Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The current author would like to acknowledge B U. K. Li and Katja Kovacic for their significant contributions to the previous version of this chapter.
It is accepted that the ability to vomit developed as a protective mechanism to rid the body of ingested toxins. Unfortunately, vomiting also frequently occurs unrelated to the ingestion of noxious agents, a circumstance that produces several clinical challenges. First, vomiting can be a sign of many diseases that affect different organ systems. Therefore determining the cause of a vomiting episode can be difficult. On the other hand, vomiting can be present in a patient without any evidence of an underlying inflammatory, anatomic, neoplastic, or metabolic process. Second, vomiting can produce several complications (e.g., electrolyte derangement, prolapse gastropathy, and blood loss) that demand diagnosis and treatment. Third, vomiting is a frequent complication of medical therapy (surgical procedures, cancer chemotherapy). Fourth, selection of appropriate therapy for this distressing problem is essential to improve patient comfort and avoid the additional medical complications associated with the vomiting.
Vomiting (emesis) is a complex reflex behavioral response to a variety of stimuli and has three phases: (1) a prodromal period consisting of the sensation of nausea and signs of autonomic nervous system (ANS) activation, (2) retching, and (3) vomiting or forceful expulsion of the stomach contents through the oral cavity. Although the overall sequence of these three phases is stereotypical, each can occur independently of the others. For example, nausea does not always progress to vomiting, and pharyngeal stimulation can induce vomiting without a prodrome of nausea. It is important to note that vomiting and regurgitation (defined as effortless reflux of the intragastric contents into the esophagus) are not synonymous. Clinically, vomiting can be distinguished from regurgitation, because regurgitation is not preceded by prodromal events, retching does not occur, and gastric contents are not forcibly expelled. The differentiation between vomiting and regurgitation is critical because each has different causes and is produced by distinct physiologic mechanisms. Furthermore, retching and vomiting, commonly thought to occur in tandem, can also occur independently of one another.
The events that herald the onset of the act of vomiting are nausea and several autonomic manifestations. , , Nausea is a subjective experience that is difficult to define. It is usually described as an unpleasant but painless sensation associated with the feeling that vomiting is imminent. This wavelike, aversive feeling is usually referred to the stomach, but some individuals perceive it in the back of the throat or head. The autonomic signs include cutaneous vasoconstriction, sweating, dilation of pupils, increased salivation, and tachycardia. Several gastrointestinal (GI) motor events characterize the emetic prodrome. , There is inhibition of spontaneous contractions within the GI tract and dilation of the proximal stomach. The esophageal skeletal muscle shortens longitudinally, pulling the relaxed proximal stomach (hiatus and cardia) into the thoracic cavity, with loss of the abdominal segment of the esophagus. These anatomic changes result in the free flow of gastric contents into the esophagus. Soon after, a single large-amplitude contraction is initiated in the jejunum and propagates toward the stomach at 8 to 10 cm/s. , This retropulsive event is termed the retrograde giant contraction (RGC). It propels the duodenal contents into the stomach before the onset of retching. , The RGC is followed by a brief period of moderate-amplitude contractions in the distal small intestine and a second period of inhibition lasting several minutes.
The two major somatic motor components of vomiting (retching and expulsion) are produced by the coordinated action of the respiratory, pharyngeal, and abdominal muscles, resulting in rhythmic changes in intrathoracic and intraabdominal pressures. , During each cycle of retching, the glottis closes and the diaphragm, external intercostal muscles, and abdominal muscles contract, , producing large negative intrathoracic and positive intraabdominal pressure spikes. The esophagus dilates and the atonic proximal stomach continues to be displaced into the thoracic cavity. The antireflux mechanisms are overcome, and the gastric contents move back and forth into the esophagus with each cycle of retching.
