Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
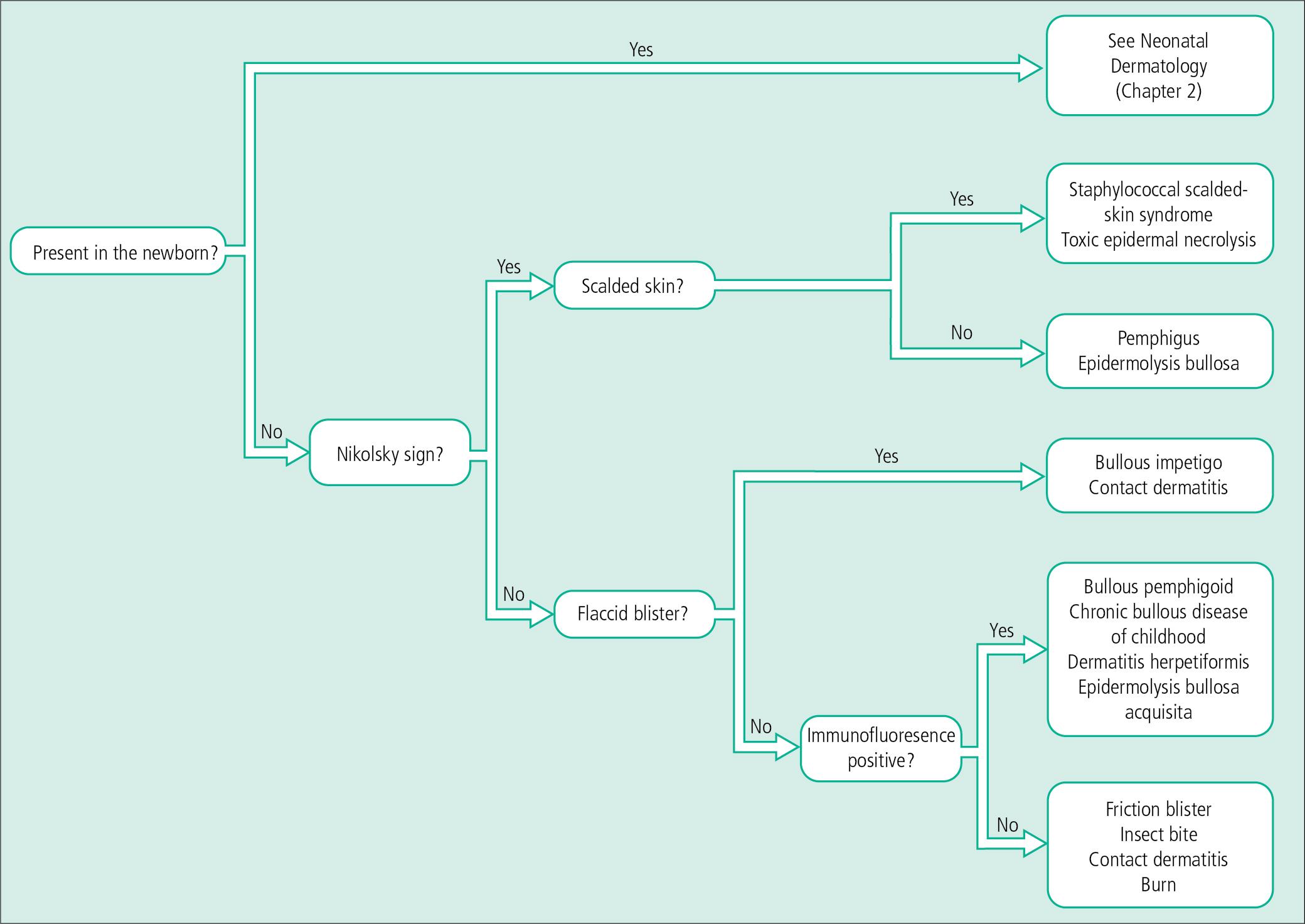
Vesiculopustular eruptions range from benign, self-limited conditions to life-threatening diseases. Early diagnosis, especially in the young or immunocompromised child, is mandatory. An algorithmic approach to diagnosis for vesiculopustular dermatoses is summarized at the end of the chapter (see Fig. 4.29 ).
An understanding of the structures that account for normal epidermal and basement membrane zone adhesion provides clues to the clinical diagnosis and pathogenesis of blistering diseases ( Fig. 4.1 ). Epidermal cells are held together by desmosome–tonofilament complexes. Electron-dense tonofilaments insert into desmosomes in the keratinocyte plasma membrane and project toward the nucleus. Intercellular bridges extend between keratinocytes and are associated with a sticky, glycoprotein-rich, intercellular cement substance. In the basement membrane zone, tonofilaments insert into hemidesmosomes, which are attached to the lamina densa by anchoring filaments that traverse an electron-lucent layer known as the lamina lucida. The electron-dense lamina densa is, in turn, affixed to the dermis by anchoring fibrils. Elastic microfibril bundles, which arise in the upper dermis, also insert into the lamina densa.
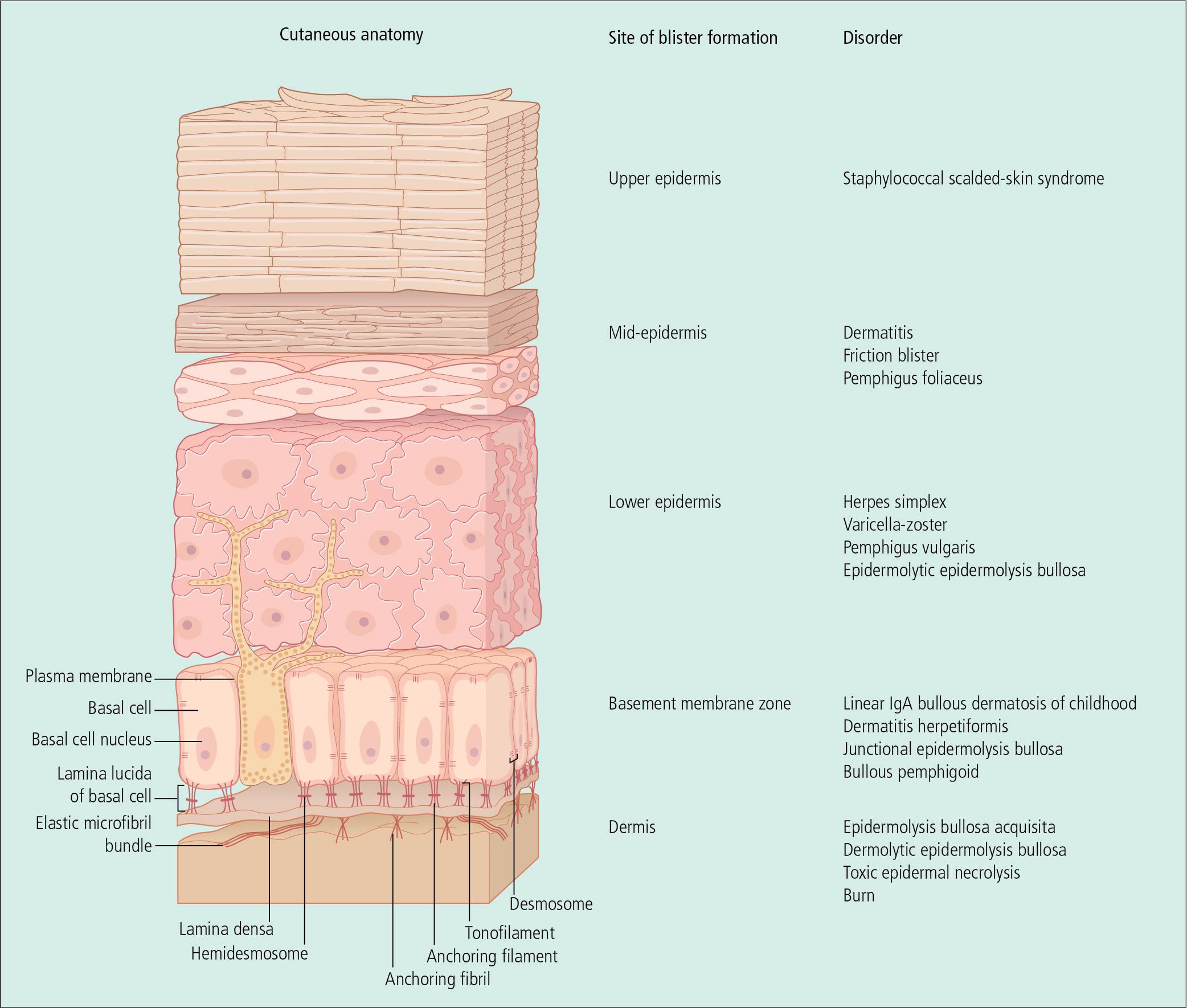
A number of proteins that play a role in the structural integrity of the skin have been identified in the basement membrane zone, and hereditary-or autoantibody-induced defects in these proteins may lead to clinical disease. Bullous pemphigoid (BP) antigen appears on the bottom of the basal cell plasma membrane and within the lamina lucida. Laminin is present within the lamina lucida, and type IV collagen has been isolated to the lamina densa. In epidermolysis bullosa acquisita (EBA), antibodies against type VII collagen have been identified in the dermis, just beneath the lamina densa. Fluorescein-tagged antibodies directed against these proteins may be used to identify the site of blister formation in disorders that involve the dermal–epidermal junction. Salt-split skin can be used as a substrate to more specifically localize antibodies above or below the basal lamina region.
