Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The vertebrobasilar system supplies blood to the brain stem, cerebellum, and occipital lobes via paired vertebral arteries. Posterior circulation ischemia is most often related to atherosclerosis involving the vertebrobasilar arteries, but may also be related to other vasculitides. Although vertebrobasilar ischemia (VBI) is less common than ischemic episodes related to internal carotid artery disease, approximately 25% of all ischemic strokes do occur in the posterior brain circulation; these events should be diagnosed appropriately as they may be the result of a treatable vasculopathy. For patients who experience posterior circulation transient ischemic attacks, disease in the vertebral arteries portends a 22% to 35% risk of stroke over 5 years. The mortality is 20%–30%, higher than for anterior circulation events.
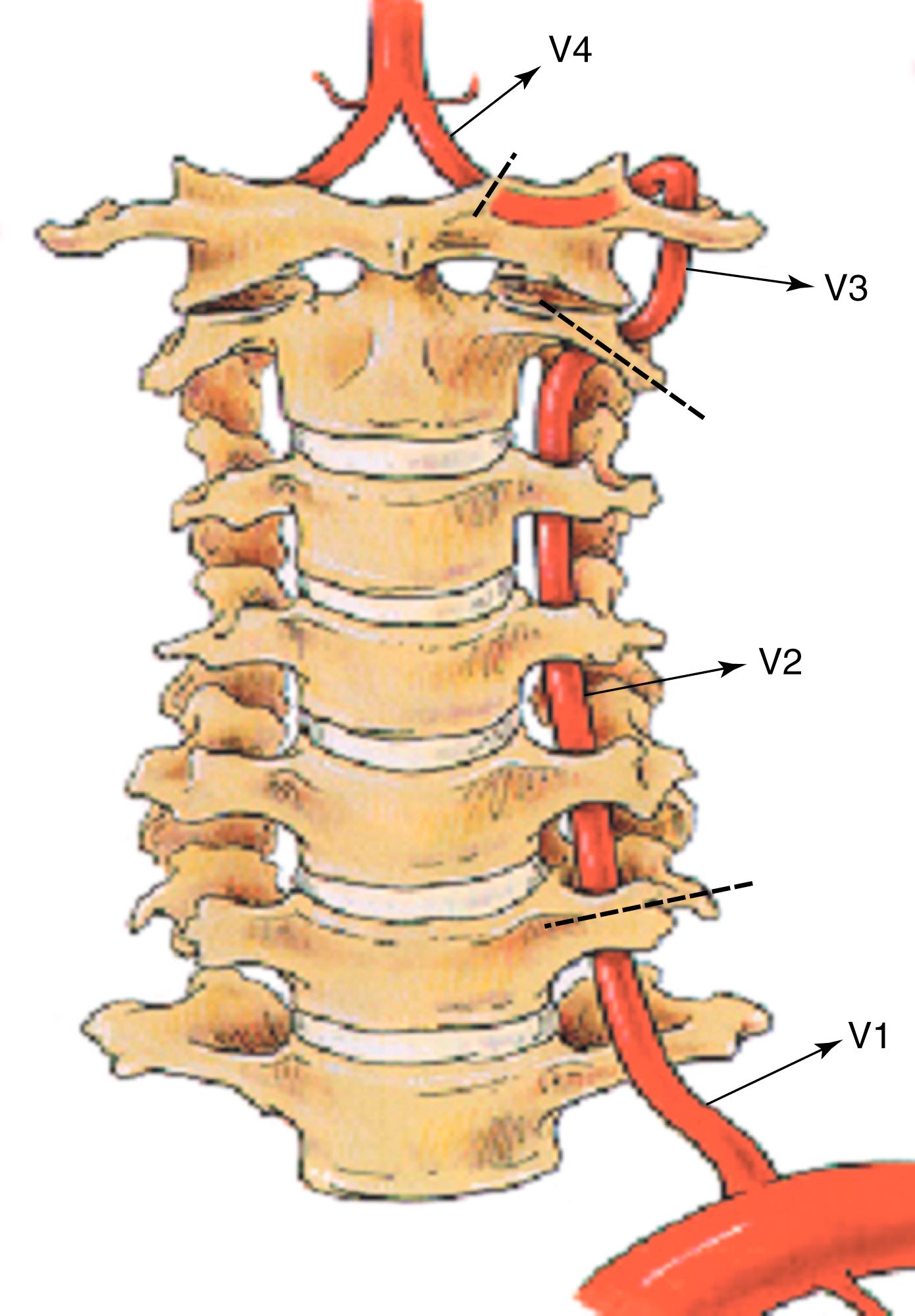
The surgical anatomy of the paired vertebral arteries is divided into four segments: segment V1 includes the origin of the vertebral artery as it arises from the subclavian artery to where it enters the C6 transverse process; segment V2 includes the segment of the artery buried within the intertransversarium muscle and the cervical transverse processes of C6 to C2; segment V3 includes the segment of the vertebral artery that extends from the top of the transverse process of C2 to the atlanto-occipital membrane at the base of the skull; and segment V4 includes the intracranial, intradural portion of the vertebral artery beginning at the atlanto-occipital membrane and terminating as the paired vertebral arteries converge to form the basilar artery ( Fig. 100.1 ).