Sometime after the onset of retching, expulsion or vomiting occurs. During this event the external intercostal muscles and the diaphragmatic crura relax and the abdominal muscles and costal diaphragm contract violently, , producing positive pressures in both abdomen and thorax, resulting in oral propulsion of the gastric contents. Retrograde contraction of the cervical esophagus assists in oral expulsion. After expulsion, antegrade peristalsis in the esophagus clears the lumen of residual material and the proximal stomach returns to its normal intraabdominal position, restoring the normal antireflux anatomy.
GI motor activity during the emetic reflex is mediated by the vagus nerve. Vagal preganglionic parasympathetic fibers activate both inhibitory and excitatory pathways in the enteric nervous system. A wide range of stimuli induce nausea and vomiting. However, these GI motor events do not appear to be the cause of the sensation of nausea, which may be a physiologic process separate from vomiting. Moreover, the programmed somatic pattern of retching and vomiting continues even when the GI motor correlates of vomiting are prevented by disruption of the vagal efferents. ,
Although GI motor activity is not necessary for retching and vomiting, the motor changes that do occur may serve a significant role. As a defense against noxious ingested agents, relaxation of the stomach can confine a toxin before it is expelled, and the RGC can move toxins and alkaline duodenal secretions to the stomach to buffer and dilute gastric irritants (e.g., vinegar, hypertonic saline) in preparation for expulsion. The buffering of the gastric contents can also serve to protect the esophagus from acid injury. Finally, changes in the position of the stomach can place it in an advantageous position for compression by the abdominal musculature.
