macrophages and platelets. As the macrophage produces TNF-α, it orchestrates angiogenesis during the inflammatory phase. Heparin, which can stimulate the migration of capillary endothelial cells, binds with high affinity to a group of angiogenic factors.
VEGF, a member of the PDGF family of growth factors, has potent angiogenic activity. It is produced in large amounts by keratinocytes, macrophages, endothelial cells, platelets, and fibroblasts during wound healing. Cell disruption and hypoxia, hallmarks of tissue injury, appear to be strong initial inducers of potent angiogenic factors at the wound site, such as VEGF and its receptor. VEGF family members include VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor. VEGF-A promotes early events in angiogenesis and subsequently is crucial to wound healing. It binds to tyrosine kinase surface receptors Flt-1 (VEGF receptor-1) and kinase insert domain receptor (VEGF receptor-2). Flt-1 is required for blood vessel organization, whereas kinase insert domain receptor is important for endothelial cell chemotaxis, proliferation, and differentiation. Animal studies have shown that VEGF-A administration restores impaired angiogenesis found in diabetic ischemic limbs; however, other studies have shown that exogenous VEGF results in vascular leakage and disorganized blood vessel formation. VEGF-C, which is also elevated during wound healing, is primarily released by macrophages and is important during the inflammatory phase of wound healing. Although it works primarily through VEGF receptor-3, which is expressed in macrophages and lymphatic endothelium, it can also activate VEGF receptor-2, increasing vascular permeability. In vivo administration of VEGF-C in an animal model using an adenoviral vector to genetically diabetic mice resulted in accelerated healing. Placental growth factor is another proangiogenic factor that is elevated after wounding. It is involved in inflammation and expressed by keratinocytes and endothelial cells. It is believed to work synergistically with VEGF, potentiating its proangiogenic function.
Both acidic and basic FGFs (FGF-1 and FGF-2) are released from disrupted parenchymal cells and are early stimulants of angiogenesis. FGF-2 provides the initial angiogenic stimulus within the first 3 days of wound repair followed by a subsequent prolonged stimulus mediated by VEGF from day 4 through day 7. There is a dose-dependent effect of VEGF and FGF-2 on angiogenesis. Both TGF-α and EGF stimulate endothelial cell proliferation. TNF-α is chemotactic for endothelial cells; it promotes formation of the capillary tube and may mediate angiogenesis through its induction of hypoxia-inducible factor 1 (HIF-1). It regulates the expression of other hypoxia-responsive genes, including inducible NO synthase and VEGF. HIF-1α mRNA is prominently present in wound inflammatory cells during the initial 24 hours, and HIF-1α protein is present in cells isolated from the wound 1 and 5 days after injury in vitro. Data also suggest that there is a positive interaction between endogenous NO and VEGF, with endogenous NO enhancing VEGF synthesis. Similarly, VEGF has been shown to promote NO synthesis in angiogenesis, suggesting that NO mediates aspects of VEGF signaling required for endothelial cell proliferation and organization.
TGF-β is a chemoattractant for fibroblasts and probably assists in angiogenesis by signaling the fibroblast to produce FGFs. Other factors that have been shown to induce angiogenesis include angiogenin, IL-8, and lactic acid. Several of the matrix materials, such as fibronectin and hyaluronic acid from the wound site, are angiogenic. Fibronectin and fibrin are produced by macrophages and damaged endothelial cells. Collagen appears to interact by causing the tubular formation of endothelial cells in vitro. Angiogenesis results from the complex interaction of ECM material and cytokines.
Fibroblasts are specialized cells that differentiate from resting mesenchymal cells in connective tissue; they do not arrive in the wound cleft by diapedesis from circulating cells. After injury, the normally quiescent and sparse fibroblasts are chemoattracted to the inflammatory site; they divide and produce the components of the ECM. After stimulation by macrophage-derived and platelet-derived cytokines and growth factors, the fibroblast, which is normally arrested in the G 0 phase, undergoes replication and proliferation. Platelet-derived TGF-β stimulates fibroblast proliferation indirectly by releasing PDGF. The fibroblast can also stimulate replication in an autocrine manner by releasing FGF-2. To continue proliferating, fibroblasts require further stimulation by factors such as EGF or IGF-1. Although fibroblasts require growth factors for proliferation, they do not need growth factors to survive. Fibroblasts can live quiescently in growth factor–free media in monolayers or three-dimensional cultures.