Ischemia affecting the temporo-occipital cerebral hemispheres or brain stem and cerebellum characteristically produces bilateral symptoms. Classic VBI symptoms are dizziness, vertigo, drop attacks, diplopia, perioral numbness, alternating paresthesia, tinnitus, dysphasia, dysarthria, and ataxia. When two or more symptoms are present, likelihood of VBI is high ( Box 100.1 ).
Disequilibrium
Vertigo
Diplopia
Cortical blindness
Alternating paresthesia
Tinnitus
Dysphasia
Dysarthria
Quadriplegia
Drop attacks
Ataxia
Perioral numbness
Posterior circulation ischemia and strokes occur most commonly in relation to large-artery diseases ( Table 100.1 ), with the vertebral artery (VA) being the most common vessel, and atherosclerosis the most common pathology leading to stroke in this distribution ( Table 100.2 ). Less common processes include dissection, trauma, fibromuscular dysplasia, Takayasu disease, osteophyte compression, aneurysms, and other arteritides.
| Mechanism | Number (%) |
|---|---|
| Large-artery occlusive disease | 132 (32) |
| Embolism – cardiac source | 99 (24) |
| Embolism – arterial source | 74 (18) |
| Penetrating artery disease | 58 (14) |
| Vasospasm/migraine | 10 (2) |
| Other causes | 34 (8) |
| Lesion | Number |
|---|---|
| Innominate artery | 2 |
| Subclavian artery | 5 |
| Extracranial vertebral artery | 131 |
| Intracranial vertebral artery | 132 |
| Basilar artery | 109 |
| Posterior cerebral artery | 38 |
In general, VBI ischemic symptoms are due either to hemodynamic or embolic etiologies. In contrast to ischemia due to carotid artery occlusive disease, the low-flow mechanism of ischemia is better recognized and more frequent than in VA disease.
Patients with low-flow ischemia have transient symptoms in the cerebral territories supplied by the basilar artery because they lack appropriate inflow from the vertebral arteries and have inadequate collateral compensation. Hemodynamic symptoms occur as a result of transient end-organ (brain stem, cerebellum, occipital lobes) hypoperfusion, and can be precipitated by postural changes or transient reduction in cardiac output. Ischemia from hemodynamic mechanisms rarely results in tissue infarction. Rather, symptoms are short-lived and repetitive. Some patients may be prone to traumatic injuries from loss of balance or consciousness, resulting in falls. For hemodynamic symptoms to occur in direct relation to the VA, significant occlusive disease must be present in both paired vertebral vessels, in the dominant VA, or in the basilar artery itself. In addition, compensatory contribution from the carotid circulation via the circle of Willis must be incomplete. Alternatively, hemodynamic ischemic symptoms may result from proximal subclavian artery occlusion and subclavian/vertebral artery steal.
Embolic causes of VBI are generally from the heart, aortic arch, subclavian, vertebral, or the basilar arteries. Approximately one-third of VBI episodes are caused by distal embolization from plaque or mural lesions from subclavian or more distal vessels. Emboli arise from atherosclerotic lesions, intimal defects from extrinsic compression or repetitive trauma, and rarely fibromuscular dysplasia, aneurysms, or dissections. While fewer patients suffer from embolic phenomena than from hemodynamic ischemia, tissue infarction is more common with embolic events. Emboli are much more likely to cause fatal events or debilitating strokes. The importance of the embolic mechanism as a cause of vertebrobasilar symptoms has been emphasized in clinical and anatomicopathologic studies. This information has been derived from autopsy and magnetic resonance imaging (MRI) studies, which have identified small infarcts in the brain stem and cerebellum and shown their source, via arteriography, to be lesions in the subclavian or vertebral arteries. As opposed to patients with hemodynamic symptomatology, multiple and multifocal infarcts in the brain stem, cerebellum, and occasionally the posterior cerebral artery territory more often develop in patients with embolic ischemia. ,
The most common atherosclerotic lesion of the VA is origin stenosis, with a prevalence of 20%–40% in patients with cerebrovascular disease. The lesions are usually smooth and fibrotic with low embolic potential. Redundancy and kinks are common in the V1 segment, but only severe kinks with poststenotic dilatations can be responsible for hemodynamic symptoms.