A different pattern of GI motor activity is observed in circumstances in which nausea is induced by motion. Before the onset of nausea, an increase occurs in the gastric slow waves from 3 to 9 cycles/min. , This phenomenon, known as tachygastria , is controlled by central cholinergic and α-adrenergic pathways. In motion-induced nausea, the GI motor activity appears to play a role in the induction of symptoms. ,
The emetic reflex consists of an afferent limb (receptor and pathway), central integration and control, and an efferent limb (pathway and effector) ( Fig. 8.1 ). , This reflex can be induced by visceral pain and inflammation, toxins, motion, pregnancy, radiation exposure, postoperative states, and unpleasant emotions. The diverse afferent receptors and pathways may originate within the gut, oropharynx, heart, vestibular system, or central nervous system (e.g., area postrema, hypothalamus, and cortical regions). These multiple afferent pathways are integrated within the brainstem, and the emetic reflex is completed through a common integrated efferent limb consisting of multiple pathways and effectors.
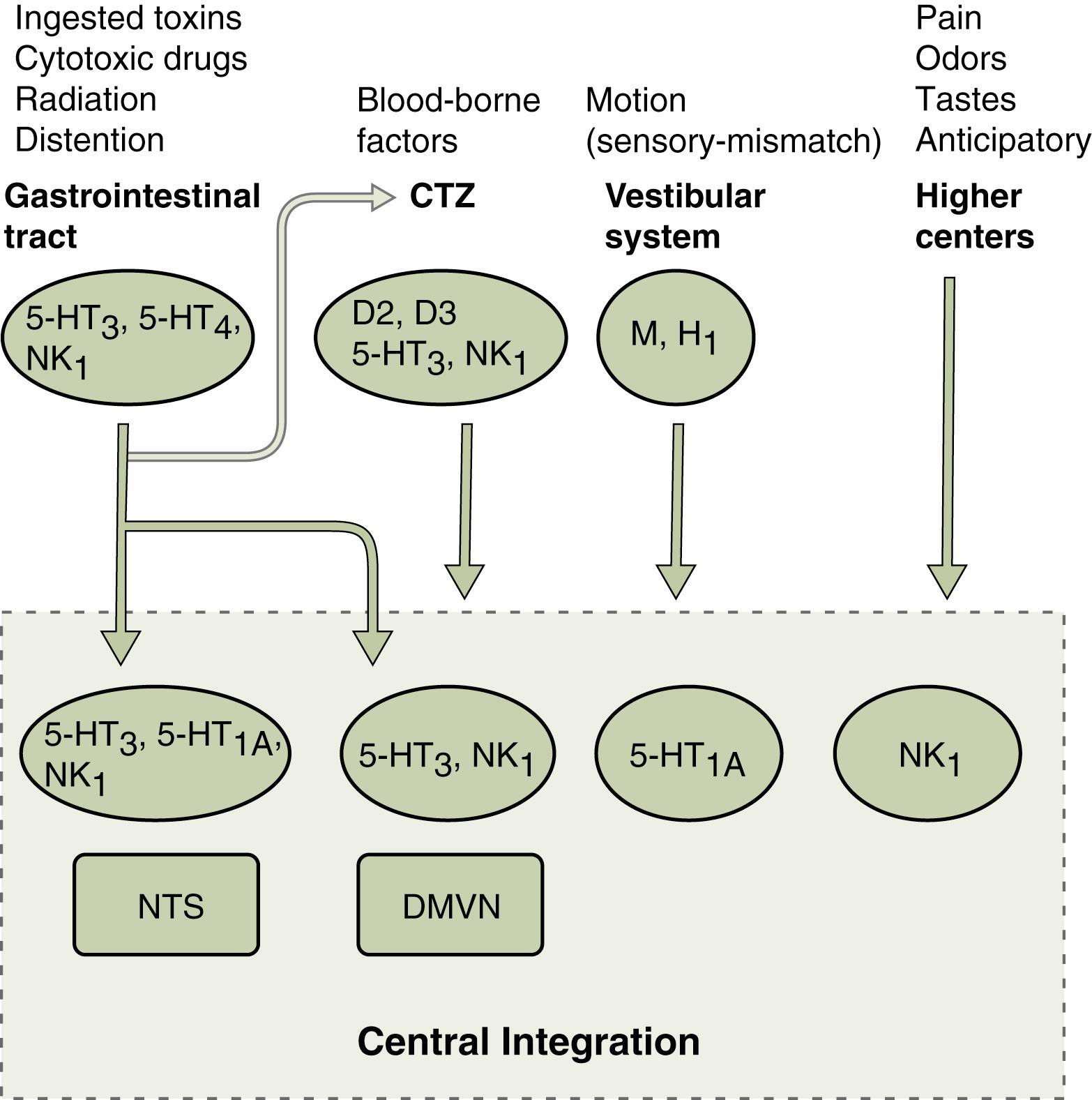
Within the GI tract, multiple receptors are capable of initiating the emetic reflex. , Mechanoreceptors present within the muscularis are activated by changes in tension and may be stimulated by passive distension or active contraction of the bowel wall. These conditions are present in bowel obstruction, a clinical state that causes vomiting. Chemoreceptors within the mucosa of the stomach and proximal small bowel respond to a wide range of chemical irritants (hydrochloric acid [HCl], copper sulfate, vinegar, hypertonic saline, syrup of ipecac) and are involved in radiation-and chemotherapy-induced emesis. The afferent pathways from the GI tract are mediated principally via the vagus nerves; the splanchnic nerves play a minor role. Vagal afferent fibers project centrad principally to the dorsomedial portion of the nucleus of the solitary tract (NTS), and to a lesser extent to the area postrema and the dorsal motor vagal nucleus.
