Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Certain viruses seem to have a predilection for the fetus and may cause abortion, stillbirth, intrauterine infection, congenital malformations, acute disease during the neonatal period, or chronic infection with subtle manifestations that may be recognized only after a prolonged period. It is important to recognize the manifestations of viral infections in the neonatal period not only to diagnose the acute infection but also to anticipate the potential abnormal growth and development of the infant.
The herpesvirus family consists of numerous closely related viruses. They all have a DNA core and are enveloped in an icosahedral (20-sided) nucleocapsid. Eight viruses in the family infect infants: herpes simplex virus (HSV) types 1 and 2; cytomegalovirus (CMV); varicella-zoster virus (VZV); Epstein-Barr virus (EBV); and human herpesviruses (HHV) 6, 7, and 8. These viruses are also characterized by the development of latent states after the primary infection.
Neonatal HSV infections are being diagnosed with increasing frequency, at a rate of 1 in every 3200 live births per year in the United States, resulting in an estimated 1500 new cases per year. The primary source of HSV infection for neonates is acquisition of the virus during delivery, and yet infected genital tract secretions in women are more common compared with neonatal infections. In addition, in numerous countries, neonatal infection is less common than in the United States despite the high prevalence of genital infections, suggesting other unknown means of protection to the neonate.
HSVs are a group of large double-stranded DNA viruses within an icosahedral nucleocapsid and a lipid envelope. There is considerable cross-reactivity between the two serotypes, HSV-1 and HSV-2. Glycoprotein G is responsible for the antigenic specificity between them, as shown by the antibody response. The seroprevalence of HSV in the United States has continued to increase for HSV-1 and HSV-2. HSV-1 is now responsible for 50% of neonatal infections, probably resulting from its increased seroprevalence and its increased maternal-to-neonatal transmission during reactivation of genital infection. Maternal HSV-2 infections recur more commonly than HSV-1, and there is more viral shedding in HSV-2 than in HSV-1 with recurrences, but the antibody to HSV-2 is more protective. The virus enters via breaks in the skin and mucous membranes, attaches to the epithelial cells, and begins to replicate. The virus is transported by retrograde axonal flow from the sensory nerve endings in the dermis to the sensory ganglia, where at least some portion of the viral DNA persists for the lifetime of the individual. HSV-1 usually persists in the trigeminal ganglion, whereas genital herpes persists in the sacral dorsal root ganglia or other local sensory ganglia.
Fever, ultraviolet light, stress, and many undetermined sources may cause the virus to reactivate, at which time the virus is transported antegrade down the sensory nerve axon to the skin or the mucous membrane, where it again results in either symptomatic or asymptomatic disease. Either way, the virus is infectious. Analyses of viral DNA from individuals with recurrent lesions indicate that the identical virus is virtually always responsible. Superinfection with a different viral strain is uncommon. Infection is not seasonal, and humans are the only known carriers of the infection.
HSV infections are labeled as first-episode, primary infections when the individual who has neither HSV-1 nor HSV-2 antibody (indicating prior infection) acquires either HSV-1 or HSV-2 in the genital tract. A first-episode, nonprimary infection occurs when an individual who already has HSV-1 antibody acquires HSV-2 genital infection, or vice versa. Recurrent infections occur with reactivation of latent infections.
Labial and oropharyngeal infections are predominantly caused by HSV-1 and may be transmitted by respiratory droplet spread or direct contact with infected secretions or vesicular fluid. Most HSV-1 infections occur in childhood and are usually asymptomatic, sometimes causing gingivostomatitis or mononucleosis-like syndrome. Girls have a higher seroprevalence compared with boys. Black children have a 35% seroprevalence by age 5 years compared with 18% in white children, and the seroprevalence remains twice as high through the teen years but is equivalent by age 60 years.
Because genital infections are usually transmitted by direct sexual contact with HSV-2, transmission most often occurs during or after adolescence. Most genital herpes infections are asymptomatic, but symptomatic and asymptomatic individuals may transmit infection. Primary genital infection may cause localized pain and burning of the labia and vaginal mucosa 2-14 days after contact. After a period of paresthesia, vesicles full of seropurulent fluid develop. These vesicles break down easily, forming shallow ulcers and releasing numerous infectious virus particles. There is often copious watery vaginal discharge, edema, dysuria, and bilateral pelvic and inguinal lymphadenopathy, accompanied by systemic symptoms of fever, malaise, and headache. Healing may occur several weeks later. Many primary genital infections, however, are asymptomatic. Children may infect themselves genitally by autoinoculation from an oral HSV-1 infection, but sexual abuse should always be considered.
Seroprevalence rates among pregnant women have indicated that at least 30% of women have serologic evidence of infection with HSV-2, but most of these women lack a history or symptoms of infection. Not only was this seroprevalence largely unsuspected among these pregnant women, but asymptomatic viral shedding also occurred among them at a rate (roughly 1% viral shedding at any time in pregnancy) similar to that in women with symptomatic recurrences. Seroprevalence increases dramatically as the number of sexual partners increases. Recurrence is higher among women with genital HSV-2 infection than among women with HSV-1, probably because HSV-2 is more likely than HSV-1 to establish latency in the inguinal dorsal root ganglia. When viral replication is not medically suppressed, there are four HSV-2 recurrences, on average, in the first year after a primary infection. HSV-1 recurs about once a year. Finally, for 70% of all women whose infants develop HSV infection, there is no history of infection or symptoms or of intercourse with a partner who has had the infection.
About 10% of HSV-2 seronegative women have HSV-2 seropositive partners. These women may remain uninfected with HSV-2 over prolonged periods despite continued, unprotected contact with a partner who is HSV-2 seropositive. Because the seroconversion rate is 20% per year for these women and most genital HSV-2 infections are asymptomatic, these women are at high risk of an unsuspected, primary HSV-2 genital infection during pregnancy. Seroconversion rates are similar among pregnant and nonpregnant women. It is estimated that about one third of women who asymptomatically shed HSV during labor have been recently infected and that their infants have a 10-fold or greater risk of being infected than the infants of mothers with recurrent disease.
Women who are seronegative for HSV-1 and HSV-2 and have HSV-1–seropositive partners may acquire HSV-1 genital infection through oral sex. Oral sex has become more popular among teens because they believe it is safer sex. Overall, a woman who is seronegative for HSV-1 and HSV-2 with a discordant partner has a 3.7% chance of acquiring infection with either virus during pregnancy, and a woman who is seropositive for HSV-1 and seronegative for HSV-2 with an HSV-2–seropositive partner acquires HSV-2 infection in 1.7% of pregnancies.
Transmission from mother to infant may occur via many different routes, including transplacental, intrapartum, and postnatal transmission. Transplacental transmission (5%), which is responsible for in utero infection, is inferred by the documentation of HSV skin lesions and viremia at birth and by elevated specific cord immunoglobulin M (IgM) levels. Primary and recurrent maternal infections have been associated with congenital infection.
Intrapartum transmission is responsible for 85% of neonatal infections. The actual transmission is influenced by the type of maternal infection. A high titer of viral particles (>10 6 /0.2 mL inoculum) is excreted for about 3 weeks with a primary maternal infection, which is more likely to involve cervical shedding than a recurrent maternal infection, in which 10 2 to 10 3 viral particles per 0.2 mL inoculum are shed for only 2-5 days. Maternal neutralizing antibodies may also be partially protective for a newborn in recurrent infections and may not yet be present (and available to cross the placenta) in a primary maternal infection. In a 20-year trial, 0.3% of women were found to be shedding either HSV-1 or HSV-2 asymptomatically at delivery. Neonatal disease resulted in 57% of first-episode primary maternal infections (defined as HSV-1 or HSV-2 isolated from genital secretions without having concurrent HSV antibodies), 25% of first episode nonprimary maternal infections (defined as HSV-2 isolated from genital secretions of a woman with only HSV-1 antibodies, or HSV-1 isolated from a woman with only HSV-2 antibodies), and 2% of recurrent maternal infections (present when the virus isolated from genital secretions was the same type as antibodies present in the serum at the time of labor).
Because recurrent infections are so much more common, half of all neonatal HSV-2 infections occur secondary to recurrent maternal infection, even though transmission from mother to infant occurs in only 2% of the cases. The amount of neutralizing antibody also affects the severity of neonatal disease. Infants who do not receive much transplacental transfer of antibody are more likely to develop disseminated disease. Prolonged rupture of membranes (>4-6 hours) also increases the risk of viral transmission, presumably from ascending infection. Delivery via cesarean section, preferably before rupture of membranes, but at least before 4-6 hours of rupture, can reduce the risk sevenfold.
Neonatal infection does still occur, however, even with cesarean delivery. Fetal scalp electrodes may accelerate transcervical infection and breach the infant's skin barrier, also increasing risk of infection. It was shown more recently that vacuum extraction may cause scalp lacerations resulting in HSV skin lesions at the site of application. The relative risk of vacuum extraction resulting in HSV infection was nearly 7.5 times that of spontaneous vaginal or cesarean delivery. Antenatal maternal viral culture screening for HSV shedding is of no predictive value in determining who will be shedding virus at delivery.
Finally, transmission may occur postnatally (10%). Restriction enzyme DNA analysis has been used to document postnatal acquisition of HSV and its spread within a nursery by identifying infection with the same herpes strain in infants of different mothers. The father and the mother, as well as maternal breast lesions, have been implicated in neonatal infections. There is also concern regarding symptomatic and asymptomatic shedding among hospital personnel, one third of whom may have a history of HSV-1 lesions and 1% of whom still have recurrent labial lesions. Individuals with a herpetic whitlow should be removed from the nursery. Removal of health care workers with other lesions would pose significant risk to neonates because it would cause significant disruption of care. Orolabial lesions should be covered with a mask, and skin lesions should be covered with clothing or a bandage. Workers should be counseled on good hand hygiene and to not touch a lesion.
Congenital infection was found in 5% of the infants in the National Institute of Allergy and Infectious Disease (NIAID) Collaborative Antiviral Study Cohort. These infants with growth restriction characteristically have skin lesions, vesicles and scarring, neurologic damage (intracranial calcifications, microcephaly, hypertonicity, and seizures), and eye involvement (microphthalmia, cataracts, chorioretinitis, blindness, and retinal dysplasia). Congenital infections are described throughout pregnancy and after primary and recurrent infections but are most likely with a primary infection or if the mother has disseminated infection and is in the first 20 weeks of pregnancy. Most cases are caused by HSV-2. The manifestations probably result from destruction of normally formed organs rather than from defects in organogenesis because the lesions are similar to lesions of neonatal herpes. A few children have isolated skin lesions, usually in association with prolonged rupture of membranes, and these lesions may be more amenable to antiviral therapy.
Although asymptomatic HSV infections are common in adults, they are exceedingly rare in neonates. Of all neonatal infections, 50% are caused by HSV-1 rather than HSV-2. Half of the infants are born prematurely, usually between 30 and 37 weeks of gestation, and many have complications of prematurity, particularly respiratory distress syndrome. Two thirds of term newborns have a normal neonatal course and are discharged before the onset of disease. They may also have simultaneous bacterial infections. One fourth of the infants have symptoms on the first day of life and two thirds by the end of the first week.
