Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Large artery atherothrombosis, cardiac embolism, and small artery disease are the important mechanisms of infarction in vertebrobasilar artery territory.
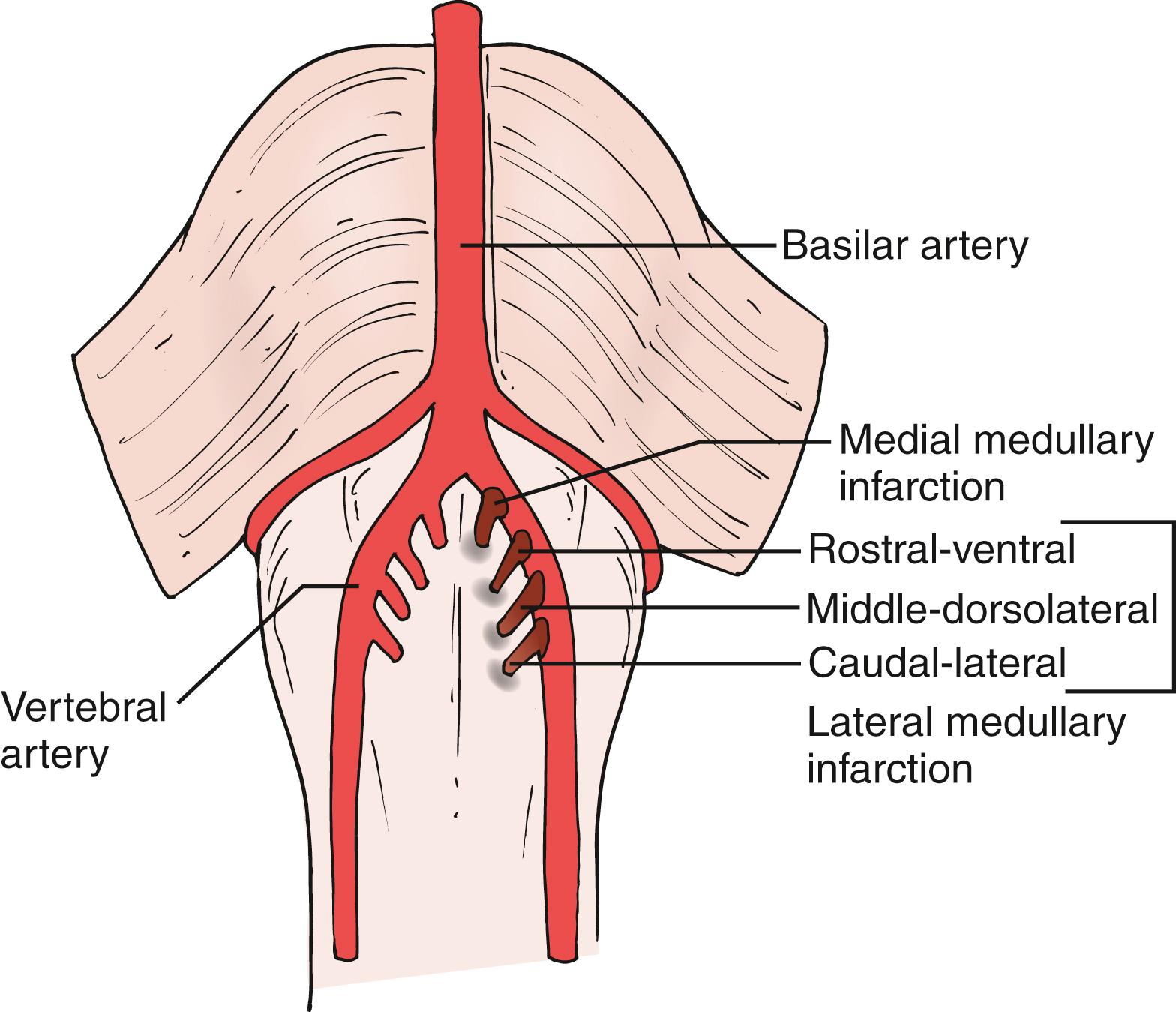
In lateral medullary infarction, clinical manifestations vary according to rostro-caudal and ventrodorsal topography. Rostral-ventral infarcts are associated with severe dysphagia, dysarthria, and contralateral trigeminal sensory involvement, whereas caudal-lateral lesions are characterized by severe gait ataxia, absent dysphagia, and sensory symptoms worse in the lower extremities.
Medial medullary infarction occurs mostly in the rostral medulla, and presents with unilateral motor, sensory, and ocular motor disturbances depending on ventrodorsal topographic involvement.
Branch occlusion associated with intracranial vertebral artery disease is the most important cause of medullary infarction.
Unilateral paramedian infarction is the most common pattern of pontine infarction. Basal involvement is associated with motor syndromes such as pure motor stroke, ataxic hemiparesis, and dysarthria clumsy hand, while tegmental lesions produce sensory symptoms and/or ocular motor disturbances, notably internuclear ophthalmoplegia.
Bilateral pontine infarcts result in quadriparesis, horizontal gaze palsy, and locked in syndrome and are usually associated with significant basilar artery thrombotic occlusion.
The most common topographic pattern of midbrain infarction is anteromedial followed by anterolateral; the former is characterized by ocular motor dysfunction (the 3rd nerve palsy or internuclear ophthalmoplegia), whereas the latter lesions produce various motor syndromes.
Cerebellar infarcts generally follow the topography of three major arteries: posterior inferior cerebellar artery, superior cerebellar artery, and anterior inferior cerebellar artery.
Basilar top occlusion is mostly embolic and results in peculiar syndromes associated with paramedian midbrain, and diencephalic infarctions, with occasional involvement of the occipital and cerebellar regions.
The two vertebral arteries (VAs), which often differ in size, are traditionally divided into four segments ( Fig. 26.1 ). In the first segment, the artery courses directly cephalad from its origin as the first branch of the subclavian artery, riding anterior to the transverse foramen until it enters the costotransverse foramen usually at C6 or C5. The second segment runs entirely within the transverse foramina from C5–C6 to C2. The third segment emerges from the transverse foramen of C2 and has a complex course posteriorly and laterally toward the costotransverse foramen of the atlas. There it circles the posterior arch of C1 and passes between the atlas and occiput within the suboccipital triangle. During its course, the third segment of the VA is covered by muscles and nerves and is pressed against bone while being covered by the atlantooccipital membrane. Its intracranial portion constitutes the fourth segment of the VA. It pierces the dura mater to enter the foramen magnum, where its adventitial and medial coats become less thick, and there is a gross reduction in elastic lamina.
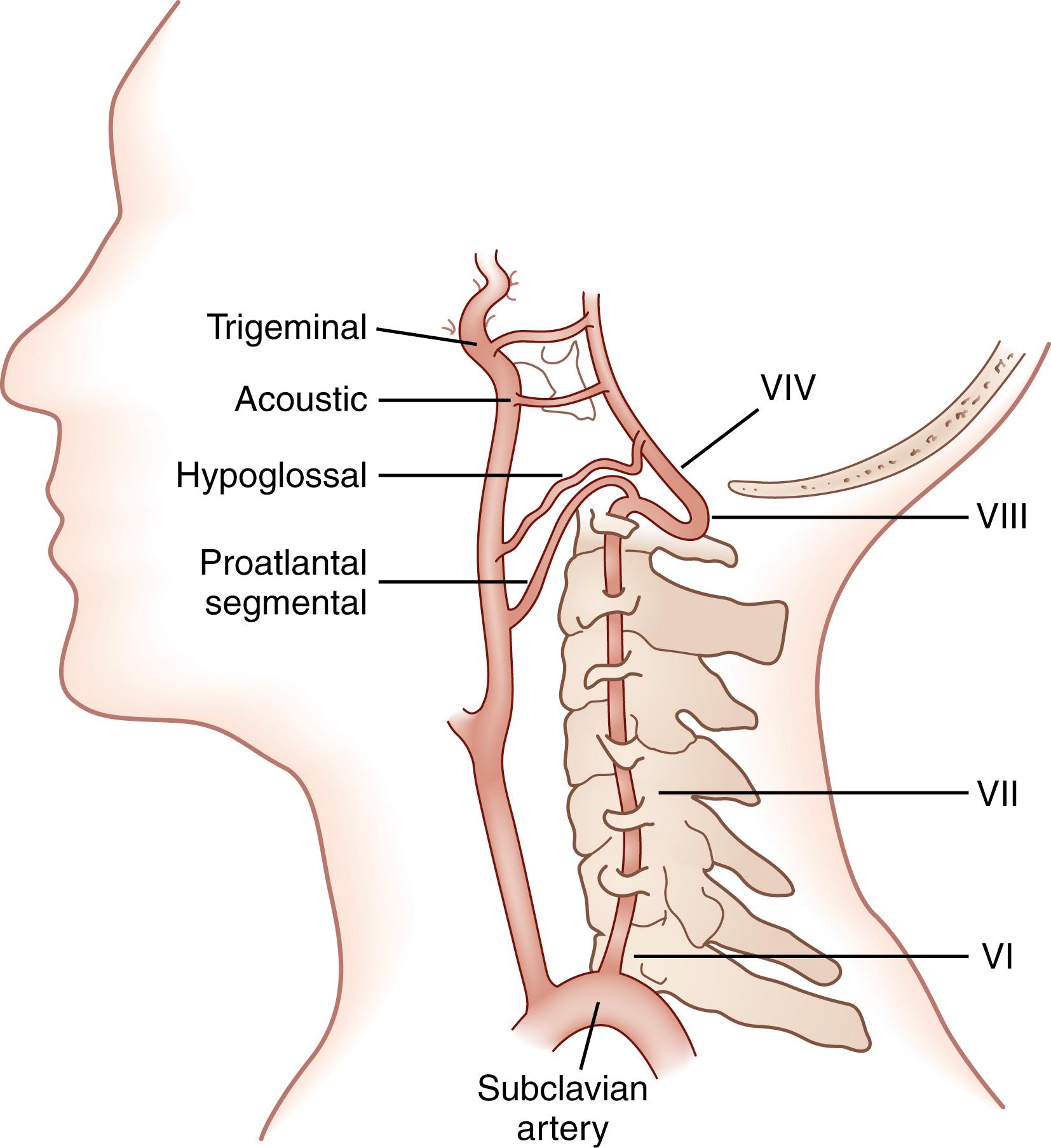
At the level of the pontomedullary junction, the two VAs merge to form the basilar artery (BA), but the exact site varies; the site is sometimes high enough on the brainstem for the VA to supply the middle and lower pons. The BA becomes somewhat smaller as it travels distally, frequently curving slightly away from the larger VA. It divides near the pontomesencephalic junction to form the two posterior cerebral arteries (PCAs).
Variations are relatively common. In approximately 8% of humans, the left VA originates directly from the aortic arch and not from the subclavian artery. Rarely, the right VA arises as a separate branch from the innominate artery and not from the subclavian artery. The VAs are commonly asymmetric. Depending on the criteria, the prevalence of a hypoplastic VA ranges from 1.9% to 7.8%. In 45% of people the left VA is larger, in 21% the right VA is larger, and in 24% the arteries are of equal size.
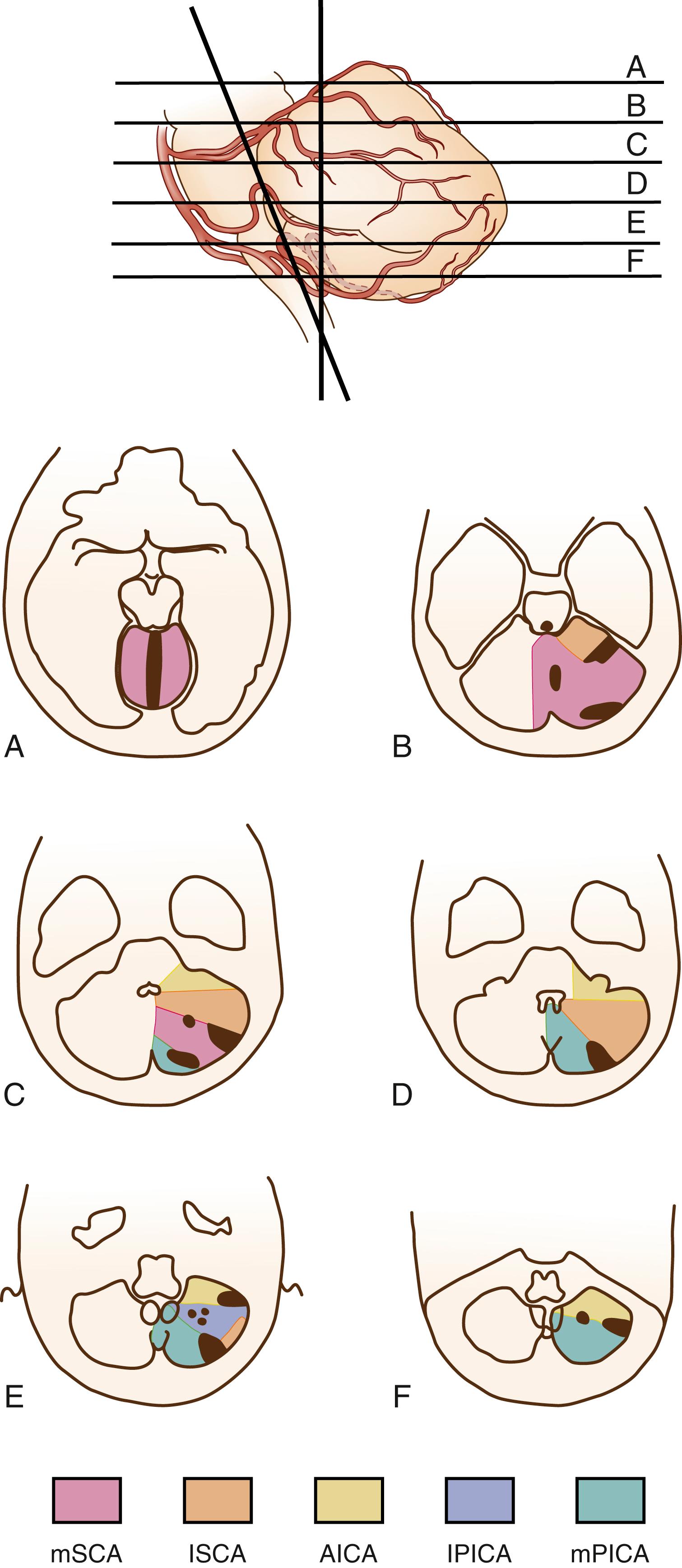
The cerebellum is mainly supplied by the three long, circumferential arteries ( Figs. 26.2 and 26.3 ).