Circulating toxins can trigger the emetic reflex. The major detector of blood-borne noxious agents is the chemoreceptor trigger zone (CTZ), which is located within the area postrema on the floor of the fourth ventricle, outside of the blood–brain barrier. Substances in the cerebrospinal fluid and bloodstream can be detected by the cells of this region. Several types of receptors for endogenous neurotransmitters and neuropeptides have been localized to the CTZ. , Intravenous (IV) infusion or direct application of these neuroactive agents (dopamine, acetylcholine, enkephalin, peptide YY, and substance P) to the CTZ can induce vomiting. Stimulation of the CTZ is essential for the induction of vomiting by these and other agents (apomorphine, cisplatin) but not for that induced by the stimulation of abdominal vagal afferents or motion. In addition to playing a role in vomiting, the area postrema is involved in taste aversion, control of food intake, and fluid homeostasis. ,
Activation of the afferent limb of the vomiting reflex may also occur through real or apparent motion of the body. Motion-induced vomiting is the result of a sensory mismatch involving the visual, vestibular, and proprioceptive systems, although an intact vestibular system is a necessary component. Histamine (H 1 ) and cholinergic muscarinic receptors are involved in the afferent limb of this pathway. In addition to the foregoing afferent pathways, higher cortical centers can also activate the emetic reflex when stimulated by unpleasant situations or in instances of conditioned vomiting (e.g., anticipatory vomiting in chemotherapy).
Although no single central locus has been identified as a “vomiting center,” two models of central coordination of the emetic reflex have been proposed: (1) a group of nuclei (paraventricular system of nuclei, defined by their connection to the area postrema) form a linked neural system whose activation can account for all of the phenomena associated with vomiting, and (2) vomiting is produced by the sequential activation of a series of discrete effector (motor) nuclei as opposed to being activated in parallel by a single locus. Furthermore, the concept of a localized “vomiting center” has been refuted by recent anatomic studies implicating multiple groups of neurons within the medulla (nucleus ambiguus) activated in sequence by a “central pattern generator” that controls the emetic reflex.
A wide variety of neurotransmitters, neuroactive peptides, and hormones are involved in the emetic reflex. As investigations proceed into the physiology of vomiting and the pharmacology of antiemetic agents, the role of these and other mediators will continue to be defined.
Dopaminergic pathways have long been known to participate in the emetic reflex. Apomorphine, a commonly used experimental emetic agent, acts through the dopamine (D 2 subtype) receptor. Furthermore, several clinically effective antiemetic agents (e.g., metoclopramide) are D 2 receptor antagonists. The site of action of these agents (agonists and antagonists) is the CTZ, where a high density of D 2 receptors is present. , These receptors participate in the emetic reflex induced by several, but not all, noxious agents acting through the CTZ. In addition to this subclass of receptors, recent evidence has implicated D 3 receptors within the area postrema as having a role in the emetic reflex.
The importance of serotonin (5-hydroxytryptamine or 5-HT) and serotonin receptors in the emetic reflex has been demonstrated by the observation that cisplatin-induced vomiting can be prevented by blockade of 5-HT 3 receptors. In addition to its involvement in mediating the emetic response to several chemotherapeutic agents, 5-HT 3 receptors play an important role in vomiting induced by radiation therapy and noxious substances in the GI tract. , The 5-HT 3 receptors are present on vagal afferent fibers in the GI tract and the presynaptic vagal afferent terminals within the central nervous system, specifically in the NTS and CTZ in the area postrema. , Current evidence indicates that chemotherapeutic agents, irradiation, and various noxious substances act directly on the GI mucosa, inducing release of serotonin from enterochromaffin cells. Terminal vagal afferents in proximity are stimulated, producing afferent activation of the emetic reflex. The precise role of the 5-HT 3 receptors on the presynaptic vagal afferents within the central nervous system has not been fully elucidated, but they appear to facilitate the emetic reflex induced by some afferent pathways (e.g., cranial irradiation, chemotherapeutic agents within the cerebrospinal fluid). Other members of the 5-HT receptor family also may be involved in the emetic reflex. The 5-HT 4 receptor has been shown to be necessary in the afferent limb of the emetic reflex induced by at least one GI irritant. Blockade of central 5-HT 1A receptors, located primarily in the NTS, prevents emesis induced by a broad range of stimuli. ,
Animal studies have convincingly linked physical and psychological stress to gastric stasis via central corticotropin-releasing factor (CRF) acting on CRF-R2 at the dorsomotor nucleus of the vagus. During exposure to stress, CRF initiates the hypothalamic-pituitary-adrenal (HPA) axis and could play an initiating role in emesis. The role of CRF in humans remains to be established, but its effects can produce the behavioral, neuroendocrine, autonomic, immunologic, and visceral responses to stress. Given its link between stress and GI motility, CRF may also be responsible for stress-induced nausea and dyspepsia.