The primary function of fibroblasts is to synthesize collagen, which they begin to produce during the cellular phase of inflammation. The time required for undifferentiated mesenchymal cells to differentiate into highly specialized fibroblasts accounts for the delay between injury and the appearance of collagen in a healing wound. This period, generally 3 to 5 days depending on the type of tissue injured, is termed the lag phase of wound healing. Fibroblasts begin to migrate in response to chemotactic substances such as growth factors (PDGF, TGF-β), C5 fragments, thrombin, TNF-α, eicosanoids, elastin fragments, leukotriene B4, and fragments of collagen and fibronectin.
The rate of collagen synthesis declines after 4 weeks and eventually balances the rate of collagen destruction by collagenase (MMP-1). At this point, the wound enters a phase of collagen maturation. The maturation phase continues for months or years. Glycoprotein and mucopolysaccharide levels decrease during the maturation phase, and new capillaries regress and disappear. These changes alter the appearance of the wound and increase its strength.
The epidermis serves as a physical barrier to prevent fluid loss and bacterial invasion. Tight cell junctions within the epithelium contribute to its impermeability, and the basement membrane zone gives structural support and provides attachment between the epidermis and the dermis. The basement membrane zone consists of several layers that secure the epidermodermal interface and connect the lamina densa to the dermis: (1) lamina lucida (electron clear), consisting of laminin and heparan sulfate; (2) lamina densa (electron dense), containing type IV collagen; and (3) anchoring fibrils, consisting of type IV collagen.
The basal layer of the epidermis attaches to the basement membrane zone by hemidesmosomes. Reepithelialization of wounds begins within hours after injury. Initially, the wound is rapidly sealed by clot formation and then by epithelial (epidermal) cell migration across the defect. Keratinocytes located at the basal layer of the residual epidermis or in the depths of epithelium-lined dermal appendages migrate to resurface the wound. Epithelialization involves a sequence of changes in wound keratinocytes—detachment, migration, proliferation, differentiation, and stratification. If the basement membrane zone is intact, epithelialization proceeds more rapidly. The cells are stimulated to migrate. Attachments to neighboring and adjoining cells and to the dermis are loosened, as demonstrated by intracellular tonofilament retraction, dissolution of intercellular desmosomes and hemidesmosomes linking the epidermis to the basement membrane, and formation of cytoplasmic actin filaments.
Epidermal cells express integrin receptors that allow them to interact with ECM proteins such as fibronectin. The migrating cells dissect the wound by separating the desiccated eschar from viable tissue. This path of dissection is determined by the integrins that the epidermal cells express on their cell membranes. Degradation of the ECM, required if epidermal cells are to migrate between the collagenous dermis and fibrin eschar, is driven by epidermal cell production of collagenase (MMP-1) and plasminogen activator, which activates collagenase and plasmin. The migrating cells are also phagocytic and remove debris in their path. Cells behind the leading edge of migrating cells begin to proliferate. The epithelial cells move in a leapfrog and tumbling fashion until the edges establish contact. If the basement membrane zone is not intact, it will be repaired first. The absence of neighboring cells at the wound margin may be a signal for the migration and proliferation of epidermal cells. Local release of EGF, TGF-α, and KGF and increased expression of their receptors may also stimulate these processes. Topical application of keratinocyte growth factor-2 (KGF-2) in young and aged animals accelerates reepithelialization. Basement membrane proteins, such as laminin, reappear in a highly ordered sequence from the margin of the wound inward. After the wound is completely reepithelialized, the cells become columnar and stratified again while firmly attaching to the reestablished basement membrane and underlying dermis.
The ECM exists as a scaffold to stabilize the physical structure of tissues, but it also plays an active and complex role by regulating the behavior of cells that contact it. Cells within it produce the macromolecular constituents, including (1) glycosaminoglycans (GAGs), or polysaccharide chains, usually found covalently linked to protein in the form of proteoglycans and (2) fibrous proteins such as collagen, elastin, fibronectin, and laminin.