In general, flaccid bullae arise within the epidermis, and tense lesions involve the dermis. Specific diagnoses, however, rely on identification of clinical patterns, histopathology, and immunofluorescent findings. A few rapid diagnostic techniques also aid in developing a working diagnosis.
Herpes simplex virus (HSV) is a common cause of oral lesions in toddlers and children of school age. Primary herpetic gingivostomatitis begins with extensive perioral vesicles and pustules and intraoral vesicles and erosions ( Fig. 4.2 ). The gingivae become edematous, red, and friable and bleed easily. Epithelial debris and exudates may form a membrane on the mucosal surfaces. The eruption is usually accompanied by fever, irritability, and cervical adenopathy. Lesions may also be scattered on the face and upper trunk. In infants and toddlers, lesions are frequently auto-inoculated onto the hands. Patients are observed for dehydration as symptoms abate over 7–10 days.
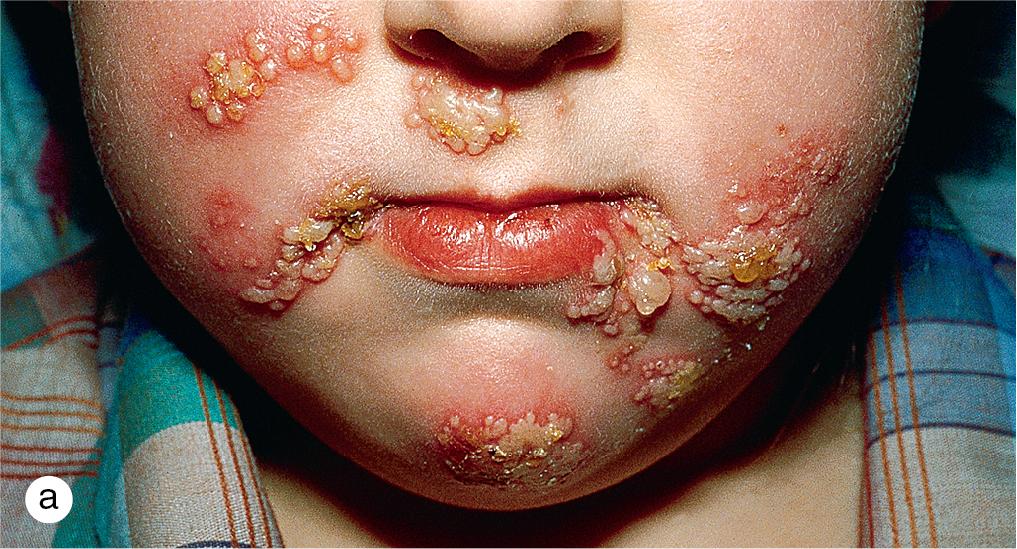
Herpetic gingivostomatitis can be differentiated from enteroviral infections, which usually produce vesicles, ulcerations, and petechiae on the hard palate and spare the gingivae. Although aphthae may be very painful, they are usually isolated lesions that lack the diffuse inflammation associated with herpes.
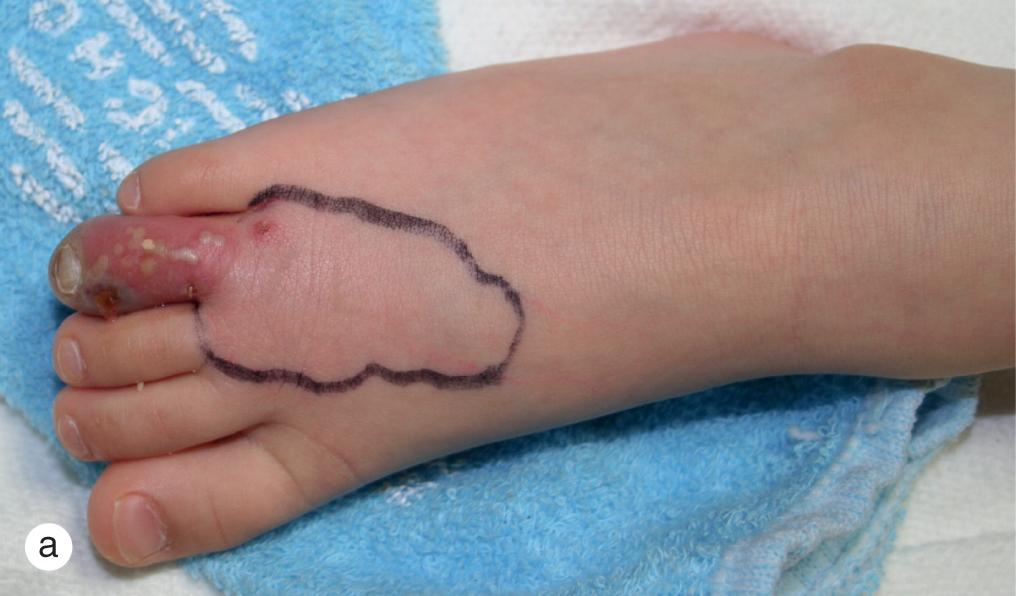
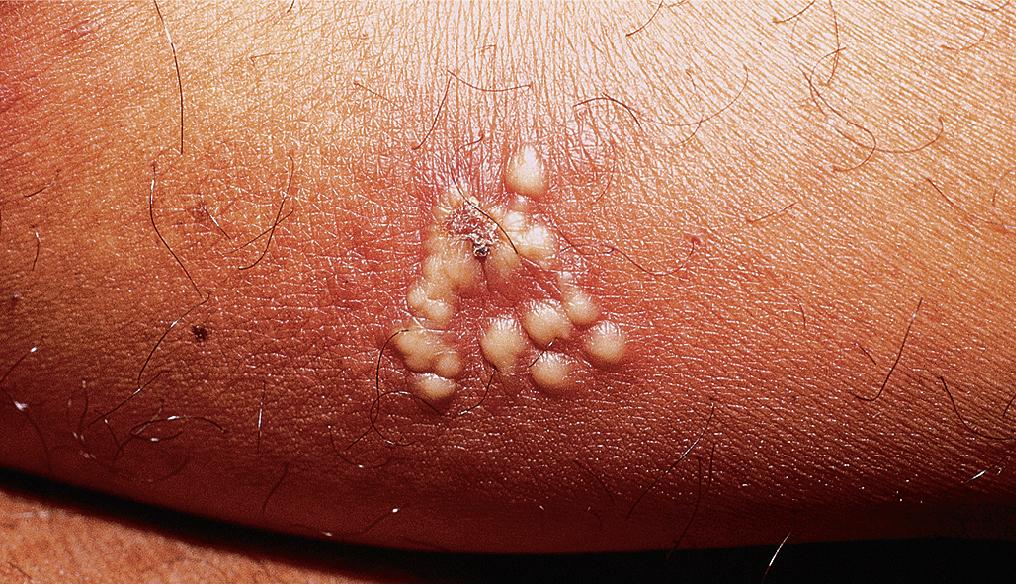
Primary herpes simplex infections may involve any cutaneous or mucous membrane surface and generally result from direct inoculation of previously injured sites. Lesions consist of herpetiform or clustered red papules, which evolve into vesicles and pustules in 24–48 h ( Figs. 4.2–4.5 ). During the following 5–7 days, vesicles rupture and crust over. Desquamation and healing are complete in 10–14 days. Primary herpes infection on the distal finger is called herpetic whitlow ( Fig. 4.3 b,c). Just as in herpetic gingivostomatitis, primary infections at other sites may be associated with painful local adenopathy and flu-like symptoms. When an HSV-1 and HSV-2 naive individual acquires either HSV type 1 or HSV type 2, a first-episode primary infection ensues. Likewise, when an individual with pre-existing HSV-1 antibody acquires an HSV-2 infection (or vice versa), a first-episode non-primary infection results.