Substance P (a member of the neurokinin family of peptides) and its receptor neurokinin NK 1 (tachykinin) are widely distributed in the central nervous system and peripheral neural and extraneural tissues. Evidence in animal models of vomiting has demonstrated that this ligand and receptor are critical to the emetic response produced by a wide range of stimuli. , NK 1 receptor antagonists prevent vomiting produced by IV (morphine) and intragastric toxins (ipecac, copper sulfate), chemotherapeutic agents (cisplatin), and motion. The site of action of these antagonists is believed to be NK 1 receptors located in the central nervous system (NTS, dorsal motor vagal nucleus). , Because blockade of this receptor prevents emesis induced by both peripheral and central-acting agents, it has been suggested that NK 1 receptors are critical elements in the central integration or effector pathway common to all emesis-inducing stimuli. Tachykinin receptor antagonists (aprepitant and its newer IV prodrug fosaprepitant) are now included in clinical treatment guidelines for moderate to highly emetogenic chemotherapy-induced vomiting.
Cannabinoid CB1 receptors located in the dorsal vagal complex inhibit the emetic reflex. , Cannabinoid agonists also modulate 5-HT 3 ion channels. Thus the CB and 5-HT 3 receptor systems colocalize and interact in the brainstem and thus contribute to the circuitry of the emetic reflex.
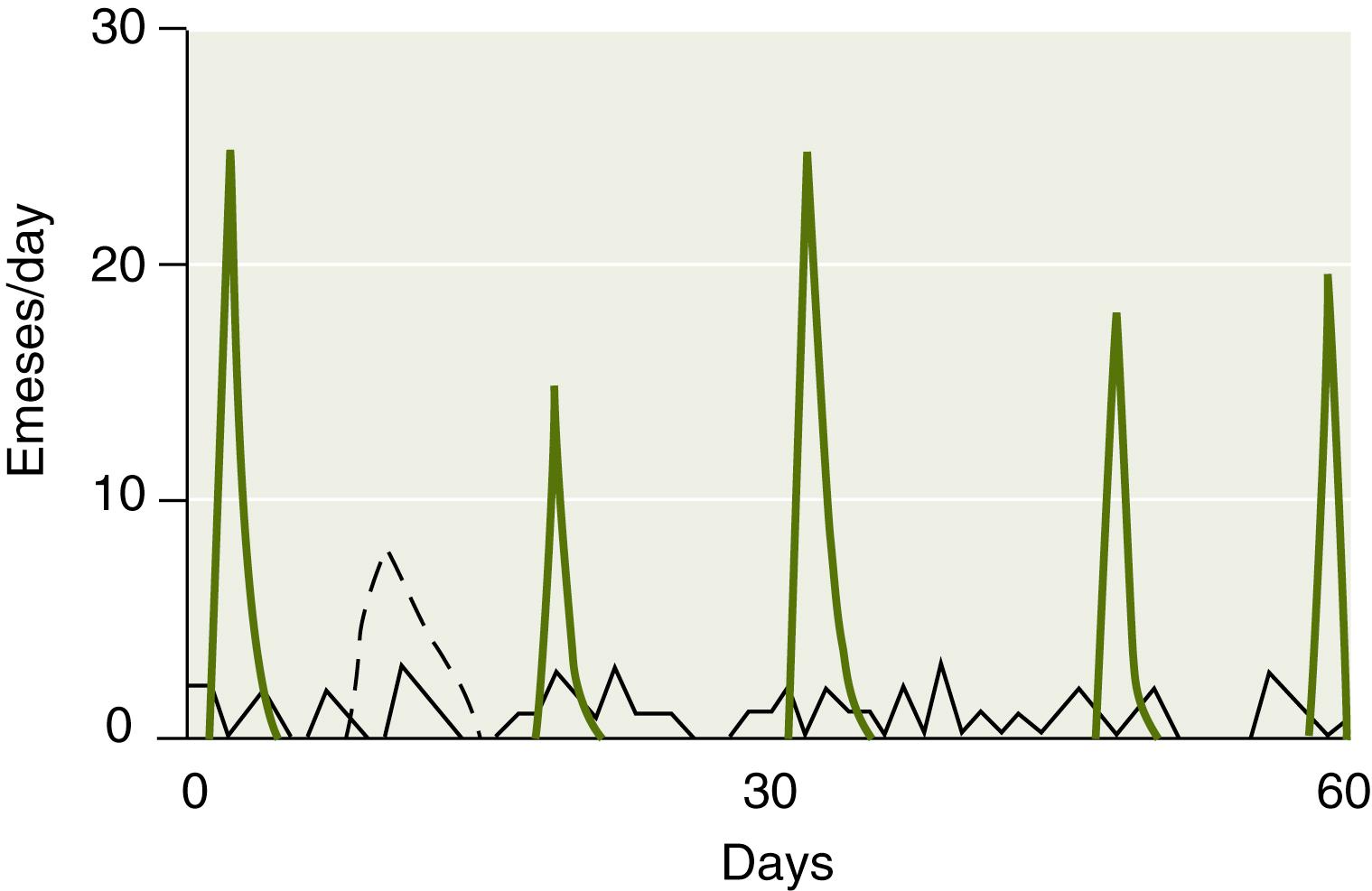
There are three temporal patterns of vomiting: one acute and two recurrent, chronic and cyclic ( Fig. 8.2 ). Because of its frequent association with infections of childhood such as viral gastroenteritis, the acute form is the most common and is characterized by an episode of vomiting of moderate to high