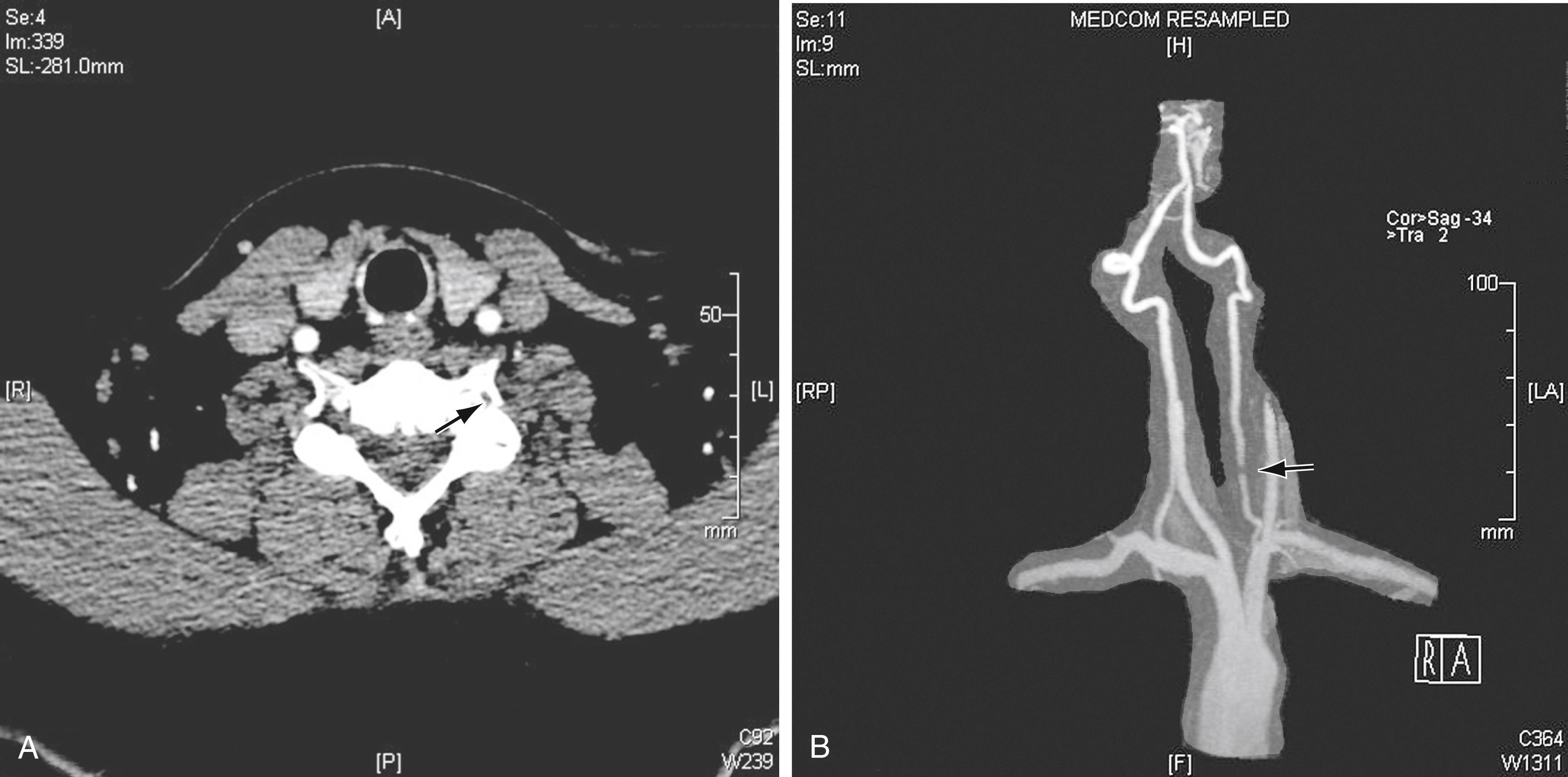
The most common pathology of the V2 segment is extrinsic compression by either bone or tendon. Compression can be by osteophytes, the edges of the transverse foramina ( Fig. 100.2 ), or intervertebral joints. Rotation or extension of the neck usually triggers compression of the VA in this segment. Extrinsic VA compression by musculotendinous structures is common in patients with an abnormally high level of entry into the transverse processes of the spine, usually C4 or C5, due to sharp angulation resulting from the abnormal level of entry ( Fig. 100.3 ).
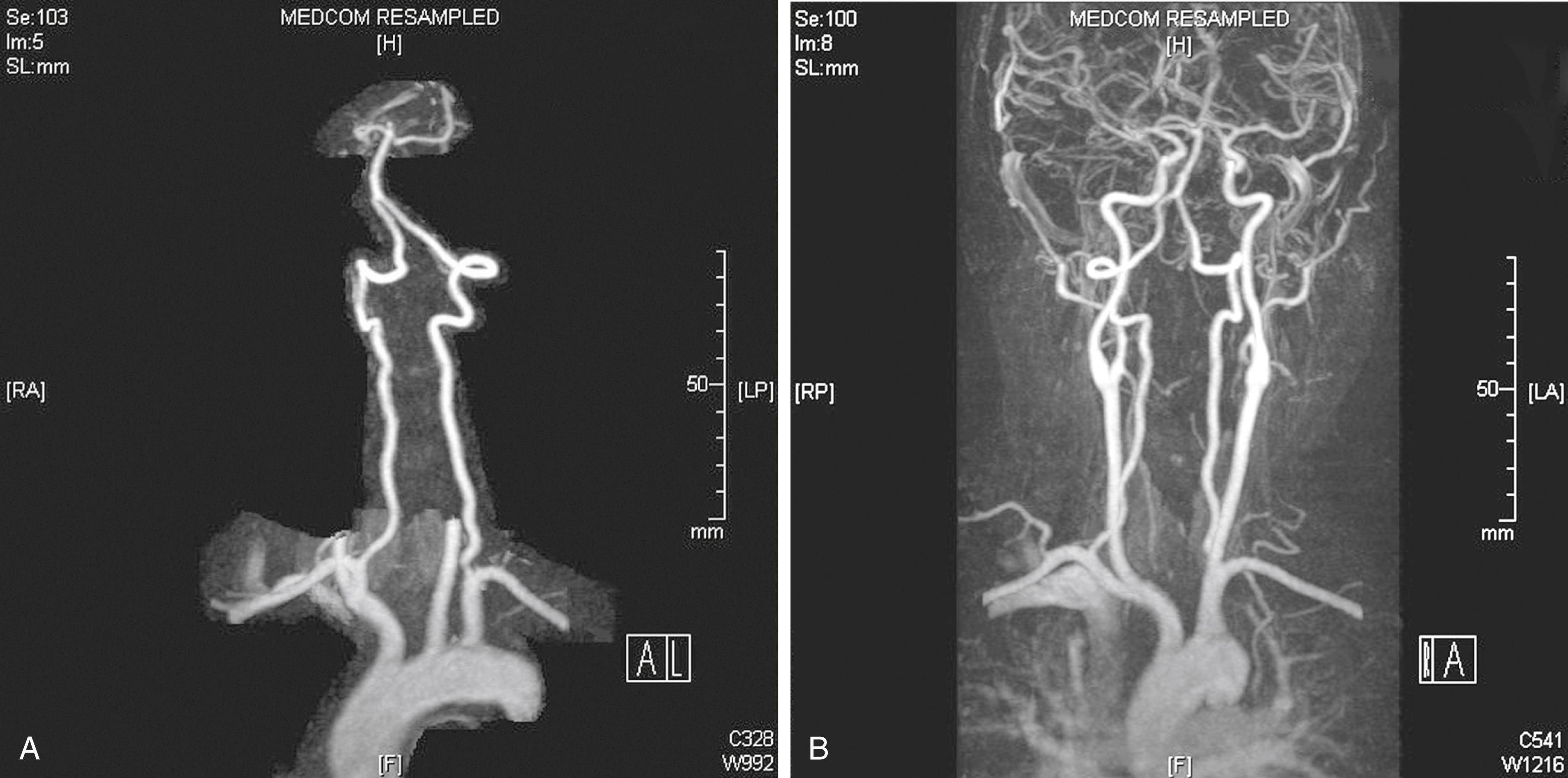
