Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is an inherited cardiomyopathy that is characterized by ventricular arrhythmias, an increased risk for sudden cardiac death (SCD), and abnormalities of right ventricular (RV) structure and function. Although structural involvement of the RV predominates, it is now recognized that involvement of the left ventricle (LV) is not uncommon. In most patients in the United States, structural abnormalities of the RV predominate . The pathologic hallmark of ARVD/C is RV myocyte loss with fibrofatty replacement ( Fig. 90.1 ). Since the first detailed clinical description of the disorder in 1982, significant advances have been made in our understanding of all aspects of this disease. Since the beginning of the 21st century, mutations in most desmosomal proteins and also some nondesmosomal proteins have been identified as the genetic basis of ARVD/C. Because a pathogenic mutation can be identified in more than 60% of affected individuals, genetic testing has emerged as an important diagnostic tool. , This chapter provides a concise and up-to-date review of the current state of knowledge regarding the natural history, clinical presentation, pathogenesis, diagnosis, and treatment of patients with ARVD/C. Specific emphasis is placed on exploring the relationship between ARVD/C and exercise.
ARVD/C is an unusual condition with an estimated prevalence of 1 out of every 5000 people in the general population. This is roughly 10-fold less common than hypertrophic cardiomyopathy (HCM), which occurs in about 1 out of every 500 people in the general population. Although ARVD/C is uncommon, it is a more common cause of SCD than HCM.
Patients usually present during the second to fifth decade of life with palpitations, lightheadedness, syncope, or SCD ( Figs. 90.2 and 90.3 ). , In our experience, it is extremely rare to manifest clinical signs or symptoms of ARVD/C before the age of 12 years or after the age of 60 years.
Although ARVC/D is predominantly a disease of the RV, it is now well established that involvement of the LV is not uncommon, particularly when magnetic resonance imaging (MRI) is used to detect subtle abnormalities in LV function and also in patients with advanced disease. , Left-dominant arrhythmogenic cardiomyopathy (ACM) also occurs and is defined by early disease of the LV, often affecting the posterolateral wall, in the absence of significant RV systolic dysfunction . Left-dominant disease is more commonly seen in patients with desmoplakin mutations.
In most cases, ARVD/C is inherited in an autosomal dominant pattern with significantly variable penetrance and expressivity. Among probands diagnosed with this disease, screening of first-degree relatives identifies other affected individuals in approximately 50% of cases. In a minority of cases, ARVD/C is inherited in an autosomal recessive pattern as part of a cardiocutaneous syndrome, such as Naxos disease or Carvajal syndrome, which is also characterized by wooly hair and palmoplantar keratodermia. , ,
Linkage mapping and candidate gene evaluation studies performed on patients with the autosomal dominant form of ARVD/C was not initially productive because of significant variability in the penetrance and expressivity of the disease. It was the evaluation of patients with Naxos syndrome, a disease with 100% penetrance by adolescence, that identified a disease-causing mutation: a homozygous deletion of two base pairs found in the plakoglobin gene located in the 17q21 locus. , The gene encodes a key component of desmosomes, which are complex intercellular adhesion structures found in stratified epithelial cells of the skin and in myocytes. Desmosomes are composed of three major groups of proteins: Cadherins are transmembrane proteins that provide the actual mechanical coupling between individual cells and include desmoglein and desmocollin. Desmoplakin is a plakin-family protein that serves to anchor the desmosomal structure to the intermediate filaments of the cell. Lastly, armadillo proteins, including plakoglobin and plakophilin, link desmoplakin and the cadherin tails.
The identification of defective desmosomal proteins in Naxos syndrome led to studies investigating their role in other ACMs. Carvajal syndrome, another cardiocutaneous syndrome that has left-dominant ACM, was shown to be associated with a recessive mutation in desmoplakin . Other genetic mutations were subsequently identified in the autosomal-dominant form of ARVC/D and include desmoplakin, desmoglein-2, desmocollin-2, and plakophillin-2 (PKP2) genes . Mutations in several extradesmosomal genes, such as those encoding transforming growth factor β3 (TGFβ3), the cardiac ryanodine receptor (RyR2), Titin, and transmembrane protein 43 (TMEM43), have also been implicated in specific types of atypical forms of ARVC/D. In the United States a desmosomal protein mutation can be identified in approximately 50% of ARVD/C patients. , The most commonly mutated genes are PKP2 (45%) and desmoglein-2 (9%). Importantly, although 86% of patients in this series had a single heterozygous gene mutation, 7% showed compound heterozygosity, and another 7% showed digenic heterozygosity. This is important because clinicians need to be aware that some individuals with ARVD/C may have more than one mutation in one or more of the many desmosomal proteins. Other candidate genes are likely to be identified in the future. Thus it is possible that the affected individual (the proband) will have more than one defective gene. Because all the genetic abnormalities have not yet been identified, the first-degree relatives may not have the known gene but could have inherited the unknown gene mutation. Therefore finding a pathogenic gender mutation in the proband but not in the first-degree relative does not completely exclude the possibility of desmosomal mutation in the first-degree relative. It is also important to recognize that not all variants identified in a desmosomal protein are causal mutations.