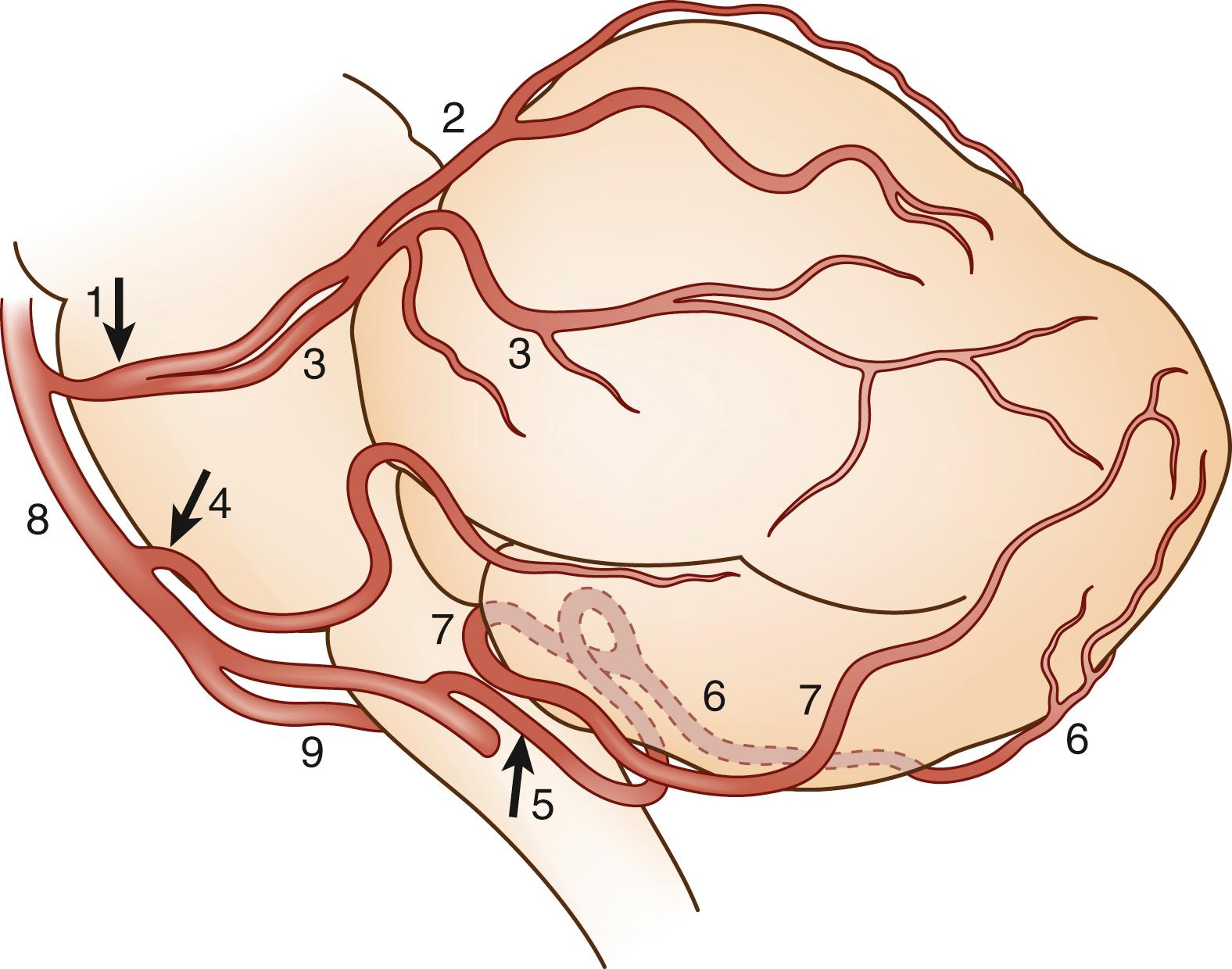
The posterior inferior cerebellar artery (PICA), usually the largest branch of the VA, arises from its intradural segment approximately 10–20 mm from the origin of the BA. This site is an average of 8.6 mm above the foramen magnum, but variations occur: it may originate from the VA as low as 24 mm below the foramen magnum. Sometimes, the PICA arises extracranially and courses cephalad within the spinal canal. In some subjects, PICAs are a branch of the ascending pharyngeal artery.
Although a branch of the VA, the PICA may be its termination, in which case the VA segment distally is a hypoplastic link to the BA or even nonexistent. When the VA ends in the PICA, it is smaller than the contralateral VA. The PICA has a medial branch, which also arises directly from the VA. Its lateral branch may be from the BA or, more commonly, from the anterior inferior cerebellar artery (AICA).
In 10%–20% of individuals, PICA is hypoplastic or absent, typically accompanied by a prominent ipsilateral AICA. The PICA and AICA are often reciprocally related in size; a large PICA may supply most of the inferior surface of the cerebellum, and the AICA on the same side is quite small with little cerebellar supply. When the AICA on one side is large, the ipsilateral PICA is frequently small. Duplication of the PICA and double origin with distal arterial convergence of the PICA may occur. Other variations include posterior spinal arteries taking their origin from the PICA instead of the VA. The lateral medulla is primarily supplied by direct lateral medullary branches of the VA rather than PICA. The only part of the medulla that is constantly supplied by the medial PICA branches together with the posterior spinal arteries is the dorsal tegmental area.
The PICA course encircles the medulla and supplies the suboccipital surface in the caudal part of the cerebellum. The PICA has a sinuous course with several loops (see Fig. 26.3 ). It travels dorsally, lateral to the medulla, below the roots of the 9th and 10th cranial nerves. From there, it courses inferiorly, makes the first (caudal) loop at a variable level, and goes up onto the posterior surface of the medulla in the sulcus, separating the medulla and the tonsil. At the top of the tonsil, the PICA makes a second (cranial) loop around the tonsil and then goes downward to the inferior part of the vermis. Sometimes the second loop occurs at the midpoint of the tonsil. Thus, the PICA has lateral medullary, dorsal medullary (ventral tonsillar), superior tonsillar, and dorsal tonsillar segments.
The PICA divides into two main branches, the medial and the lateral PICA. The medial PICA climbs along the inferior and dorsal surface of the vermis and the internal part of the hemispheres, making a third loop. The lateral PICA most often arises from the upper part of the dorsal medullary segment of the parent trunk, between the first and second loops, and then gives rise to several terminal branches to the caudal hemisphere. Sometimes it arises from the first loop. The caudal loop is usually found at the level of the foramen magnum. It can be found below this level, but rami from the lateral PICA that supply the tonsil are always above the foramen magnum. Rami from the cranial loop supply the choroid plexus of the fourth ventricle.
Two main areas of supply can be distinguished within the PICA territory. The dorsomedial area is supplied by the medial PICA, whose territory includes the dorsolateral portion of the medulla. The anterolateral area is supplied by the lateral PICA, which does not supply the medulla (see Fig. 26.2 ).
The AICA arises from the caudal third of the BA in 74% of people, sometimes from the middle third and occasionally from its inferior limit. The AICA is absent in 4% of individuals and can arise from the VA or the BA by a common trunk together with the PICA. Rarely, several small vessels arising directly from the BA or from the internal auditory artery may replace the AICA. The AICA winds around the lower pons and supplies the middle cerebellar peduncle, flocculus, and a small portion of the anterior inferior cerebellum. Proximal branches of the AICA usually supply the lateral portion of the pons, including the facial, trigeminal, vestibular, and cochlear nuclei, the root of the 7th and 8th cranial nerves, and the spinothalamic tract ( Fig. 26.2 ).
The superior cerebellar artery (SCA) takes its origin from the rostral BA just before its bifurcation into the PCAs. In some individuals there are two parallel SCAs rather than a single artery.
Each SCA has a short trunk that divides into two main branches, a medial branch and a lateral branch. They follow the pontomesencephalic sulcus and pass around the superior cerebellar peduncle to ramify onto the rostral cerebellum (see Fig. 26.3 ). The SCA courses along the anterosuperior margin of the cerebellum. The medial SCA starts with a course parallel to that of the lateral SCA but turns medially to reach the lateral surface of the mesencephalon and the inferior colliculus; from there, the medial SCA makes a rostral loop along the superior margin of the colliculus and then courses over the superior vermis (see Fig. 26.3 ). The SCA supplies the rostral half of the cerebellar hemispheres as well as the dentate nucleus. Along the course of the SCA, branches supply the laterotegmental portion of the rostral pons (see Fig. 26.2 ).
These three major arteries (the PICA, AICA, and SCA) and their branches are connected by numerous anastomoses, which limit infarct size in patients who have VA or BA occlusions. Drawings of the territory of each cerebellar artery and their branches conform to the computed tomographic (CT) and magnetic resonance imaging (MRI) horizontal axial sections ( Fig. 26.4 ).
The BA extends from the unification of the two VAs at the pontomedullary junction to the terminal bifurcation of the PCAs at the rostral midbrain. The length of BA is approximately 25–35 mm, and the diameter is about 2.7–4.3 mm at the proximal portion. The luminal diameter tends to taper toward the distal end. The largest branches of the BA are SCA and AICA. In addition, there are numerous smaller perforating arteries.
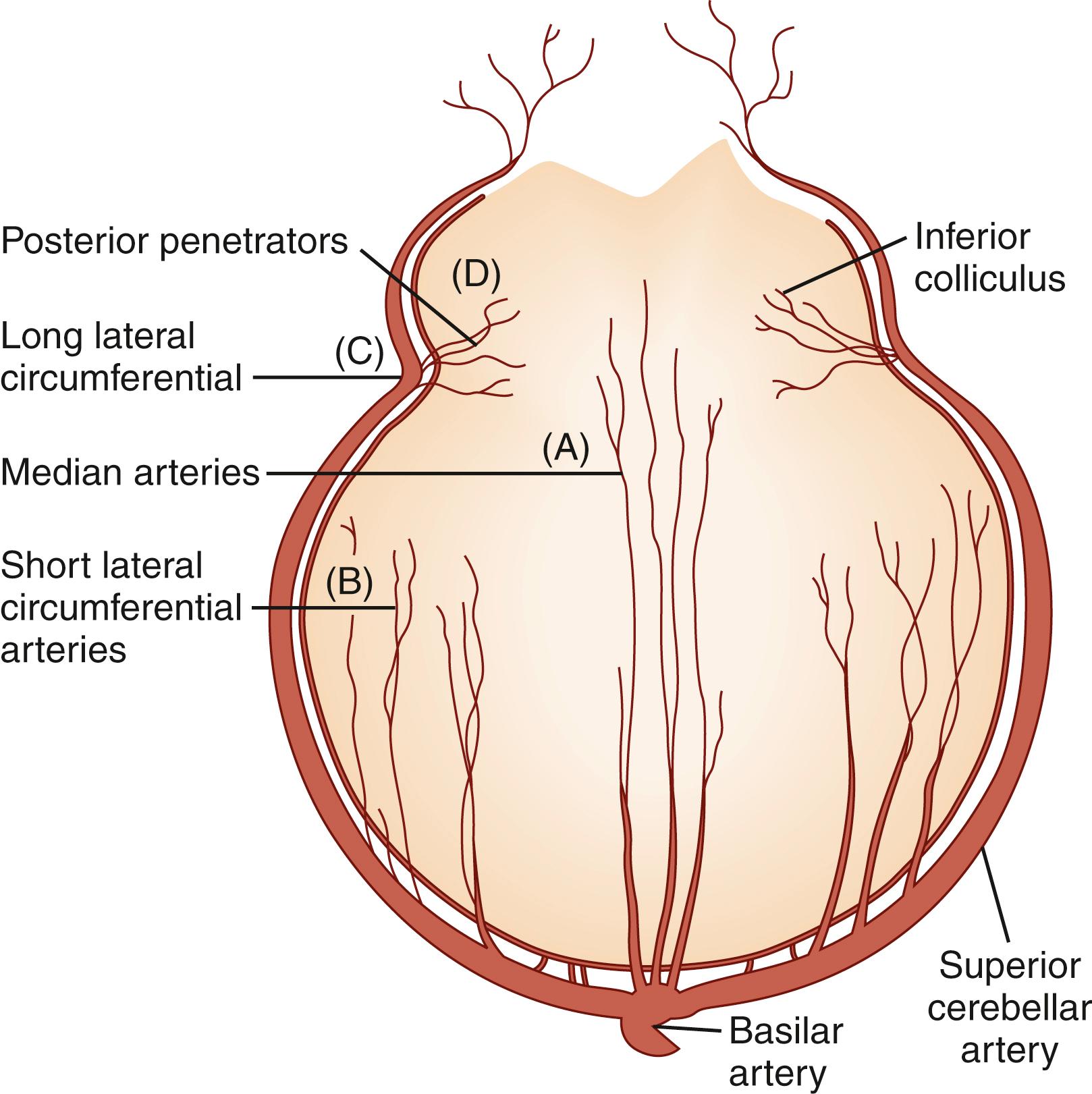
The three groups of arterial penetrators ( Fig. 26.5 ) are (1) median arteries, which usually take a slightly caudal course and then penetrate the brainstem and supply the paramedian basal and tegmental regions, (2) short lateral circumferential arteries, which give rise to branches that penetrate the brainstem and supply the intermediate tegmental and basal regions, and (3) long lateral circumferential arteries, which course around the brainstem and supply the lateral basal and tegmental regions. There are also posterior branches, which arise from the long lateral circumferential cerebellar vessels (the SCA, PICA, and AICA), course in a horizontal and dorsoventral direction, and supply the lateral tegmentum (see Fig. 26.5 ). Penetrating vessels are usually less than 100 μm in diameter, and their size is roughly proportional to their length. The medial penetrating vessels arise from the VA and BA as well as from the AICA and PCA; lateral penetrators frequently enter the brainstem along the laterally emerging nerve roots and arise from the VA, PICA, AICA, BA, SCA, and posterior choroidal arteries.