In children, most infections are caused by HSV type 1, whereas HSV type 2 is most commonly found in genital infections in adolescents and adults ( Fig. 4.4 ). However, HSV type 2 can also be found in non-genital areas, and type 1 virus may be spread from mouth to hand to genital sites. Although sexual contact must be considered in any child who develops genital herpes, non-venereal sources are probably most common. Confirmed genital HSV type 2 in children should prompt a referral to child abuse specialists and can put the child at risk for HSV type 2 meningitis. Both HSV type 1 and HSV type 2 can cause neonatal herpes, and higher risk of acquisition is associated with first-episode primary maternal infection, followed by non-primary infection, and finally recurrent infections. In the United States, neonatal herpes is estimated to affect 1500 neonates annually, with a 4% mortality rate. Vesicles are usually restricted to the perineum and genital skin and quickly ulcerate. Erythema and edema may result in severe dysuria and urinary retention.
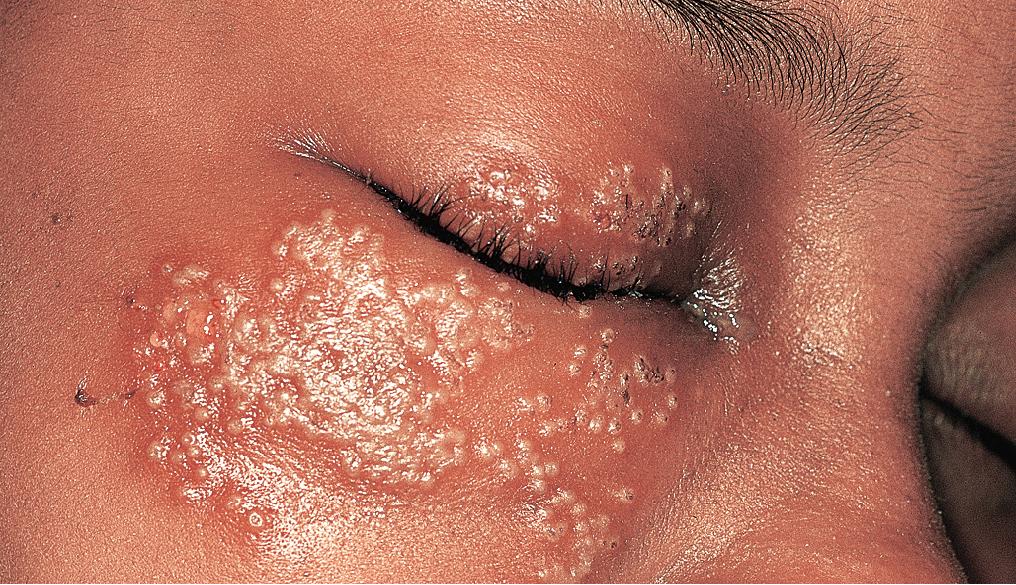
In immunocompromised children or patients with certain skin conditions, such as atopic dermatitis, seborrheic dermatitis, and immunologic blistering disorders, herpes simplex may disseminate over the entire skin surface (eczema herpeticum or Kaposi varicelliform eruption; and to the lungs, viscera, and central nervous system (CNS). HSV may also produce sepsis and/or meningitis with neurologically disabling or life-threatening disease in the nursery. Early diagnosis and antiviral therapy can reduce morbidity and mortality in these clinical settings.
After the initial episode, HSV enters a dormant state. A number of endogenous and environmental factors may trigger reactivation of the virus, such as a Streptococcus infection of the throat, an upper respiratory infection, sunburn, and surgery ( Fig. 4.5 ). Lesions usually occur near the site of the primary eruption, mucous membranes are not usually involved, systemic symptoms are absent, and the rash heals in less than a week. Although recurrences are unpredictable, disease-free periods tend to increase with time, even in individuals who initially experience frequent recurrences.
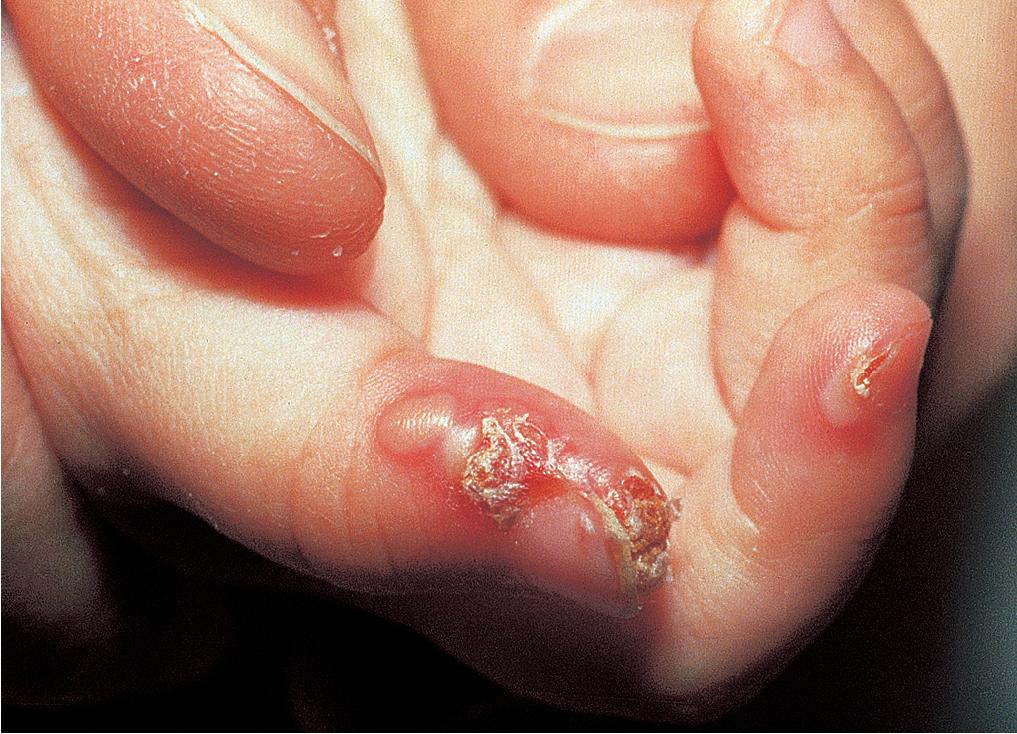
A clinical suspicion of herpes simplex can be confirmed quickly by performing a Tzanck smear at the bedside. Viral culture is rarely used and has been replaced by polymerase chain reaction (PCR) studies or direct immunofluorescence for rapid and reliable confirmation. PCR of the cerebrospinal fluid (CSF) has revolutionized diagnosis of CNS herpes disease by identifying patients who appear to have disease localized to skin, eye, and mouth and triaging appropriate treatment. If CSF PCR is found to be positive, patients are treated with a 21-day course of acyclovir as opposed to the 14-day course with disease localized to skin, eye, and mouth.