Initial attempts to explain the pathogenesis of ARVC/D produced several hypotheses, including the dysplastic theory, which held that the atrophy and fibrofatty replacement of the RV myocardium in ARVD/C was a congenital, developmental defect. This led to the original description of the syndrome as ARVD. It is now clear, however, that the structural defects in ARVD/C are not present at birth but actually develop progressively throughout childhood and early adulthood.
The genetics of ARVD/C has provided support for the hypothesis that the disease may be caused by desmosomal dysfunction. The pathogenic mechanisms are not fully clear, but several theories have been posited. Defective desmosomal proteins may lead to impaired mechanical coupling between individual cells, leading to myocyte uncoupling, especially under conditions that increase myocardial strain. The resulting inflammation, fibrosis, and adipocytosis may be a nonspecific response to injury similar to that seen in other forms of myocardial damage. This pathogenic model can explain the observation that prolonged strenuous exertion, which increases myocardial strain, significantly increases the risk for an earlier clinical onset of the disease and augments the risk for SCD. It also explains why the RV, which is more distensible than the LV because of its thinner wall and asymmetrical shape, is more often involved in ARVC/D, especially in its early stages . Furthermore, defects in mechanical coupling of myocytes may also lead to impairment in electrical coupling . Ultrastructural evaluation of the myocardium of patients with ARVC/D has shown reduced expression of several intercalated disk proteins, including connexin-43, a key component of gap junctions. This finding may account for the development of conduction delay and arrhythmias, even in the absence of significant structural defects in the early “concealed” phase of the disease. Recent studies have also shown that PKP2 haploinsufficiency leads to sodium current (INa) deficit in murine hearts. The results of this study suggest that there is cross-talk between the desmosome and sodium channel complex. The resultant sodium channel dysfunction may contribute to the development of ventricular arrhythmias in patients with ARVD/C.
The mechanisms that lead to the variability in penetrance and expressivity of disease are still not fully understood. Family members with identical genotypes and even monozygotic twins show significant differences in symptomatology, presence and distribution of structural changes, and rate of disease progression. This observation has led to the “second hit” hypothesis, which suggests that modifier genes and/or environmental factors are likely responsible for phenotypic heterogeneity.
The observation that there is a disproportionate number of athletes among patients with ARVD/C led to the hypothesis that exercise is an environmental factor in the pathogenesis of this disease. , A series of recent studies has proven beyond a doubt that this hypothesis is correct. Exercise has now been demonstrated to be the single most powerful environmental factor that impacts both the development and clinical course of the disease.
One of the first studies to highlight the relationship between exercise and ARVD/C was by Corrado et al., who reported that young athletes had a fivefold risk of dying from ARVD/C compared with nonathletes. A subsequent study confirmed this finding when they found that implementing a preparticipation screening decreased the incidence of SCD in competitive athletes.
