Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Venous thromboembolism (VTE) is a challenge in the management of injured patients. The mainstay of management requires thoughtful decision making regarding prevention, diagnosis, and appropriate therapy both while hospitalized and post discharge. Concomitant injuries frequently delay initiating VTE prophylaxis or interrupts chemoprophylactic therapy. The trauma population has a varied risk tolerance (e.g., immobility, spinal injury, hemorrhage, intracranial hemorrhage), which makes general algorithms difficult to apply. In the past decade there have been significant changes in the prevention and treatment of VTE, types and timing of chemoprophylaxis, as well as utilization of chemoprophylaxis post hospitalization. Additionally, there has been a significant decrease of enthusiasm for vena cava filters (VCFs), with a much narrower indication for usage.
Acute trauma resulting in hospitalization is an independent risk for developing VTE, with a hazard ratio of 4.6. Venous thrombosis occurs in as many as 58% of injured patients who do not receive prophylaxis, and pulmonary embolism (PI) occurs in up to 11% of these patients. Most of these thromboses (98%) are initially asymptomatic. Patients with lower extremity fracture (69%), spinal cord injury (62%), and traumatic brain injury (TBI, 54%) have the highest incidences of VTE. Furthermore, other factors such as older age (odds ratio [OR] 1.05), blood transfusion (OR 1.74), and surgery (OR 2.3) contribute to the risk of VTE development. Fatal pulmonary embolism occurs with a frequency 0.4% to 4.2% but accounts for 12% of all deaths after major trauma. Failing to provide VTE prophylaxis to patients at risk and hospitalized is even considered a medical error by the Institute of Medicine. Importantly, VTE can occur early after injury, with 37% of symptomatic pulmonary emboli (PE) occurring within the first 4 days after trauma. Although thromboprophylaxis, specifically, has contributed to a decrease of VTE in the past 2 decades, other aspects of patient management such as early mobility and decreased time in the operating room have improved patient outcomes overall and likely contributed to the decreased incidence of VTE.
Patients that sustain multisystem trauma typically present with all three components of Virchow’s triad (stasis, endothelial injury, hypercoagulable state), which explains the prevalence of VTE after injury. Immobility of the patient as a whole or of injured extremities leads to stasis within the venous system. Stasis is more pronounced in the intensive care unit, particularly in patients requiring neuromuscular blockade. This is the premise by which intermittent pneumatic compression (IPC) devices were initially created, although their contribution to fibrinolysis extends beyond their local effect. Direct injury to the venous system can occur with and without hemorrhage. Patients with vascular injury have insult to the endothelium causing release of tissue factor. Stretch, compression, and crush injury from impacts, fractures, and shear stress from cavitation due to gunshot wounds can cause intimal injury to the venous system without disruption of the vein. However, direct venous injury is not required to put injured patients at increased risk of thrombosis. Posttraumatic cytokine release from injury at a remote location causes activation of procoagulant factors, reduction in anticoagulant factors, and a relative hypercoagulable state. As the body recognizes the need to stop bleeding, the development of thrombus begins, and VTE can form within minutes of the trauma.
Prevention of VTE is the mainstay in management, and simultaneously, the most controversial aspect of the disease. Specifically, low-molecular-weight heparin (LMWH, enoxaparin 30 mg subcutaneously twice daily) and low-dose unfractionated heparin (LDUH, 5000 units subcutaneously every 8 hours) are the most effective prophylaxis against VTE, yet have the risk of contributing to hemorrhage in a patient population that is already among the highest risk for bleeding. The use of heparin (LMWH or LDUH) is the most important and primary modality supported by multiple organizations such as Eastern Association for the Surgery of Trauma (EAST), American College of Chest Physicians (ACCP), the Brain Trauma Foundation, the Spinal Cord Injury Consortium (SCIC), and the Trauma Quality Improvement Project (TQIP). For example, the ACCP guidelines state, “For major trauma patient, we suggest use of LDUH (Grade 2C), LMWH (Grade 2C), preferably with intermittent pneumatic compression (IPC, Grade 2C),” and the EAST guidelines state, “Trauma patients with an ISS [Injury Severity Score] greater than 9, who can receive anticoagulants, should receive LMWH as their primary mode of VTE prophylaxis (Level III).” Importantly, LDUH was previously considered to be inferior to LMWH for VTE chemoprophylaxis in trauma patients based on head-to-head trial with every-12-hour dosing. Increasing LDUH dosing to every 8 hours, the current edition of the ACCP guidelines consider LDUH and LMWG to be equal. In patients who have a low creatinine clearance (less than 20 to 30 mL/minute) prohibiting the use of LMWH, LDUH is the preferred agent. Additionally, dalteparin ( Table 1 ) is an LMWH that has demonstrated efficacy in VTE prophylaxis and therapy after the publication of the ninth edition of the ACCP guidelines.