The distal basilar segments are also the source of occasional variations. During early fetal life, the internal carotid artery (ICA) supplies the posterior hemispheres and brainstem via posterior communicating arteries. In one third of humans, this primitive vascular pattern persists, and the connecting segment from the BA to the PCA remains vestigial. In these patients, the PCA may fill from carotid injection and not after VA opacification. In 2% of humans, this primitive circulatory pattern is bilateral; the BA may be hypoplastic in its distal segment and end in the SCAs. Penetrating branches from the posterior communicating artery, SCA, and proximal PCA supply the paramedian midbrain and thalamus; details are described in Chapter 25 .
Occasionally, primitive connections from the ICA to the posterior circulation vessels persist into adult life. The most common persisting channel is the trigeminal artery, which remains in 0.1%–1.0% of adults. The trigeminal artery arises from the ICA as it enters the cavernous sinus proximal to the carotid siphon, penetrates the sella turcica or the dura near the clivus, and joins the BA between the AICA and SCA branches. In such cases, the VAs and proximal BA are commonly small or hypoplastic. Persistence of the hypoglossal artery is the next most common variant. This vessel originates from the ICA in the neck, usually between C1 and C3, and courses posteriorly to enter the hypoglossal canal, from which it joins the BA.
A persistent otic artery is a rarer anomaly; this vessel leaves the ICA within the petrous bone and enters the posterior fossa with the 7th and 8th cranial nerves at the internal acoustic meatus, later to join the mid-BA. The rarest fetal communicating channels are persistent proatlantal intersegmental arteries, which originate from the nuchal internal or external carotid artery at C2 and C3 and join the horizontal (third) segment of the VA suboccipitally. Isolated reports describe communications between the common or proximal ICA and the lower VAs.
Vertebro-BA territory ischemia is caused by large artery disease, small artery disease, cardiogenic embolism, and other uncommon diseases.
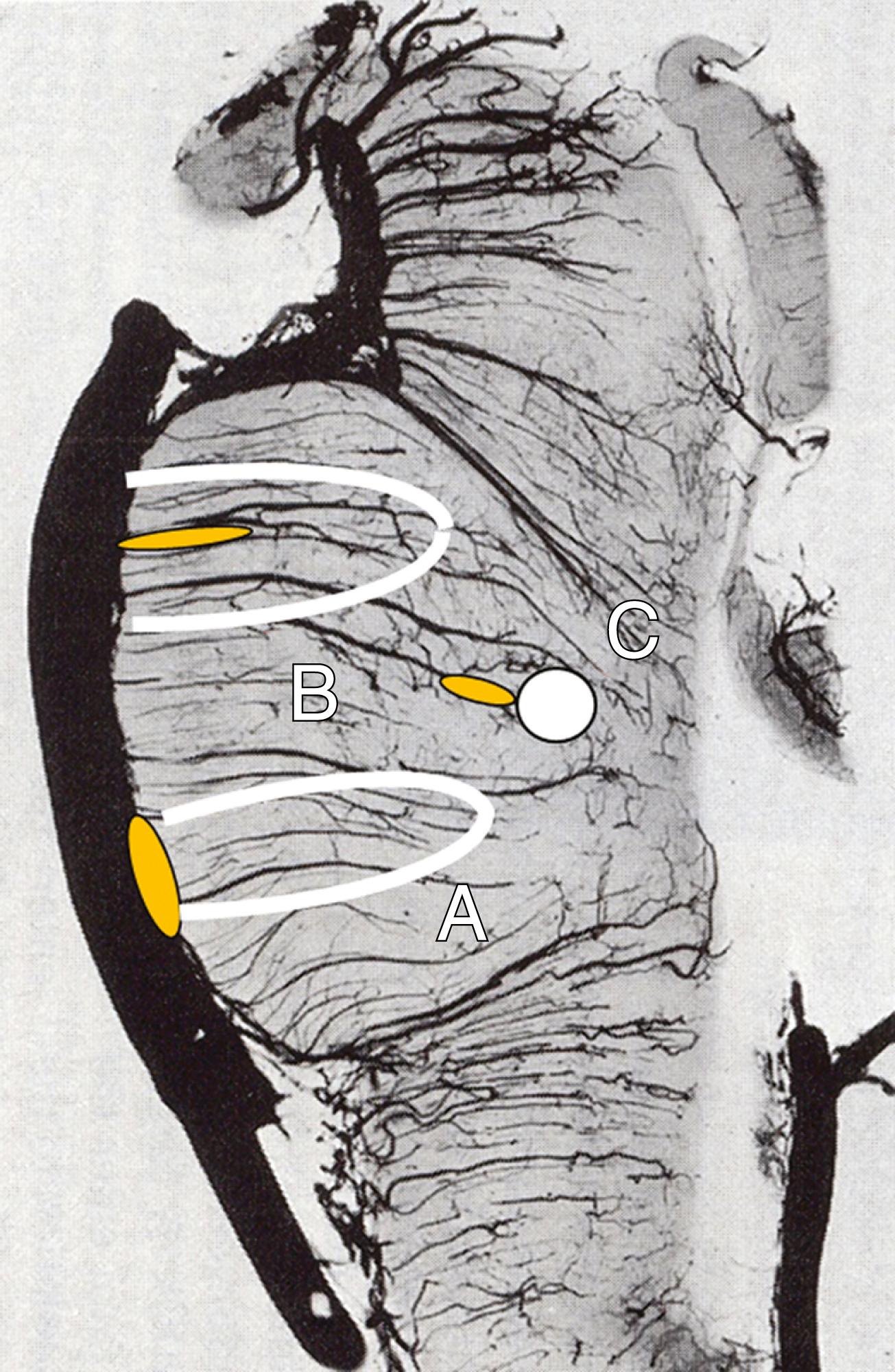
The main pathology of large artery disease consists of thrombosis superimposed on atherosclerosis. In the posterior circulation, atherosclerosis is prone to occur in the proximal extracranial VA (ECVA), distal intracranial VA (ICVA), lower-middle portion of the BA, and proximal PCA ( Fig. 26.6 ). , The histologic features of posterior circulation atherosclerosis do not differ qualitatively from atherosclerosis elsewhere. ,
Thrombosis occurring within the ECVA seldom forms a long anterograde or retrograde extension, which differs from thrombus in the ICA. Extensive collateral channels in the VA system may explain the difference. Thrombus formed within the ICVA, however, frequently extends into the proximal BA. Within the BA, atherosclerotic stenosis is common in the proximal 2 cm, more often seen on the ventral than in the dorsal side. , Thrombi within the BA tend to have limited propagation, occasionally extending only to the orifice of the next long circumferential cerebellar artery (the AICA or SCA).
Detailed stroke mechanisms of large artery disease include artery-to-artery embolism, in situ thrombotic occlusion, branch occlusion, hypoperfusion, and their combinations.
Atherosclerotic plaques with erosion and ulceration often generate embolism. , Emboli arising from the ECVA occlude distal arteries such as the PCA, SCA, PICA, and distal BA. Stenoses in the ICVA or BA also produce embolism, although they more often cause infarction by way of branch occlusion. , Embolism seems to occur more frequently in the setting of posterior fossa hypoperfusion associated with significant bilateral VA occlusive disease, due in part to ineffective washout of emboli in hypoperfused areas. Embolisms may develop from more proximal arteries, such as the subclavian artery, the ascending aorta, and aortic arch.
In patients with intracranial artery atherosclerosis, thrombus formation in areas of plaque can result in total arterial occlusion, leading to infarction. In the posterior circulation, in situ thrombotic occlusion is often observed in the territories of PCA, and BA branches such as AICA or PICA. , In situ thrombotic occlusion produces relatively large territorial infarction. However, unlike cardiogenic embolism, it less often produces “malignant” infarction because of relatively well-developed collateral circulation in the setting of the chronic atherosclerotic process. With persistent occlusion, the initial infarct frequently grows leading to progressive neurologic worsening. Thus, the ultimate size of the infarct varies according to the size of the occluded vessel, the speed of arterial occlusion, and the status of the collateral circulation.
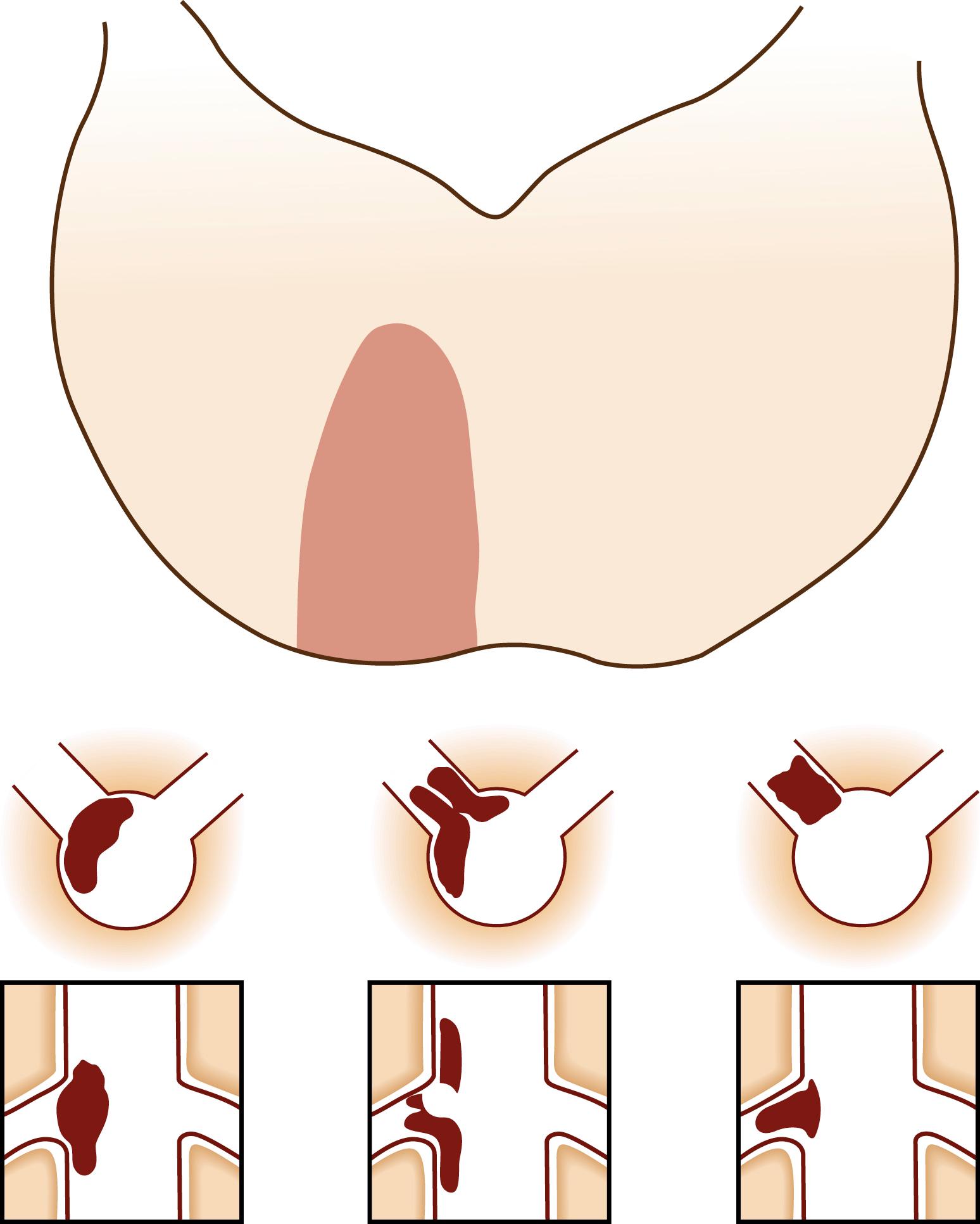
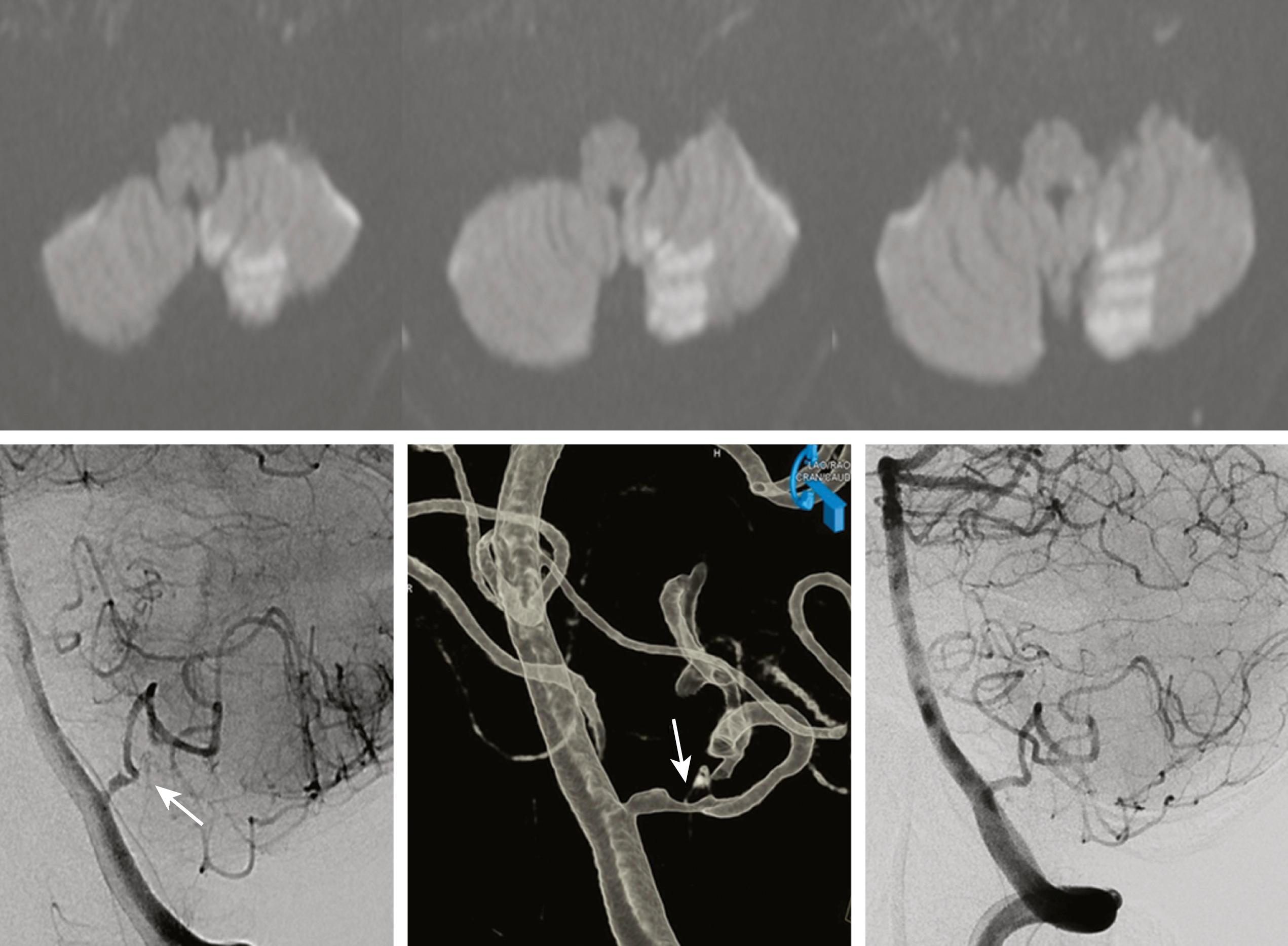
Atherosclerotic plaques in an intracranial artery can occlude the orifice of one or several perforators, causing infarcts limited to the perforator territory ( Fig. 26.7A ). Pathologic features of this “atheromatous branch occlusion” were described. , Branch occlusion is more often observed in posterior than in anterior circulation stroke and is the major mechanism of brainstem (pontine and medullary) infarctions. , ,
Brainstem infarcts associated with branch occlusion tend to extend to the basal surface (see Fig. 26.7A and B ), whereas those caused by small artery lipohyalinotic disease (see below) produce a small island of infarction within the parenchyma ( Fig. 26.8C ). The former is more often associated with atherosclerotic characteristics, larger lesion volume, and an unstable and unfavorable clinical course than the latter. , , Compared with arterial lesions producing embolism, branch occlusion is associated with less severe stenosis. Nowadays, high-resolution vessel wall MRI (HR-MRI) can identify the small plaque that occludes the perforator, even in patients with normal MRA findings. ,
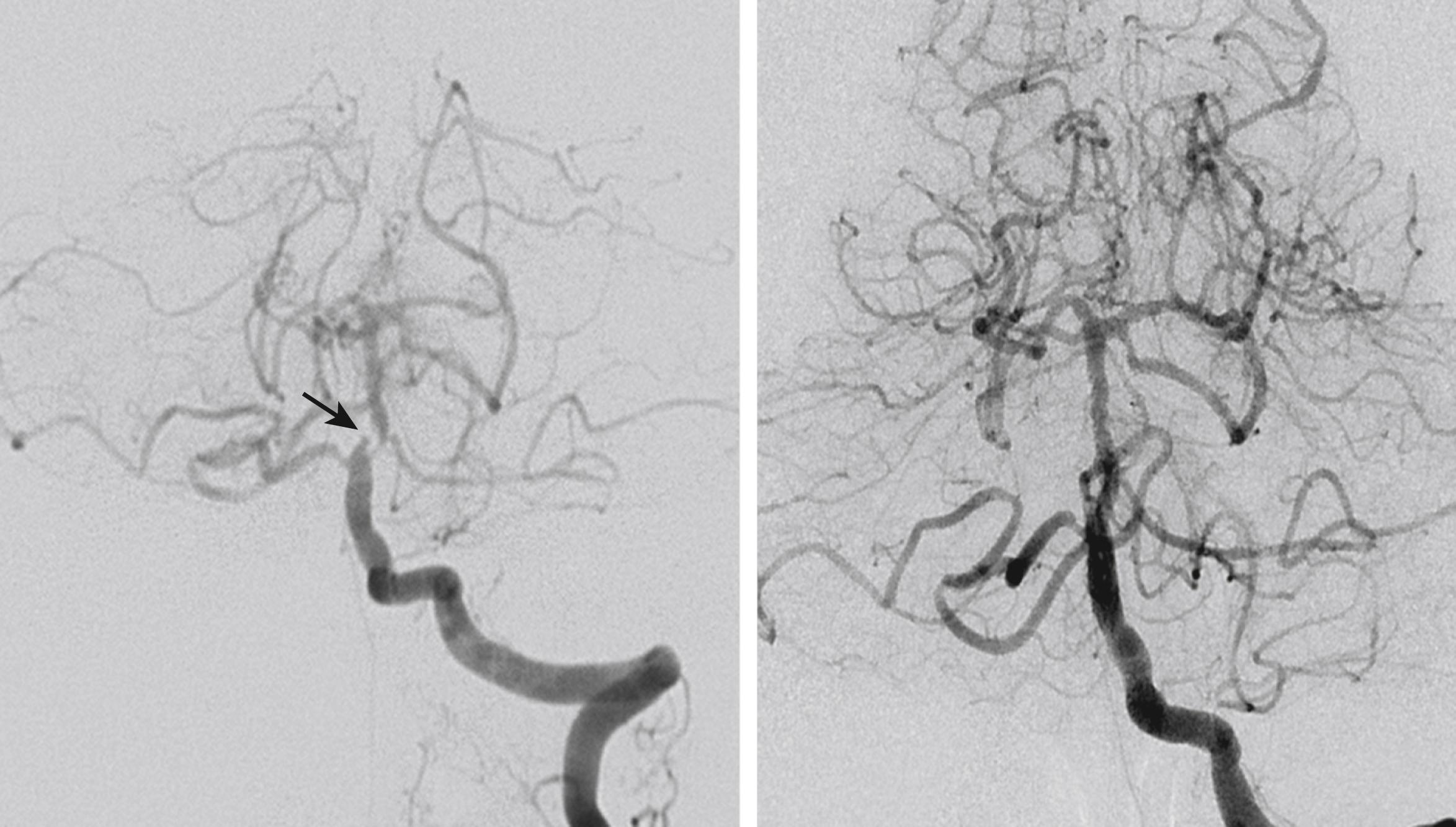