The Tzanck smear is obtained by removing the roof of a blister with a scalpel or scissors and scraping its base to obtain the moist, cloudy debris. This is spread onto a glass slide with the scalpel blade, desiccated with 95% ethanol, and stained with Giemsa or Wright stain. The diagnostic finding in viral blisters is the multinucleated giant cell, which is a syncytium of epidermal cells with multiple, overlapping nuclei; hence, it is much larger than other inflammatory cells. Unfortunately, a positive Tzanck smear cannot be used to differentiate between HSV and varicella, and a viral PCR should be obtained when the clinical situation dictates.
In general, management of herpes infections is symptomatic with cool compresses, lubricants, and oral analgesics. Topical antiviral agents are of little use in the treatment of uncomplicated infections in normal hosts. Immunocompromised children with primary or recurrent herpes simplex infections and patients with severe primary herpetic gingivostomatitis or genital herpes usually improve quickly with parenteral acyclovir. In some children, especially older patients without oral involvement, oral acyclovir may be administered. Several orally administered drugs, which include valaciclovir hydrochloride and famciclovir, achieve consistent blood levels. Valaciclovir is U.S Food and Drug Administration (FDA) approved for the treatment of herpes labialis in children 12 and older and genital HSV in adolescents. This medication is preferred to acyclovir given its better bioavailability and less frequent dosing. In recurrent disease, initiation of oral acyclovir with the prodromal tingling in the skin before the appearance of blisters may abort the episode. In selected children with frequent, multiple, widespread recurrent eruptions, long-term, suppressive therapy may be necessary. Extended oral suppressive therapy for infants following parental treatment for neonatal HSV infection can reduce the risk of late neurologic sequelae.
Herpes simplex infections are usually differentiated from other blistering eruptions by the typical clustering of lesions, the clinical course, a positive Tzanck smear and viral PCR, and characteristic skin-biopsy findings. Impetigo may mimic herpes. However, bullae tend to be relatively large, with a central crust and peripheral extension, and a Gram stain demonstrates Gram-positive cocci. Blistering distal dactylitis, which is caused by Group A β-hemolytic streptococci or Staphylococcus aureus , may be mistaken for herpetic whitlow ( Fig. 4.6 ). In staphylococcal or streptococcal infection, the lesions on the finger tips usually coalesce to form one or several 5–10 mm diameter blisters, and Gram stains and cultures demonstrate the causative bacterium. Unlike impetigo at other sites, blisters on the fingers tend to be tense because the stratum corneum is thick on acral surfaces. Occasionally, the eruption of herpes simplex may form a dermatomal pattern. In this situation, a viral PCR is required to exclude herpes zoster.
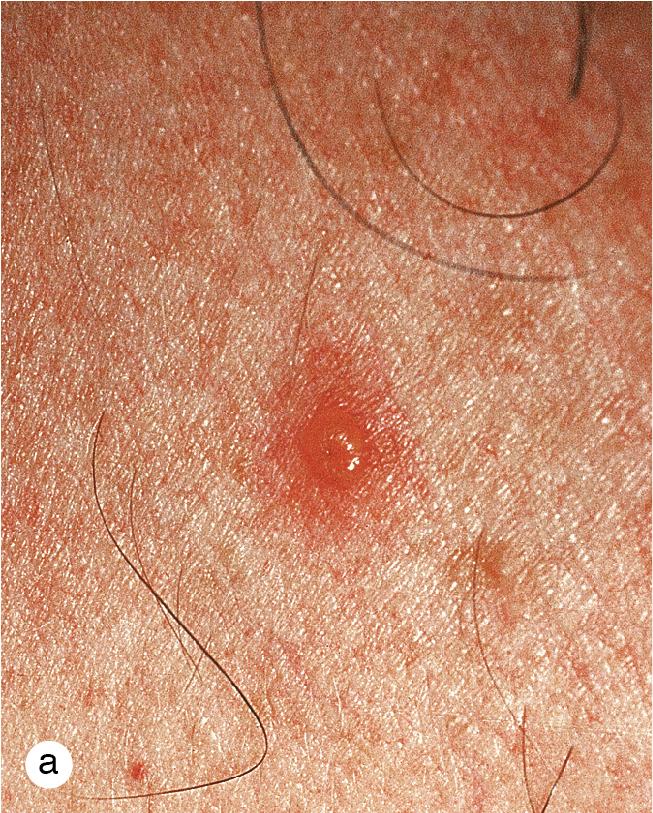
Varicella is a mild, self-limited infection in most children. However, disseminated disease is a problem in the neonate and immunosuppressed child. Early administration of varicella zoster immune globulin to immunocompromised children exposed to varicella may be preventive, and antiviral therapy in patients with disseminated lesions may be life-saving. The introduction of the varicella vaccine in 1995 reduced varicella mortality and morbidity, with no reported pediatric deaths since 2010, as well as reduced the likelihood of herpes zoster in children. Congenital varicella syndrome can occur when women are infected with the varicella virus during pregnancy, causing multiple anomalies in the newborn. Treatment involves antivirals and varicella immunoglobulin irrespective of gestational age.
After exposure to varicella, the incubation period varies from 7 to 21 days. Fever, sore throat, decreased appetite, and malaise precede the skin lesions by several days. Early cutaneous findings vary from a few scattered, pruritic, red papules to generalized papules that evolve in 24 h to vesicles on a bright red base (dew drops on a rose petal, Fig. 4.7 ). Central umbilication of blisters follows rapidly, and crusting and desquamation occur within 10 days. New papules and vesicles continue to appear for 3–4 days. The rash often begins on the head and trunk, moving to the extremities but can involve any area including mucosal surfaces, particularly the buccal mucosa and gingivae. Although the blisters are intraepidermal and usually heal without scarring, some develop deep inflammation or become secondarily infected and heal with pitted or hypertrophic scars ( Fig. 4.8 a,b).