| Heparin | Dose | Indications | Contraindications |
|---|---|---|---|
| Enoxaparin (Lovenox) | Prophylaxis: 30 mg SC bid or 40 units SC daily * Therapy: 1 mg/kg SC bid |
Appropriate for VTE prophylaxis and treatment as well as unstable angina and MI | High risk of bleeding Daily dosing recommended in spinal and epidural anesthesia |
| Dalteparin (Fragmin) | Prophylaxis: 5000 units SC daily Therapy: 100 anti-factor Xa U/kg SC bid |
Appropriate for VTE prophylaxis and treatment as well as unstable angina and MI | High risk of bleeding |
* Increase dosing to 40 mg subcutaneously twice a day in patients > 150 kg.
Nonpharmacologic modalities such as IPC, TED hose (graded compression stockings), or foot pumps have limited efficacy but can be used in patients felt to be at bleeding risk for using pharmacologic prophylaxis. It is our practice to use these methods regardless of chemoprophylaxis. This is especially true for patients with temporary limitations to the use of VTE chemoprophylaxis (e.g., patients with solid organ injury, hemorrhage from long-bone fractures, TBI), mechanical prophylaxis should be used. Additionally, foot pumps can be used as an alternative to IPC in patients with external fixators or casts. ACCP guidelines state, “For trauma patients in whom LMWH and LDUH are contraindicated, we suggest mechanical prophylaxis, preferably with IPC, over no prophylaxis (Grade 2C) when not contraindicated by lower-extremity injury. We suggest adding pharmacologic prophylaxis with either LMWH or LDUH when the risk of bleeding diminishes or the contraindication to heparin resolves (Grade 2C).” The caveat to IPC is that they have to be worn to be efficacious! It is not uncommon that patients can be less than compliant with their use, and it is incumbent on the providers to emphasize their importance ( Fig. 1 ). Probably the most important nonpharmacologic prophylactic measure is ambulation. Here lies the challenge of motivating patients and providing adequate pain control.
The obvious remaining question is, when is the risk of bleeding diminished? Determining parameters that assess the degree of ongoing hemorrhage is helpful to establish patterns of management. In the authors’ institution, a decrease in hemoglobin (Hb) of less than 1 g/dL over a 24-hour period is considered cessation of hemorrhage and the indicator to initiate chemoprophylaxis. An exception to this practice is an injury to the central nervous system, specifically TBI, spine fracture, and spinal cord injury. Initiation of chemoprophylaxis in this population requires greater caution. Relatively minimal additional hemorrhage into the brain or spinal cord can cause catastrophic changes in outcomes.
Despite standardization of pharmacologic prophylaxis, VTE continues to cause significant morbidity in critically ill patients, with DVT rates in excess of 10% to 15% with adequate chemoprophylaxis. There are several potential causes. First, unlike elective surgery, the injury has already occurred, and Virchow’s triad is already underway. Another possible etiology has been linked to inadequate anti-Xa levels achieved with standard dosing. Integrated pharmacist rounding in the intensive care unit can aid in achieving adequate anti-Xa levels in the trauma population. However, there is increasing evidence that VTE rates are not reduced with anti-Xa guided dosing. This suggests that an additional target such as platelets may be necessary to optimize thromboprophylaxis in the trauma population. Of perhaps greater significance than anti-Xa level dosing is the avoidance of missed standard prophylaxis dosing. A 2014 study found that interrupted LMWH therapy was an independent risk factor for DVT formation. Since missed doses occur commonly, this is a risk factor that can be easily addressed at the physician level, and all effort should be made to standardize perioperative practices to avoid missed doses of LMWH. The initial studies on the development of enoxaparin showed consistent efficacy in subjects 50 to 150 kg. Our practice has been to increase to 40 mg subcutaneously every 12 hours with patients greater than 150 kg.