In patients with severe vascular stenosis/occlusion and insufficient collaterals, hemodynamic transient ischemic attacks (TIAs) can occur. Typically, symptoms such as dizziness, diplopia, and visual disturbances occur briefly and stereotypically in patients who are dehydrated or fatigued. When stroke develops, the symptoms may fluctuate widely according to the degree of hydration, blood pressure, and the position of the patient’s head. Improving perfusion with hydration or induced hypertension may be of help in such patients. Although the efficacy has not yet been proven, revascularization therapies, such as angioplasty/stenting, may rapidly relieve these symptoms (see Fig. 26.8 ).
Unlike anterior circulation diseases, MRI lesion patterns of hemodynamic infarction are not clearly established in the posterior circulation, due in part to considerable normal variations and collateral patterns that influence perfusion. Small infarcts occurring in the cerebellar borderzone (areas bordering PICA, AICA, and SCA) may be attributed to hypoperfusion associated with cardiac arrest or severe VA or BA occlusive disease. However, these infarcts are also produced by embolism to small arteries within the borderzone areas. The stroke mechanism is often difficult to assess partly because severe vertebrobasilar atherosclerosis can induce both hemodynamic and embolic strokes and partly because the territory of each cerebellar artery often overlaps. Posterior circulation infarcts solely attributable to hemodynamic failure seem to be distinctly uncommon. More often, hypoperfusion plays an additive role in the development of stroke, together with other major stroke mechanisms, for example, small embolic infarcts in the borderzone areas, progressive enlargement of infarction in patients with in situ thrombotic occlusion (see above).
The most frequent location for ECVA atherosclerotic disease is at the origin from the subclavian arteries. Atheromas may originate in the subclavian artery and spread to the proximal ECVA. ECVA atherosclerosis shares epidemiologic features with its close cousin, atherosclerosis of the ICA origin; the two sites are frequently affected in the same individuals. Despite the high incidence of ECVA atherosclerosis, serious posterior circulation strokes are relatively uncommon in this condition. When stroke develops, it is almost always related to embolism from thrombi formed in the proximal ECVA. , , Compared to unilateral VA lesions, bilateral lesions (contralateral occlusion or hypoplasia) seem to generate embolism more often, probably related with hypoperfusion in the posterior fossa that may promote thrombus generation, and inefficient washout of emboli. Hypoperfusion in turn is related to the effective development of collateral circulation, especially when the VA occlusion occurs gradually. Important sources of collaterals include occipital branches of the external carotid artery, the ascending cervical and transverse cervical branches of the thyrocervical trunk, and retrograde flow from the contralateral VA or from the posterior communicating system.
Generally, ICVA occlusive disease is a more serious condition than ECVA disease. Unilateral ICVA disease may produce medullary infarction or PICA cerebellar infarction through branch occlusion, that is, occlusion of the medullary perforators and the ostium of the PICA, respectively. Thrombi within the stenosed ICVA may also generate emboli that occlude distal vessels (artery-to-artery embolism). Bilateral ICVA occlusion is less well tolerated and often leads to TIAs or cerebellar and brainstem infarction, although some patients who have adequate collateral circulation may survive without major infarction.
Pathologically and angiographically documented BA occlusion often leads to catastrophic bilateral pontine infarction, but some patients have only limited or transient deficits. The variable outcome depends on extensiveness of the thrombus and the status of collateral circulation (e.g., backward flow from the well-developed posterior communicating artery) ( Fig. 26.9 ). The collateral status may in turn be influenced by the extent of the atherothrombotic diseases in individuals. For example, collateral circulation through the PICA would be poor when the ICVA is also obstructed. When thrombus propagates to the distal BA, collateral circulation from the SCAs and the posterior communicating arteries becomes limited. The speed of BA occlusion also matters; BA embolism and dissection tend to result in sudden coma and quadriparesis, while progression of brainstem ischemia related to atherothrombosis is slow, progressive, and earns time for collateral development. Early plaques associated with mild stenosis generally produce unilateral pontine infarcts through the mechanism of branch occlusion.
A single subcortical or brainstem infarct usually results from disease of penetrating arteries ( Fig. 26.7C ). Its pathologic hallmarks include irregular cavities, less than 15–20 mm in size, located in subcortical, brainstem, and cerebellar areas. Penetrating arteries associated with these lesions have disorganized vessel walls, fibrinoid material deposition, and hemorrhagic extravasation through arterial walls, first called “segmental arterial disorganization” and then lipohyalinosis by Fisher. , These vascular changes develop in arteries or arterioles 40–400 μm in diameter and frequently affect the perforating arteries from the PCA or BA. Penetrating artery disease is the main mechanism of brainstem infarction although brainstem infarctions may also be caused by atheromatous branch occlusion as discussed before ( Fig. 26.7A and B ).
A thrombus arising in the heart more often travels to the anterior circulation than to the posterior circulation system. Nevertheless, previous studies showed that about one-fifth of posterior circulation infarcts result from cardiogenic embolism. These emboli commonly occlude the PCA, rostral BA, SCA, PICA, and the ICVA. Infarcts are typically larger than those associated with large artery atherosclerotic disease, partly because the clots are larger and partly because of the insufficiently developed collateral circulation. The onset is usually abrupt. Additional infarcts may be seen in the anterior circulation as well. The occluded artery is often spontaneously recanalized, and hemorrhagic transformation of an infarct is common, which may cause worsening headache or neurologic deterioration ( Fig. 26.10 ). Atrial fibrillation is the most common cause of embolic infarction, and among stroke patients with atrial fibrillation, slightly less than one-fourth develop infarction in the vertebrobasilar territory.