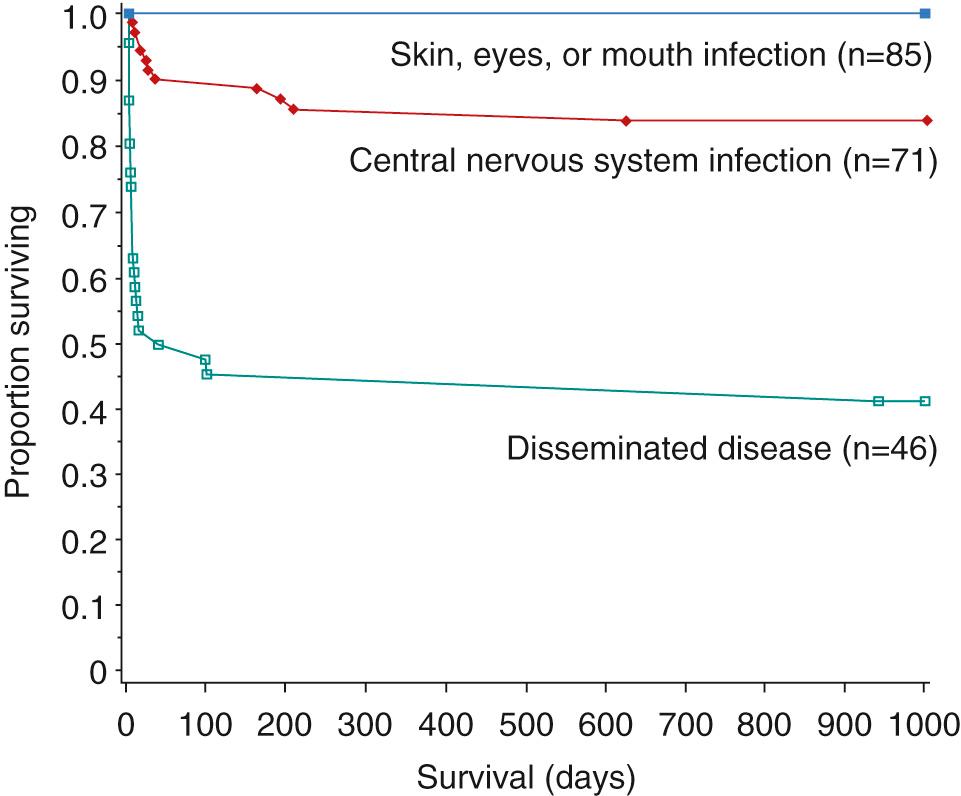
Clinically, neonatal infections are classified as (1) disseminated, involving multiple organs, with or without central nervous system (CNS) involvement; (2) encephalitis, with or without skin, eye, or mouth involvement; and (3) localized to the skin, eyes, or mouth. Approximately 25% of cases are disseminated; 30% have CNS involvement; and 45% are localized to the skin, eyes, or mouth.
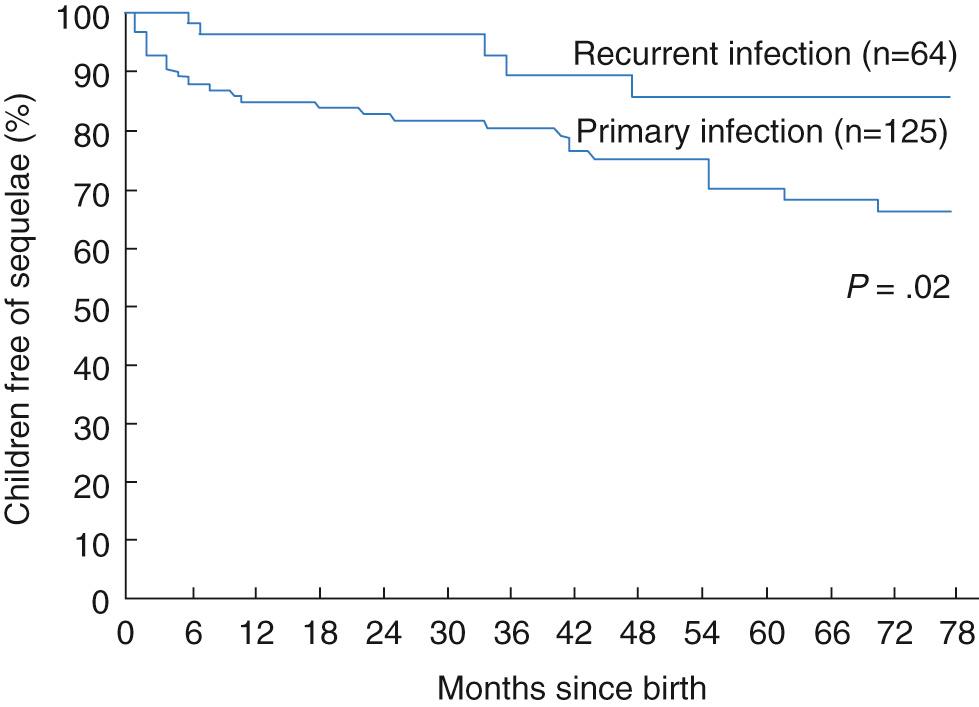
Among the 202 infants with HSV infections followed up in the NIAID Collaborative Antiviral Study Group, mortality was significantly greater with disseminated infection (57%) than with encephalitis (15%) and did not occur with disease limited to the skin, eyes, or mouth. The relative risk of death was 5.2 for infants in or near coma at onset of treatment, 3.8 for those with disseminated intravascular coagulopathy, and 3.7 for premature infants. Among infants with disseminated disease, mortality was higher among those with pneumonitis. Sequelae among survivors were more common with encephalitis or disseminated infection, particularly with HSV-2 infection, or in the presence of seizures but also were more likely in infants with SEM infection who had three or more recurrences of vesicles within 6 months. Sequelae were found in 75% of survivors with HSV-2 and in only 27% of survivors with HSV-1 infection, and this may be related to the in vitro susceptibility of HSV-1 to acyclovir.
Infants with disseminated infections have the worst prognosis. Disseminated infections may involve virtually every organ system, but predominantly involve the liver, adrenal glands, and lungs. Infants usually present by 10-12 days of life with signs of bacterial sepsis or shock but often have unrecognized symptoms several days earlier. Although the presence of cutaneous vesicles is helpful in diagnosis, 20% of infants never develop vesicles. Disseminated intravascular coagulation (DIC) with decreased platelets and with petechiae and purpura are common, and bleeding often occurs in the gastrointestinal tract. Pneumatosis intestinalis may also be present. Hepatomegaly or hepatitis, or both, is usually present, with or without jaundice. Respiratory distress, often with pneumonitis or pleural effusion seen on the chest radiograph, has a poorer prognosis.
Many infants die before manifesting symptoms of CNS involvement, which is common (60%-80%). These infants may present with irritability, apnea, a bulging fontanel, focal or generalized seizures, opisthotonos, posturing, or coma. Cerebrospinal fluid (CSF) may be normal or may show evidence of hemorrhage. Virus can be isolated from CSF of only one third of infants with CNS symptoms. The routine use of polymerase chain reaction (PCR) on CSF has aided considerably in recognition of the disease. Death usually occurs at about age 2 weeks, roughly 1 week from the onset of symptoms, and often involves respiratory failure, liver failure, and DIC with shock.
Encephalitis may occur as a component of disseminated disease, via blood-borne seeding of the brain, resulting in multiple lesions of cortical hemorrhagic necrosis often in association with oral, eye, or skin lesions, at 16-19 days of life. Brain involvement results from neuronal transmission of the virus. Regardless of the source of neurologic infection, only about 60% of infants have skin vesicles, and less than half have virus isolated from CSF. Although CSF is occasionally normal, it usually shows mild pleocytosis, with predominance of mononuclear cells, elevated protein concentration, and normal glucose concentration. Infections that are discovered late may have significant numbers of erythrocytes in CSF. Lethargy, poor feeding, irritability, and localized or generalized seizures may be the presenting manifestations. Nearly all electroencephalograms show nonspecific abnormalities.
In 12 infants with HSV-2 encephalitis, diffusion-weighted magnetic resonance imaging (MRI) showed extensive, often bilateral changes that were not visible on computed tomography (CT) or conventional MRI in eight infants. Disease was found in the temporal lobes, cerebellum, brainstem, and deep gray nuclei. Hemorrhage and watershed distribution ischemic injury were also seen. These areas progressed to cystic changes on follow-up imaging. Nearly half of untreated children die from neurologic deterioration 6 months after onset, and virtually all survivors have severe sequelae (microcephaly and blindness or cataracts).
Fever is a known symptom of HSV infection and is a common reason for an infant in the first month of life to be taken to the emergency department. The American Academy of Pediatrics (AAP) Committee on Infectious Diseases recommends considering HSV infection in neonates with fever, irritability, and abnormal CSF findings, especially in the presence of seizures. In a study of nearly 6000 infants with laboratory-confirmed viral or serious bacterial infections admitted from the emergency department, only 30% of the infants with HSV infections were febrile; 50% were fever free, and 20% were hypothermic. Of the febrile infants with CSF pleocytosis, bacterial meningitis (1.3%) was more common than HSV infection (0.3%), but not statistically so. Similarly, febrile infants with mononuclear CSF pleocytosis were not statistically more likely to have HSV infections (1.6%) than bacterial meningitis (0.8%), and 1.1% of hypothermic infants presenting with a sepsis-like syndrome had HSV infection. In all of these infants, HSV infection should be considered when they present in the first month of life, especially if they fail to improve on antibiotics and bacterial cultures remain negative for the first 24-48 hours.
Infants with disease localized to the skin, eyes, or mouth usually present by 10-11 days of life. Greater than 90% of these infants have skin vesicles, usually over the presenting part at birth and appearing in clusters. Recurrences are common for at least 6 months. Infants at risk should be monitored for localized infections (vesicles) of the oropharynx. Either HSV-1 or HSV-2 can cause keratoconjunctivitis, chorioretinitis, microphthalmos, and retinal dysplasia, later possibly leading to cataracts. One third of these infants later develop neurologic sequelae indicative of undiagnosed neurologic involvement.
Isolation of virus is definitive diagnostically. Cultures of the newborn (scrapings of mucocutaneous lesions, CSF, stool, urine, nasopharynx, and conjunctivae) should be delayed to 24-48 hours after birth to differentiate viral replication in the newborn from transient colonization of the newborn at birth. The specimens for culture may be combined to save money because it is not important where the virus is located, but whether the virus is present, with the exception of CSF specimens, which are needed to determine CNS involvement. If the culture shows cytopathic effects, typing should be done. Serologic testing is not useful in neonatal disease because transplacentally transferred maternal antibody confounds the interpretation, but finding low-avidity HSV-2 IgG in the maternal serum near term does indicate an elevated risk of neonatal disease. PCR testing has become invaluable, especially for CSF, which has a very low recovery rate for HSV cultures. PCR can also be used to test blood, scrapings of lesions, the conjunctiva, or the nasopharynx. However, PCR has detected HSV DNA in the amniotic fluid of women with symptomatic infection, and yet the infants were uninfected and healthy. In addition, all three forms of neonatal disease (disseminated, CNS, and skin, eyes, and mouth [SEM] disease) may have viremia, so blood PCR should not solely be relied upon to determine extent of disease, and blood PCR should not be used to monitor response to therapy.