In connective tissue, proteoglycan molecules form a gel-like ground substance. This highly hydrated gel allows the matrix to withstand compressive force while permitting rapid diffusion of nutrients, metabolites, and hormones between blood and tissue cells. Collagen fibers within the matrix serve to organize and strengthen the matrix, whereas elastin fibers give it resilience (matrix proteins have adhesive functions).
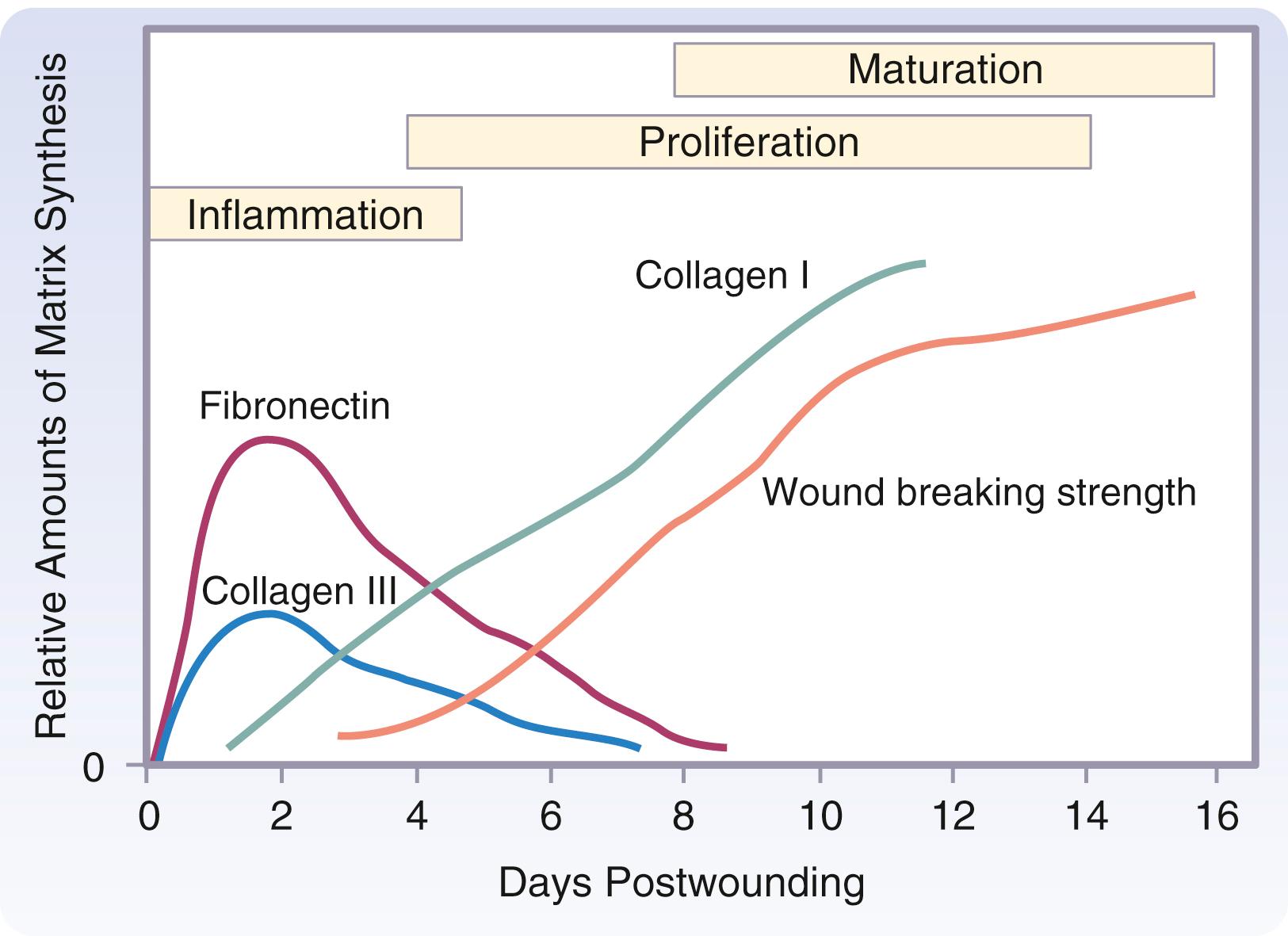
The wound matrix accumulates and changes in composition as healing progresses, balanced between new deposition and degradation ( Fig. 6.4 ). The provisional matrix is a scaffold for cellular migration and is composed of fibrin, fibrinogen, fibronectin, and vitronectin. GAGs and proteoglycans are synthesized next and support further matrix deposition and remodeling. Collagens, which are the predominant scar proteins, are the end result. Attachment proteins, such as fibrin and fibronectin, provide linkage to the ECM through binding to cell surface integrin receptors.