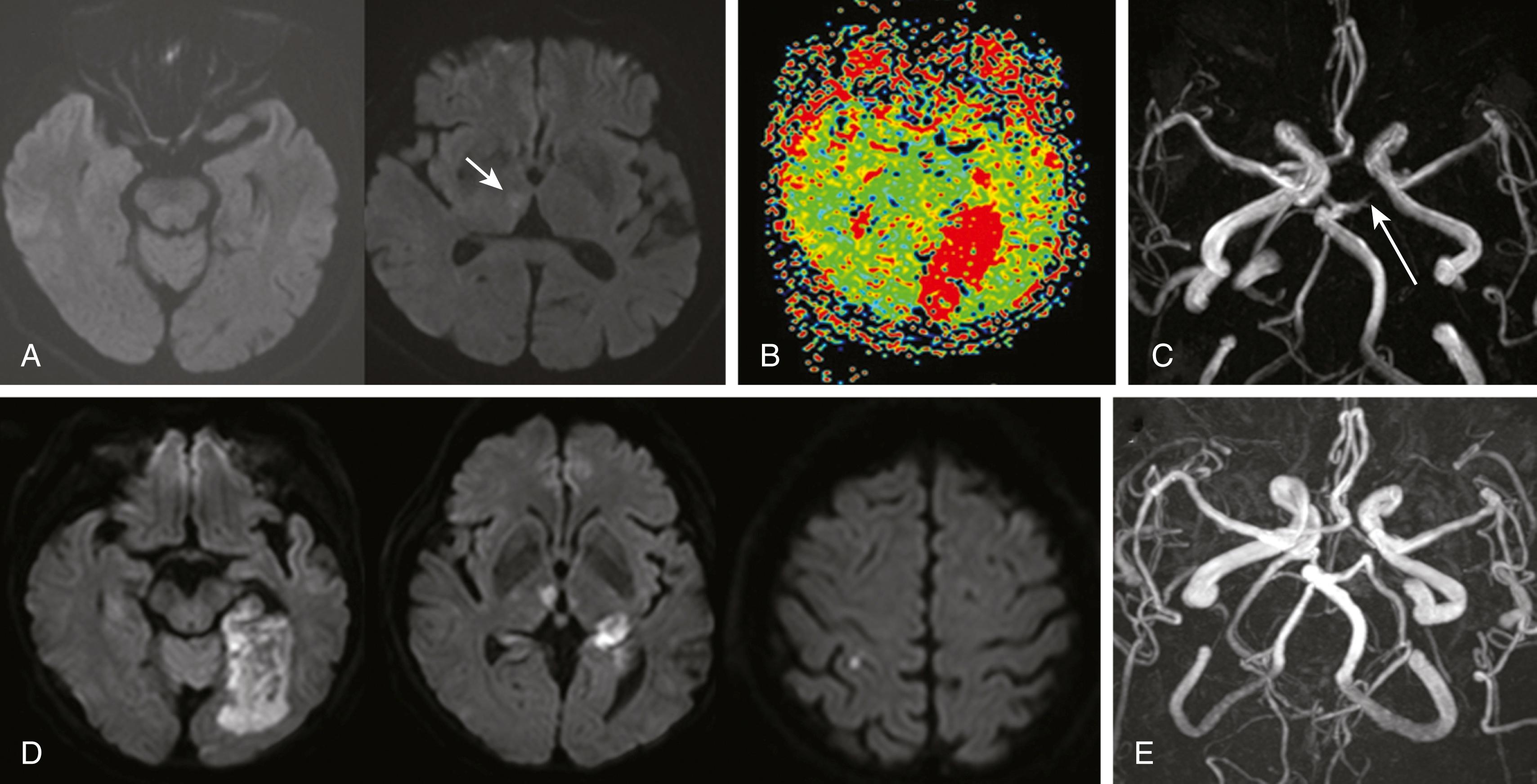
Although there still are debates, patent foramen ovale (PFO) with a large amount of shunt may be an etiology of embolic infarcts (paradoxical embolism). , The posterior circulation seems to be a predilection site for embolism in patients with PFOs. , A recent study showed that embolic infarctions associated with PFO more often occurred in the vertebrobasilar artery territory than those associated with atrial fibrillation (44.4% vs. 22.9%). Relatively poor adrenergic innervation in the vertebrobasilar circulation, and inefficient response to sympathetic stimuli at the time of Valsalva maneuvers may explain the increased chance of blood clot to travel to the vertebrobasilar system. Given the evidence that PFO closure is effective in the prevention of PFO-related stroke in patients with a large amount of shunt, PFO has been increasingly recognized as a treatable cause of stroke, especially in young patients.
Embolic infarction associated with cardiac catheterization also occurs preferentially in the posterior circulation. ,
Cervicocranial artery dissections occur in 1%–2% of patients with ischemic strokes, but are one of the major causes (10%–25%) of stroke in young patients. In the posterior circulation, dissection most commonly develops in the VA. Although ECVA dissection has been traditionally emphasized, , recent reports from Asia suggest that ICVA dissection may be equally prevalent, or even more frequent.
Dissection of the ECVA is often associated with trauma associated with rotating neck motion such as chiropractic procedures or other neck manipulations. Some arise from very minor trauma such as riding on a rollercoaster, coughing, falling on the back, and turning the head to back a car. VA dissections are associated with conditions such as Marfan syndrome, Ehlers-Danlos syndrome, pseudoxanthoma elasticum, systemic lupus erythematosus, fibromuscular dysplasia (FMD), and congenital bicuspid aortic valves. However, in most cases, dissection occurs in otherwise normal persons. Patients with family history of dissection have a higher risk of recurrent dissection. ,
The most common symptom of ECVA dissection is pain in the posterior head or neck. Ischemic symptoms and signs may develop at the same time or after a delay of hours to a few days. The lateral medulla and cerebellum are the regions most susceptible to ischemia from ECVA dissection ( Fig. 26.11 ). , ICVA dissections present either as ischemia, subarachnoid hemorrhage (SAH), or headache and most often occur near the origin of the PICA. The dissection often extends into the BA. ICVA dissections are important causes of medullary and cerebellar infarction. Less commonly, a dissecting aneurysm forms and acts as a mass lesion compressing the brainstem, cranial nerves, or other vessels. In one study, among 31 patients with ICVA dissections, 55% had headache, 48% had brainstem or cerebellar infarction, and 10% presented with SAH.
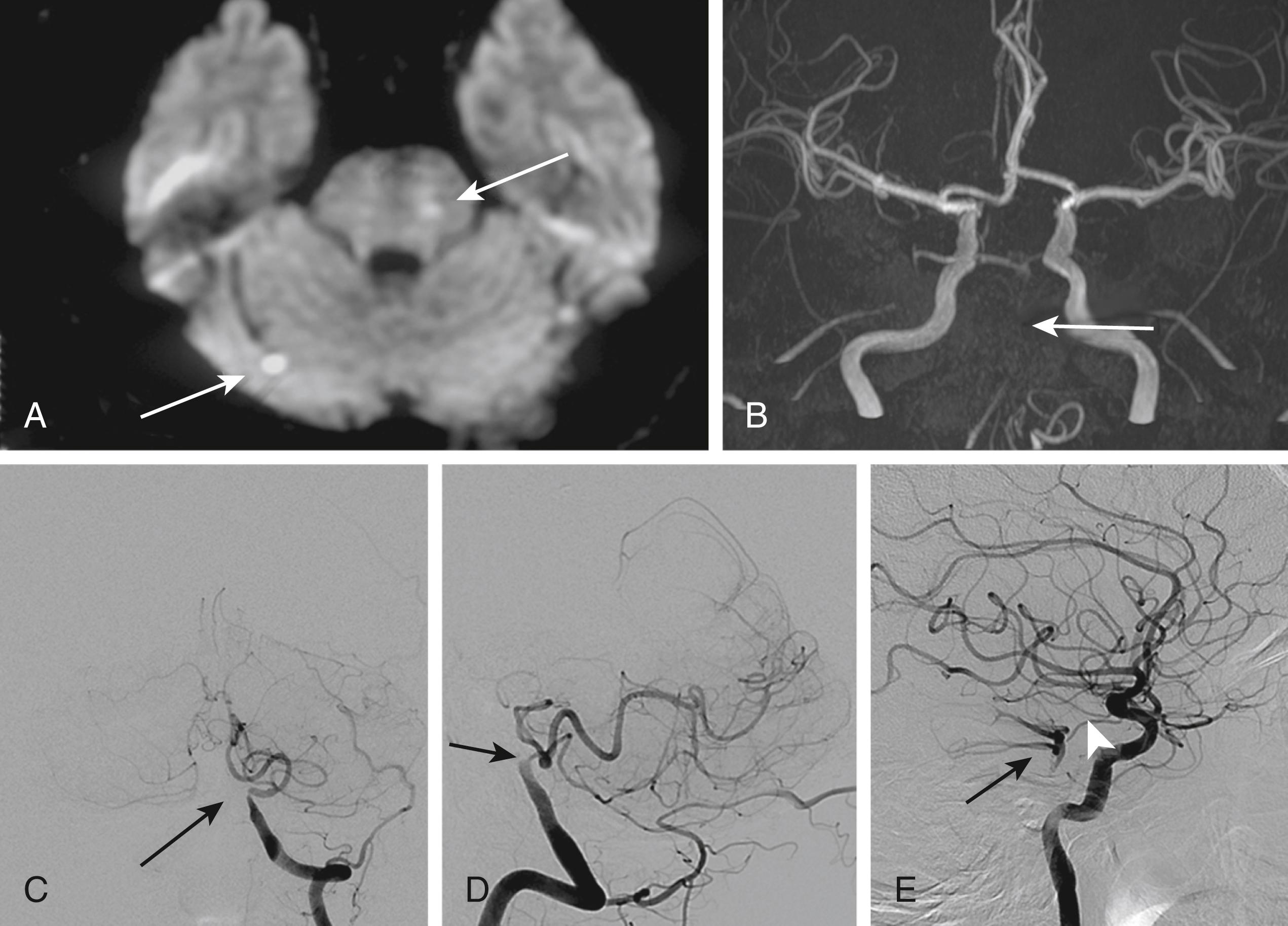
BA dissections are less common and carry a graver prognosis than VA dissections. They often produce extensive, bilateral pontine infarction presenting with altered consciousness and quadriparesis. In a review of 38 patients with BA dissections, 27 had brainstem infarctions, 5 had SAH, and 6 patients had both. Thirty patients (79%) died. However, a more recent study emphasized that unilateral pontine infarctions with a relatively favorable outcome are relatively common. Dissections occurring in the PCA are uncommon and present with PCA territory infarction, SAH, and mass effect. Dissections in the PICA present with either cerebellar infarction or SAH ( Fig. 26.12 ). , Dissections occurring in the proximal PICA appear to produce ischemic symptoms, while those in the distal portion tend to cause SAH. Although a PICA dissection has been considered a rare condition, a recent study showed that at least 6% of PICA territory infarction was caused by PICA dissection. Dissection occurring in the SCA is rare and seems to produce SAH more often than infarction.
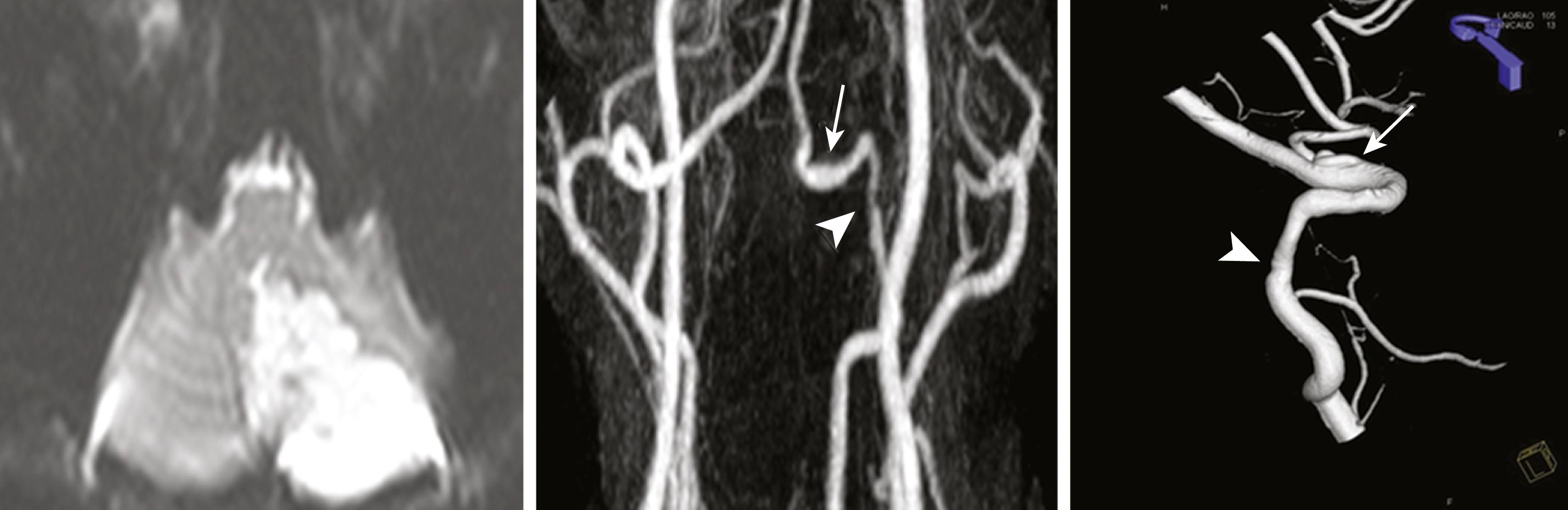
With the advent of imaging techniques, fusiform, tortuous, elongated, ectatic arteries (dolichoectasia) are increasingly recognized. They are found in the distal ICA and middle cerebral artery (MCA), but are most often observed in the BA. The ICVA may also be affected.
