Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
directly acting antiviral agent
hepatitis C virus
interferon
interleukin
interferon-stimulated gene
natural killer
regulated upon activation, normally T cell expressed, and presumably secreted
RNA-dependent RNA polymerase
tumor necrosis factor–related apoptosis-inducing ligand
T-regulatory cell
Hepatitis C virus (HCV) infection is a prominent cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC), with up to 185 million chronically infected individuals worldwide, most of whom are undiagnosed and untreated. A protective vaccine is not available, and those who clear infection spontaneously or with treatment are susceptible to reinfection. The number of untreated patients presenting with long-term seqeula of chronic hepatitis C infection, including hepatocellular carcinoma, is expected to increase during the next decade. Recent advances in HCV therapeutics with the development and clinical application of directly acting antiviral agents (DAAs) have transformed HCV management in the developed world, although DAAs are not yet widely available worldwide. This chapter will provide an overview of the principles of HCV virology and the immunopathogenic mechanisms of disease.
HCV was identified in 1989 as the most common etiologic agent of posttransfusion and sporadic non-A, non-B hepatitis, a clinical entity first reported in 1975. Despite difficulties propagating HCV infection in vitro , marked scientific progress has been made during the past 26 years with use of heterologous expression systems, functional infectious complementary DNA clones in chimpanzees, the replicon system, retroviral pseudoparticles displaying functional HCV glycoproteins, and complete cell culture systems.
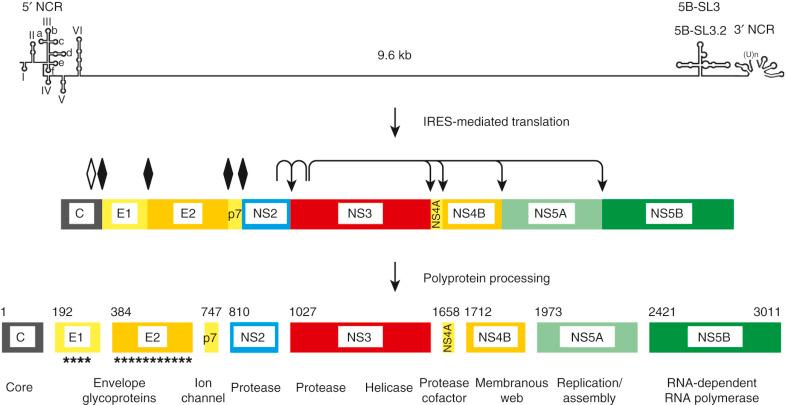
HCV is classified in the genus Hepacivirus within the family Flaviviridae, which also includes yellow fever virus, dengue virus, and West Nile virus. The genetic organization and polyprotein processing of HCV are illustrated in Fig. 28-1 . HCV contains a 9.6-kb positive-strand RNA genome composed of a 5′ noncoding region with an internal ribosome entry site, an open reading frame that encodes the structural and nonstructural proteins, and a 3′ noncoding region. The structural proteins include the core and envelope glycoproteins E1 and E2, and the p7 viroporin. The nonstructural proteins include the NS2-3 and NS3-4A proteases, the NS3 RNA helicase, the NS4B and NS5A proteins, and the NS5B RNA-dependent RNA polymerase (RdRp). Infectious virus is associated with low-density and very low density lipoproteins, as well as host apolipoproteins B, C1, and E. HCV infection is a highly dynamic process, with an estimated serum HCV half-life of less than 1 hour, an estimated infected hepatocyte half-life of approximately 1 week, and production and clearance of up to 10 virions per day in an infected individual. High replicative activity and the lack of a proofreading function of the viral RdRp is the basis of the high genetic variability of HCV. HCV isolates are classified into genotypes and subtypes. The seven HCV genotypes differ in their nucleotide sequence by 30% to 35%, and within an HCV genotype, subtypes (designated a, b, etc.) can be defined, and differ in nucleotide sequence by approximately 20%. Genotype distribution varies globally, and HCV genotype is associated with variable responses to interferon (IFN)-based and IFN-free treatment with DAAs.