The duration of thromboprophylaxis necessary for injured patients is not clearly defined. It is reasonable to continue chemoprophylaxis throughout the hospitalization and rehabilitation. Both ACCP and EAST guidelines support this practice. It should also be emphasized that VTE risk extends beyond hospitalization. In the largest study to date looking at VTE development following trauma admission discharge, 62% of the cases that developed VTE did so after hospital discharge. Associated risk factors were increasing patient age, male sex, increasing Injury Severity Score (ISS), immobility prior to trauma, soft tissue injury to the leg, and prior superficial vein thrombosis. These factors all increased risk of VTE within 92 days of hospitalization independent of surgery for acute trauma admissions. Although there are no data to provide guidance for extended VTE prophylaxis, it is certainly worthwhile to consider extended LMWH or a vitamin K antagonist (VKA, e.g., warfarin) until the patient is mobile.
The “prophylactic” (without a diagnosis of DVT or PE) insertion of vena cava filters (VCFs) in trauma patients was popularized in the 1990s. Rogers et al demonstrated a benefit to selected high-risk trauma patients for a “prophylactically” inserted VCF. Multiple studies (class 3 evidence at best) corroborated this paradigm, which led to several trauma-related organizations cautiously supporting the use of VCF incorporating VCF into their guideline statements (e.g., EAST, TQIP, and the SCIC). The Spinal Cord Injury Consortium (SCI Consortium) statement supports the consideration of using a VCF when prophylactic doses of anticoagulation are not safe within 72 hours from injury. In contrast, the ninth edition of the ACCP guidelines stated, “For trauma patients, we suggest that an inferior vena cava filter should not be used for primary VTE prevention (Grade 2C).” Therefore, VCFs should not be used exclusively for the prophylaxis of DVT. In accordance with this, in the last 10 years we have seen a significant decreased use of these devices for “prophylaxis.” The initial decline in prophylactic VCF use was likely linked to the French PREPIC (Prévention du Risque d’Embolie Pulmonaire par Interruption Cave) trial. This group found there was no difference in mortality rate and an increased rate of DVT in patients who had VCFs placed, in addition to VTE anticoagulation. The fact that VCFs were inserted despite therapeutic anticoagulation prohibits generalization of this study to practice in the United States. A landmark study that Rogers authored reversed his earlier findings in a large randomized multicenter trial—finding no decrease in incidence of symptomatic pulmonary embolism or death at 90 days, with or without filter placement. We additionally published our own 20-year experience evaluating 35,000 patients, demonstrating overall no benefit of decreasing PE rates with prophylactic VCF use except ventilator days > 3. While there may still be a very small subset of patients where a prophylactic VCF would benefit, such as high-risk intracranial hemorrhage that cannot receive pharmacologic prophylaxis due to fear of progression of intracranial hemorrhage, these two studies have demonstrated there is likely no role for widespread utilization of prophylactic VCF, where there is no evidence of VTE. In our practice, this remains a multispecialty discussion regarding PE risk and prophylactic anticoagulant risk, but in a very limited population.
The diagnosis of DVT and superficial venous thrombosis (SVT) is made using duplex ultrasound (DUS), which has almost totally supplanted venography as a diagnostic tool for this disease. DUS should be performed when signs of thrombus occur (unilateral extremity edema). Several reports have stated DUS as a useful screening tool; however, routine serial DUS examinations are not cost-effective, thus the routine screening of asymptomatic patients is not supported by the ACCP or EAST. Specifically, the ACCP guidelines state, “For major trauma patients, we suggest that periodic surveillance with VCU [venous compression ultrasound] should not be performed (Grade 2C).” EAST guidelines recognize that some patients at high risk may benefit from routine screening for DVT. Our practice has been the use of selective DUS in high-risk patients such as complex pelvic fractures with planned operative intervention that have had a contraindication to anticoagulant prophylaxis. It is important to recognize that DUS has little role in detection of VTE in pelvic vessels. If central DVT is suspected, CT venography is the modality of choice.