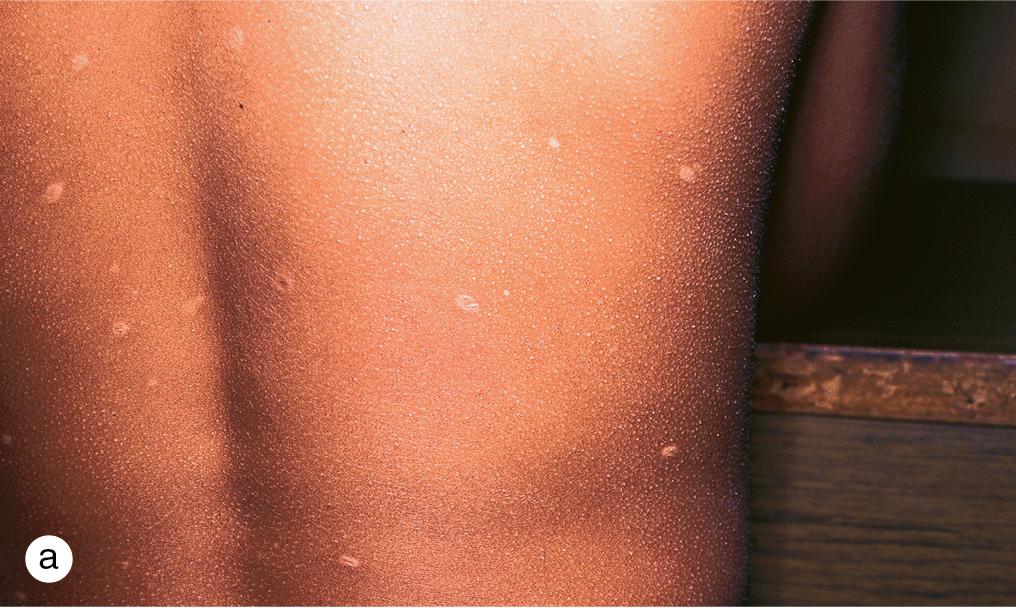
Oral or parenteral acyclovir is indicated for treatment of varicella in immunocompromised patients and normal hosts with severe disease. However, famciclovir and Valaciclovir provide better bioavailability and more convenient dosing. Valaciclovir is FDA approved for treatment of varicella in immunocompetent children 2 years and older. Unfortunately, the newer antiviral agents are not routinely available in liquid preparations. However, special formulating pharmacies may be able to prepare Valaciclovir syrup for children who cannot swallow capsules. Pruritus may be intense and responds to cool compresses, calamine lotion, and oral antihistamines. Topical products containing antihistamines should be avoided because of the risk of significant percutaneous absorption and systemic toxicity. Secondary infection is usually caused by staphylococci and is treated with oral antibiotics such as dicloxacillin, cefalexin, or erythromycin ( Fig. 4.8 c). When cellulitis or other deep, soft-tissue infection complicates chickenpox, hospitalization and parenteral therapy may be required ( Fig. 4.8 d). Rarely, progressive purpura and necrosis of large areas of skin herald the onset of disseminated intravascular coagulation ( Fig. 4.8 e). This phenomenon, referred to as purpura fulminans, occurs in fewer than 1 in 20 000 cases of varicella.
Administration of systemic corticosteroids, even in normal children, during varicella infection may result in severe blistering, disseminated viral infection, and increased risk of complications. As a consequence, it is important to differentiate chickenpox from contact dermatitis, insect bites, or other viral infections. The Tzanck smear may be particularly useful early in the course of disease, when only a few skin lesions are present. PCR confirmation for varicella infection is useful in immunocompromised hosts or when the diagnosis is in question.
Although the world was declared officially free of smallpox (variola) in 1980, this virus has resurfaced as a potential biological warfare agent. Chickenpox can be distinguished from smallpox in which lesions develop on the face and distal extremities and spread centrally. Early red macules and papules become vesicular and pustular over several days, and adjacent lesions tend to be in the same stage of development. New lesions, which are firm and deep-seated, may appear for 1–2 weeks, become confluent, and often heal with scarring. Patients are usually ill-appearing, and the rapid diagnostic Tzanck smear is negative for multinucleated giant cells. Many medical centers have developed protocols for isolating and evaluating patients with suspected smallpox infection.
Herpes zoster or shingles represents a reactivation of the dormant varicella virus from the sensory root ganglia. The most commonly involved sites include the head and neck and the thoracic sensory nerves ( Fig. 4.9 ). After a variable prodrome, which may include mild constitutional symptoms and localized itching and burning, linear, clustered, red papulovesicles appear in a unilateral linear pattern in one or several dermatomes. In some children, the eruption may be completely asymptomatic. Over 3–5 days, the rash reaches its full extent, and during the ensuing 1–2 weeks, vesicles and erosions develop umbilicated crusts and desquamate, much like in chickenpox.
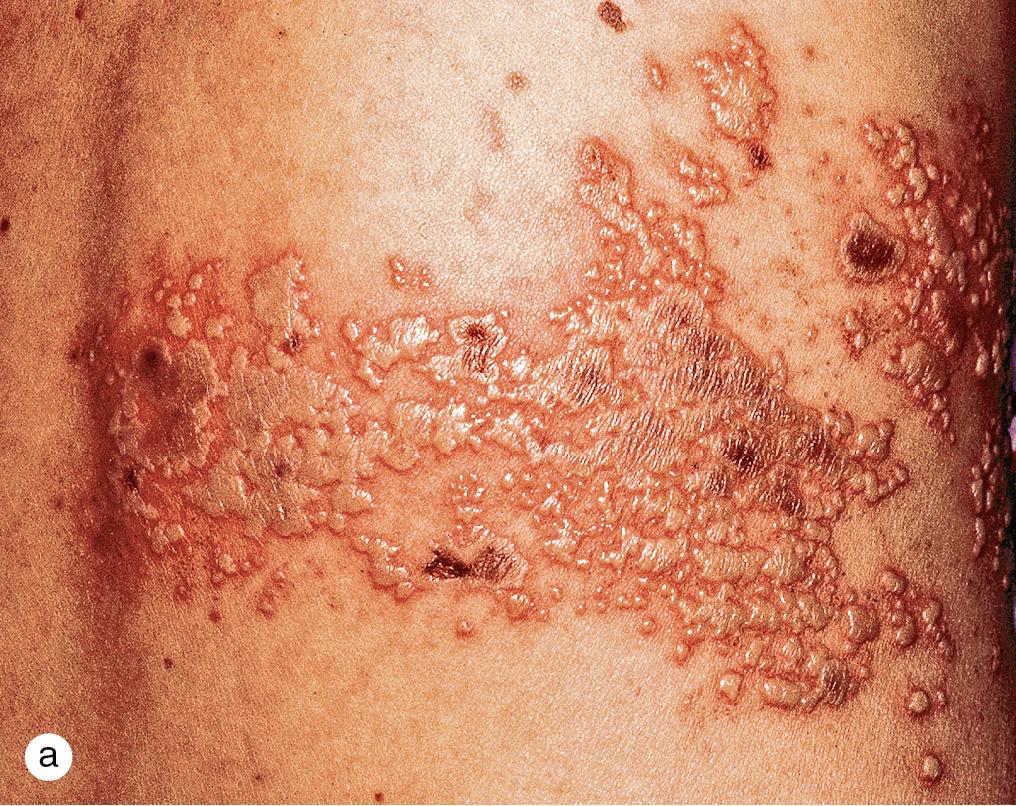
Although 6–10 lesions may appear outside the primary dermatomes in normal individuals, the development of widespread cutaneous lesions suggests the possibility of an immunodeficiency state and an increased risk of visceral involvement. Herpes zoster is rare in healthy children but can occur and is not a sign of immunodeficiency in the setting of just one episode.; however, immunocompromised children or children with cancer are at a greater risk for zoster. Varicella can also spread into cerebral and extracranial arteries, producing varicella zoster virus vasculopathy, manifesting as stroke, giant cell arteritis, aneurysms, and sinus thrombosis. In children, stroke can occur 4 months after varicella infection and is often monophasic.
The presence of a dermatomal bullous eruption is virtually diagnostic for herpes zoster. However, herpes simplex occasionally presents in a dermatomal pattern. Moreover, zoster may be confused with herpes simplex early in the course, when only a few clustered vesicles are present and the dermatomal organization is not yet apparent. In the event of diagnostic uncertainty, Varicella zoster virus (VZV) and HSV PCR should be obtained.
Management of shingles is usually limited to supportive measures similar to those for chickenpox. In disseminated disease, administration of parenteral acyclovir may be life-saving. Oral acyclovir, Valaciclovir, or famciclovir can also be used in immunocompromised hosts and normal patients with symptomatic disease. Immunocompetent individuals who develop eye involvement also benefit from antiviral therapy and are followed closely by an ophthalmologist. The high risk of zoster in patients undergoing bone marrow transplantation or other elective immunosuppression may require antiviral prophylaxis in these children. Although there is no approved herpes zoster vaccine for children, and the risk of infection is generally low, zoster vaccines exist in the market for prophylaxis against zoster in adults over age 50. In 2008, the live attenuated zoster vaccine, ZOSTAVAX (Merck & Co. Inc.) was licensed in the United States; however, it only demonstrates effectiveness of 70% in adults aged 50–59, and less than 38% in adults aged 70 and older. In 2017, the FDA approved a recombinant zoster vaccine, Shingrix (GlaxoSmithKline), which demonstrates an overall efficacy of 97% without decline in efficacy with increasing age.