Vidarabine was the first antiviral agent used to treat HSV and was efficacious despite its toxicity. Acyclovir, a deoxyguanosine analogue, is preferentially taken up by virus-infected cells and phosphorylated by thymidine kinase, which is encoded in the virus. Host cell enzymes then effect di- and triphosphorylation. Acyclovir triphosphate prevents DNA polymerase and is a chain terminator, preventing viral DNA synthesis. Acyclovir is the only drug recommended for use in neonates. When a low-dose acyclovir (30 mg/kg/day in three divided doses) was compared with vidarabine, the morbidity and mortality were equivalent, but the ease of acyclovir administration and decreased toxicity resulted in it readily supplanting vidarabine in use. High-dose intravenous (IV) acyclovir (60 mg/kg/day in three divided doses) was then compared with low-dose acyclovir for a longer treatment duration (21 days for disseminated or CNS disease and 14 days for disease localized to the skin, eyes, or mouth). High-dose acyclovir resulted in a much improved survival rate: Infants with disseminated infection had an odds ratio of survival of 3.3, and infants with CNS disease had a similar survival. The likelihood of developmentally normal survival had an odds ratio of 6.6 compared with infants treated with the lower dose.
Infants with an abnormal creatinine clearance need to have the acyclovir dose adjusted, and all infants need to be monitored for neutropenia. All neonates should have ophthalmologic and MRI examinations. CT and ultrasonography may be used alternatively but are not as sensitive to abnormalities. Infants with CNS disease need to have a repeat lumbar puncture at the end of the course of treatment. Treatment should be continued until CSF is PCR negative. Infants who continue to have detectable HSV DNA in CSF by PCR at the end of therapy are more likely to die or have moderate to severe impairment. Poor prognostic indicators are lethargy and severe hepatitis in disseminated disease, and prematurity and seizures in CNS disease.
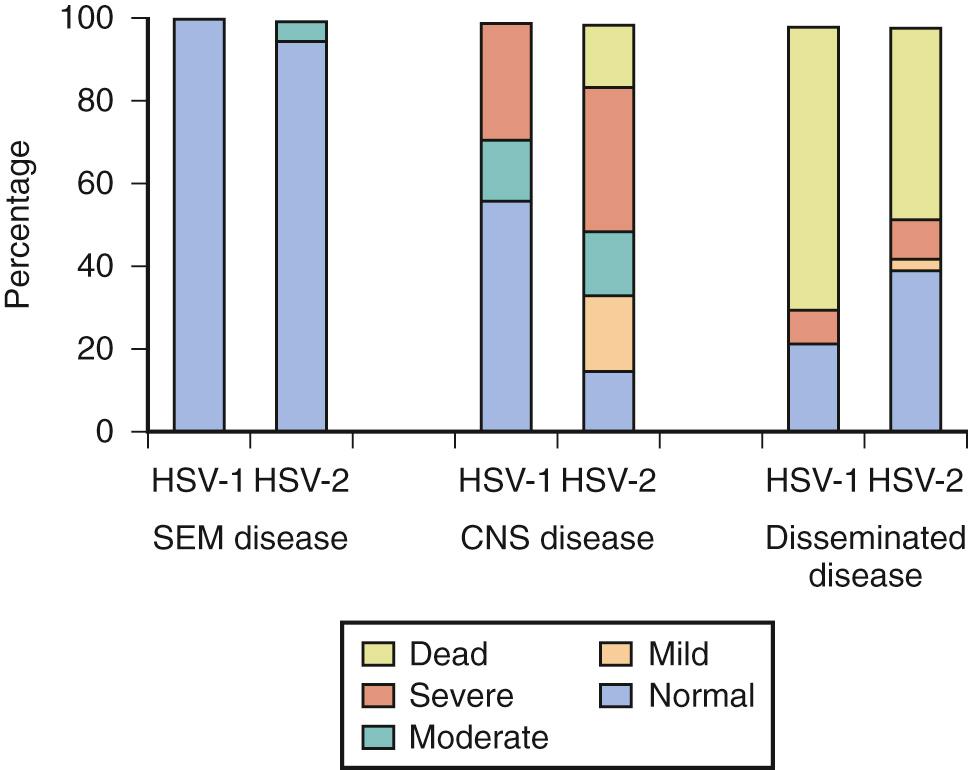
Mortality has been tremendously decreased with high-dose acyclovir and is now 29% for disseminated disease; 4% for CNS disease; and 0% for SEM disease. Although the percentage of survivors with normal development (31%) has not changed for CNS disease, normal development among survivors of disseminated disease is now 83%, and for SEM disease is greater than 98% ( Figs. 50.1 and 50.2 ). Neonates with SEM disease with neurodevelopmental abnormalities on follow-up may represent undetected CNS disease, adverse effects of inflammation secondary to disease, or seeding from recurrent skin lesions. In a study of 77 neonates with culture-proven HSV disease, CSF PCR detected HSV DNA in 7 of 29 infants who had been classified as having SEM disease, 13 of 14 classified as having disseminated disease, and 26 of 34 who had been classified as having CNS disease. HSV DNA remains in CSF for an average of 10 days after the onset of CNS disease. It has also been shown that infants with fewer than 100 copies of HSV DNA per microliter of CSF after 4 days of treatment had improved survival and neurologic outcome. To improve outcome, earlier recognition and treatment of infection are needed. Initiation of therapy in the high-dose acyclovir trial usually began 4-5 days after onset of symptoms, which is no better than that in the low-dose trial. Topical ophthalmic antiviral drugs should be given to infants with ocular involvement in addition to parenteral therapy. This may include 1% trifluridine, 0.1% iododeoxyuridine, or 3% ganciclovir. All other therapy is supportive. Early data indicate that quantitative PCR for HSV DNA in blood may be useful in determining outcome and treatment, but there are not enough data to recommend this currently. Infants with disseminated disease have higher viral loads compared with infants with CNS disease and infants with SEM disease. The viral load is also higher in those infants who die than in those who survive with neurologic disease or those who survive without neurologic disease.
Oral suppressive acyclovir therapy for 6 months after completion of treatment has been used to decrease recurrences in infants, which occurred in 50% of infants within 1-2 weeks of stopping the initial acyclovir course. There is a significant reduction in the recurrence of skin lesions in infants with any of the three disease classifications and improved neurodevelopmental outcomes with CNS HSV disease. Infants are treated with three doses per day at 300 mg/m 2 /dose, and the neutrophil counts need to be checked at 2 and 4 weeks and then monthly during therapy.
Resistance to acyclovir is a concern and has been seen in mothers who have had multiple recurrences treated with suppressive acyclovir, mothers who have taken oral acyclovir for suppression in the later part of pregnancy, and even in a few infants on suppressive therapy for the 6 months after the end of treatment.
In 1999, the American College of Obstetrics and Gynecology recommended that cesarean delivery be performed if a mother has HSV genital lesions or prodromal symptoms at the time of delivery. Seventy percent of mothers of infants with neonatal disease do not have a history or symptoms of HSV infection, however, and their partners do not have a history of HSV infection, and neonatal infection may still occur even if a cesarean delivery is performed. Repetitive cervical cultures do not predict whether a mother will be shedding virus at delivery. Mothers should be counseled regarding the signs and symptoms of disease, and some may then recognize infection. If rupture of membranes has been present longer than 6 hours, some experts still recommend cesarean delivery in the face of genital lesions, but data are lacking, and controversy exists. Scalp electrodes should be avoided. There is also no consensus about treatment when a mother has genital lesions and ruptured membranes, except in the case of a very immature fetus.
If an infant is delivered vaginally to a mother with recurrent genital lesions (5% risk of infection), most experts do not recommend treating the infant. The infant does not need contact precautions. Cultures and PCR of the neonate should be obtained at 24 hours of life, and the infant should be observed carefully. Circumcision should be delayed until cultures are known to be negative. Hand washing should be emphasized. The infant should be managed with contact precautions. If the mother has an active herpes labialis or stomatitis, she should wear a disposable surgical mask while handling her infant until the lesions have crusted and dried. She should not kiss the infant. Breastfeeding may be allowed if there are no lesions on the breast. The mother needs to be taught the signs and symptoms of neonatal disease because culture does not always detect neonatal disease.
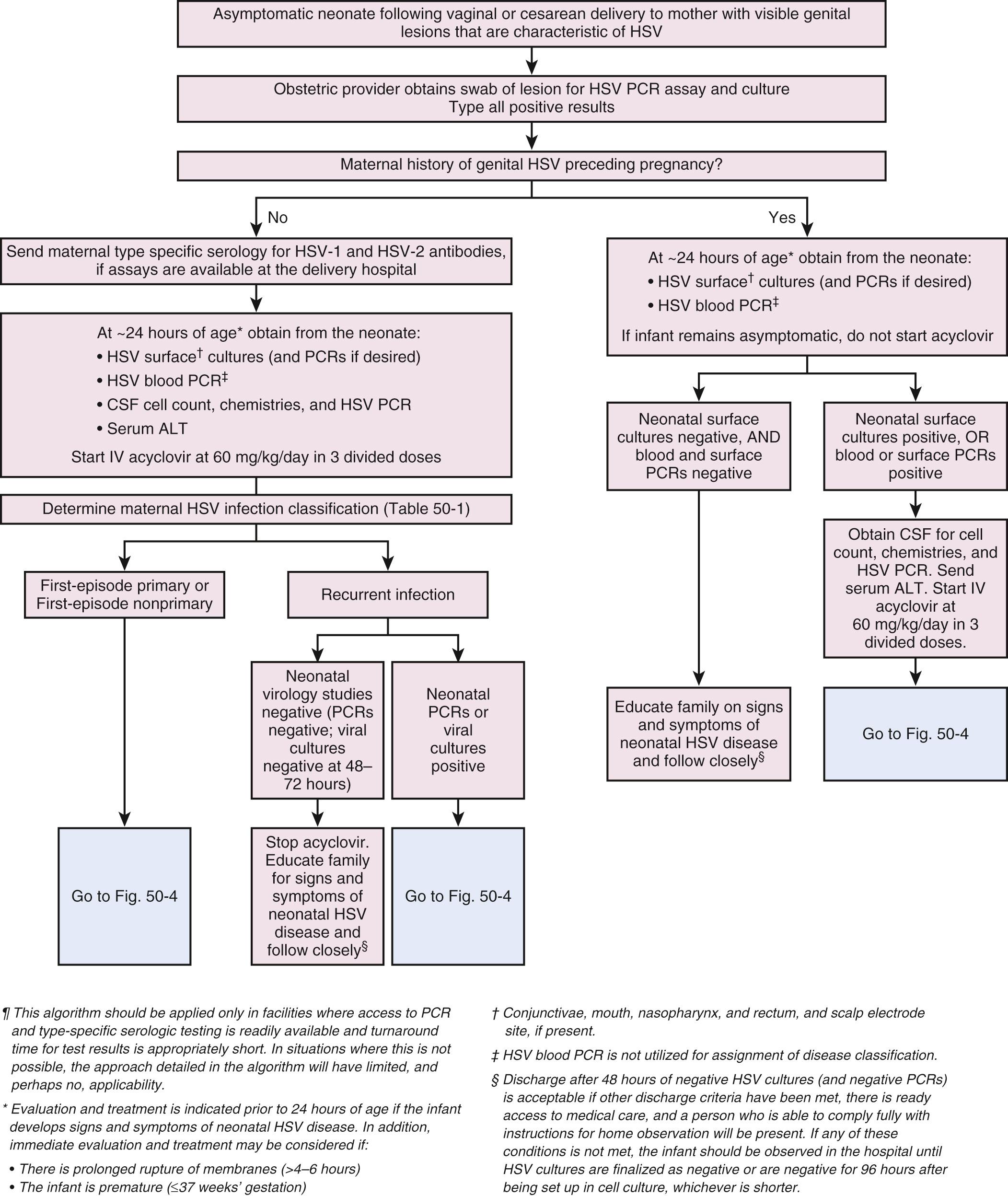
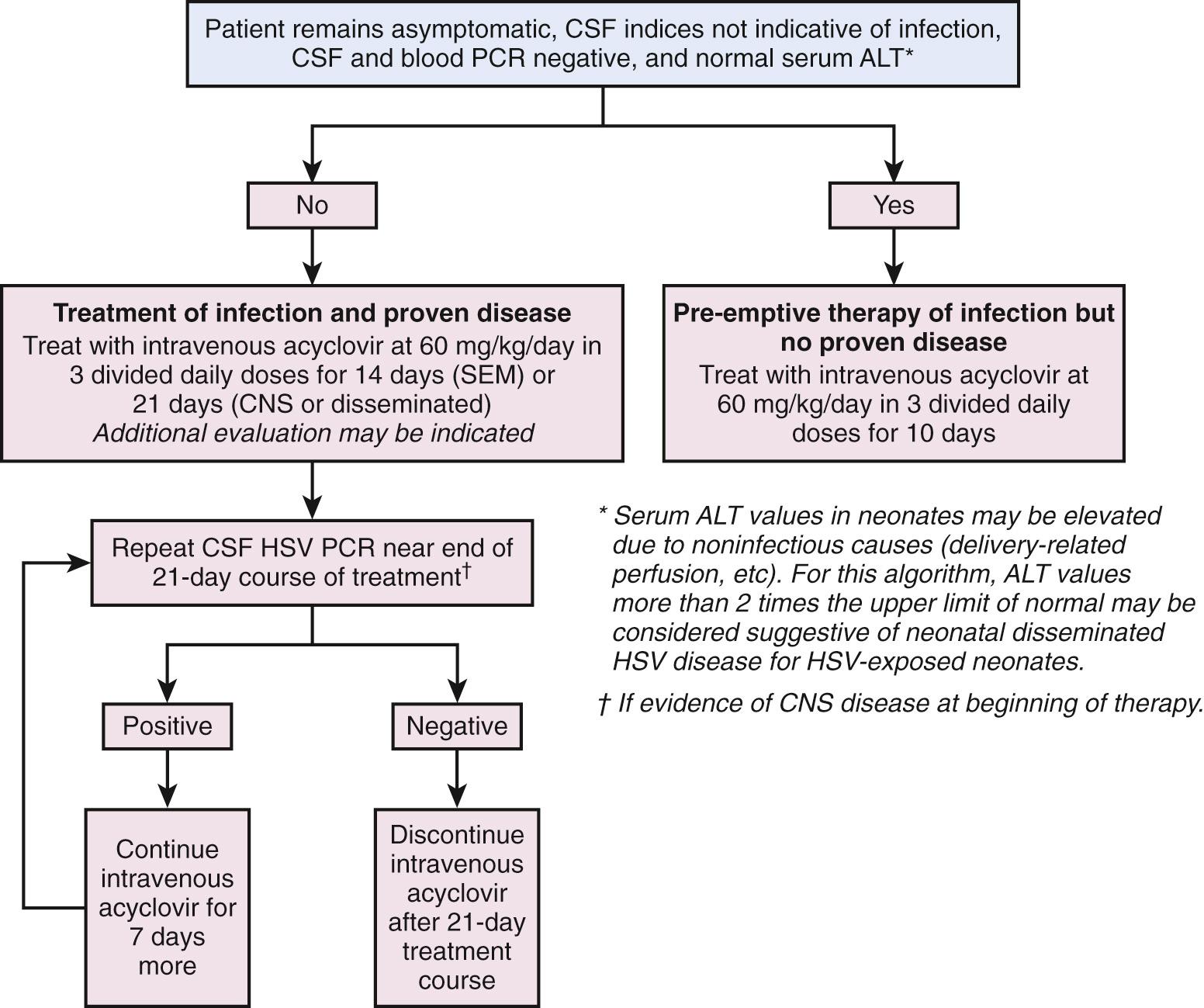
Whenever a mother has active genital lesions at the time of delivery, and she has no history of prior herpetic infection, both herpes culture and PCR need to be performed, regardless of whether the birth is via cesarean section or vaginal delivery ( Figs. 50.3 and 50.4 ). Type-specific serology can be used to determine if this is a recurrent infection or is a first episode infection ( Table 50.1 ). Because the risk of infection to the newborn is greater than 50% in a primary, first-episode infection and 25% in a nonprimary, first-episode infection in the mother, these infants should have surface cultures and blood and surface PCR for HSV, serum alanine aminotransferase (ALT) and CSF cell count, chemistries, and PCR for HSV performed at 24 hours of life, and earlier if the baby is ill or premature or there was prolonged rupture of membranes. Acyclovir should be started after the evaluation. If it is a recurrent infection, the acyclovir may be discontinued after a negative evaluation. Empiric acyclovir treatment should be considered for 10 days for any first-episode infection, whether primary or nonprimary, even if the baby's evaluation is negative. If a CSF infection is suspected, treatment should be continued for 21 days, after which a repeat CSF PCR needs to be sent. Acyclovir should be given for another 7 days whenever the PCR is positive for HSV.