intensity. Recurrent vomiting is also a common problem encountered by pediatricians and gastroenterologists and could be further subclassified: into chronic , a low-grade (1 to 2 times/day) daily pattern, and cyclic , an intensive but intermittent pattern ( Table 8.1 ). Because both the acute and cyclic patterns can produce intense vomiting, until the repetitive nature (more than three episodes) becomes evident, the cyclic pattern is understandably misclassified as an acute one and thus is typically misdiagnosed as a bout of viral gastroenteritis or food poisoning.
| Clinical Feature | Acute | Chronic Recurrent | Cyclic Recurrent |
|---|---|---|---|
| Epidemiology | Most common | Two-thirds of recurrent vomiting cohort | One-third of recurrent vomiting cohort |
| Acuity | Moderate-severe, ± dehydration | Not acutely ill or dehydrated | Severe, dehydrated |
| Vomiting intensity | Moderate to high | Low, 1 to 2 emeses per hour at the peak | High, ∼6 emeses per hour at peak |
| Recurrence, rate | No | Frequent, >2 episodes per week | Infrequent, ≤2 episodes per week |
| Stereotypy | Unique— if child has had three similar episodes, consider cyclic pattern | No | Yes |
| Onset | Variable | Daytime | Early morning |
| Symptoms | Fever, diarrhea | Abdominal pain, diarrhea | Pallor, lethargy, nausea, abdominal pain |
| Household contacts affected | Usually | No | No |
| Family history of migraine headache | 14% positive | 82% positive | |
| Causes | Viral infections | Ratio of GI to extra-GI causes 7:1; upper GI tract mucosal injury most common (esophagitis, gastritis) | Ratio of extra - GI to GI causes 5:1; cyclic vomiting syndrome most common (also hydronephrosis, metabolic, cannabinoid hyperemesis syndrome) |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here