Although systemic corticosteroids may be administered to reduce the risk of postherpetic neuralgia in adults over 60 years of age, this treatment has not been shown to be beneficial in young adults or children. Children with shingles must be isolated from individuals who are susceptible to chickenpox because of the risk of acquiring infection from direct contact with skin lesions.
Hand-foot-and-mouth disease (HFMD) is a distinctive, self-limited viral eruption mostly occurring in children younger than 10 years old caused most frequently by Coxsackie virus A16 and enterovirus 71 (EV-71). The disease is highly infectious, and, like other enteroviruses, peak incidence occurs in the late summer and fall. After exposure, the incubation period is 4–6 days. A 1–2-day prodrome of fever, anorexia, and sore throat is followed by the development of 3–6 mm diameter elongated, gray, thin-walled vesicles on a red or non-inflamed base. As the name suggests, lesions appear most commonly on the palms, soles, and sides of the hands and feet, but red papules and vesicles may also erupt on the buttocks, trunk, face, arms, and legs ( Fig. 4.10 a,b). The enanthem is characterized by vesicles that rapidly ulcerate to leave sharply marginated erosions on a red base on the tongue, buccal mucosa, and posterior pharynx ( Fig. 4.10 c). Most cases do not involve internal findings and are limited to fever, malaise, and herpangina, ulcerations of oropharyngeal surface without papulovesicular eruptions on the skin. Although cutaneous and mucosal lesions may be completely asymptomatic, pruritus and burning can be severe. Systemic symptoms, which include fever, diarrhea, sore throat, and cervical adenopathy, may be absent or mild, and treatment is supportive. The eruption usually clears in less than a week. Inactivated monovalent vaccines against EV-A71 were introduced in China in 2016 in efforts to lower the burden of HFMD among children aged 6–71 months.
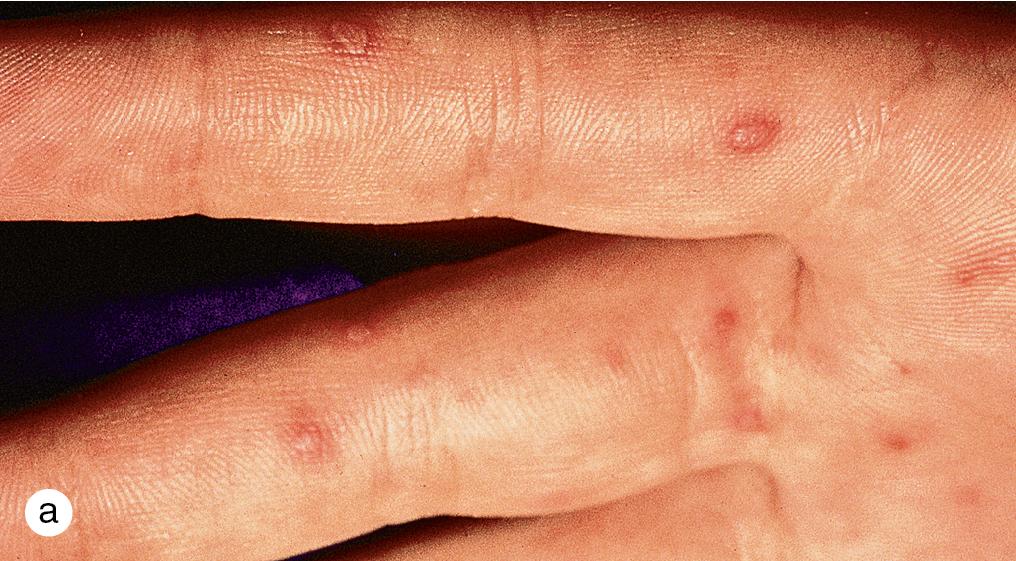
In late 2011 and spring 2012, a new clinical variant caused by Coxsackie A6 virus, previously reported in Asia and Africa, spread to North America. Although symptoms and the rash were also self-limited, high fever and more widespread skin lesions are characteristic. The skin eruption is reminiscent of disseminated herpes simplex infection, but identification of the typical lancet-shaped enteroviral vesicles on the hands and feet provides a clue to the diagnosis ( Fig. 4.11 ). Typical HFMD may not require viral testing; however, atypical strains such as CVA6 may be subjected to enteroviral PCR to differentiate from herpes virus.
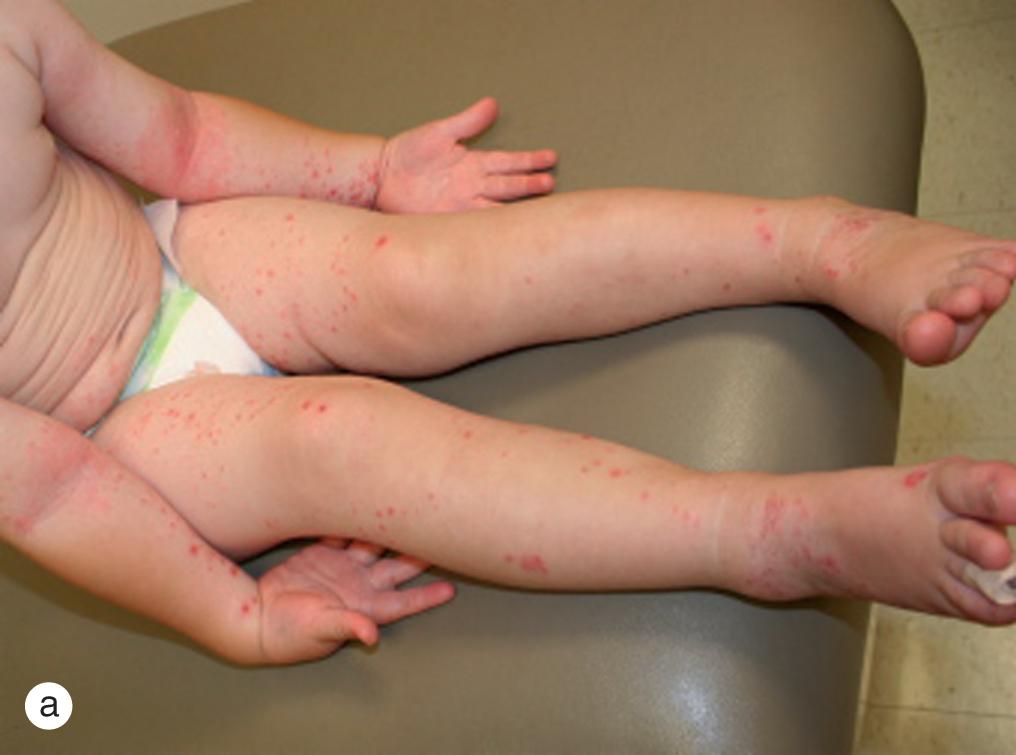
Expanding, honey-colored, crusted patches or bullae with a central crust suggest the diagnosis of impetigo ( Fig. 4.12 ). In older children in temperate climates, lesions appear most commonly on exposed skin during the summer months. Although Group A β-hemolytic streptococci were the predominant organisms 25–30 years ago, Staphylococcus aureus alone or in combination with streptococci is recovered from a majority of cultures. As a consequence, anti-staphylococcal antibiotics are now the drugs of choice for treatment.