| Classification of Maternal Infection | PCR/Culture From Genital Lesion | Maternal HSV-1 and HSV-2 IgG Antibody Status |
|---|---|---|
| Documented first-episode primary infection | Positive, either virus | Both negative |
| Documented first-episode nonprimary infection | Positive for HSV-1 Positive for HSV-2 |
Positive for HSV-2 AND negative for HSV-1 Positive for HSV-1 AND negative for HSV-2 |
| Assume first-episode (primary or nonprimary) infection | Positive for HSV-1 OR HSV-2 Negative OR not available † |
Not available Negative for HSV-1 and/or HSV-2 OR not available |
| Recurrent infection | Positive for HSV-1 Positive for HSV-2 |
Positive for HSV-1 Positive for HSV-2 |
* To be used for women without a clinical history of genital herpes.
† When a genital lesion is strongly suspicious for HSV, clinical judgment should supersede the virologic test results for the conservative purposes of this neonatal management algorithm. Conversely, if in retrospect, the genital lesion was not likely to be caused by HSV and the PCR assay result or culture is negative, departure from the evaluation and management in this conservative algorithm may be warranted.
There is also considerable controversy concerning the prevention of a primary HSV genital infection in a seronegative pregnant woman. Some authorities advocate for type-specific serologic screening for HSV in all pregnant women, arguing that many mothers want the information; that they may be counseled against oral or unprotected sex; and that strategies may be devised from the data that are collected. However, others argue against testing, stating that it is not cost effective, that there is no recommended intervention, and that the unexpected positive test result can cause significant psychological and social distress. Targeting for testing only women who are at high risk for infection misses too many seronegative women. Some treatment strategies do exist, however. It has been shown that the use of condoms in at least 70% of sexual intercourse between a woman seronegative for HSV and a man seropositive for HSV reduced transmission by greater than 60%. Antiviral suppression with valacyclovir for 8 months in seropositive male partners reduced the transmission of HSV-2 infection to pregnant women by 48% and symptomatic infection by 75%.
Obstetricians now recommend antiviral prophylaxis (usually valacyclovir or acyclovir) from 36 weeks of pregnancy onward to suppress viral recurrences. No major malformations have been associated to date with the use of acyclovir in pregnancy. In a Cochrane Database meta-analysis of third-trimester antiviral prophylaxis, women were less likely to have recurrence at delivery (relative risk 0.28), cesarean delivery for genital lesions (relative risk 0.30), and HSV detected at delivery (relative risk 0.14). Although there were no cases of neonatal disease among the infants, there were too few patients to draw a conclusion about neonatal risk. Neonatal infection may occur because viral shedding still does occur. There has been some success in vaccine development for women who are seronegative for HSV-1 and HSV-2, but the trials still need to be performed.
CMVs are the largest viruses in the Herpesvirus family and are noted for their worldwide distribution among humans and animals. The virus is highly species specific, and humans are the only known reservoir for disease. Infection occurs throughout the year. After primary infection, the virus enters a latent state, and reactivation may frequently occur. Reinfection may also result from any of the thousands of human strains, which are homologous, but not identical. The differing antigenic makeup of the various strains may make it possible to identify the source of the viral infection. It also allows re-infection to occur with other strains in an already seropositive individual.
The virus has a double-stranded DNA core surrounded by an icosahedral, or 20-sided, capsid. This capsid is surrounded by amorphous material, which, in turn, is surrounded by a lipid envelope, probably acquired during budding through the nuclear membrane. The virus is named for the intranuclear and paranuclear inclusions seen with symptomatic disease—cytomegalic inclusion disease. These inclusion bodies often yield an “owl's eye” appearance to the cells. The virus does not code its own thymidine kinase or DNA polymerase, which is important when considering treatment. The virus is cultured in the laboratory only in human fibroblasts, although it replicates in vivo primarily in epithelial cells.
CMV is currently the most common intrauterine infection. CMV is responsible for congenital infection in 0.15%-2% of newborns, and it is the leading cause of nongenetic deafness and learning disability. Infection is more prevalent in underdeveloped countries and among lower socioeconomic groups in developed countries, where crowding and poor hygiene are more common. Each year, 2% of middle- to high-socioeconomic-class women of childbearing age seroconvert compared with 6% of women from lower socioeconomic groups. Seropositivity also increases with age, breastfeeding for longer than 6 months, non-white race, number of sexual contacts, and parity. A study of US national death certificate and census data from 1990-2006 documented that African Americans and Mexican Americans were at increased risk for congenital CMV infection and that African Americans and Native Americans had a much greater rate of mortality among infants under age 1 year because of congenital CMV. Of note, the highest rate of congenital CMV infections occurs in populations with the highest rates of maternal seroprevalence rather than in countries with only moderate seroprevalence among pregnant women, as in the United States. Primary and nonprimary maternal infections may contribute differently to the development of congenital CMV in different populations.
Transmission of CMV requires close contact with contaminated secretions because the virus is not very contagious. The virus can be cultured from urine, cervical secretions, saliva, semen, breast milk, blood, and transplanted organs, and all these sites intermittently excrete virus. Viral excretion is particularly prolonged after primary infection, but it also occurs with reactivation of infection or re-infection with a different strain. Congenitally infected infants may shed the virus for years and serve as a large reservoir for spreading infection to others. Toddlers also may shed the virus for prolonged periods compared with adults, in whom the humoral and cellular defense mechanisms lead to a latent state within a few months.
Transplacental transmission is responsible for congenital infection in 1% of newborns, and intrauterine fetal death is more likely. Primary maternal infection during pregnancy is more likely to result in maternal-to-fetal transmission, but fetal infection may also result in women with pre-existing antibody to CMV. This may occur via reactivation of a prior maternal infection, or infection with a newly acquired strain. Roughly two thirds of infants with congenital CMV worldwide are born to mothers with pre-existing antibody to CMV because of the high prevalence of CMV in the population. Overall, only 5%-10% of congenital infections are symptomatic, and these are more likely after a primary maternal infection, although symptomatic infections have been reported in women with pre-existing antibody. Symptomatic infants have a mortality of 20%-30%, and two thirds of survivors may have sequelae. The 90% of infants with asymptomatic infection at birth, however, also have a 5%-15% risk of later sequelae.