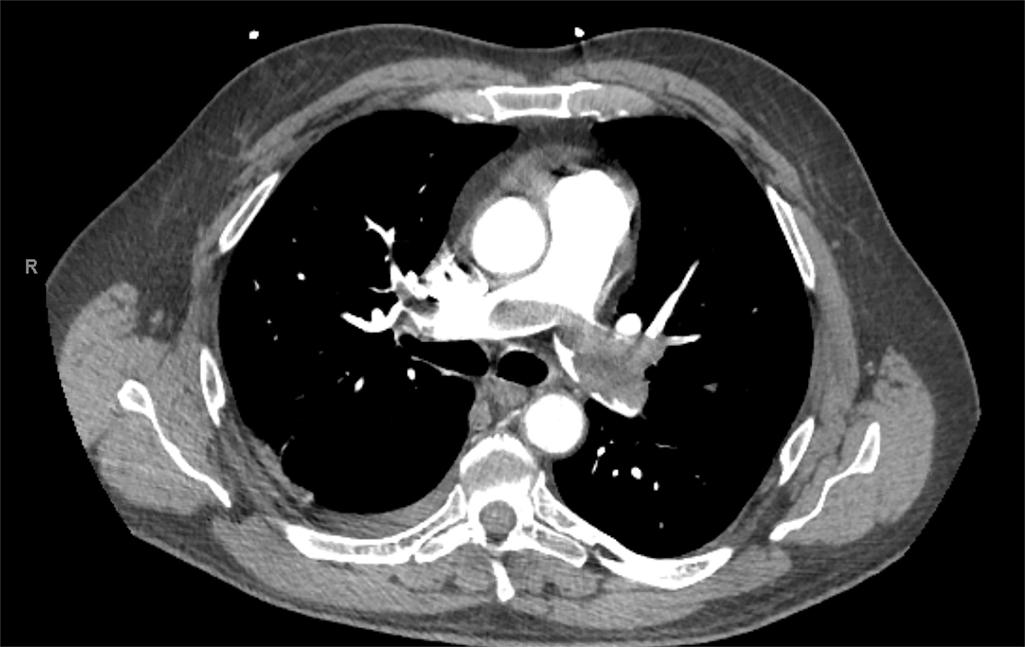
CT angiography (CTA) has become the gold standard for the diagnosis of PE ( Fig. 2 ). It is widely available, is minimally invasive compared with conventional pulmonary angiography, and can be rapidly obtained compared with ventilation-perfusion scanning, which also has very limited accuracy in the trauma population. It also allows for the evaluation and diagnosis of other thoracic pathology such as atelectasis, pneumonia, effusions, etc. Another important attribute of CTA is its ability to assist in prognosis. A simple assessment of the right ventricular to left ventricular diameter ratio (RV/LV) greater than 1.0 is predictive of an adverse outcome compared with ratio less than 1.0.
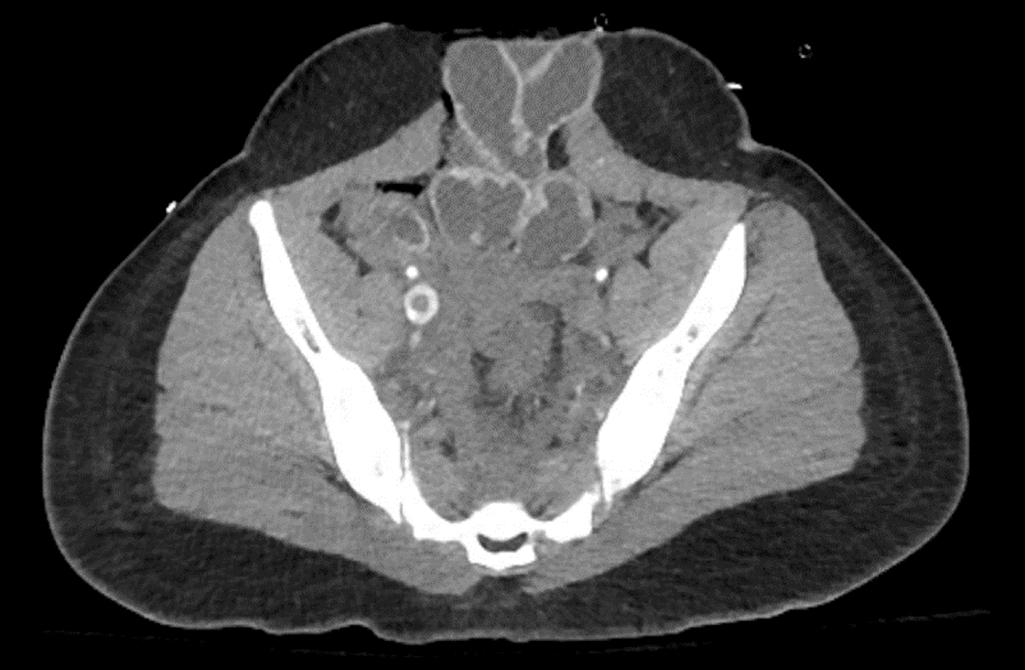
Although CTA is limited by a relative contraindication in patients with low creatinine clearance or with intravenous contrast allergies, it has superior sensitivity and specificity compared with alternative modalities. Occult proximal DVT are not uncommonly seen on contrast-enhanced CT of the chest or abdomen ( Fig. 3 ).