The etiologies of fusiform arterial dilatation remain unclear but may involve degenerative processes under genetic influences that lead to structural arterial defects characterized by fibrous dysplasia, internal elastic lamina degeneration, and fibrous and collagen replacement of the media. In adults, atherosclerotic changes in the vessels may interact with congenital structural defects to augment fusiform dilatation. A genetic deficiency in α-glucosidase was found in patients with fusiform BA aneurysms. In some, the arterial abnormalities are widespread and affect other vessels such as the abdominal aorta. ,
In the dilated vessel, blood flow is slowed, which predisposes to thrombus formation. Artery-to-artery embolism or branch occlusion may lead to infarction ( Fig. 26.13 ). One study reported that vertebrobasilar dolichoectasia was present in 6.4% of patients with cerebral infarction. Symptoms may occur due to compression and traction on posterior fossa structures. , Occipital–nuchal pain is common, and cranial nerve damage may result in 7th and 8th cranial nerve palsies, hemifacial spasms, tinnitus, deafness, vertigo, trigeminal pain, and hypoglossal paralysis. , , Large BA aneurysms can compress the cerebral peduncle leading to hydrocephalus, or may manifest as cerebellopontine angle masses. ICVA aneurysms may compress the medulla resulting in hemiparesis and other neurologic deficits.
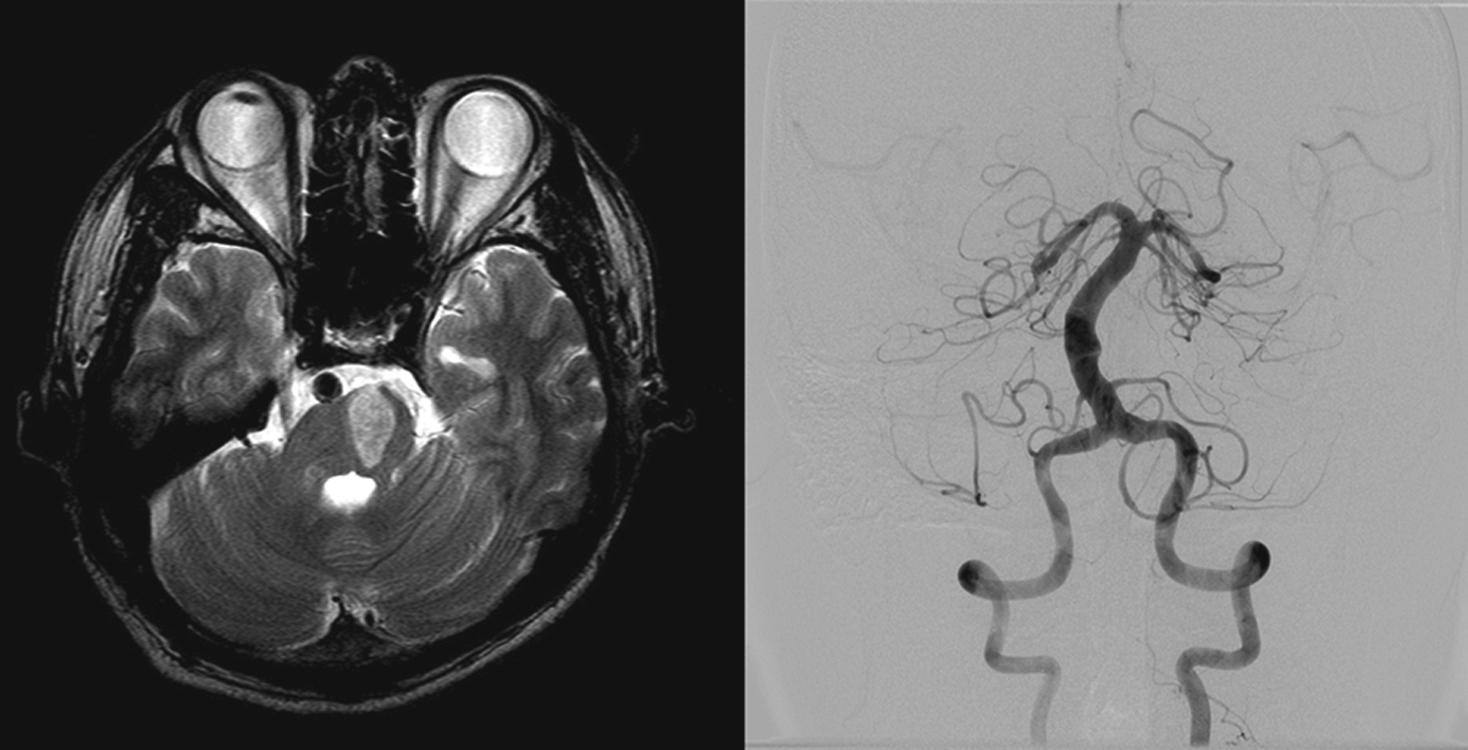
Spondylitic osteophytes that project from the vertebral joints adjacent to the transverse foramina can compress the VA, usually at C1,2 level on neck rotation, and lead to recurrent TIAs or even strokes. In many cases, the contralateral VA is hypoplastic, ends at PICA or previously occluded, rendering the compressed VA the major supply to the posterior circulation. Symptoms are precipitated by turning or rotation of the neck, during which the VA may become temporarily occluded. According to the recent series of 21 patients with this “rotational VA occlusion” syndrome, all patients developed vertigo accompanied by tinnitus (38%), fainting (24%), or blurred vision (19%). The induced nystagmus was mostly downbeat with horizontal and torsional components beating toward the compressed VA side. Although artery-to-artery embolism arising from stenosed VA was proposed, more recent studies suggest that symptoms are attributed to asymmetric excitation of the labyrinth induced by transient ischemia. , The prognosis is more benign than previously thought and conservative treatment is usually sufficient.
Posterior fossa ischemic strokes may occur during or after surgical or interventional procedures, such as aneurysm clipping, stenting, or surgical removal of tumor. In patients with aneurysms, blockage of the orifice of tributary vessels secondary to clot within the aneurysm may be responsible for ischemic stroke. Other mechanisms include (1) dissection or intimal tear due to forceful retraction of the artery or interventional procedure; (2) sudden decompression of the artery by tumor removal, which had been encased and compressed for a long time, resulting in turbulent blood flow and subsequent thrombus formation; and (3) vasospasm due to localized SAH secondary to vessel injury.
FMD is a vasculopathy of unknown cause characterized by hyperplasia of the intima and media of arteries, adventitial sclerosis, breakdown of normal elastic tissue, without inflammation. Thickened septa and ridges protrude into the lumen. The most common angiogram findings are a “string of beads” appearance: segments of constriction alternating with normal or dilated segments. Renal arteries and cervicocranial arteries are the two most commonly involved sites. In patients with cervicocranial artery involvement, lesions are present in the ICA in 95% and are bilateral in 60%–85% of cases. ECVA involvement has been reported in 12%–43% of affected patients.
FMD affecting the ECVA may cause TIA or stroke via artery-to-artery embolism, hemodynamic insufficiency, or their combination. Although rare, FMD may occur in the intracranial arteries such as BA or PCA, resulting in posterior circulation TIA and infarctions. , Intracranial FMDs accompanied by dissection or pseudoaneurysm formation , tend to carry a relatively poor prognosis. Hypertension secondary to the renal involvement also contributes to the development of stroke in patients with FMD.
Moyamoya disease is characterized by progressive occlusion of the distal ICA or proximal MCA, with the development of fine meshworks of basal collateral vessels. Posterior circulation stroke is rarely a presenting manifestation of moyamoya disease, except for PCA infarction in patients with PCA involvement (for details, see Chapter 25 ).
Giant cell arteritis is a systemic vasculitis characterized by subacute granulomatous inflammation of the aorta and its major branches (large and medium vessels) with particular tropism for the extracranial carotid artery branches. Headache and visual loss, the most common clinical manifestations, are caused by involvement of the superficial temporal arteries and ophthalmic branches/central retinal arteries, respectively. Stroke is a rare complication of giant cell arteritis, occurring in about 3% of patients. , Giant cell arteritis may involve ECVA and produce TIAs or brainstem/cerebellar infarctions. Occasionally the subclavian arteries become occluded. Because this condition is treatable, and the prognosis is poor if untreated, this possibility should be suspected in elderly patients with extensive ECVA steno-occlusive disease when they have prolonged unexplained fever,headache, malaise, anemia, and erythrocyte sedimentation rate and/or C-reactive protein elevation.
Vasculitis may be caused by infectious (e.g., bacterial, tuberculous, spirochetal, fungal, viral) and immunologic (e.g., lupus, polyarteritis nodosa) disorders. Vasculitis more often involves the anterior circulation, but involvement of posterior circulation is not an exception. Takayasu disease primarily involves the aorta and its branches, and involvement of the subclavian artery and ECVA may produce subclavian steal syndrome or vertebrobasilar ischemia. ,
A persistent trigeminal artery (PTA) is the most common embryonic carotid-basilar anastomosis, occurring in 0.1%–1.0% of the general population. It usually forms a connection between the cavernous part of the ICA and the upper third of the BA. A high incidence (85%) of VA hypoplasia or atresia is associated. The severe VA hypoplasia may lead to vertebrobasilar ischemic symptoms when collaterals are insufficient. In addition, brainstem TIA or infarction may occur due to emboli that originate from an ICA plaque or diseased heart and traverse through the PTA.
The medulla is mainly supplied by a number of penetrating arteries arising from the ICVAs. The dorsal tegmental area is also supplied by branches arising from the medial PICA and posterior spinal artery. The most rostral part may be supplied by branches from the BA or AICA. The caudal part of the anterior medulla is supplied by penetrating arteries arising from the anterior spinal artery (ASA).
Since the first description of Wallenberg syndrome more than 100 years ago, clinical and pathologic findings of lateral medullary infarction (LMI) have been reported. However, pre-mortem lesion identification became possible only after MRI was introduced. Recent studies using MRI , have rapidly expanded our understanding of LMI syndromes. One study reported that LMI represents 1.9% of all admitted acute stroke.
The onset may be sudden, but in more than half of the patients, symptoms/signs develop progressively or stutteringly; the first symptoms/signs are usually headache, vertigo, nausea/vomiting, or gait instability, whereas hiccup tends to occur later. Occasionally, some of these symptoms/signs develop days or even weeks after the onset. The progressive onset is often associated with enlargement of ischemic area detected by follow-up MRI, associated with persistent or progressive thrombosis. Thus, progressive addition of symptoms (e.g., initial dizziness followed by sensory loss of the limb and then dysphagia) seems to be equivalent to the progressive worsening of motor dysfunction in patients with internal capsular infarction. Symptoms/signs of LMI are summarized in ( Table 26.1 ).
| Items | N = 130 |
|---|---|
| Sensory symptoms/signs | 125 (96) |
| Ipsilateral trigeminal | 34 (26) |
| Contralateral trigeminal | 32 (25) |
| Bilateral trigeminal | 18 (14) |
| Isolated limb/body | 27 (21) |
| Isolated trigeminal | 13 (10) |
| Gait ataxia | 120 (92) |
| Severe gait ataxia a | 79 (61) |
| Dizziness | 119 (92) |
| Horner sign | 114 (88) |
| Hoarseness | 82 (63) |
| Dysphagia | 84 (65) |
| Severe dysphagia b | 52 (40) |
| Dysarthria | 28 (22) |
| Vertigo | 74 (57) |
| Nystagmus | 73 (56) |
| Limb ataxia | 72 (55) |
| Nausea/vomiting | 67 (52) |
| Headache | 67 (52) |
| Neck pain | 9 (7) |
| Skew deviation of eyes | 53 (41) |
| Diplopia | 41 (32) |
| Hiccup | 33 (25) |
| Facial palsy | 27 (21) |
| Forced gaze deviation | 8 (6) |
A dizzy sensation and gait instability are the most common symptoms occurring in more than 90% of patients. Whirling vertigo, a symptom attributed to involvement of vestibular nuclei and their connections, occurs in approximately 60%. Vertigo is an early sign that usually improves within days or weeks, even if dizziness and gait instability persist. Vertigo is usually accompanied by nystagmus and vomiting.
Gait instability and dizziness can be attributed either to dysfunctional vestibular or cerebellar system. In the acute stage, approximately 60% of patients are unable to stand or walk. Gait ataxia is usually more common and severe than limb incoordination. , Lateropulsion (forced to sway when patients stand or sit) seems to be attributed to lesions affecting the vestibular nuclei and vestibulospinal projections, while limb and gait ataxia are related to damage to the inferior cerebellar peduncle, spinocerebellar fibers, or the cerebellum itself. , Occasionally, patients fall to any direction, which may be related to involvement of the ventral spinocerebellar tract, which conveys proprioceptive information from both sides. Limb ataxia is usually milder than that shown in SCA territory cerebellar infarction, and may be described as “weakness” or “clumsiness” by the patient.