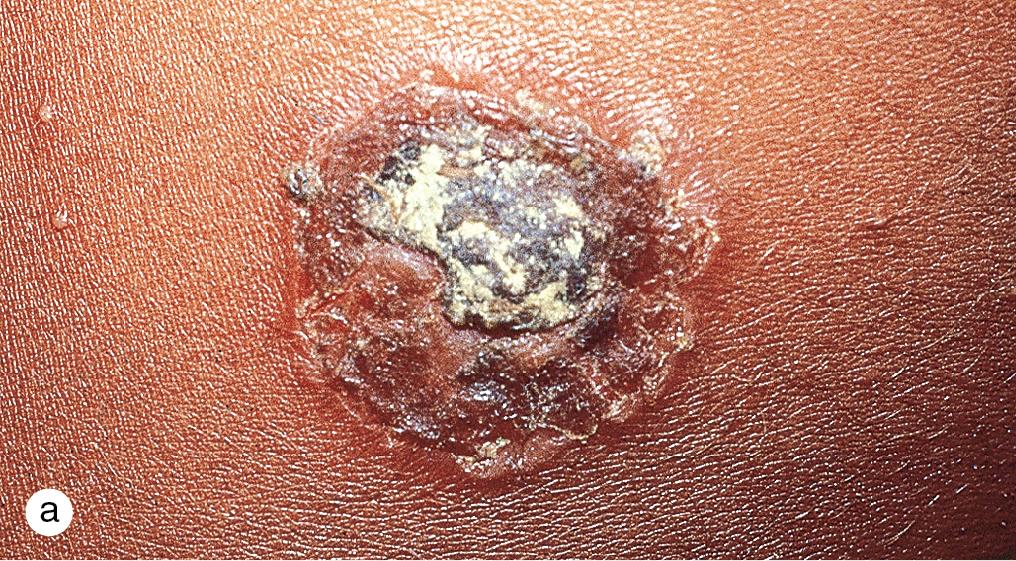
Impetigo is often a self-limiting process, likely to resolve in 4 weeks; however, the lesions are highly contagious and subject to social stigma. Complications, which include cellulitis and disseminated infection, as well as spread to family members and classmates, can be limited by antibiotic therapy. Topical antibiotics, such as bacitracin, polymyxin B, neomycin, and mupirocin may be used in localized disease, but increasing resistance to traditional topical antibiotics makes retapamulin a better first-line agent. More recently, ozenoxacin 1% cream has demonstrated efficacy in treating impetigo with activity against methicillin-resistant Staphylococcus aureus (MRSA) and low probability of spontaneous mutants in quinolone-resistant and sensitive stains. Widespread lesions have been treated with oral agents such as cephalexin, amoxicillin-clavulanate, and erythromycin. Unfortunately, there is increased evidence for resistance against erythromycin, clindamycin, cephalexin, and cloxacillin. Previously, MRSA was confined to health care facilities; however, its prevalence is increasing in community settings. Consequently, the practitioner must select antibiotic coverage based on the resistance patterns in their respective communities (e.g. clindamycin, trimethoprim-sulfamethoxazole, tetracyclines) and consider resistance when patients fail to respond to appropriate doses of anti-staphylococcal antibiotics.
Impetigo should be contrasted from the rarer variant, bullous impetigo (BI), which is characterized by fragile fluid-filled vesicles caused by the staphylococcal exfoliative toxin . As opposed to the hematogenous spread of toxin in staphylococcal scalded-skin syndrome, the toxin in BI is restricted to the site of infection. Hence, wound culture is encouraged to guide treatment specific to antibiotic sensitivity. The first-line agent for localized BI is mupirocin, whereas the first-line agent for widespread BI is cephalosporins.
Staphylococcal scalded-skin syndrome (SSSS), also known as Ritter disease, occurs almost exclusively in infants and toddlers ( Fig. 4.13 ), with an incidence of about 7.5 per 100 000 infants <1 year old. It has, however, been reported with increasing frequency in older children and adults, particularly in debilitated patients with decreased renal function. The in-hospital mortality rate for SSSS in children is low (0.31%), whereas the mortality rate for SSSS in adults is 4.3%. This process is considered in any child who develops a generalized, tender erythema associated with a Nikolsky sign. When a Nikolsky sign is present, a minimal shearing force produced by finger pressure induces a skin slough or blister formation. The diagnosis is mainly clinical with the presence of tender erythroderma, positive Nikolsky sign, desquamation particularly at friction zones, perioral crust, and absence of mucosal involvement; however, cultures may be necessary to guide antibiotic choice.
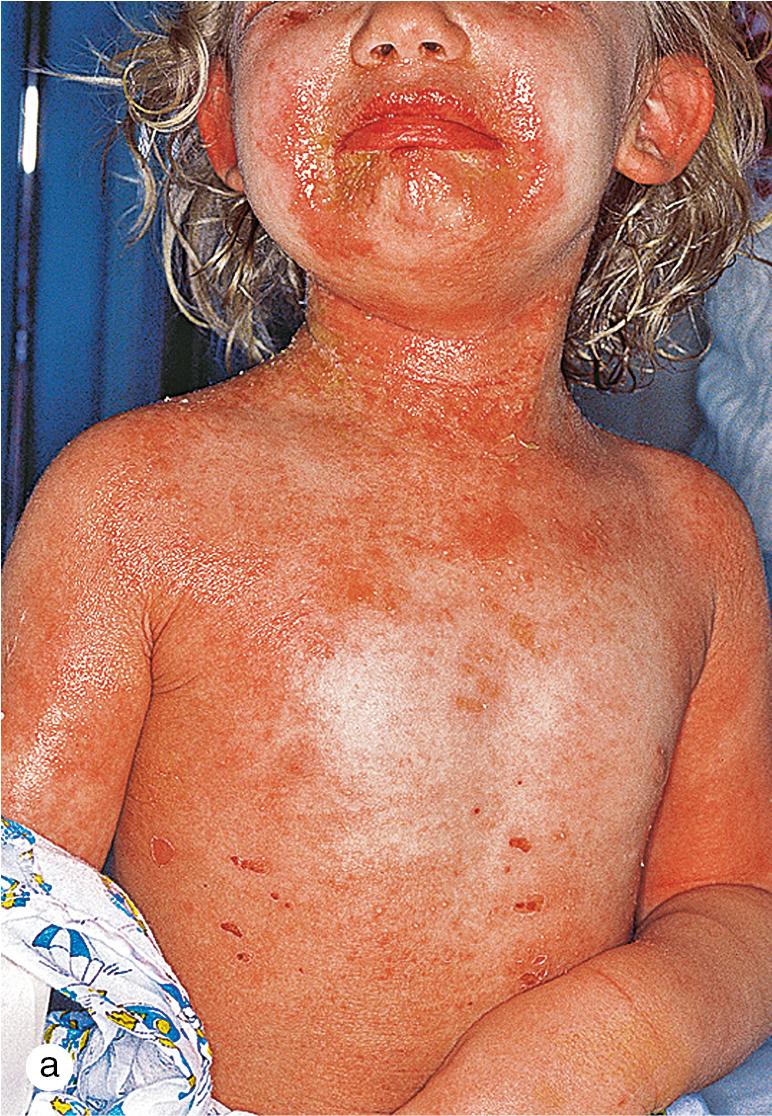
SSSS occurs when exfoliative toxin produced by certain staphylococci binds to a desmosomal adhesion molecule desmoglein-1 in the subgranular region of the epidermis, resulting in cleavage at this site high in the epidermis. Interestingly, this is the same adhesion molecule that is targeted by autoantibodies in pemphigus foliaceus.
Although SSSS is usually self-limiting in healthy children who rapidly develop antibodies to the toxin, immunocompromised patients may develop complications related to their primary staphylococcal infection. Moreover, renal excretion of the toxin may be slowed in patients with impaired renal function (e.g. chronic renal failure) or renal clearance of the toxin (e.g. infants). Most SSSS is associated with a primary cutaneous infection. However, the soluble toxin that causes the rash may be produced by an occult infection, such as osteomyelitis, septic arthritis, pneumonia, or meningitis. Healthy children respond to oral anti-staphylococcal antibiotics, which eradicate the infection and subsequent toxin production; however, there is an increase in mupirocin and fusidic acid resistant strains. Infants, severely ill older children, and patients with occult infection require appropriate culturing and parenteral therapy. Consider MRSA in patients not clinically improving or critically ill, and use vancomycin. Parents are also counseled about the generalized desquamation that develops 10–14 days after the acute infection.