Since then, many studies followed, and their aim has been to determine the role of exercise as a disease modifier in ARVD/C. La Gerche et al. are credited with recognizing that the ARVD/C phenotype can be acquired through intense exercise. They studied 47 athletes with definite or probable ARVD/C. The majority ( n = 41) practiced endurance sports (cyclists, 72%; distance runners, 6%; triathletes, 6%; and kayakers, 2%). They identified desmosomal mutations in only six athletes, and only two patients in the group had family history of ARVD/C. They found that the group that performed the most exercise had lower rates of desmosomal mutations, yet many of them met diagnostic criteria for definite or probable ARVD/C. On the basis of these results, they concluded that high-intensity endurance exercise may be sufficient to evoke an ARVD/C phenotype, even in mutation-negative individuals. Saverniak et al. investigated the impact of exercise on myocardial function. The study included 110 patients, 65 of them with ARVD/C and 45 who were mutation-positive family members. Exercise participation was measured by metabolic equivalent (MET) minutes per week. Athletes were classified as those participating in vigorous exercise (at least 6 METs) for at least 4 hours per week. The authors found that athletes were more likely to meet diagnostic criteria, they had a lower biventricular function, and they had an earlier onset of life-threatening ventricular arrhythmias compared with nonathletes. Ruwald et al. studied 108 probands that met Task Force Criteria (TFC) for ARVD/C diagnosis; they assessed the effects of competitive and recreational sports on age of onset and risk for ventricular arrhythmias (VAs) or death. Via a questionnaire, patients self-reported the age at which they initiated the sport and what the sport was. They found that the patients who engaged in competitive exercise had an earlier presentation of the disease and also had a twofold increase in the risk for life-threatening arrhythmias and death compared with inactive patients and those practicing recreational sports.
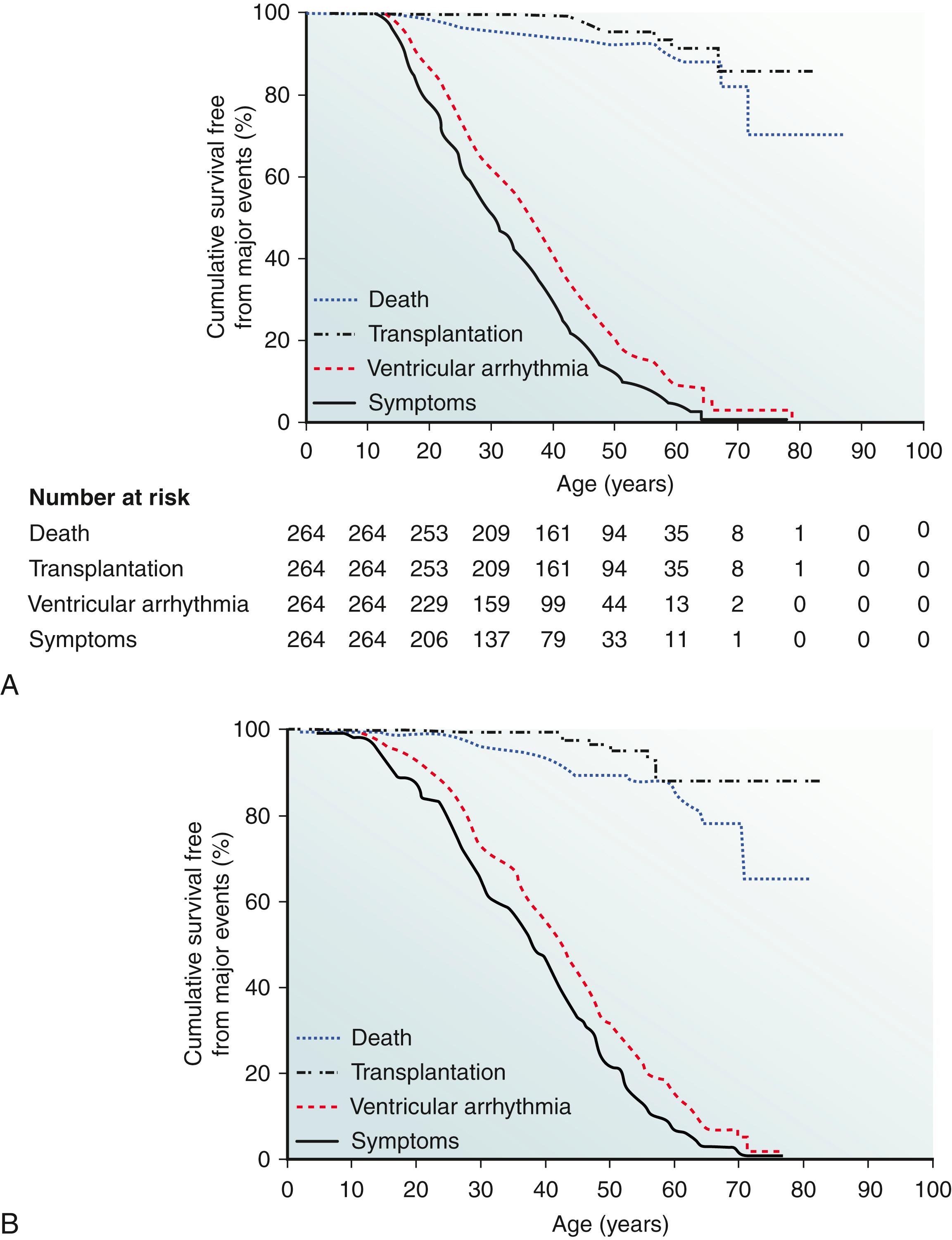
The Johns Hopkins ARVD Program has played an important role in defining the link between exercise and ARVD in patients with and without mutations, as well as examining the important question of a safe exercise threshold. James et al. were the first to study the role of exercise in ARVD/C patients who had inherited a pathogenic desmosomal mutation ( Fig. 90.4 ). They evaluated 87 probands and family members from the Johns Hopkins ARVD/C Registry. All patients participated in an exercise interview detailing their exercise history for leisure/recreation, work, and transportation. Participants were classified as an endurance athlete if they performed 50 hours per year or more of vigorous intensity sports with a high dynamic demand as defined by the 36th Bethesda Conference Classification of Sports (Task Force 8). The study had several key findings. First, ARVD/C patients who were endurance athletes became symptomatic at an earlier age. Furthermore, endurance exercise and higher duration of exercise participation were both associated with an increased likelihood for developing manifest ARVD/C. Endurance exercise was also associated with worse survival from a VA and heart