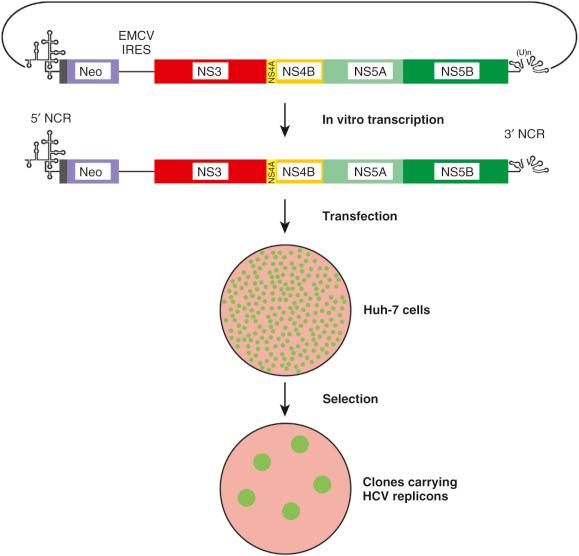
The development of selectable subgenomic, dicistronic HCV RNA replicons, capable of autonomous replication in Huh-7 cells (a human hepatocyte-derived cellular carcinoma cell line) transformed opportunities to conduct HCV research ( Fig. 28-2 ). This model allows in vitro study of HCV RNA, genetic dissection of HCV RNA elements and proteins, and biochemical and ultrastructural characterization of the viral replication complex, and has facilitated the discovery and development of DAAs and the investigation of antiviral resistance in vitro . Furthermore, it has been used to study interactions between viral and host cell components. A major breakthrough came with the discovery that a genotype 2a strain of virus, derived from a Japanese patient with a rare case of fulminant hepatitis and designated JFH-1 (for Japanese fulminant hepatitis 1), was able to replicate and release infectious particles in cell culture, which was subsequently also achieved with the H77 genotype 1a isolate, facilitating studies to explore the viral life cycle. Cell culture–derived HCV particles were found to be infectious in vivo in chimpanzees and immunodeficient mice with chimeric human livers, and viral inocula derived from these animal models were infectious for naïve Huh-7 cells in vitro . This enabled the study of the life cycle steps, including viral entry, genome packaging, virion assembly, maturation, and release. The challenge remains to develop in vivo and ex vivo models that mimic the natural environment of the liver, as primary HCV isolates show a poor ability to replicate in tissue culture.
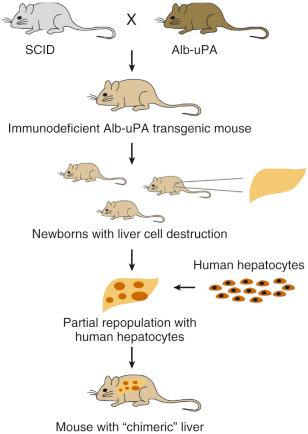
Progress in the development of a small animal model of HCV replication has been achieved with the successful infection of immunodeficient mice reconstituted with human hepatocytes. Inoculation with serum from patients with hepatitis C results in persistent HCV viremia in mice with high-level human hepatocyte engraftment, with viral titers similar to those found in infected humans ( Fig. 28-3 ). This model has been used successfully to investigate the in vivo phenotype of specific mutations introduced into the HCV genome, selected aspects of viral pathogenesis, and neutralization of viral entry, and to evaluate novel antiviral strategies. Table 28-1 summarizes the current model systems and the advantages and limitations of each of them.
| Description | Advantages | Limitations | |
|---|---|---|---|
| Subgenomic replicon | Bicistronic RNA construct, in vitro cell line transfection model | Allows study of nonstructural proteins in the life cycle, crucial for DAA development (NS3-4A, NS5A, NS5B), amenable for study of all HCV genotypes, capable of autonomous replication | Lacks structural proteins, no infectious virus release, does not recapitulate the entire HCV life cycle |
| HCV pseudotyped particles | HIV/MLV-defective retroviral particles expressing HCV E1/E2, in vitro production using producer cell line | Allows study of HCV entry, attachment factors, receptors | No infectious particles, lacks nonstructural HCV proteins, does not allow study of the entire HCV life cycle |
| Cell culture–derived HCV | JFH1 genotype 2a clinical isolate, replicates in vitro in cell lines | Can study the entire HCV life cycle, including entry, replication, assembly, and egress, infectious in humanized mouse models and chimpanzees, can study genotypic chimeras | A single unique clinical isolate, questions of generalizable findings relative to clinical isolates |
| Primary human hepatocytes, induced pluripotent stem cells, human embryonic stem cells | In vitro , nonimmortalized cell culture models, support various levels of replication and infected cell frequencies | Innate immune signaling pathways intact, can study the entire HCV life cycle | Restricted levels of replication and infection, poor replication of primary clinical isolates |
| Chimpanzee, humanized mouse models | In vivo infection models, multiple mouse models with genetic expression of HCV entry factors, or chimeric human liver and/or immune systems | In vivo infection models, support entire HCV life cycle and amenable for longitudinal study, afford study of genetic modulation of HCV genome | Question of recapitulation of human disease and generalizability of findings |
The structures and membrane association of HCV proteins are illustrated in Figs. 28-4 and 28-5 .