The V2 segment is a frequent site for development of traumatic or spontaneous arteriovenous fistulas. This occurs because fixation of the adventitia of the VA to the periosteum of the foramina makes the vessel vulnerable to luxation/subluxation injuries in this area. The close proximity of the artery to its surrounding venous plexus results in an arteriovenous fistula when the artery and vein are damaged in continuity. The VA may tear completely or incompletely (dissection) as a consequence of stretch injury after brisk neck rotation or hyperextension.
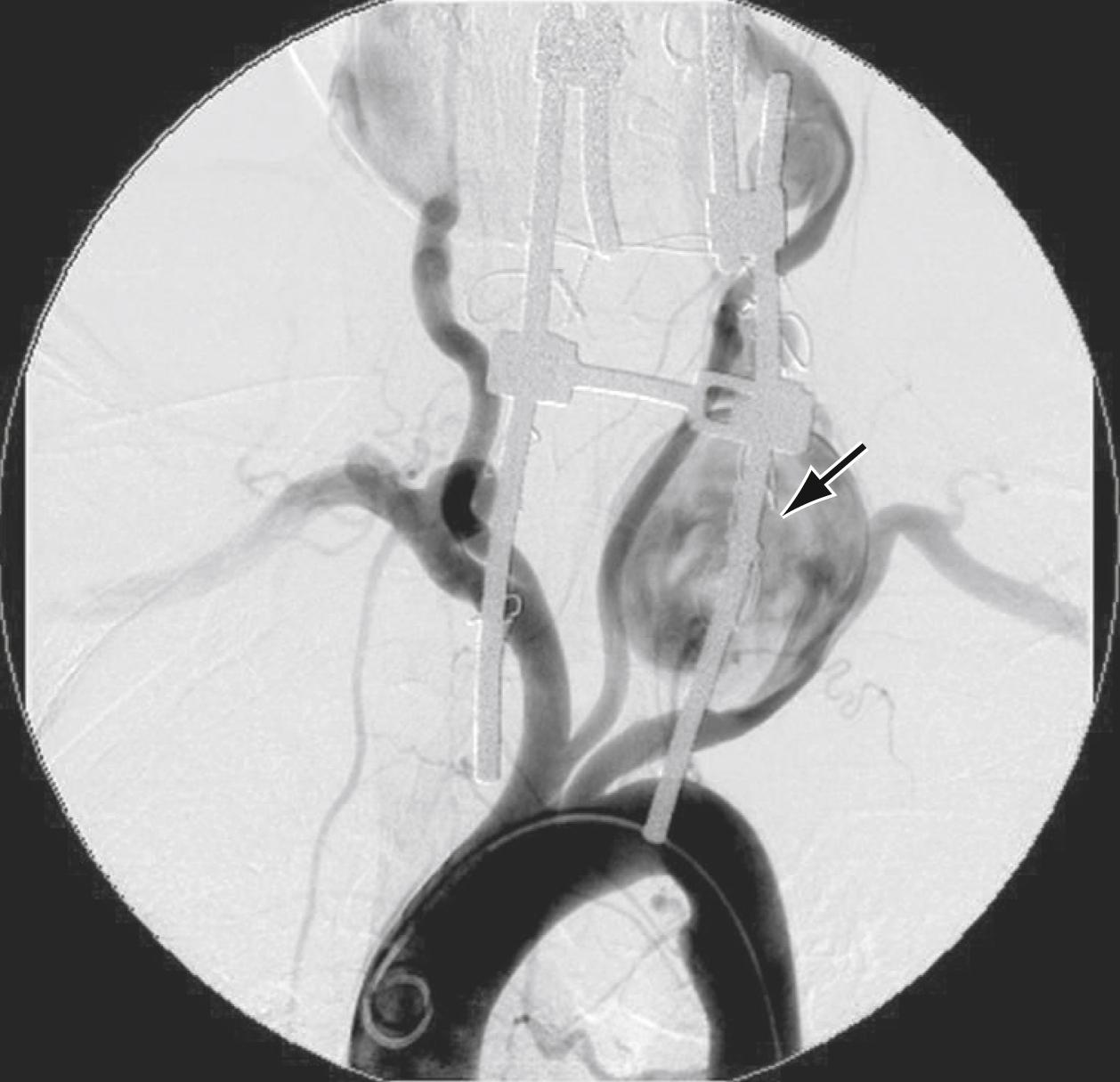
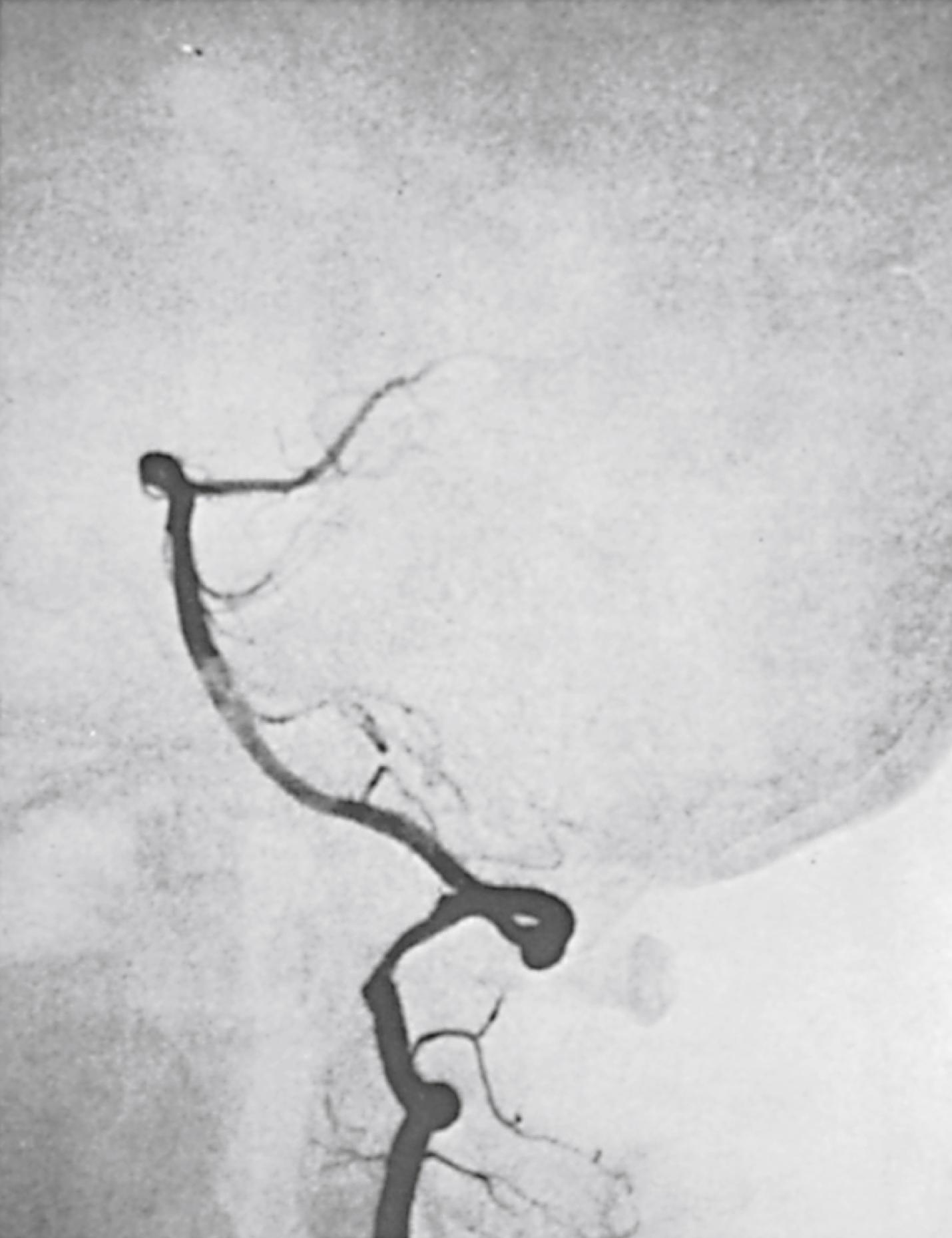
Although most vertebral artery aneurysms occur intracranially, the V2 segment is the most common site of extracranial aneurysmal degeneration ( Fig. 100.4 ). Two-thirds of all extracranial VA aneurysms involve the V2 segment, with most as pseudoaneurysms secondary to trauma or dissection. True degenerative extracranial VA aneurysms are rare, accounting for less than 1% of all VA lesions. In one review, all patients with a true extracranial VA aneurysm had some form of connective tissue disorder. Most extracranial vertebral aneurysms are asymptomatic and found incidentally. Some present with symptoms including: a palpable neck mass, dizziness, headaches, and/or neurologic deficit from cerebral ischemia or nerve compression. Rupture is rare. Because the natural history is unknown, ligation with or without reconstruction is most often recommended for large extracranial vertebral aneurysms.