failure. Lastly, those individuals who continued to participate in the top quartile for hours of annual exercise after presentation had worse survival from first VA compared with individuals who reduced their exercise after presentation. The role of exercise in ARVD/C patients without mutations was subsequently studied by Sawant et al. , They assessed the role of duration and intensity of exercise participation among 43 probands without a desmosomal mutation and compared it with 39 probands with desmosomal mutations, all of whom met the 2010 TFC. It was found that among nondesmosomal mutation patients, those participating in the highest quartile of MET-hours per year exercise presented at a significantly earlier age, had worse structural abnormalities on cardiovascular magnetic resonance (CMR), and had significantly poorer ventricular arrhythmia-free survival. Next, exercise participation stratified by mutation status and family history was compared, and it was found that although nondesmosomal ARVD/C patients had performed a similar duration of annual exercise as desmosomal carriers, their exercise was significantly more intense, with significantly higher participation in endurance sports and expenditure of greater MET hours per year of exercise compared with desmosomal patients ( Fig. 90.5 ). Lastly, ARVD/C nondesmosomal mutation patients with no family history performed the highest MET hours per year of exercise compared with nondesmosomal mutation patients with family history and desmosomal mutation carriers ( Fig. 90.6 ). Because the nondesmosomal mutation patients without family history did the highest intensity exercise, they concluded that exercise may be the key environmental factor driving disease pathogenesis in that subgroup. Interestingly, when they compared annual prepresentation MET hours and endurance exercise participation during youth (age ≤25 years), it was found that higher intensity exercise during youth was associated with an earlier age of presentation in both desmosomal and nondesmosomal ARVD/C patients. Lastly, the Johns Hopkins group assessed the safety of the American Heart Association (AHA) minimum exercise recommendations for overall health (390–690 metabolic equivalent hours per year) for healthy desmosomal mutation carriers. To accomplish this, they interviewed 28 family members of 10 probands with a PKP2 mutation. They reported that probands had done more exercise than family members, and the family members that were athletic had worse outcomes; however, there were no life-threatening VAs in the healthy carriers that restricted exercise at or below the upper bound of the AHA minimum exercise recommendation. Furthermore, at the time of ARVD/C diagnosis and their first VA, patients had accumulated a 2.8-fold and a 3.5-fold greater MET-hours exercise, respectively, than that recommended by the AHA ( Fig. 90.7 ). Their conclusion was that although it is clear that unaffected PKP2 mutation carriers should refrain from endurance and high-intensity exercise, it might be potentially safe for them to practice the minimum exercise for healthy adults recommended by the AHA. A more recent study from the Hopkins group revealed that the risk for VAs is dramatically reduced in patients who markedly reduce their exercise level once the diagnosis is established.