Stimulation of fibroblasts by growth factors induces upregulated expression of integrin receptors, facilitating cell-matrix interactions. Ligand binding induces clustering of integrin into focal adhesion sites. Regulation of integrin-mediated cell signaling by the extracellular divalent cations Mg 2+ , Mn 2+ , and Ca 2+ perhaps is caused by induction of conformational changes in the integrins.
A dynamic and reciprocal relationship exists between fibroblasts and the ECM. Cytokine regulation of fibroblast responses is altered by variations in the composition of the ECM. For example, expression of matrix-degrading enzymes, such as the MMPs, is upregulated after cytokine stimulation of fibroblasts. Collagenolytic MMP-1 is induced by IL-1 and downregulated by TGF-β. Activation of plasminogen to plasmin by plasminogen activator and procollagenase to collagenase by plasmin results in matrix degradation and facilitates cell migration. Modulation of these processes provides additional mechanisms whereby the cell-matrix interaction can be regulated during wound healing. Matrix modulation is also seen in tumor metastasis. Neoplastic cells lose their dependence on anchorage, mediated mainly by integrins; this is probably caused by decreased production of fibronectin and subsequent decreased adhesion, and, as a result, these cells can break away from the primary tumor and metastasize.
An example of the necessary dynamic interactions occurring in the provisional matrix during wound healing is the effect of TGF-β on incisional wounds sealed with fibrin sealant. Fibrin sealant is a derivative of plasma components that mimics the last step in the coagulation cascade. Commercially available fibrin sealant has an approximately ten fold greater concentration of fibrin than plasma and consequently provides a more airtight, waterproof seal. Fibrin sealant may serve as a mechanical barrier to the early cell-mediated events occurring in wound healing. Supplementation of fibrin sealant with TGF-β has been demonstrated to reverse the inhibitory effects of fibrin sealant on wound healing and increase tensile strength compared with sutured wounds. The increased tensile strength may be a result of improved cell migration into the wound site, more rapid clearance of fibrin sealant, suppression of gelatinase (MMP-9), and enhancement of ECM synthesis in TGF-β–supplemented wounds.
Collagens are found in all multicellular animals and are secreted by various cell types. They are a major component of skin and bone and constitute 25% of the total protein mass in mammals. The proline-rich and glycine-rich collagen molecule is a long, stiff, triple-stranded helical structure that consists of three collagen polypeptide α chains wound around one another in a ropelike superhelix. With its ringlike structure, proline provides stability to the helical conformation in each α chain, whereas glycine, because of its small size, allows tight packing of the three α chains to form the final superhelix. There are at least 20 types of collagen, the main constituents of connective tissue being types I, II, III, V, and XI. Type I is the principal collagen of skin and bone and is the most common. In adults, the skin is approximately 80% type I and 20% type III. In newborns, the content of type III collagen is greater than that found in adults. In early wound healing, there is also increased expression of type III collagen. Type I collagens are the fibrillar, or fibril-forming, collagens. They are secreted into the extracellular space, where they assemble into collagen fibrils (10–300 nm in diameter), which then aggregate into larger, cable-like bundles called collagen fibers (several micrometers in diameter).
Other types of collagens include types IX and XII (fibril-associated collagens) and types IV and VII (network-forming collagens). Types IX and XII are found on the surface of collagen fibrils and serve to link the fibrils to one another and to other components in the ECM. Type IV molecules assemble into a meshlike pattern and are a major part of the mature basal lamina. Dimers of type VII form anchoring fibrils that help attach the basal lamina to the underlying connective tissue and are especially abundant in the skin.
Type XVII and type XVIII collagens are two of a number of collagen-like proteins. Type XVII has a transmembrane domain and is found in hemidesmosomes. Type XVIII is located in the basal laminae of blood vessels. The peptide endostatin, which inhibits angiogenesis and shows promise as an anticancer drug, is formed by cleavage of the C-terminal domain of type XVIII collagen.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here