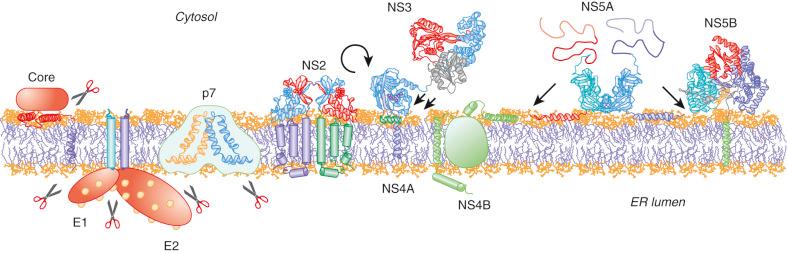
The first structural protein encoded by the HCV open reading frame is the core protein, which forms the viral nucleocapsid. The mature core is a dimeric α-helical membrane protein composed of two domains. The association of the core with lipid droplets and interaction with NS5A play central roles in viral assembly. In addition, the interaction of the core with lipid droplets may affect lipid metabolism, contributing to the development of hepatic steatosis, particularly observed in patients with HCV genotype 3 infection.
The envelope proteins E1 and E2 are glycosylated and form a noncovalent heterodimer, which is believed to represent the building block for the viral envelope. E1 and E2 are anchored to the endoplasmic reticulum membrane by C-terminal transmembrane domains, which are also involved in the heterodimerization and endoplasmic reticulum retention of the proteins. Recent insights into the structure and function of the E1 and E2 have been gained through crystal structure analysis. The genes encoding E1 and E2 are notably variable. A hypervariable region (HVR) of approximately 28 amino acids in the N-terminal domain of E2 has been termed hypervariable region 1 and differs by up to 80% among HCV isolates.
p7 is a hydrophobic 63–amino acid polypeptide that is not required for RNA replication in vitro but is essential for the assembly and release of infectious HCV in vitro , potentially through interaction with NS5B and modulation of virion sphingolipid content, as well as productive infection in vivo , although it is not incorporated into mature virions.
Cleavage of the polyprotein precursor at the NS2-NS3 junction is accomplished by a cysteine protease encoded by NS2, whose function is strongly enhanced by the N terminus of NS3. The NS2-3 protease itself is dispensable for RNA replication, but cleavage at this junction is essential. NS2 plays an essential role in HCV infectious virus assembly independently of its protease activity, which may involve a series of interactions with structural and other nonstructural viral proteins.
NS3 is a multifunctional protein that has proven to be a viable target for antiviral intervention. The N terminus of NS3 and the cofactor NS4A constitute the serine-type protease NS3-4A. NS3-4A can cleave and thereby inactivate select cellular proteins, including two crucial adaptor proteins in innate immune sensing—mitochondrial activating signaling protein (MAVS) (also known as CARDIF or IPS-1 ) and toll-interleukin (IL)-1 receptor domain–containing adaptor molecule (TICAM1) inducing IFN-β, also known as TRIF , as well as T-cell protein tyrosine phosphatase, thereby facilitating viral replication, persistence, and pathogenesis. The low substrate specificity of NS3-4A has made the development of NS3-4A-targeting therapy challenging. However, after NS3-4A-mediated cleavage, the product derived from the N-terminal fragment remains bound to the active site, blocking the enzyme. This feature has been utilized in the development of potent direct-acting antivirals (DAAs).
NS4B is an integral membrane protein that induces the formation of the membranous web, a specific membrane alteration that serves as a scaffold for the HCV replication complex. In addition, NS4B harbors nucleoside triphosphatase activity and may have a role in assembly of HCV virions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here