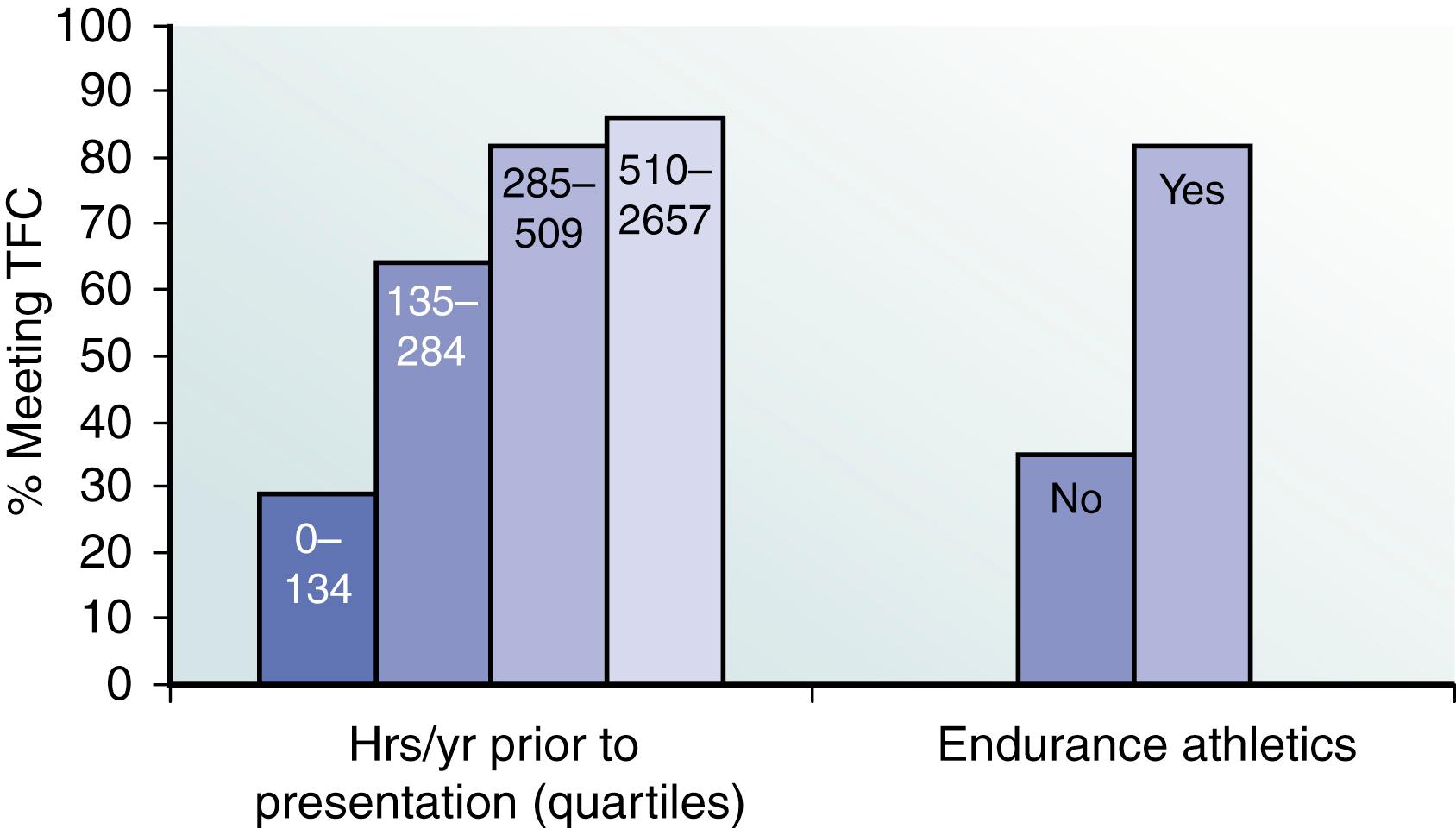
![Fig. 90.5, Exercise intensity (median metabolic equivalent hours [MET hours] per year) stratified by (A) quartiles of age of clinical presentation and (B) age of clinical presentation before and after age 25 years. Gene-elusive patients had done more intense exercise regardless of age of presentation. Among patients presenting by age 25 years, there is a fivefold difference in intensity. Fig. 90.5, Exercise intensity (median metabolic equivalent hours [MET hours] per year) stratified by (A) quartiles of age of clinical presentation and (B) age of clinical presentation before and after age 25 years. Gene-elusive patients had done more intense exercise regardless of age of presentation. Among patients presenting by age 25 years, there is a fivefold difference in intensity.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/VentricularTachycardiasinArrhythmogenicRightVentricularDysplasiaCardiomyopathy/3_3s20B9780323757454000901.jpg)
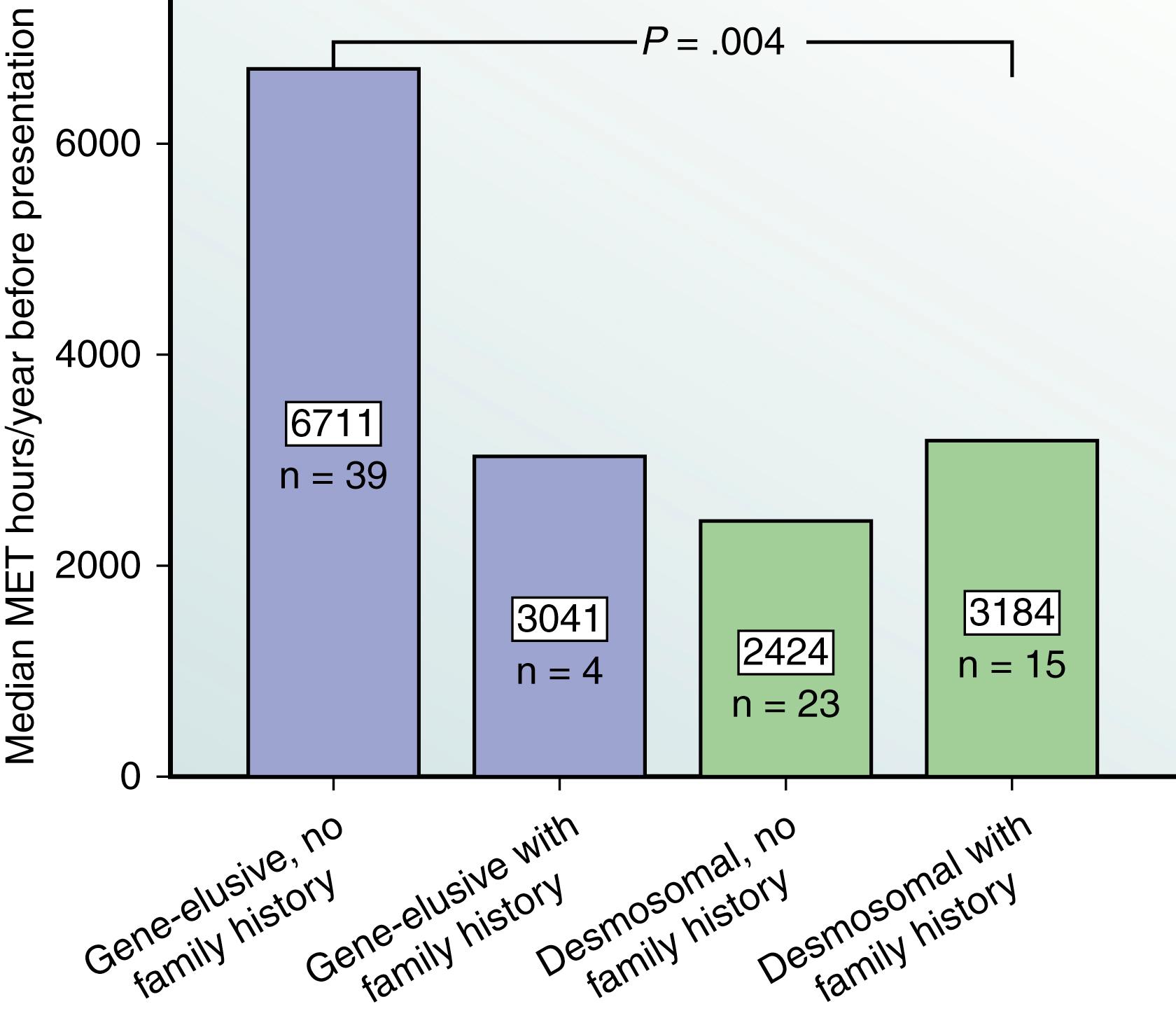
![Fig. 90.7, Median exercise intensity (metabolic equivalent hours [MET hours] per year) before diagnosis in 5-year age increments compared with the American Heart Association (AHA)-recommended minimum stratified by diagnosis by 2010 Task Force Criteria (TFC) (A) and ventricular tachycardia/ventricular fibrillation (VT/VF) history (B). Dotted black line represents the upper boundary of the AHA-recommended minimum exercise (650 MET hours per year). Error bars depict interquartile range. Statistically significant P < .05. Fig. 90.7, Median exercise intensity (metabolic equivalent hours [MET hours] per year) before diagnosis in 5-year age increments compared with the American Heart Association (AHA)-recommended minimum stratified by diagnosis by 2010 Task Force Criteria (TFC) (A) and ventricular tachycardia/ventricular fibrillation (VT/VF) history (B). Dotted black line represents the upper boundary of the AHA-recommended minimum exercise (650 MET hours per year). Error bars depict interquartile range. Statistically significant P < .05.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/VentricularTachycardiasinArrhythmogenicRightVentricularDysplasiaCardiomyopathy/5_3s20B9780323757454000901.jpg)