Staphylococcal folliculitis is a common problem in older children and adults. Red papules and pustules on an inflamed base erupt in a follicular pattern, most frequently on the buttocks, thighs, back, and upper arms ( Fig. 4.14 ). Occasionally, superficial follicular pustules evolve into painful, deep-seated furuncles or spread to neighboring follicles and soft tissue to create an abscess or carbuncle. Abscesses and resultant cellulitis may be associated with fever, malaise, and sepsis. The incidence of furunculosis and abscess formation has increased dramatically with the spread of MRSA and should not be mistaken for insect or spider bites.
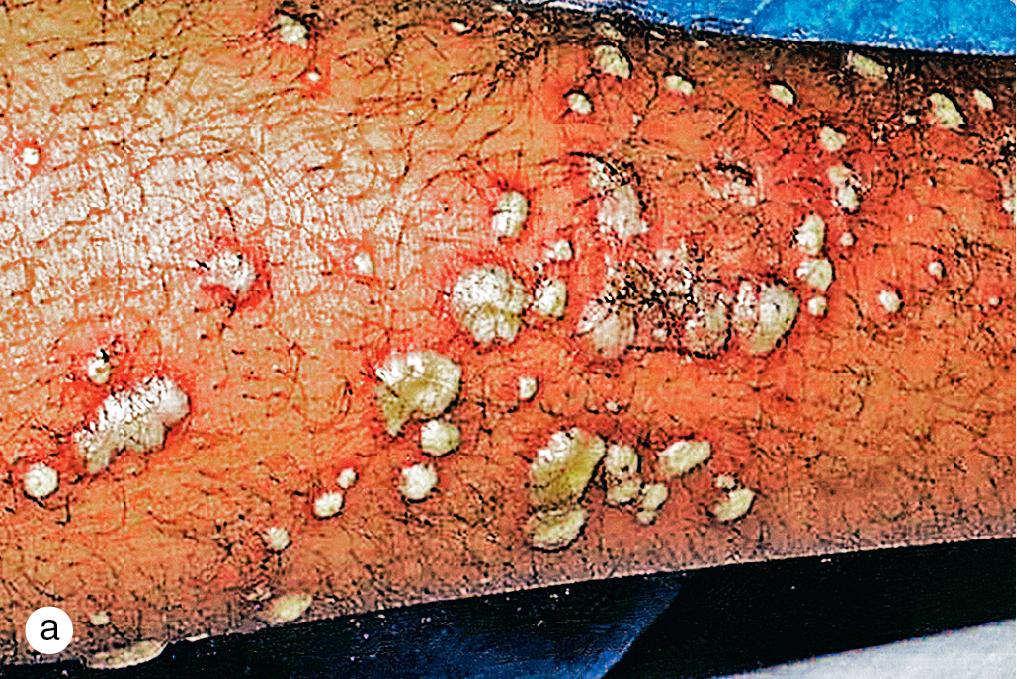
Localized folliculitis may improve with topical antibiotics. However, widespread lesions respond best to topical benzoyl peroxide wash or systemic antibiotics. Additionally, abscesses are incised and drained, and cellulitis may require parenteral antibiotics.
Children with Down syndrome are particularly prone to follicular occlusion disorders such as folliculitis, acneiform eruptions, and pilonidal cysts. Further, immunosuppressive states and isotretinoin use resulting in reduction of white blood cell count have been associated with an increased risk for staphylococcal folliculitis. Long-term use of antiseptic soaps, topical antibiotics (e.g. clindamycin, tetracycline, erythromycin), dilute bleach baths, and peeling agents (e.g. benzoyl peroxide, retinoic acid, salicylic acid) may reduce the risk of recurrent infection. Persistent or difficult-to-treat folliculitis, especially when associated with furunculosis, should prompt culturing for MRSA infection.
Regardless of the etiology, infectious or non-infectious, folliculitis responds well to normal warm saline compresses, followed by topical erythromycin or bacitracin ointment and sterile gauze dressing. Certain hydrating lotions and creams that are also designed to remove scale (e.g. Carmol and Ureacin with urea, LactiCare, AmLactin and Lac-Hydrin with lactic acid) may be beneficial. Patients are also instructed to avoid tight clothing and occlusive moisturizers. In toddlers, folliculitis may improve after toilet training. Older children with enuresis should also be encouraged to remove wet clothing as soon as possible.
Although there is clinical overlap among the various immunologically mediated vesiculobullous dermatoses, they can be differentiated on the basis of specific clinical patterns, histopathology, immunopathology, and response to therapy ( Table 4.1 ). In pemphigus, blisters form within the epidermis. Chronic bullous dermatosis of childhood (CBDC), BP, dermatitis herpetiformis (DH), and EBA are characterized by subepidermal blisters.
| Immunoglobulin | Location in Skin | Target Antigen | Clinical Findings | |
|---|---|---|---|---|
| Intraepidermal | ||||
| Pemphigus foliaceus | IgG | Intercellular, upper epidermis | Desmoglein-1 | Seborrheic distribution  |
| Pemphigus vulgaris | IgG |
|
|
|
| Subepidermal | ||||
| Linear IgA bullous dermatosis (CBDC) | IgA | Linear pattern in lamina lucida | 97 kDa cleaved ectodimer of BP180 antigen | Vesicles, bullae, lower trunk, thighs, occasionally disseminated  |
| Bullous pemphigoid (BP) | IgA C3 |
Epidermal side of lamina lucida | BP230 (BPA1) BP180 (BPA2) |
Bullae tend to be >2.0 cm but can mimic CBDC  |
| Dermatitis herpetiformis | IgA | Granular deposits in basement membrane zone at dermal papillary tips | Extremely pruritic papules, crusted vesicles on extensor surfaces of extremities, buttocks  |
|
| Epidermolysis bullosa acquisita (EBA) | IgG IgA |
Subbasement zone in lamina lucida | Collagen VII | May mimic dystrophic EB or CBDC, mucous membranes often involved  |
Childhood pemphigus refers to a group of rare, chronic, potentially life-threatening immunobullous disorders characterized by flaccid intraepidermal bullae that erupt on normal-appearing or erythematous skin. Clinically and histologically, pemphigus can be divided into two types: pemphigus vulgaris (PV) and pemphigus foliaceus (PF). Though observed around the world, the geographic distribution of PV is highest in Ashkenazi Jews of Mediterranean origin. In most countries, PV is more prevalent than PF except for Finland, Brazil, and Tunisia.
Although the pathogenesis of pemphigus is still not completely understood, immunoglobulin (Ig)G, IgA, IgM, and complement have been identified in blister fluid. In pemphigus, the target antigen was identified as a desmoglein, one of several cadherin-type adhesion molecules that comprises desmosomes. Disruption of desmosomes results in cleavage of the epidermis and blister formation. Desmoglein-1 is found throughout the epidermis and only minimally in the oral mucosa, whereas desmoglein-3 occurs primarily in the oral mucosa and the lower half of the epidermis. As a consequence, patients with autoantibodies directed against desmoglein-1 develop superficial lesions within the epidermis resulting in PF. In contrast, patients with autoantibodies directed against desmoglein-3 develop PV of the oral mucosa and limited or no lesions in the skin because of normally functioning desmoglein-1 in the upper half of the epidermis. Finally, individuals with autoantibodies directed against both desmoglein-3 and -1 develop deep epidermal skin blisters, as well as oral mucous membrane involvement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here