Dissection, fibromuscular disease, arteritis, and embolizing atherosclerotic plaques can also be identified in the V2 segment. Disease in the second segment of the VA can result in extracranial occlusion or stenotic lesions in the intraforaminal segment, which commonly give rise to emboli.
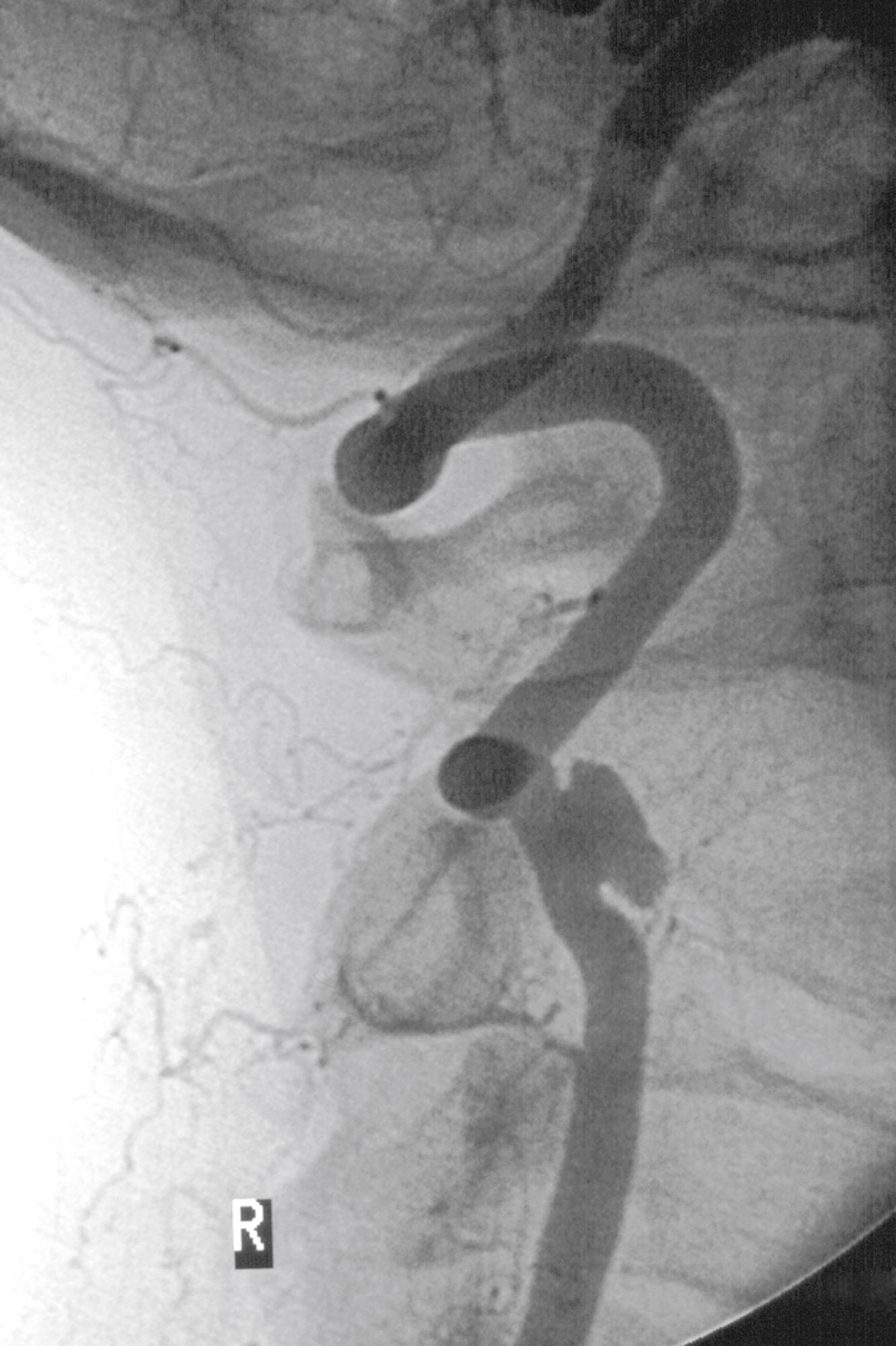
The most common problems at the V3 level relate to trauma and dissection; they include occlusive lesions, arteriovenous fistulas, and pseudoaneurysms. Extracranial VA dissection occurs most commonly in the V3 segment because the artery is mobile and, therefore, vulnerable to mechanical injury. The first two cervical vertebrae are the most mobile of the spine, 50% of neck rotation occurs between C1 and C2. The VA is redundant at this level to allow the arc of displacement of the transverse process of the atlas (80 degrees), to which the VA is attached. Clinically, extracranial vertebral dissection may present with severe occipitocervical neck pain with associated dizziness, vertigo, double vision, ataxia, and dysarthria. Dissection may be associated with fibromuscular dysplasia; but can also occur in a normal artery after seemingly trivial trauma ( Fig. 100.5 ). VA dissection often follows neck hyperextension or rotation and has been associated with practicing yoga, painting a ceiling, coughing, vomiting, sneezing, and cervical chiropractic manipulation. , Vertebral dissection is rare, with an incidence of 1 per 100,000, but it is a significant cause of stroke in a young and otherwise healthy population. In one study, 62% of dissections observed serially with angiography completely resolved. The remaining dissections went on to vessel occlusion, remained stenotic, or developed pseudoaneurysmal dilation and/or AV fistulas ( Fig. 100.6 ). Half of the patients diagnosed with extracranial VA dissection will be asymptomatic, 21% will experience mild neurologic sequelae, 25% will suffer moderate to severe deficits, and 4% will die. Notably, there appears to be no relationship between recanalization and neurologic outcome.