The link between exercise and ARVD/C has also been advanced through important research in animal models. The production of transgenic animals is a very important tool that has been used to understand ARVD/C. They have helped us not only understand how desmosomal mutations produce the ARVD/C phenotype but also provided more evidence of the relationship between exercise and the disease. In 2006, Kirchhof et al. used heterozygous plakoglobin-deficient mice and their wild-type siblings to study whether plakoglobin and training have a functional role in the development of ARVD/C. Endurance training with 8 weeks of daily swimming was used to test the hypothesis. Using murine echocardiography, electrocardiogram (ECG), MRI, and positron-emission tomography (PET), they measured cardiac dimensions and function as well as myocardial glucose uptake. They found that plakoglobin deletion is sufficient to produce an ARVD/C-like phenotype. Furthermore, endurance training produced altered RV contractile and electrophysiologic function in plakoglobin-deficient mice, manifested by increased RV volume, reduced RV function, spontaneous VAs, and prolonged conduction times compared with their wild-type counterparts. Another group evaluated the impact of exercise on ARVD/C cardiac manifestations. To accomplish this, they used an adeno-associated virus (AAV)-mediated PKP2 mutant gene to produce an ARVC/D-causing mutation in the cardiac tissue of wild-type mice. Subsequently, the mice were divided into two groups, trained and sedentary, and they were euthanized to obtain heart samples. Left and right end-diastolic volume, end-systolic volume, ejection fraction, and wall motion abnormalities were