Even with primary maternal CMV infection during pregnancy, transplacental infection occurs in only 30%-40% of the fetuses, and only 10%-15% of these infected fetuses develop symptomatic disease. With recurrent maternal CMV infection or re-infection with a new strain during gestation, only 1%-3% of fetuses are infected. Although the risk of transmission seems to be increased in the third trimester, the risk of malformations (which occur during the period of organogenesis) and developmental disabilities lessens. The fetus may be infected throughout gestation. The rate of intrauterine transmission was 9% preconceptually, and 31%, 34%, and 40% in the first, second, and third trimesters, respectively. Maternal IgG crosses the placenta and provides passive immunity for the fetus but may also facilitate transport of CMV across the placenta, as IgG-virion complexes use the fetal F C receptor on the syncytiotrophoblast for transcytosis. Villus core macrophages can neutralize complexes formed with high-avidity antibodies, but low-avidity antibody allows the virus to escape and seems to be more significant in the first half of pregnancy when the virus has considerable teratogenic potential. Neurons migrate from the periventricular germinal matrix to the cortex between 12 and 24 weeks of gestation. This process may be interrupted by infection, resulting in CNS malformations. In the second half of pregnancy, during the period of myelination, white matter lesions may develop, as seen on MRI.
Perinatal infection is responsible currently for an additional 3%-5% of infections among newborns, resulting from exposure to cervical secretions and blood during delivery or via breast milk. Transmission in early childhood may occur from child to child and from child to other family members. There is also an implication that infection may occur via fomites because virus may survive in urine for hours on plastic surfaces and has been cultured from toys in daycare centers. Nearly half of mothers of premature infants infected in the nursery seroconvert within 1 year, and the same proportion of susceptible family members seroconvert when a single family member is infected.
Daycare compounds the problem. There is a 15% rate of infection among parents of children in daycare, particularly if the child is younger than 18 months. Mothers of children in daycare, particularly if they are of middle socioeconomic status and previously seronegative, are at significant risk of developing a primary CMV infection in a subsequent pregnancy; this may account for 25% of symptomatic congenital infections. Seronegative women who work at daycare centers have an 11% seroconversion rate per year, well above any predictable rate, and are at considerable occupational risk. Among children attending daycare, 30%-70% excrete the virus.
Similar concern has been expressed about female health care workers and their occupational risk. Early data did not support an increased risk of transmission of the virus, but current infection control measures and diagnostic methodologies did not exist at that time, so the risk is not really known.
After early childhood, viral transmission seems to be minimal until puberty, when sexual activity begins. Infection rates are highest among adults with multiple sexual partners. An additional risk of infection occurs with blood transfusions and organ transplantation, as the virus is present within the leukocytes and tissues. Transmission may be prevented by mandating that all blood products come from seronegative donors. Alternatively, the white blood cells carrying the virus may be removed by using frozen, deglycerolized red blood cell transfusions or by using filters to remove the leukocytes. However, neither the use of only seronegative blood nor the use of filters completely protects against transmission of virus. Thus, some recommend using both mechanisms.
CMV transmission via human breast milk feeding has been reported to result in infection in premature infants (<32 weeks’ gestation), resulting in a sepsis-like infection. The virus is found in the whey portion of the milk, and mothers may excrete virus in their milk when they are not excreting the virus elsewhere, such as in urine or saliva. The long-term outcome has not yet been determined, but in a report of 40 preterm infants who developed viruria in the nursery, most likely from breast milk feedings, neonatal outcome was not different from that in control infants. The infants exhibited cholestasis, elevation of C-reactive protein, mild neutropenia, and thrombocytopenia, but these symptoms resolved. There is still debate as to whether the virus contributes to bronchopulmonary dysplasia or whether the most immature infants will develop hearing, neurologic, or developmental abnormalities.
Most women are asymptomatic during primary infection and even more so with re-infection or recurrent infection, and pregnancy does not alter the clinical picture. A mononucleosis-like illness, which is heterophil negative, may develop in 5%-10% of women. Other manifestations are rare.
CMV frequently infects the decidua of the placenta, causing fibrosis and edema that result in intrauterine hypoxia and the release of cytokines further stimulating the placenta. This placental injury and hypoxia can cause fetal intrauterine growth restriction (IUGR), even without transmission of the virus to the fetus.
Although 85%-90% of all infants with congenital CMV are asymptomatic at birth, 15% may be at risk for later sequelae. The results of follow-up of 330 infants with asymptomatic infection who were mostly of low socioeconomic status are shown in Table 50.2 . The most important sequela seems to be sensorineural hearing loss, which is often bilateral and may be moderate to profound. The presence of periventricular radiolucencies or calcifications on CT is highly correlated with hearing loss. The hearing loss may be present at birth or may appear only after the first year of life and is frequently progressive as a result of continued growth of the virus in the inner ear. There is a very low risk of chorioretinitis, which may not be present at birth but may develop later secondary to continued growth of the virus. A further finding may be a defect of tooth enamel in the primary dentition, leading to increased caries. Neurologic handicap may occur but is uncommon. Premature infants are most at risk.
| Sequelae | Symptomatic Infection (%) | Asymptomatic Infection (%) |
|---|---|---|
| Sensorineural hearing loss | 58 | 7.4 |
| Bilateral hearing loss | 37 | 2.7 |
| Speech threshold, moderate to profound | 27 | 1.7 |
| Chorioretinitis | 20.4 | 2.5 |
| IQ ≤70 | 55 | 3.7 |
| Microcephaly, seizures, or paresis/paralysis | 51.9 | 2.7 |
| Microcephaly | 37.5 | 1.8 |
| Seizures | 23.1 | 0.9 |
| Paresis/paralysis | 12.5 | 0 |
| Death | 5.8 | 0.3 |
Cytomegalic inclusion disease occurs in only 10%-15% of infected infants and results in multiorgan involvement, particularly of the reticuloendothelial system and the CNS. Death may occur at birth or months later, resulting in an overall mortality of 20%-30%, usually from DIC, bleeding, hepatic failure, or bacterial infection.
CNS involvement may be diffuse. Infants may be microcephalic, exhibit poor feeding and lethargy, and be hyper- or hypotonic. Intracranial calcifications of the basal ganglia and cortical and subcortical regions, ventricular enlargement, cortical atrophy, or periventricular leukomalacia may also be present. Most commonly, an infant who is small for gestational age or premature has hepatosplenomegaly and abnormal liver function tests. Hyperbilirubinemia, which occurs in more than half of the infants, may be transient but is more likely to be persistent, with a gradual increase in the direct component. Petechiae, purpura, and thrombocytopenia (direct suppression of megakaryocytes in bone marrow) usually develop after birth and may persist for weeks. Approximately one third of infants with congenital infection have thrombocytopenia, and one third of those have severe thrombocytopenia, with platelet counts less than 10,000/dL. There may also be a Coombs-negative hemolytic anemia. Diffuse interstitial or peribronchial pneumonitis is possible, but less common than with perinatally acquired disease. Table 50.3 lists the clinical findings in 24 newborns with symptomatic CMV infection. Persistent CMV may be responsible for a severe necrotizing pneumonitis in preterm infants and is hypothesized to result in a greater severity of bronchopulmonary dysplasia.
| Finding | No. (%) |
|---|---|
| Jaundice | 15 (62) |
| Petechiae | 14 (58) |
| Hepatosplenomegaly | 12 (50) |
| Intrauterine growth restriction | 8 (33) |
| Preterm birth | 6 (25) |
| Microcephaly | 5 (21) |
| Hydranencephaly | 1 (4) |
| Death | 1 (4) |
Fowler et al. showed that although maternal antibody may not prevent congenital CMV infection, it lessens the severity ( Table 50.4 ). Sequelae were found in 25% of infants after primary infection, but in only 8% after recurrent infection. Likewise, mental impairment (intelligence quotient [IQ] <70), sensorineural hearing loss, and bilateral hearing loss were found in 13%, 15%, and 8% of infants, respectively, after primary maternal infection, but only 5% of infants born after recurrent maternal infection had sensorineural hearing loss, and none had mental impairment or bilateral hearing loss. These infants also showed the progressive nature of sequelae after primary and recurrent infection ( Fig. 50.5 ).
| Sequelae | Primary Infection * | Recurrent Infection * | P Value |
|---|---|---|---|
| Sensorineural hearing loss | 15 (18/120) | 5 (3/56) | 0.05 |
| Bilateral hearing loss | 8 (10/120) | 0 (0/56) | 0.02 |
| Speech threshold ≥60 dB | 8 (9/120) | 0 (0/56) | 0.03 |
| IQ ≤70 | 13 (9/68) | 0 (0/32) | 0.03 |
| Chorioretinitis | 6 (7/112) | 2 (1/54) | 0.20 |
| Other neurologic sequelae | 6 (8/125) | 2 (1/64) | 0.13 |
| Microcephaly | 5 (6/125) | 2 (1/64) | 0.25 |
| Seizures | 5 (6/125) | 0 (0/64) | 0.08 |
| Paresis or paralysis | 1 (1/125) | 0 (0/64) | 0.66 |
| Death | 2 (3/125) | 0 (0/64) | 0.29 |
| Any sequelae | 25 (31/125) | 8 (5/64) | 0.003 |
Ramsay et al. looked at the 4-year outcome of 65 neonates with symptomatic congenital CMV in the United Kingdom and found a better prognosis than previously reported from the United States ( Table 50.5 ). Overall, the rate of neurologic abnormalities was 45%. Infants who presented with abnormal neurologic findings other than microcephaly had the worst prognosis, with a 73% rate of gross motor and psychomotor abnormalities compared with a 30% rate among children who did not present with neurologic findings. A Japanese study of 33 congenitally infected infants found that abnormal abdominal findings (ascites or hepatosplenomegaly) on fetal ultrasonography were associated with liver dysfunction and a 53-fold increase in mortality; infants who had no abdominal findings survived. Evidence is also accumulating that neonatal viral blood load (>1000 copies per 10 5 polymorphonuclear leukocytes [PMNLs] via quantitative PCR) may also predict infants who will develop sequelae, regardless of whether the infants were symptomatic or asymptomatic after birth. Chorioretinitis, periventricular calcifications, and microcephaly remain standard predictors of poor cognitive outcome. In contrast, children who have normal development and no hearing loss at 1 year of age are unlikely to develop neurodevelopmental handicaps.
| Neonatal Presentation | No. Infants | Normal N (%) |
Disability | |
|---|---|---|---|---|
| Motor or Psychomotor N (%) |
Sensorineural Deafness N (%) |
|||
| Group 1 | 22 | 6 (27) | 14 (64) | 2 (9) |
| Group 2 | 35 | 22 (63) | 8 (23) | 5 (14) |
| Group 3 | 8 | 8 (100) | 0 | 0 |
| All groups | 65 | 36 (55) | 22 (34) | 7 (11) |
Mothers with recurrent CMV infection or re-infection usually transfer significant antibody to the infant in utero. Even if this antibody transfer does not occur, a term infant who acquires infection after birth, denoting a perinatal infection, is usually asymptomatic. Transmission may occur in passage through the birth canal, via breast milk, or secondary to blood transfusion. Breastfeeding infants largely seroconvert. The incubation period is 4-12 weeks. Term infants may develop pneumonitis secondary to CMV and present with cough, tachypnea, congestion, wheezing, and apnea. Few infants require hospitalization, and there is spontaneous resolution in most term infants. In contrast, premature infants, often infected through blood transfusion, have a high rate of serious or fatal illness. They also may develop pneumonitis, but with a picture of overwhelming sepsis, hepatosplenomegaly, thrombocytopenia, and neutropenia. There may be an increased risk in these infants of neuromuscular handicaps, although there does not seem to be a higher rate of sensorineural hearing loss, microcephaly, or chorioretinitis. Transmission via blood transfusion can now be largely eliminated, indicating that most infants are infected postnatally by maternal breast milk.