Involvement of the vestibular nuclei and their connections lead to nystagmus. The nystagmus is mostly horizontal or horizontal-rotational to the side opposite the lesions. , It normally improves over time, but in rare cases can be permanent. Although forced conjugate eyeball deviation to the lesion side (ocular lateropulsion) is uncommon, milder degree of eyeball deviation is frequently observed when patients are ordered to close and then open the eyes, when correctional eyeball movements occur. Skew deviation, with the ipsilateral eye going down, is also frequent, and ocular torsion is often accompanied by head tilt (ocular tilt reaction [OTR]). Most often, lateral gaze is characterized by hypermetric saccades to the side of the lesion and hypometric saccades to the opposite side. These ocular manifestations are caused by dysfunctional vestibulo-ocular reflex pathways and can present clinically as blurred vision, diplopia, oscillopsia (a sense of moving objects), or tilting of visual images. ,
Nausea/vomiting is usually an initial and transient symptom closely associated with vertigo, nystagmus, and gait instability and is probably caused by involvement of vestibular nuclei and their connections. It may also be caused by involvement of a putative vomiting center near the nucleus ambiguus or the tractus solitarius. ,
Elements of Horner syndrome are frequent, occurring in about 90% of patients. This is caused by involvement of the descending sympathetic fibers in the lateral reticular substance. A constricted pupil with ipsilateral palpebral fissure narrowing is more frequently observed than facial anhidrosis.
Involvement of the nucleus ambiguus results in paralysis of the ipsilateral palate, pharynx, and larynx producing dysphagia, dysarthria, and hoarseness. Dysarthria may also be attributed to the concomitant involvement of the cerebellum. Dysphagia is present in approximately two-third of LMI patients, among whom about 60% require a nasogastric tube for feeding. , Swallowing difficulty usually improves within days or months, but rare patients require persistent assistance in feeding. Dysphagia in LMI is more often associated with problems in the range of, than timing of, hyolaryngeal excursion. During eating, foods are often stuck in the piriform recess of the pharynx, and patients attempt to extricate the material by a crowing-like cough. Hoarseness is equally common, while dysarthria is less common occurring in about one-fourth of patients with pure LMI patients. Some patients may show paralysis of the ipsilateral vocal cord.
Approximately one-fourth of patients develop hiccup, , often days after stroke onset. Hiccup usually goes away within a few days but can persist for weeks or even months when it becomes an annoying symptom. Involvement of the dorsal motor nucleus of the vagus, solitary tract, and neurons related to expiration and inspiration in the reticular formation near the nucleus ambiguous may be responsible for hiccup. ,
Sensory symptoms/signs are one of the most common manifestations of LMI. In the largest series, sensory function was intact in only 4% of patients. Sensory symptoms/signs occur more frequently in the contralateral body/limbs (in ≈85%) than in the face (58%–68%), , and the sensory defect in the face usually clears more quickly than that on the body/limbs. Although a selective loss of spinothalamic sensation is a rule, vibration sensation is occasionally involved as well in the hypalgic body/limbs, due probably to the fact that some of vibratory sensations are carried through the lateral column.
Crossed (ipsilateral trigeminal-contralateral limb/body) sensory changes have been considered a classical sensory pattern in LMI. However, recent studies have identified that sensory findings are much more diverse in the acute stage. In the largest series, the patterns included ipsilateral trigeminal-contralateral limb/body in 26%, contralateral trigeminal-contralateral limb/body in 25%, bilateral trigeminal-contralateral limb/body in 14%, limb/body involvement without trigeminal involvement in 21%, and trigeminal involvement without limb/body involvement in 10%. Although the arm and leg are usually equally involved, in approximately 30% of the patients there is a discrepancy; some have more severe sensory deficits in the arm while others have more severe deficits in the leg. The latter occasion is more common, and some may have a sensory level on the trunk mimicking a spinal cord syndrome.
These diverse sensory manifestations are related to the varied patterns of involvement of the spinothalamic tract, descending trigeminal tract, and ascending secondary trigeminal fibers ( Fig. 26.14 ). In addition, approximately 7% of LMI patients have additional ipsilateral tingling sensation often associated with mild lemniscal sensory deficits, more marked in the arm than in the leg. Due to the lemniscal sensory impairment, these patients often have feeling of “clumsiness” or “weakness” in the ipsilateral extremities. This sensory pattern is related to involvement of the lower-most medulla and explained by partial involvement of lemniscal sensory fibers at the upper-most part of the fasciculus cuneatus or crossing fibers to the medial lemniscus.
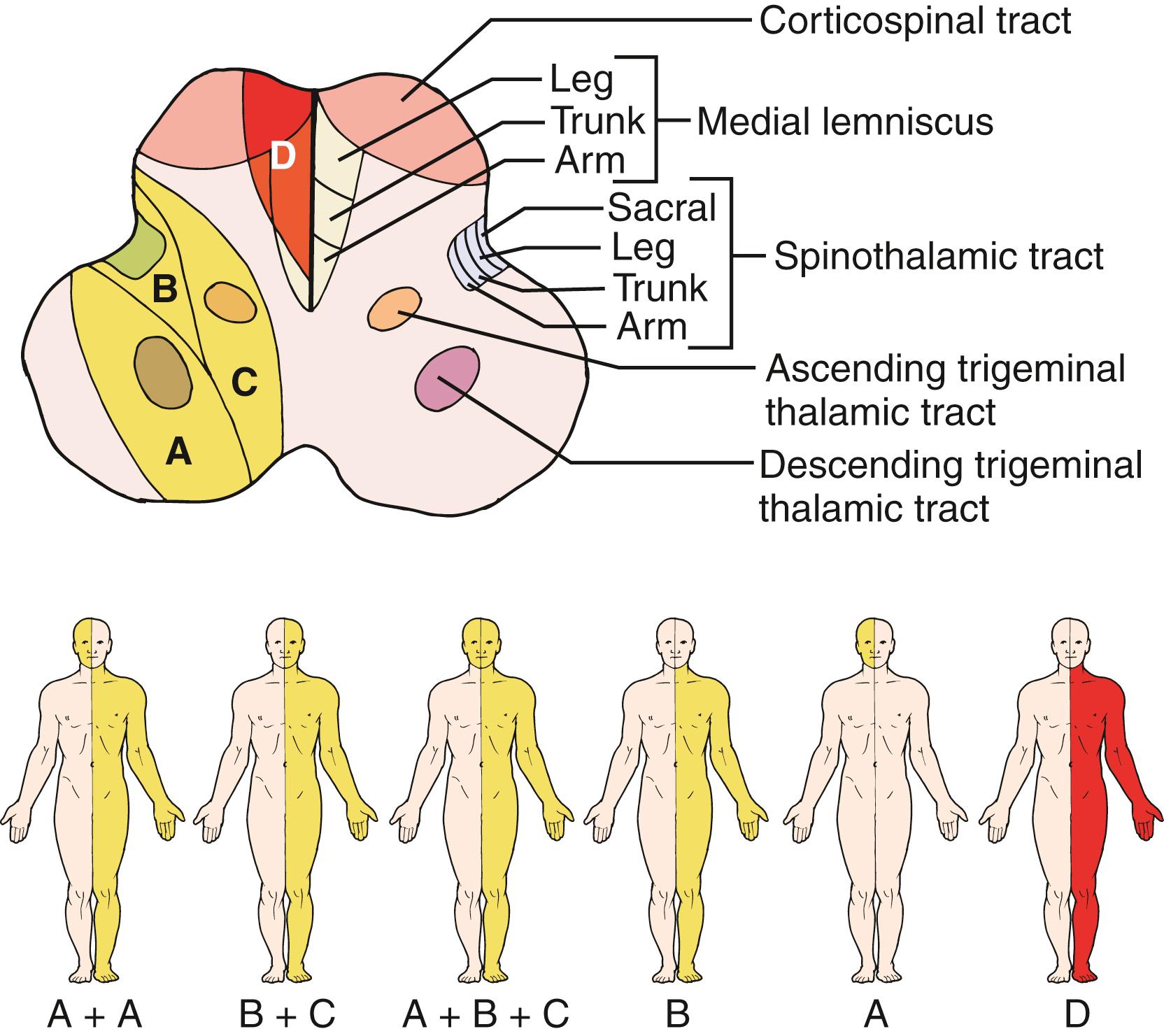
In the descending trigeminal tract, V3-representing area is located most dorsally and V1 most anteriorly, but in the ascending secondary trigeminal tracts, V3 is located most medially and V1 most laterally. Therefore, trigeminal sensory involvement is often inhomogeneous, more so on the contralateral side than ipsilateral side. On both sides, the inhomogeneity is either divisional or segmental (onion-skin) pattern. , , Bilateral perioral paresthesia, often observed in patients with large infarcts extending medially, may be caused by the simultaneous involvement of the descending and ascending V3 pathways near its decussation. , The perioral or V3 area is usually spared or less markedly involved on the side contralateral to the lesion, probably because V3 area is located most medially in the secondary ascending trigeminal tract, and so less often affected by the lesion primarily involving the lateral part of the medulla.
Patients occasionally complain of facial pain. The pain usually appears at onset and heralds other symptoms and signs. It is described as sharp, stinging, stabbing, or burning, tingling, and uncomfortably numb. The eyeball and surrounding area are the most commonly affected, but the entire face including the lips and inside the mouth may be involved. Although facial pain usually improves, it may last permanently in some patients. Involvement of the sensory nucleus of the descending tract of the 5th cranial nerve may explain the facial pain.
Headache occurs in about half of the patients. , It usually begins at onset or a few days before other symptoms/signs and subsides within several days. It most often occurs in the ipsilateral occipital or upper nuchal area followed by the frontal region, and is usually described as dull, aching, or throbbing. Considering that headache occurs before other symptoms and is not related to any symptoms/signs of LMI, headache seems to be caused by an ICVA pathology, possibly related to dilatation of VA or collateral vessels after VA stenosis/occlusion, rather than the medullary lesion itself. Involvement of the descending spinal tract of the fifth cranial nerve and its nucleus may also be responsible for frontal headache. Prominent and persistent nuchal pain may be a manifestation of VA dissection.
Facial palsy, usually mild and upper neuron type, is present in one-fifth to one-fourth of patients. It is presumably caused by involvement of aberrant corticobulbar fibers that loop caudally before travelling rostrally toward the facial nucleus. In patients with upper-most medullary (or pontomedullary junction) lesion, there occurs relatively severe peripheral type facial palsy due to direct involvement of facial nerve fascicles.
The medullary reticular formation contains neurons related to the control of respiration, and patients may show respiratory arrest or decreased respiratory drive especially during sleep (Ondine’s curse). Severe respiratory abnormalities calling for medical attention are uncommon unless patients have bilateral or extensive lesions. In pure LMI, aspiration pneumonia associated with dysphagia is the most common reason for respiratory care, in which case it is often difficult to assess how much respiratory control abnormality contributes to the patient’s condition. Other autonomic disturbances such as tachycardia, bradycardia, sweating, orthostatic hypotension, gastric motility dysfunction, and urinary retention are sporadically observed.
Ipsilateral hemiparesis may be associated with other typical symptoms of LMI.
The pathogenic mechanism of this so-called Opalski syndrome remains debatable. Recent imaging techniques such as diffusion-weighted MRI (DWI) and diffusion tensor imaging (DTI) showed that infarcts occurring at the lower-most medullary area or the medulla-spinal cord junction involve the ipsilateral corticospinal tract after the pyramidal decussation. , This observation corroborates with Opalski’s original patients who showed hyperreflexia and Babinski signs. Concomitant infarcts in the spinal cord may also explain ipsilateral hemiparesis.
In addition, patients with ipsilateral ataxia or lemniscal sensory dysfunction (see above) may complain of “clumsiness” or “weakness” in their limbs. In view of transient motor deficits and absent reflex abnormalities in the majority of these patients, Kim suggested that most of the patients with ipsilateral “weakness” may have a pseudoparesis unrelated to pyramidal damage. Thus, ipsilateral weakness in LMI patients may have diverse causes, and the term Opalski syndrome should be cautiously used.
The symptoms/signs of LMI differ according to the topography of lesions. Currier et al. was the first to divide the lesion topography into ventral, superficial, and dorsal and suggested that clinical features vary according to the pattern. More recently, Kim et al. , analyzed the MRI-identified lesions in a three-dimensional manner and made clinical-topographic correlation.