assessed via CMR. They found that sedentary mice did not have any sign of RV dysfunction. On the contrary, endurance exercise training resulted in RV dysfunction that was similar to the ARVD/C phenotype. Meanwhile, Chelko et al. analyzed disease progression in a mouse model with ARVD/C. They compared desmoglein-2 homozygous, heterozygous, and wild-type mice by examining the effects that exercise had on the disease. This was done by making mice swim 90 minutes per day, 5 days per week. This resulted in 6 out of 12 homozygous mutant mice surviving to 16 weeks of age, with 6 dying during exercise. With the help of echocardiography and ECG they observed that the homozygous mice had reduced ejection fraction, longer QRS duration and isovolumetric relaxation, and an increased risk for dying from a cardiac arrhythmia compared with the heterozygous and wild-type mice. In the most recent study using animal models, the investigators generated transgenic mice with overexpression of desmoplakin, both wild-type and mutant, and compared them with a nontransgenic model. The mice were divided into two groups: sedentary and those exposed to a 12-week running regimen. Their results showed that exercise accelerated the development of the disease, manifested by RV dilation and focal fat infiltration in the mutant mice compared with the wild-type and nontransgenic mice. LV function was not affected in either of the models.
These data raise a critical question of how much exercise can be considered safe in these patients. The answer is that more research is required to evaluate the threshold needed to trigger the onset of ARVD/C. Understanding the interaction of genotype and exercise, as well as other environmental factors with the potential for triggering the disease, is also required. Furthermore, the improved understanding of the molecular mechanisms through which exercise causes the pathologic features of ARVD/C is key to the improvement of patient care.