Not all infants with symptomatic disease at birth have neurologic impairment. One third of these infants may have a normal neurologic outcome, and none of 45 infants with normal ultrasonography results had long-term sequelae ( Table 50.6 ). However, 5%-15% of asymptomatic infants may have sequelae.
| No. (%) Newborns With Poor Outcome | Odds Ratio (95% CI) | P Value | PPV (%) | NPV (%) | ||
|---|---|---|---|---|---|---|
| Normal US Results | Pathologic US Results * | |||||
| DQ ≤85 | 0/45 | 8/11 (72.7%) | NE | <0.001 | 72.7 | 100 |
| Motor delay | 0/45 | 6/11 (54.5%) | NE | <0.001 | 54.5 | 100 |
| SNHL | 3/45 (6.7%) | 6/11 (54.5%) | 16.8 (3.2-89) | <0.001 | 54.5 | 93.3 |
| Death or any sequela | 3/45 (6.7%) | 11/12 (91.7%) | 154 (17.3-1219.6) | <0.001 | 91.7 | 93.3 |
* One newborn with pathologic US results died during the neonatal period. Follow-up data were available for 11 of the 12 patients who lived.
Initially, CT was considered the gold standard to diagnose cerebral involvement in congenital CMV infection, and 90% of symptomatic infants with abnormal CT findings had neurodevelopmental or neurosensory sequelae. However, MRI is much more sensitive in diagnosing white matter and migrational abnormalities. Cranial ultrasonography is more sensitive in diagnosing lenticulostriate vasculopathy and germinolytic cysts, which are especially found in the anterior temporal region along with ventriculomegaly and ventricular septations. Infection in the first trimester may result in abnormal CNS development, which, in turn, may cause abnormal neuronal migration and cerebellar hypoplasia. Infection occurring between 16 and 18 weeks’ gestation may result in lissencephaly, whereas infection occurring between 18 and 24 weeks’ gestation is related to the development of polymicrogyria. Migrational disorders correlate with poor neurodevelopment and sensorineural hearing loss, and polymicrogyria correlates with cognitive and motor delay and epilepsy. Periventricular calcifications are also a poor prognostic indicator of developmental delay.
Abnormalities in balance and posture causing delays in walking, abnormalities in gross motor function, and cerebral palsy are now being seen in children with sensorineural hearing loss. These may reflect abnormal vestibular function in the ear and/or cerebellar lesions. A few reports have noted an increase in autism spectrum disorders, but this is not universal. In contrast, a normal fetal brain MRI result generally indicates a good clinical outcome, although there may still be some cases of mild hearing loss.
Congenital CMV is the most significant cause of nongenetic sensorineural hearing loss in childhood: 10%-15% of all infants experience hearing loss, but it may also be detected in 30%-65% of symptomatic infants. One fourth of hearing loss detected by 4 years of life is secondary to congenital CMV infection. The hearing loss tends to be more severe and to occur earlier in infants with symptomatic infection. These children also tend to have bilateral loss, whereas infants with asymptomatic infection are more likely to have unilateral loss of hearing. The loss can be progressive or fluctuate and may be delayed by years. Less than half of all sensorineural hearing loss secondary to CMV is detected by newborn screening. Increased blood and urine viral load with more prolonged urinary excretion at an earlier age is associated with a higher risk of sensorineural hearing loss in both symptomatic and asymptomatic infants. These children often shed virus for greater than 4 years. The cause of this continued and fluctuating hearing loss is not well understood. It has been variably attributed to a direct cytopathic effect of the virus, to the host's immune response, and to injury to the endolymphatic structures. Rehabilitation with hearing aids or cochlear implants can provide useful speech comprehension, even if language development is not as advanced as in uninfected children who do not have other neurologic handicaps.
Chorioretinitis may be found in 15%-30% of symptomatic newborns. Children often have impairment or strabismus secondary to chorioretinitis, optic atrophy, or central cortical lesions and to macular scarring. Chorioretinal scars, resulting in visual impairment, are frequent in children receiving cochlear implants.
Because most women with CMV infection remain asymptomatic, screening for primary infection is necessary to determine risk. Detecting CMV IgG, which has high sensitivity and specificity, in a woman known previously to be seronegative reliably diagnoses primary infection. Few European or American countries routinely screen for seroconversion, however, because there is no consensus for treatment of either a newly infected pregnant woman or her infant. Detection of CMV IgM may indicate a recent infection, but IgM also increases with reactivation of latent infection or re-infection. CMV IgG avidity testing may be helpful: The binding capacity or avidity of IgG is low just after an infection and remains low for 18-20 weeks after infection and then increases with time. Also, the synthesis of IgG antibodies against glycoprotein B (gB) is delayed in a primary infection, so the absence of these antibodies in an immunoblot test is a marker for recent infection. Finally, CMV DNA may be detected during acute infection from the peripheral blood.
If a primary maternal infection is suspected, or ultrasonography shows abdominal or cerebral findings or IUGR, fetal diagnosis may be undertaken. Cordocentesis is unnecessary because the sensitivity is lower and the risks are greater than with amniocentesis. CMV may be detected in the amniotic fluid by viral culture or by PCR for CMV DNA. The amniotic fluid should only be sampled at least 6 weeks after the onset of maternal infection to allow for delayed passage of virus across the placenta and at 21-23 weeks’ gestation to allow for fetal renal maturity to be able to excrete the virus. A high viral load was initially correlated with the development of a symptomatic infection and neurodevelopmental sequelae, but this was not confirmed in subsequent studies.
CMV must be identified in an infant by 21 days of age to be certain it is a congenital infection rather than a perinatal infection. Urine culture for CMV, either by routine viral culture or by rapid shell vial culture, was the gold standard for the diagnosis of CMV infection for years. More recently, it has been replaced by PCR amplification testing of urine, saliva, or blood. PCR testing performs as well as or better than rapid culture. PCR offers more rapid turn-around, is not as affected by storage and transport conditions, costs less, and is better for high throughput than culture. It performs well in targeted testing and large volume screening testing. Both saliva and urine are acceptable in large-volume screening programs, but saliva is easier to collect and comparable in reliability. In a study in the United Kingdom, 98% of salivary swabs collected by parents from newborns who failed the routine hearing screen were successfully tested before 21 days of life for CMV. Cost benefit analyses of this program and a similar program in the United States compared well with other screening programs, suggesting a potential for targeted screening of at-risk infants who might benefit from treatment. Screening of pregnant women in general is not recommended in the United States or in many European countries because of the lack of a successful treatment modality. However, it has been suggested that screening of women undergoing assisted reproduction might identify those recently infected and should, therefore, delay their efforts. In addition, dried blood spots, as collected on the Guthrie cards in newborn screening programs, can be used to retrospectively diagnose a congenital infection in an older infant. The sensitivity of PCR testing of dried blood spots is slightly lower, making them less suitable for screening programs. Finally, the diagnosis may be made by determining a fourfold increase in specific IgG titers or presumptively by detecting anti-CMV IgM because IgM does not cross the placenta. However, there are false-positive and false-negative results with IgM, rendering it less useful.
Ganciclovir (GCV) and its oral prodrug valganciclovir (VGCV) are used to treat the newborn with symptomatic congenital infection. GCV is an acyclic deoxyguanosine nucleoside analogue, which acts as a chain terminator during elongation of the CMV DNA.
Kimberlin et al. randomly assigned neonates with symptomatic CNS disease to receive 6 weeks of therapy with ganciclovir, at 6 mg/kg/dose given every 12 hours versus no treatment and monitored hearing with brainstem auditory evoked response. When tested at 6 months, in 84% of treated infants, normal hearing was maintained or hearing was improved, in contrast to 59% of control infants. None of the treated infants had worsening of hearing at 6 months compared with 41% of untreated infants. When tested at 1 year, 21% of treated infants and 68% of control infants exhibited worsening of hearing. Neutropenia was three times more common (63%) in treated infants than in untreated infants. Maintenance of IV access was also an issue. Viral excretion in urine returned to pretreatment levels within 2 weeks after GCV was discontinued, as did the viral load. In addition, the Denver Developmental Screening Test was also performed at 6 weeks, 6 months, and 1 year of age. Infants who were treated with GCV had fewer developmental delays at 6 months and at 1 year of age.
Subsequently, it was determined that oral administration of VGCV given twice daily at 16 mg/kg/dose provided comparable systemic coverage. A second randomized, placebo-controlled trial comparing 6 weeks of oral VGCV treatment to 6 months of oral VGCV in infants with symptomatic congenital infection was begun. Although no difference in best ear hearing as determined by brainstem auditory evoked response was seen at 6 months’ follow-up, there was a small, but significant improvement in hearing at 12 and 24 months among the infants treated for 6 months. The infants treated for 6 months also performed better on the Bayley Scales of Infant and Toddler Development (3rd edition) at 24 months. Of note, neutropenia was comparable between the two groups and lower than in the GCV trial. Therefore, it is recommended that infants with symptomatic congenital disease in their first month of life be treated with 6 months of oral VGCV after counseling the parents regarding the 20% risk of neutropenia. Carcinogenicity and gonadotoxicity have been seen in animal models, but not in humans. Infants with asymptomatic congenital disease should not be treated. Recently, a small study of 54 infants with symptomatic infection and hearing impairment at birth were treated with 12 months of GCV/VGCV. Hearing improved in 65%, with return to normal hearing in most. There was no control group, and hearing was not reported beyond a year of life.
Pasteurization can be used to inactivate CMV in human milk, and freezing of the milk decreases, but does not eliminate, the virus. If a mother is known to be CMV seronegative and her infant must receive donated, fresh human milk, the use of a seronegative donor would be beneficial.
The development of a vaccine against CMV is a public health priority. Several live-attenuated vaccines have been developed, but trials have not demonstrated sufficient protection, and none of the vaccines has been licensed for use. In addition, it is now understood that natural immunity does not prevent transmission of the virus to the fetus and that a significant proportion of congenital infections result from nonprimary infections during pregnancy. Clinical isolates of CMV strains show significant genetic variation and recombination into a tremendous number of strains, implying that multivalent vaccines may be needed. These vaccines may need to target both the cellular and humoral immune responses to the virus to be effective. There is some promise in recombinant live-attenuated vaccines, which delete the genes responsible for immune evasion.
However, prevention remains the most important available means to avoid infection. CMV may be transferred to human hands by contact with mucosal surfaces, saliva, urine, or stool from small children shedding the virus or from surfaces contaminated by those children. The human skin maintains an acidic microenvironment, so the virus does not survive on human hands as long as it does on other surfaces. Nevertheless, it can be transferred from the hands to mucosal membranes, potentially resulting in infection. Hand washing with soap, antibacterial soap, or even just water removes much of the virus, and hand sanitizers and, to a lesser extent, diaper wipes can inactivate the virus. Female childcare workers, especially when their children are younger than 2 years of age, need to be counseled about the risks of CMV in pregnancy and taught good hand washing procedures, as do women health care workers, particularly in nurseries. The US Centers for Disease Control and Prevention (CDC) has developed a set of hygiene practices to reduce the risk of CMV infection for women who are pregnant or planning to become pregnant. These include thoroughly washing hands with soap and warm water after changing diapers, feeding or bathing a child, wiping the nose or drool, or handling the child's toys. The pregnant woman should not share food, drinks, eating utensils, or toothbrushes with a young child or put a pacifier in her own mouth. She should avoid saliva when kissing a young child, and she should clean toys, countertops, and other surfaces in contact with either saliva or urine. There was an 84% reduction in seroconversion among seronegative pregnant women in high-risk occupations when they were educated about CMV and hygiene in comparison with those who did not receive counseling.