An arteriovenous fistula results from rupture of the wall of the VA into its surrounding venous plexus. In long-standing fistulas, the pulsatile mass formed by the fistula and its dilated venous channels is called an arteriovenous aneurysm.
The VA may be compressed in the pars atlantica of the third segment between the occipital ridge and the arch of the atlas. In these patients, symptoms of low flow are usually precipitated by head extension or rotation. The V3 segment is rarely affected by significant atherosclerosis.
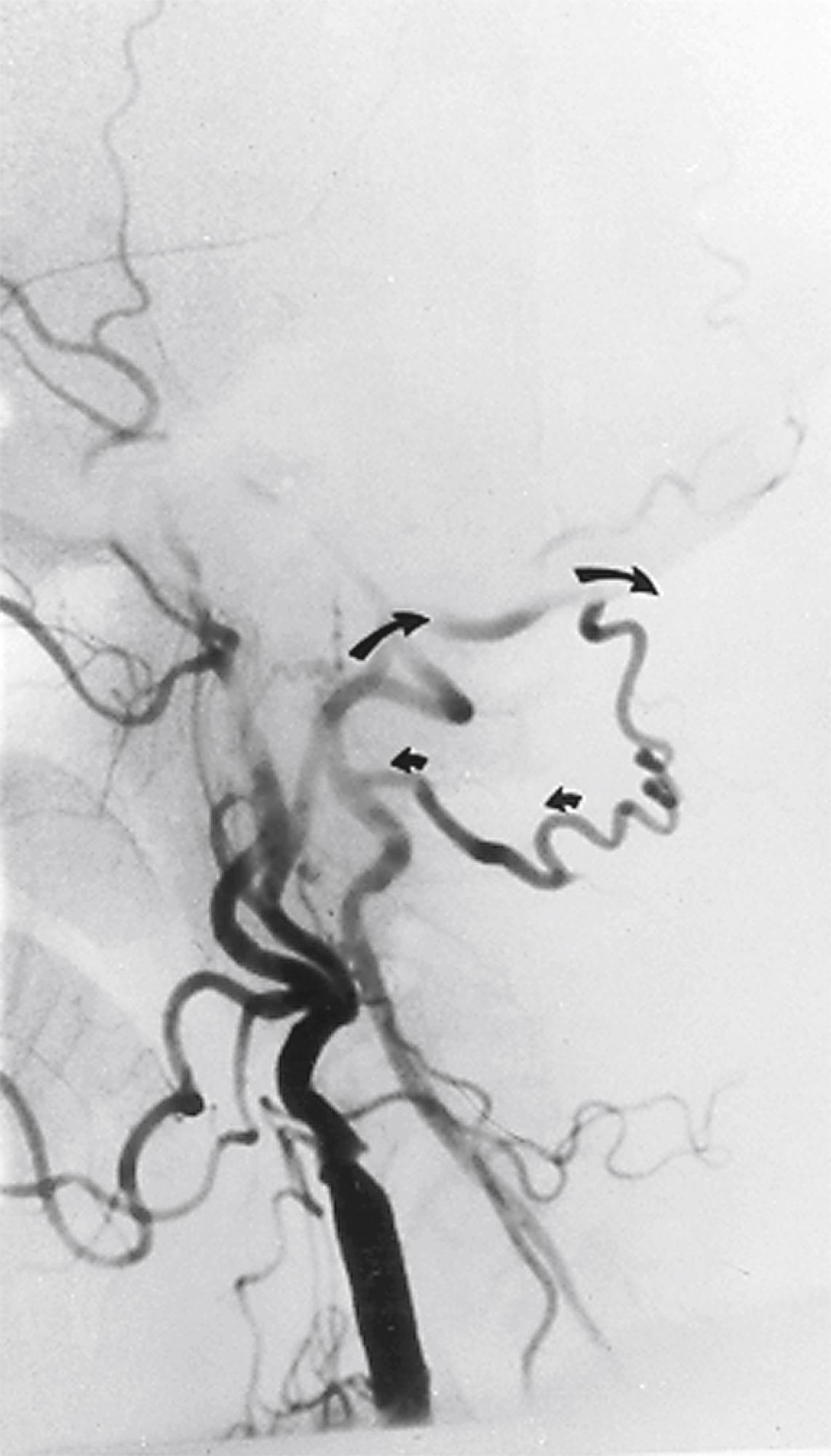
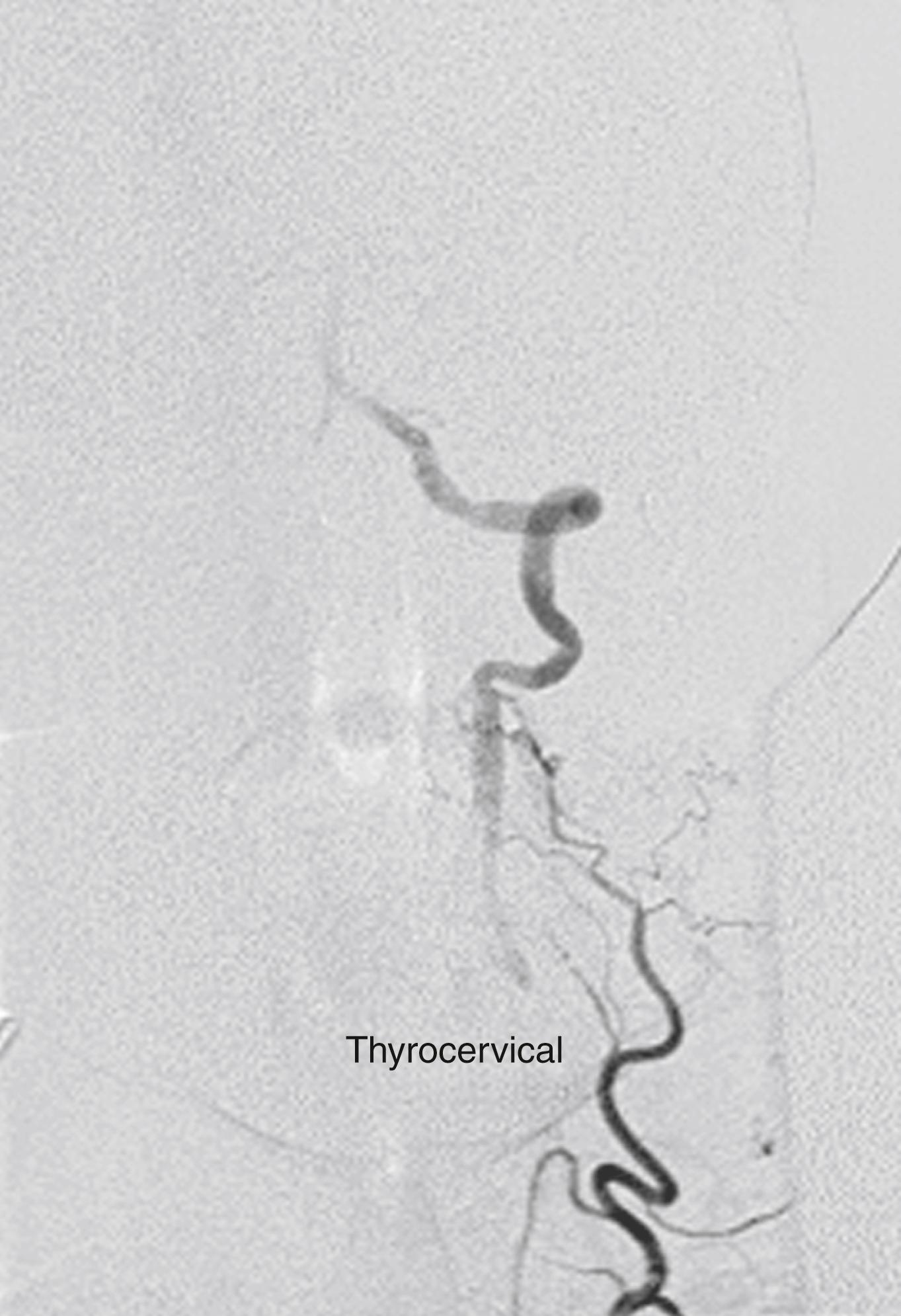
When the VA is occluded in the V1 or V2 segments, it usually reconstitutes at the V3 segment by collaterals from the external carotid via the occipital artery ( Fig. 100.7 ) or collaterals from the ipsilateral subclavian artery via branches of the thyrocervical trunk ( Fig. 100.8 ). Because of this collateral network, the distal vertebral and basilar arteries usually remain patent despite a proximal VA occlusion. This finding is crucial for developing appropriate surgical strategy.
Similar to the V3 segment, the fourth segment (V4) is infrequently affected by atherosclerosis. This segment is also prone to arteriovenous fistula formation and aneurysmal degeneration. Advanced atherosclerotic disease of the basilar artery is generally a contraindication to the reconstruction of VA lesions.
The minimal anatomic requirement to justify VA reconstruction for hemodynamic symptoms would be greater than 60% stenosis in both VAs, if both are patent and complete, or 60% stenosis in the dominant VA if the contralateral is hypoplastic, ends in a posteroinferior cerebellar artery, or is occluded. A single normal VA is typically sufficient to adequately perfuse the basilar artery regardless of the patency status of the contralateral VA. Unlike carotid artery disease, the mere presence of stenoses in an asymptomatic patient is not an indication for reconstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here