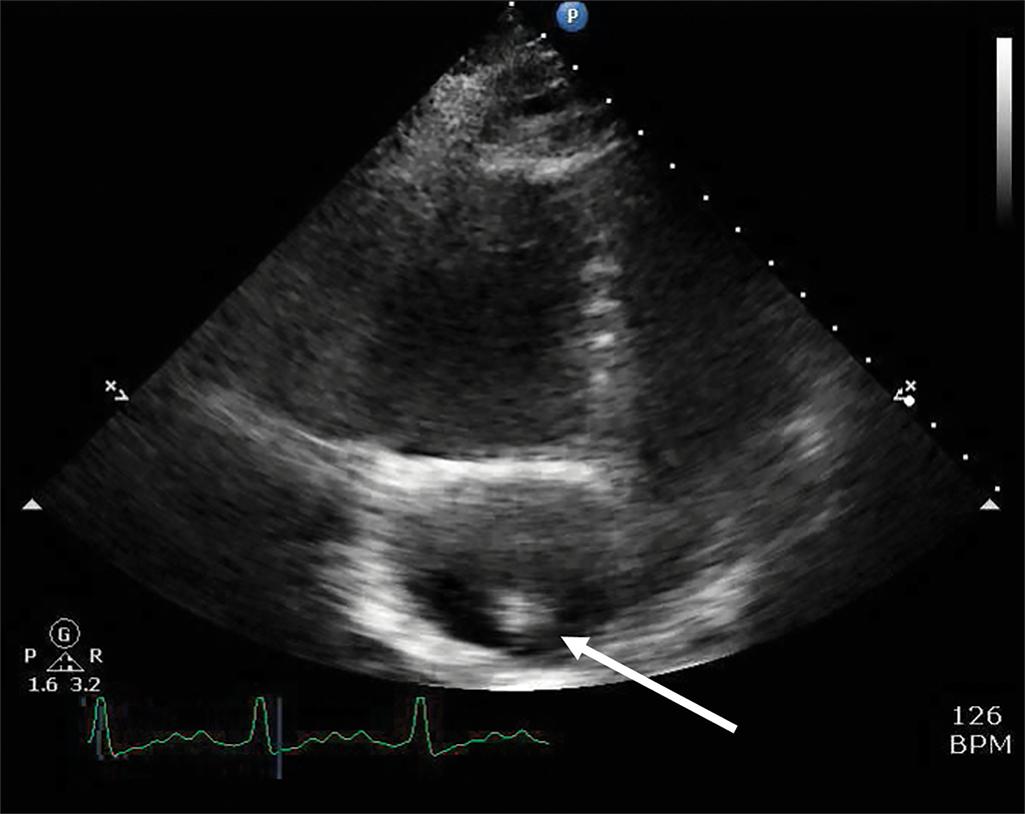
Additionally, medical prophylaxis for patients with contrast allergies can be administered prior to the study. In patients that are unable to travel, inference of suspected PE can be made by right heart strain/dilatation on echocardiogram or portable ventilation-perfusion scanning. Critical care ultrasonography/echocardiography can occasionally visualize a clot in transit.
Not all patients who have a diagnosis of injury are considered “high” risk; however, we have all experienced the “low”-risk patient with a VTE event. Reliable predictors to determine the subset of patients at increased risk and predictors of VTE have remained elusive within the trauma population. Since hospitalization for acute trauma is an independent risk factor for VTE, universal prophylaxis strategy is utilized. Certain injuries and conditions are associated with higher risk ( Table 2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here