Transmission of CMV via blood transfusions may be largely eliminated by using blood from antibody-negative donors, by using frozen, deglycerolized red blood cells, or by using leukocyte-depletion filters. Because both methods have a small failure rate, some recommend using both antibody-negative donors and leukocyte depletion filters.
When a pregnant woman has been infected with CMV and her fetus has been diagnosed with infection, few options are available other than abortion. The use of passive immunization with CMV-specific hyperimmune globulin (HIG), primarily given intravenously, initially suggested a lower rate of transmission of the virus to the fetus, but a subsequent randomized double-blind trial of HIG in 124 women found a fetal transmission rate of 30% in treated women with a primary infection, and 44% in control women, which did not reach statistical significance. There was also a higher rate of obstetric complications among treated women, so the use of HIG is not routinely advised. A large scale trial in the United States is currently in progress.
Varicella (chickenpox) is one of the most highly communicable human diseases. It is the result of a primary infection with varicella-zoster virus (VZV), which is one of the human DNA herpesviruses. After infection, latent virus persists in the dorsal root ganglia. The localized rash of herpes zoster results from a reactivation of infection, in which the virus begins to multiply within the ganglia and propagate down the sensory nerves to its dermatomes.
Humans are the only known reservoir of VZV. Immunity is lifelong and widespread. Of young adults, 70%-80% have a history of chickenpox, and this has been found to be quite reliable. Conversely, only 10%-20% of individuals lacking a history of chickenpox have been found to be seronegative, even though asymptomatic infection is believed to be unusual. Chickenpox rarely occurs in immunocompetent, seropositive individuals, although it has been reported to occur among pregnant women. Most reinfections are mild. Because of the implementation of universal immunization in the United States in 1995, as well as the recommendation of a second vaccine dose in 2006, varicella infections have decreased by greater than 90%. Primary varicella infection in pregnancy now only amounts to roughly 2-3/1000 pregnancies/year.
The initial protection against VZV depends on IgG. Neonates become ill because they are exposed to high titers of the virus from the mother and yet antibody is absent. In contrast, limitation of the severity of the infection and recovery from infection largely depend on cellular immunity, or T cells. Pregnant women and others who are immunosuppressed have decreased T-cell immunity, so disease can be very severe. Severe disease may also develop when the individual is receiving high doses of steroids.
Chickenpox is seen year round, with some increase in winter months, and is worldwide in distribution. In household contacts, 90% of susceptible individuals are infected. Transmission of the virus occurs via air-borne droplet spread or via contact with the virus in the vesicular lesions of either varicella or, rarely, zoster. Although it is well documented that susceptible individuals may develop varicella after exposure to zoster lesions, varicella-zoster does not develop after exposure to chickenpox. Transmission may occur 1-2 days before the onset of the rash until all vesicular lesions are dried and crusted, at least 6 days after onset of the rash. The incubation period is 10-21 days after exposure, unless the individual has been given varicella-zoster immunoglobulin, which may delay the onset of the infection for 28 days. When the mother has varicella around the time of delivery, the onset of the rash in the newborn is usually 9-15 days after the onset of the rash in the mother.
After replication of the virus within the nasopharynx and invasion of the local lymph nodes, there is transient viremia, which seeds the viscera. Viral multiplication continues, causing a greater viremia and widespread cutaneous involvement. Subsequent viremias result in crops of vesicles, followed by the development of latency within the dorsal root ganglia.
After a short prodrome of fever, headache, and malaise, there is generalized exanthema, which begins on the face and trunk and proceeds centripetally. Recurrent crops of vesicles appear for 2-5 days and then crust and scab, usually healing without scarring. Secondary bacterial infection of the cutaneous lesions is the most common complication and may lead to sepsis, pneumonia, and meningitis. Most other complications are rare, including encephalitis, hepatitis, myocarditis, arthritis, and glomerulonephritis. Reye syndrome, which sometimes occurred after chicken pox, has almost disappeared now that it is recommended not to use salicylate-containing products with chicken pox.
Varicella pneumonia is the most common cause of mortality. The onset of fever and cough usually occurs within 2-4 days after the development of the rash but may occur later. Radiographic changes consist of diffuse, nodular lesions with perihilar prominence. Dyspnea, cyanosis, rales, and chest pain may be severe, and there may be hemoptysis. Although only 15% of adults develop pneumonia, 90% of all cases of chickenpox pneumonia occur among adults. Immunocompromised individuals are also at increased risk.
Shingles, or varicella zoster, is characterized by clusters of vesicles in 1-3 sensory dermatomes as a result of VZV reactivating from the latent state in the sensory ganglia where it had resided. These vesicles are pruritic and painful and, occasionally, result in postherpetic neuralgia, which can last a few days to months. Occasionally, there can be reactivation of VZV viscerally without any skin lesions.
There does not seem to be an increased risk of spontaneous abortion or prematurity as a result of maternal chickenpox, although prematurity and IUGR are common among infants with congenital varicella syndrome. Mortality of 40% with chickenpox pneumonia has been reported among pregnant women who did not receive antiviral therapy. Some experts recommend oral acyclovir or valacyclovir for pregnant women with varicella, especially during the second and third trimesters, when the risk of severe infection is greatest. IV acyclovir is recommended in the presence of varicella complications.
Because of the potential for increased mortality, varicella-zoster immunoglobulin (VZIG) may be useful for passive immunization of a susceptible pregnant woman after significant exposure to varicella. Controlled trials are, however, still unavailable. VZIG needs to be administered within 96 hours of exposure and, preferably, after susceptibility has been confirmed serologically. VZIG, or Varizig, is approved by the US Food and Drug Administration (FDA) for use in newborns, premature infants, children less than 1 year of age, and pregnant women. Uncertainty continues regarding its effect on the fetus. VZIG may not protect the fetus from infection. Likewise, the absence of signs of maternal chickenpox after the use of VZIG does not indicate that the fetus is protected, and there is at least one report of congenital varicella syndrome when the mother received VZIG 4 days after exposure. Ultrasonography revealing a wasted limb may assist in the diagnosis of infection of the fetus, although this is, of necessity, a late diagnosis, which rules out the option of an abortion.
Intrauterine infection occurs as a result of hematogenous dissemination across the placenta and can occur even with mild maternal infection. The lowest risk of congenital varicella syndrome (CVS) occurs with maternal infection before 12 weeks of pregnancy (0.55%) and rises to 1.4% between 13 and 20 weeks, giving an overall risk of 0.91% in the first 20 weeks of pregnancy ( Table 50.7 ). Only a couple of cases of CVS have been described between 20 and 28 weeks’ gestation. A few infants (1.1%) may develop herpes zoster in the first 2 years of life when the mother had had varicella in the late second or third trimester of pregnancy. A later maternal infection is more likely to result in less severe fetal manifestations. Although portions of the congenital syndrome have been seen after maternal zoster, the full syndrome has not been seen, presumably because viremia is uncommon with zoster, and some pre-existing immunity is present. Theoretically, the fetal lesions result from the occurrence of zoster in utero.
| Study | First Trimester | Second Trimester | Third Trimester |
|---|---|---|---|
| Enders et al. | 1/236 * | 7/351 * | Not reported |
| Jones et al. | 1/110 * | 1/46 * | 0/13 |
| Harger et al. | 0/140 * | 1/122 * | 0/100 |
| Mean % | 0.41 | 1.73 | Not reported |
* The number of infants born with congenital varicella syndrome out of the number of women infected.
Typical features of the congenital syndrome include cicatricial cutaneous lesions, often in a zigzag shape, with limb involvement, ocular abnormalities, and severe mental retardation. The condition may progress to an early death. Skin lesions usually occur over an involved limb, sometimes over the contralateral limb, and are often depressed and pigmented in a dermatomal distribution. Cataracts, microphthalmia, chorioretinitis, cerebral atrophy, seizures, and mental retardation may occur; sometimes bowel and bladder dysfunction also may occur. Hypoplasia of the bone and muscle of a limb is prominent.
Maternal counseling is difficult because the incidence of the syndrome is so low as to preclude routinely recommending abortion. Maternal infection may have a 25% incidence of fetal varicella, but this does not indicate that the fetus will develop congenital varicella syndrome. Similarly, chorionic villus sampling with PCR to show virus and cordocentesis to show fetal VZV IgM indicate only fetal infection, not the development of the congenital syndrome.
The most helpful prenatal test is fetal ultrasonography. Unfortunately, the abnormalities may occur late, thus precluding elective abortion. Limb abnormalities carry a 50% risk of mental retardation and early death. Hydrocephalus, liver calcifications, hydrops fetalis, and polyhydramnios may also be seen.
Chickenpox is considered congenital when it occurs within the first 10 days of life, and it results from transplacental transmission of the virus from mother to fetus. Maternal varicella, particularly occurring from 5 days before delivery until 2 days after delivery, results in a higher neonatal mortality (20%-30%) because there has not been enough time for maternal antibody to develop and transfer across the placenta to the fetus. The fetal attack rate is 24%-50%. The incubation period is shorter—9-15 days from the onset of the maternal rash to the development of fetal or neonatal disease. Infants may have a very mild infection with few lesions or may have severe disease, with widespread cutaneous and visceral involvement, including encephalitis and hepatitis. Death most often occurs secondary to pneumonia. Zoster may also occur and represents an intrauterine infection.
Dried blood spots from the Guthrie cards of greater than 400 children with cerebral palsy were compared with case controls for the presence of neurotrophic viruses as determined by PCR. Although seroprevalence was high even in control infants (nearly 40%), the presence of VZV DNA nearly doubled the risk of cerebral palsy.
Postnatally acquired chickenpox occurs between 10 and 28 days of life. The infection is usually mild and is unlikely to result in death. An outbreak may occur within the neonatal intensive care unit (NICU) but is much less common than in pediatric units.
PCR of vesicular fluid or a scab, or even saliva during the acute infection, is now the preferred method for diagnosis. Culture and direct fluorescent antibody (DFA) testing are less reliable. Serologic testing for IgG can detect an increase in antibody titer and document infection retrospectively. The presence of specific IgM usually indicates recent infection but is an unreliable indicator.
The primary approach to varicella should be preventive. The vaccine seems to protect 85% of individuals vaccinated. Pregnancy should be avoided for at least 1 month after vaccination, but there have been no cases reported of fetal malformations in inadvertently immunized pregnant women. Nursing mothers may be immunized as the vaccine strain has not been detected in breast milk. Healthy children should be vaccinated promptly, according to the immunization schedule, at ages 12-15 months and 4-6 years. Parents should be counseled about the possibility of a febrile seizure resulting after vaccination. There should be at least 3 months between doses when catch-up vaccination is being given. There are separate recommendations for children with chronic disease or immunodeficiency or those who are receiving steroids. The vaccine should also be given to an otherwise healthy child at least 12 months of age within 3-5 days of exposure and repeated at the appropriate age.
VZIG should preferably be given within 96 hours after exposure. It modifies the course of infection but does not prevent it. Passive immunization should be administered to any infant born to a mother who develops varicella 5 days before delivery to 2 days after delivery. Because a high dose would be needed, it is not recommended that the mother be given VZIG before delivery for passive immunization of the infant.
Acyclovir is the antiviral drug of choice for any infant with severe or potentially severe chickenpox, whether congenital or postnatal in origin. No acute toxicity has been shown after acyclovir use for neonatal HSV infections, although the dosage for neonatal chickenpox is much higher, and long-term toxicity is unknown.