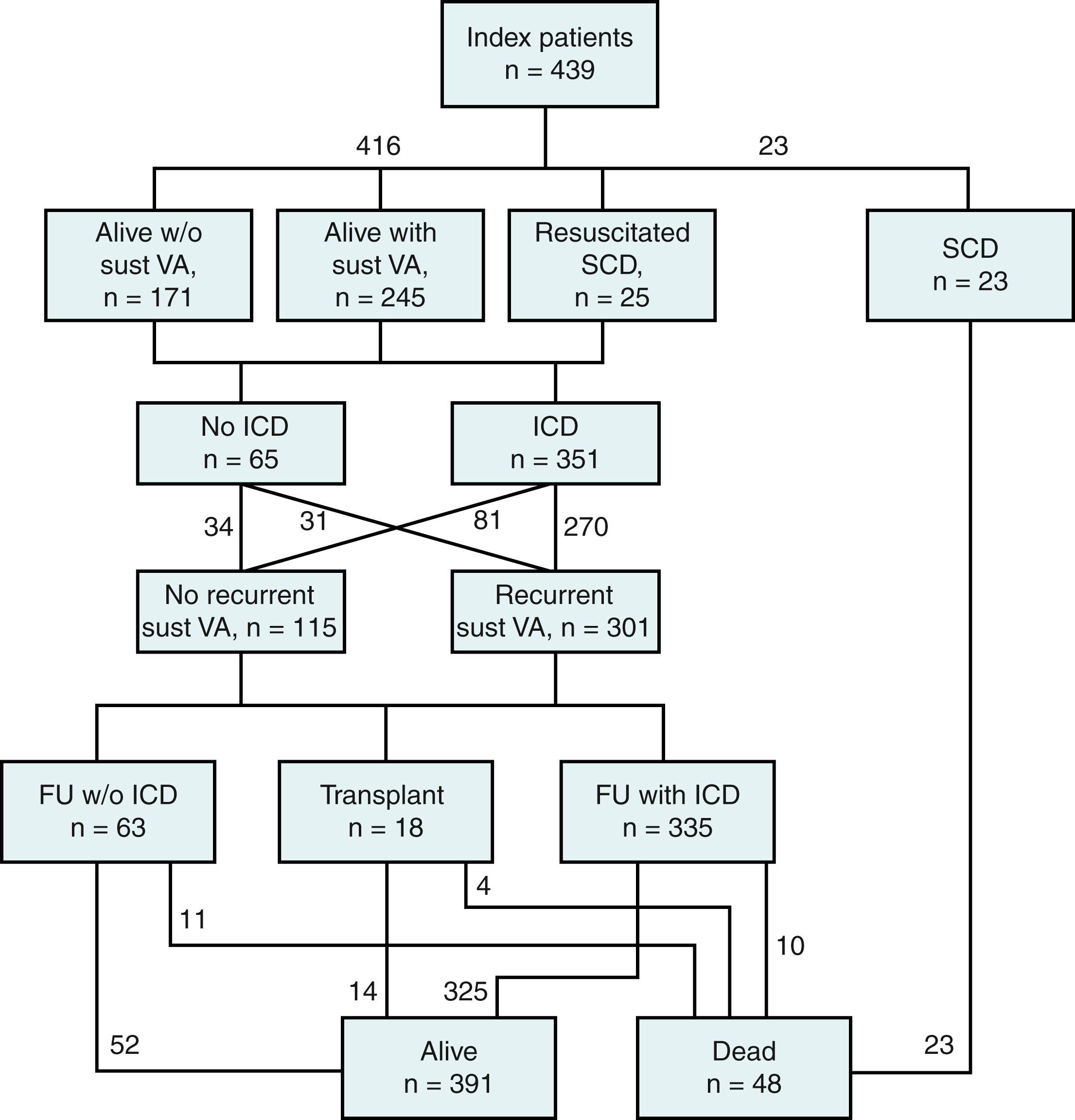
Management of patients with ARVD/C has four components. The first is getting the diagnosis correct. The second component involves accurate risk stratification for SCD and deciding whether to place an implantable cardioverter-defibrillator (ICD). The third component of management is minimizing ICD therapies. The final component of management is preventing progression of the disease.
The first step in management is making the diagnosis. Misdiagnosis of ARVD/C is common because of lack of awareness of the 2010 TFC and misinterpretation of MRI ( Box 90.1 ).
Regional RV akinesia, dyskinesia, or aneurysm and one of the following measured at end diastole:
PLAX RVOT ≥32 mm
PSAX RVOT ≥36 mm
Fractional area change ≤33%
Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and one of the following:
Ratio of RV end-diastolic volume to BSA ≥110 mL/m 2 (male) or ≥100 mL/m 2 (female)
RV ejection fraction ≤40%
Regional RV akinesia, dyskinesia, or aneurysm
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here