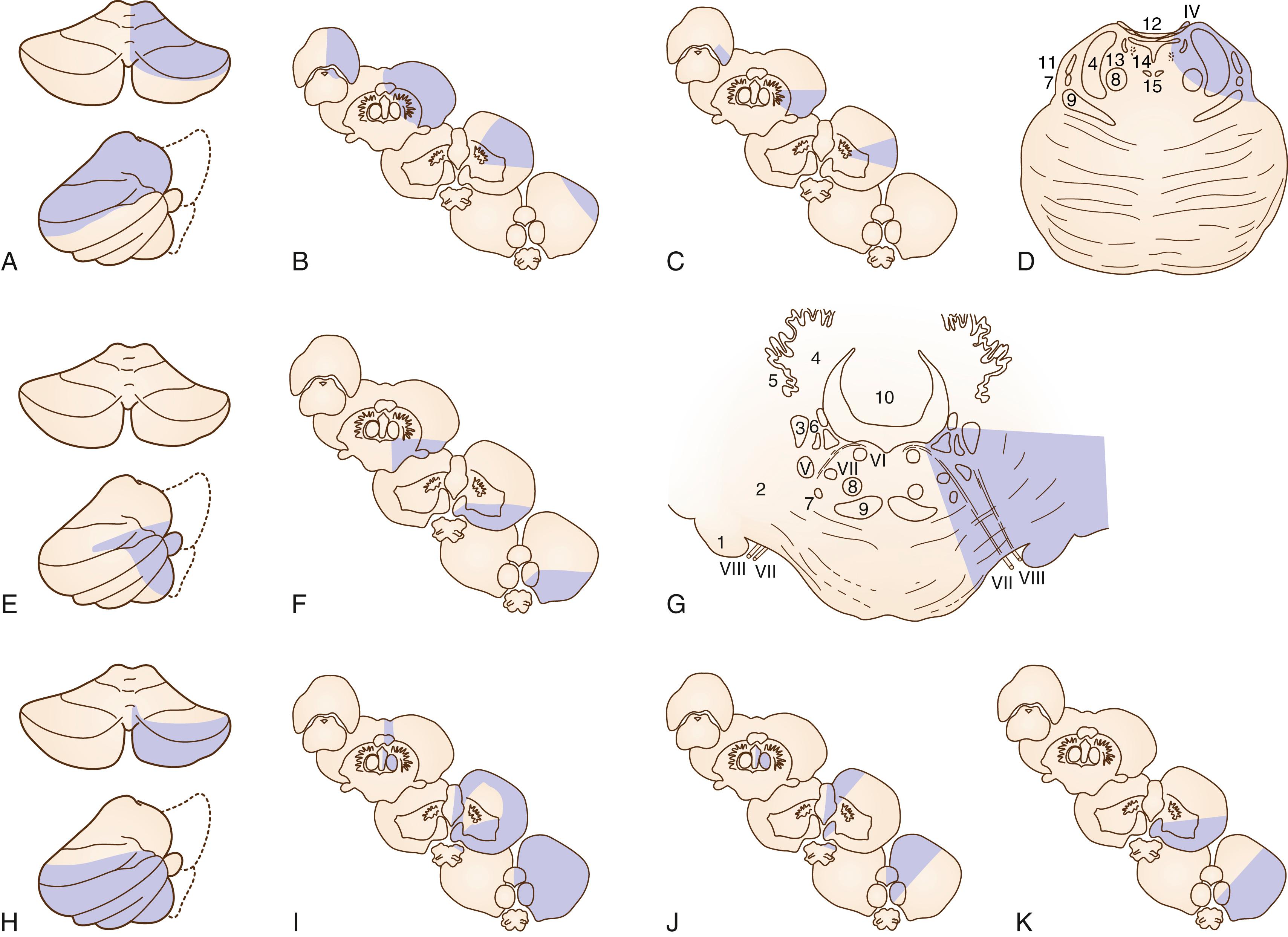
Generally, rostral lesions tend to involve ventral, deep areas while caudal lesions involve lateral-superficial regions ( Figs. 26.15–26.18 ). , This is probably related to the anatomic course of the ICVA; the ICVAs are located adjacent to the lateral surface at a caudal medulla level (see Fig. 26.16 ), which ascend ventrorostrally (see Fig. 26.18 ) to fuse into the BA at the ponto-medullary junction. The rostral-ventral lesions tend to produce ipsilateral trigeminal sensory symptoms, whereas caudal-lateral superficial lesions tend to produce sensory levels with a gradient worse in the leg. A large, wide lesion is associated with a bilateral trigeminal sensory pattern ( Fig. 26.19 ), which is quite uncommon in patients with caudal lesions.
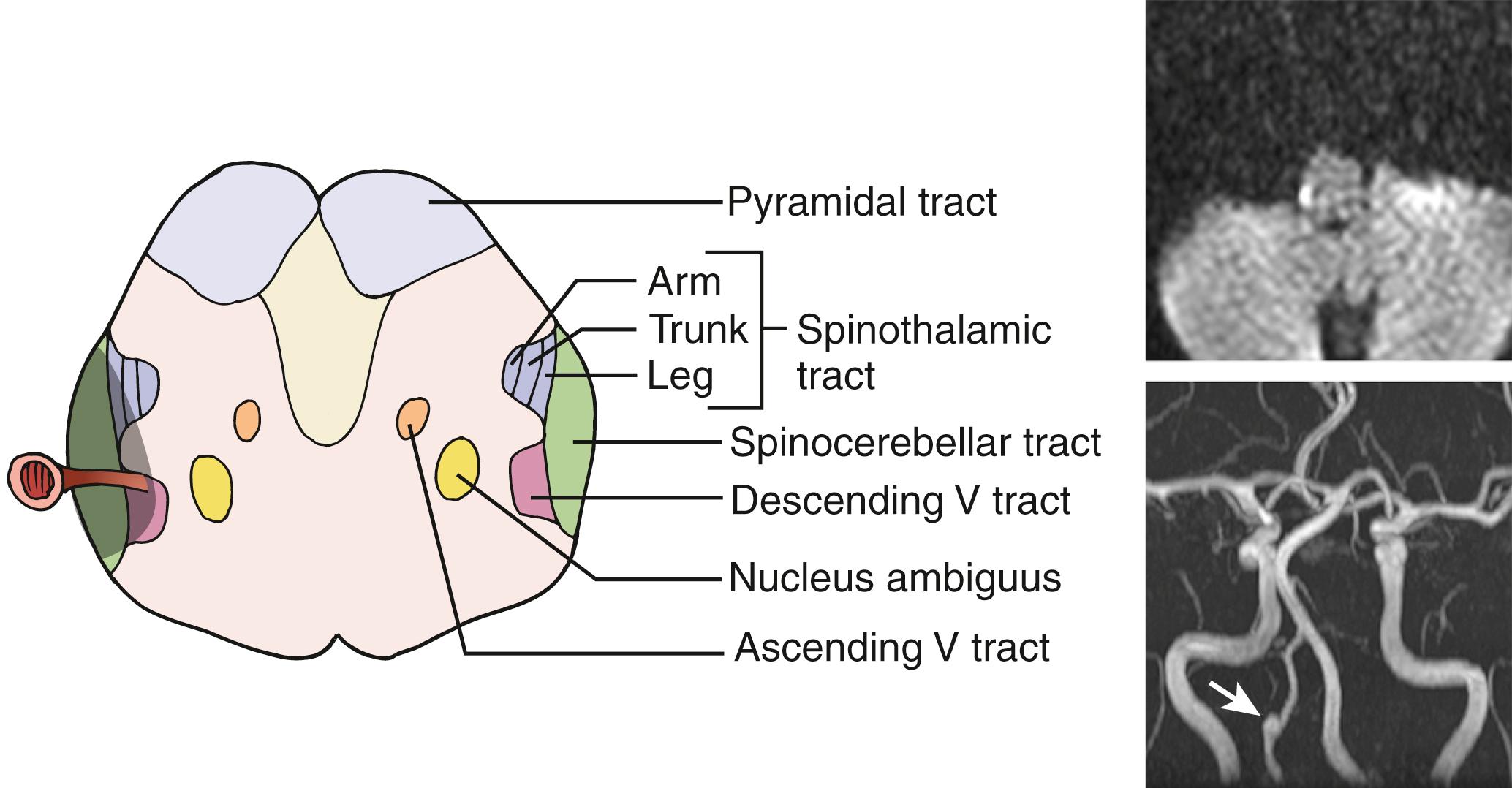
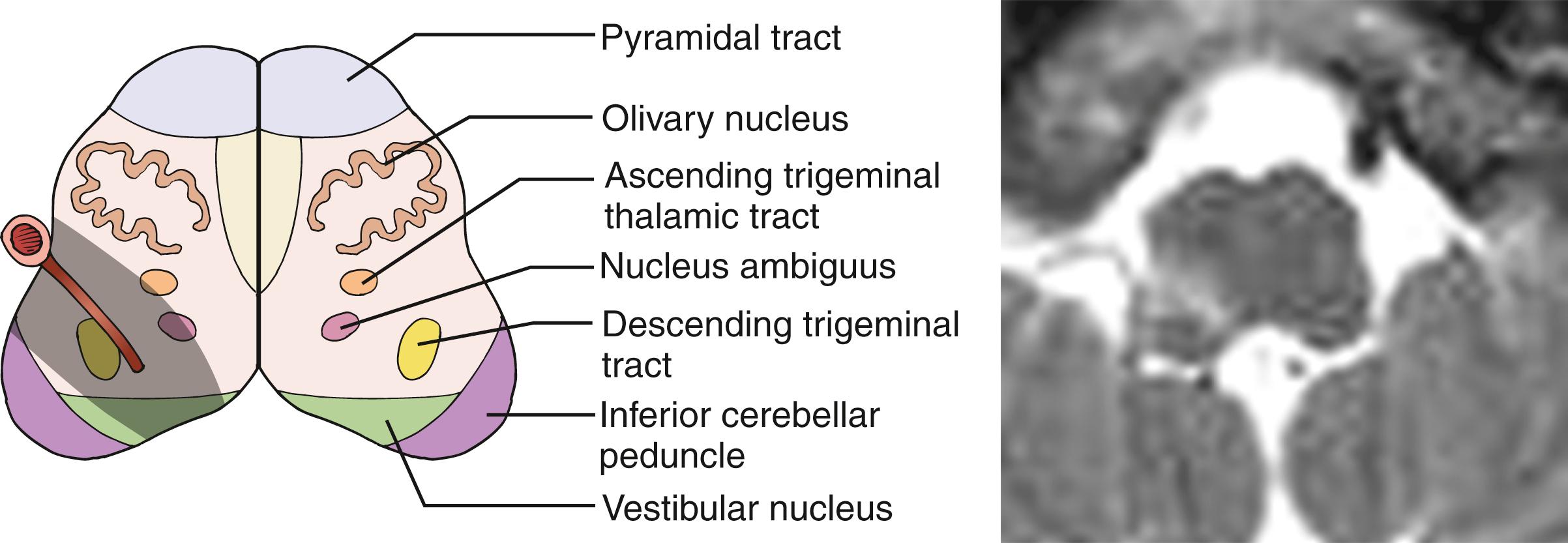
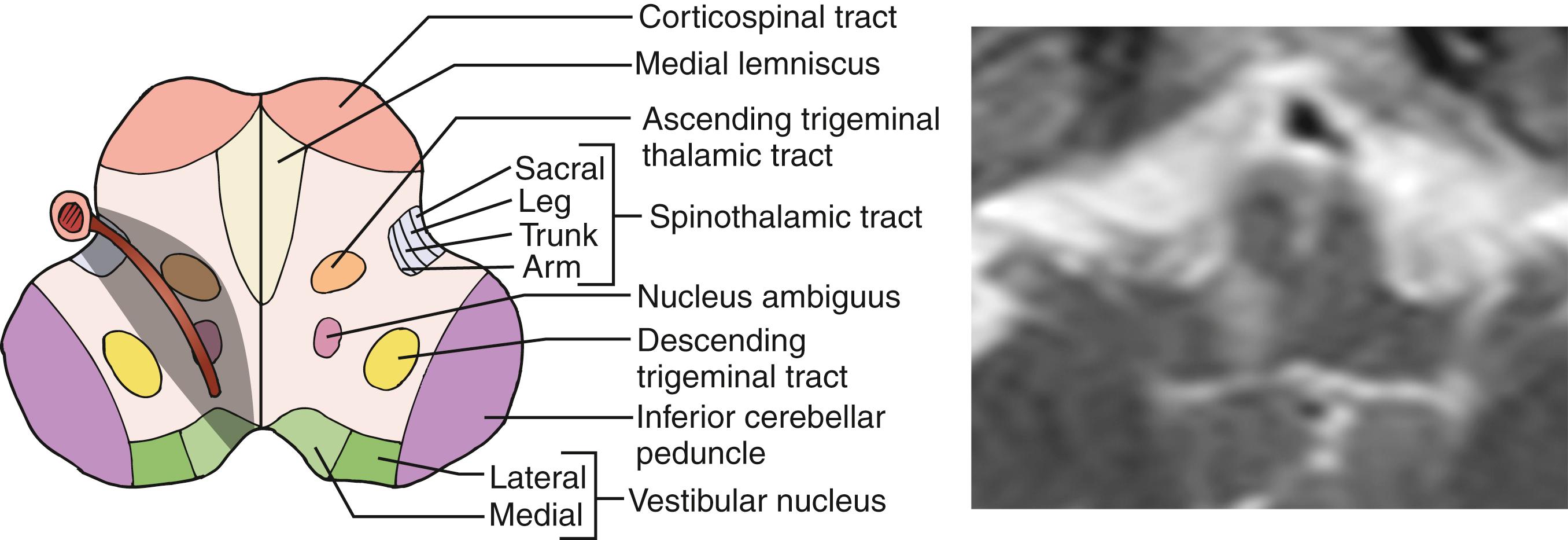
The more important rostrocaudal difference is dysphagia, which is distinctly more prevalent and severe in patients with rostral than caudal lesions. , This may be explained by (1) caudal medullary lesions are usually thin (see Fig. 26.16 ) and do not extend deeply as to involve the nucleus ambiguus and (2) the lower part of the nucleus ambiguus is not directly related to pharyngeal muscle motility. Facial palsy is also more common in rostral lesions. In patients with most rostral lesions, often at the pontomedullary junction, dysphagia is slight or absent because the nucleus ambiguus is spared at this level. These patients often show severe, ipsilateral, peripheral-type facial palsy.
The caudal lesions are closely associated with severe lateropulsion and gait ataxia probably due to frequent involvement of the latero-dorsally located spinocerebellar tract and vestibular nuclei (see Fig. 26.16 ). Dissection and headache are also more common in this group.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here