Although nosocomial cases of chickenpox are uncommon in the nursery, they may occur. Strict respiratory droplet isolation of any patients with active lesions or in the incubation period needs to be implemented, and only visitors and staff with prior immunity should be allowed in the room. Strict hand washing and contact precautions are a necessary. Postnatal exposure of a term infant 2-7 days of age should carry little additional risk. Infants with low birth weight or preterm infants may be at considerable risk, however, because they were born before the transfer of maternal antibody across the placenta. All hospitalized exposed preterm infants with birth weight 1000 g or less or gestational age less than 28 weeks should receive VZIG or IV acyclovir, regardless of the maternal immune status. Hospitalized, exposed preterm infants (≥28 weeks’ gestation) should receive VZIG or IV acyclovir if the mother lacks a reliable history of chickenpox or serologic evidence of immunity. VZIG cannot be used to control the spread of varicella in a nursery because it only modifies, but does not prevent, the course of chickenpox. Infants with congenital varicella syndrome do not need to be isolated if all the skin lesions have healed.
Strict preventive measures are also necessary in the delivery and nursery services. If the mother has active lesions of chickenpox at the time of delivery, she must be isolated from other patients and the staff. If the maternal lesions appear within 5 days before delivery and 2 days after delivery, the infant should be protected further with VZIG. If the mother had chickenpox before delivery and her lesions have healed, she need not be isolated, but the infant should be, preferably in the mother's room, during the incubation period. An infant with congenital chickenpox also needs isolation and treatment but may remain with the mother. If the mother was exposed to chickenpox 6-20 days before delivery and she has no history of chickenpox, she and her infant need to be isolated while her susceptibility is being confirmed via serology, and VZIG should be given to the infant if the mother develops lesions within 48 hours of delivery. The mother and the infant may be discharged home together. If the mother develops the varicella rash postnatally, the infant has already been heavily exposed, and transplacental transfer of maternal anti-VZV IgG and breast milk antibodies may decrease the severity of the infant's infection.
EBV, a B-lymphotropic herpesvirus, is among the most prevalent of human viruses. Greater than 95% of pregnant women are seropositive. When EBV infects adolescents, it may cause infectious mononucleosis, but most infections are asymptomatic. The development of a rash is more common when infection is treated with ampicillin or other penicillins. CNS and hematologic symptoms and, rarely, disseminated disease, which is usually fatal, may occur. Lymphoproliferative disorders may also result from infection. The virus has been associated with a few malignancies, most often in Africa and Asia. A case-control study of more than 400 children with acute lymphoblastic leukemia (ALL) showed an association of maternal infection with EBV at 12-14 weeks’ gestation. Further study indicated reactivation may also be associated with nonacute lymphoblastic childhood leukemia. However, the Guthrie cards of 54 children with ALL failed to show any DNA of EBV via PCR testing. Findings from this and other studies have left the matter open to question. Finally, using quantitative real-time PCR to detect viruses in the tissue of infants who died as a result of sudden infant death syndrome compared with controls, there was a statistical association with EBV and HHV-6 9 and sudden infant death syndrome.
Humans are the only natural hosts for the virus; as is typical of herpesviruses, the virus is excreted intermittently during reactivations for the remainder of the individual's life. Transmission, via saliva, seems to require close, personal contact. Fomites are not believed to transmit infection, even though virus may be viable for hours in saliva outside the body. Infection is endemic year round. Blood transfusion has also been documented to transmit virus occasionally.
Primary infection is rare during pregnancy because nearly all pregnant women are immune. The virus can be detected in cervical secretions by DNA hybridization. IgG and IgM to the viral capsid antigen can be detected in the acute infection, whereas IgG to the EBV nuclear antigen is detectable during latent infection.
Several studies have associated the occurrence of a primary infection in the first trimester of pregnancy with an adverse outcome, including prematurity and pregnancy-induced hypertensive diseases, although these associations remain unproven. EBV can cross the placenta and has been shown to cause placental infection, but the pregnancy outcome seems to be normal. There do not appear to be any congenital anomalies.
There is a report associating the presence of CMV and EBV viral DNA (rarely found in the blood of the newborn screening cards) with the development of cerebral palsy. The viruses were not found in the blood of the case controls.
The role of the T-lymphotropic viruses HHV-6 and HHV-7 in human infection is just being recognized because HHV-6 was identified only in 1986, and HHV-7 was identified even later. They are double-stranded DNA members of the herpesvirus family, and are closely related to CMV. Like all herpes viruses, they cause persistent and lifelong infection. They are present worldwide, and humans are the only known hosts. There is only one variant of HHV-7, but two variants of HHV-6—A and B. Reflecting the high seroprevalence in adults, most infants have maternally acquired HHV-6 and HHV-7 IgG antibody, which wanes over the first 6 months of life, at which time these infants become seronegative and more susceptible to infection. HHV-6B infection is common in infants 6-12 months of age, and most children are seropositive by age 2 years. HHV-7 is acquired later than HHV-6 but is usually acquired by age 5-6 years. After infection, the virus persists for life and is shed in saliva, which is recognized as the major source of transmission. The mean incubation period is 9-10 days for HHV-6 and not known for HHV-7.
Many infants with primary HHV-6 infection are afebrile, although infants infected at younger than 6 months of age were more likely to be febrile than infants infected after 6 months. Symptoms were present, however, in greater than 90% of cases of primary infection. These may include high fever without rash and sometimes cervical or postoccipital lymphadenopathy, otitis media, and respiratory or gastrointestinal disease. There may rarely be hepatitis or a mononucleosis syndrome. One fourth of the infants were diagnosed with infantum subitum (roseola), which causes an erythematous maculopapular rash when the fever resolves. Persistent and reactivated infection has not been shown to cause illness but does persist in the brain, and HHV-6 has been implicated in some cases of meningitis and encephalitis in infants and children younger than 2 years of age. It is also associated with febrile seizures in 10% of these febrile infants, and some of these infants develop status epilepticus. HHV-7 infections are usually asymptomatic, but a few may cause roseola and may be responsible for recurrent cases of roseola, in which case it remains unclear whether they are the primary cause or simply able to reactivate HHV-6. HHV-7 is also responsible for febrile illness and cases of febrile seizures. Again, whether the seizures are caused by the HHV-7 infection or by reactivation of HHV-6 is still being debated.
HHV-6 is the only herpesvirus that can integrate its genome into the human chromosome, and many authors have hypothesized that HHV-6 congenital infection is inherited chromosomally and not transferred across the placenta. There are no cases of congenital HHV-7, which cannot integrate into the chromosome. About one third of congenital HHV-6 infections are caused by HHV-6A. The remaining two thirds are caused by HHV-6B. All cases seem to be asymptomatic. This is in contrast to HHV-6 infections later in infancy, all of which are caused by HHV-6B. Although HHV-6 can be transmitted transplacentally from mother to fetus with maternal re-infection or reactivation of infection, congenital HHV-6 infections can be inherited from the chromosomes of either or both parents. Congenital infection was found in 1% of US infants, a rate similar to those in other western European countries, and is not known to cause any clinical disease. However, there is one report of 57 newborns with HHV-6 congenital infection and 242 control newborns without infection showing significantly lower developmental scores on the Bayley-II Mental Index at 12 months of age compared with the scores in the controls. There were no effects on the visual or hearing screens. HHV-7, but not HHV-6, has been found in human milk.
Diagnosis remains difficult. Few assays are commercially available. Indirect immunofluorescence can be used to distinguish HHV-6 from HHV-7 IgG antibody and can be followed for a fourfold rise in serial titers, but IgM antibodies cannot always be detected in young children with primary infections. Also, antigen detection can be used to determine active HHV-6 and HHV-7 infections, but the assays are not sensitive in young children. Of note, confirmation of infection does not change the management of the infant in the majority of cases.
Paramyxoviruses are being increasingly noted as significant causes of human disease. Their spectrum has been greatly expanded with the discovery of human metapneumovirus (hMPV). They are single-stranded, RNA viruses with a lipid envelope. Among the human pathogens, there are two subfamilies. The Paramyxoviridae subfamily includes the parainfluenza viruses (PIVs) and the mumps and measles viruses. hMPV, along with the related respiratory syncytial virus (RSV), belongs to the other subfamily, the Pneumovirinae.
PIVs are enveloped, single-stranded, negative-sense RNA viruses in the Paramyxoviridae family. The four antigenic types (1-4) belong to the same subfamily as the mumps and measles (rubeola) viruses. Spread occurs through direct contact with infected respiratory secretions, via either respiratory droplets or contact with contaminated secretions on fomites. PIV-3 has been shown to survive for at least 10 hours on nonabsorptive surfaces (countertops) and 4 hours on absorptive surfaces, such as hospital coats, but die within minutes on finger pads. Infection may occur year round, but seasonal or nosocomial outbreaks also occur. PIV-1 and PIV-2 outbreaks of croup or other respiratory illnesses tend to occur in the fall season, whereas PIV-3 outbreaks, which are associated with bronchiolitis and pneumonia in infants, are more likely to occur in spring and summer. Parainfluenza may also exacerbate symptoms of chronic lung disease.
Nearly everyone is infected with all PIV types by 5 years of age. PIV-3, the most common cause of respiratory illnesses of all the serotypes, is also the most common type in infants younger than 12 months of age, infecting about half of them in that first year. PIV-3 seems to spare infants in the first 4 months of life because of the presence of transplacentally acquired neutralizing antibodies, but it is the usual source of nosocomial nursery outbreaks, spread via health care workers, often in the presence of overcrowding. Older, convalescing preterm infants seem to be at increased risk of severe disease in nursery outbreaks, consistent with waning levels of maternally acquired antibody, and this may result in a high mortality rate among chronically ill infants. The incubation period is 2-6 days, and virus may be shed for 3 weeks or longer by infected infants. Immunity is incomplete, so re-infections may occur with all of the serotypes, but they mostly cause only mild upper respiratory infections.
PIVs have been regarded as second in frequency to RSV as a cause of respiratory infections. Rhinovirus seems to be more common in respiratory infections, however, and the more recently discovered hMPV is also probably more common than PIV. As a group, PIVs are responsible for upper respiratory infections, laryngotracheitis (croup), bronchiolitis, and pneumonia. PIVs may also exacerbate asthma symptoms. PIV-1 and PIV-2 are more likely to cause croup, whereas PIV-3 is highly associated with bronchiolitis and pneumonia, particularly in infants and young children. Respiratory infections from PIVs may be difficult to differentiate clinically from infections caused by RSV. In young children and infants, these infections tend to be mild with a very low mortality rate. Infected children may require supplemental oxygen but rarely need ventilatory support. A few infants are asymptomatic. PIVs have been identified as a cause of 13% of community-acquired pneumonia in infants. Co-infection with other respiratory viruses may occur in 20%-60% of infants and may increase the severity of the infection. In newborns, the infection is more similar to a severe RSV infection. Infants may have apnea, bradycardia, and pneumonia, and there may be worsening of chronic lung disease.
PIV infection may be diagnosed by isolating the virus from nasopharyngeal secretions or by rapid antigen detection techniques, such as immunofluorescence and by PCR antigen detection. Serologic detection is not helpful. Prevention of nosocomial infections requires strict adherence to contact and respiratory precautions and strict hand washing. Treatment is largely supportive. Severely ill infants must be monitored closely for need for oxygenation and ventilation. Severe laryngotracheobronchitis has been treated with racemic epinephrine and parenteral corticosteroids, which decrease symptom severity and duration of hospitalization. Antibiotics may be used if there is bacterial superinfection. The usefulness of ribavirin or IVIG remains unproven.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here