Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Between 350,000 and 600,000 people in the United States (US) develop venous thromboembolism (VTE) each year, with at least 100,000 deaths from VTE annually. The incidence of VTE is higher among currently or recently hospitalized patients, the annual cost of treatment of VTE in the US as high as $10 billion. The US Surgeon General recognized deep vein thrombosis (DVT) and pulmonary embolism (PE) as major health problems in 2008 in a call to action for prophylaxis, and the Agency for Healthcare Research and Quality updated a guide for quality improvement in preventing VTE in 2016, which identified VTE among the most common preventable causes of hospital death. This chapter provides an overview of risk factors, risk stratification models, methods of VTE prophylaxis, a discussion of specific surgical populations, and recommendations regarding VTE prophylaxis.
A number of patient-specific and procedural risk factors for VTE have been identified, allowing for individualized risk assessment. Risk factors are categorized as strong, moderate, and weak in Box 147.1 and further discussed below. There has not been much change in the specific risk factors for VTE in hospitalized patients over the past two decades, until the COVID-19 pandemic, as COVID infection is one of the diseases with highest risk for VTE.
Fracture (hip or leg)
Hip or knee replacement
Major general surgery
Major trauma
Spinal cord injury
Arthroscopic knee surgery
Central venous lines
Chemotherapy
Congestive heart or respiratory failure
Hormone replacement therapy
Malignancy
Oral contraceptive therapy
Paralytic stroke
Pregnancy/post-partum
Previous venous thromboembolism
Thrombophilia
Bed rest >3 days
Immobility due to sitting (e.g., prolonged car or air travel)
Increasing age
Laparoscopic surgery
Obesity
Pregnancy/antepartum
Varicose veins
Strong risk factors include hip or leg fracture, hip or knee replacement, major general surgery, major trauma, and spinal cord injury. An early trial on VTE prophylaxis from 1959 compared 150 patients with hip fracture who were given a vitamin K antagonist to 150 who were given no prophylaxis, and found VTE in 29% of control participants compared to 3% of the treated participants. In patients undergoing surgery, there is a linear relationship between length of surgery and incidence of VTE. In trauma patients, proximal DVT was seen in 12% of patients within the first 2 weeks and 35%–40% of patients with spinal cord injury during the first 3 months.
Moderate risk factors include cancer and chemotherapy, prior VTE, thrombophilia, congestive heart failure, respiratory failure, and hormone replacement, oral contraceptives, and postpartum status. In a study of over 2000 patients undergoing surgery for an abdominal cancer, the incidence of VTE was 2%, and 46% of deaths in the study were due to VTE. Chemotherapy also increases the risk of VTE: women undergoing chemotherapy and surgery for breast cancer had three times higher risk of VTE compared to those undergoing surgery alone. A case–control study found that patients with a history of VTE were eight times more likely to develop a new VTE during a high-risk period such as perioperatively, during an illness, or during immobilization, compared with those without a prior history. Thrombophilia naturally increases the risk of VTE. The most common inherited thrombophilias in the US are factor V Leiden (with a lifetime risk of VTE of 10%), the prothrombin gene mutation, protein C and S deficiencies, and antithrombin deficiency. Antiphospholipid antibodies are the most common acquired thrombophilia. In congestive heart failure, there is a higher incidence of VTE with a decreasing ejection fraction. Alterations in hormones, whether hormone replacement therapy, oral contraceptive use, or pregnancy and postpartum state, increase risk of VTE. It is important to note this is also a varying exposure: oral contraceptives with less than 35 μg of ethinyl estradiol are associated with a lower incidence of VTE in the community compared with those containing more than 50 μg.
Weak risk factors include bed rest, immobility, increasing age, obesity, and varicose veins. Bed rest and immobility contribute to venous stasis. The incidence of DVT is also higher among patients with leg plaster casts, leading to a recommendation they receive prophylaxis for the duration of their immobilization. Patients over 40 years of age are at higher risk of VTE with the risk approximately doubling for each decade of age. While obesity was noted as a risk factor for DVT in an early study, Heit et al. did not find an association between BMI and DVT in their study of 627 community-dwelling residents. In a study of cancer patients, presence of superficial phlebitis or varicose veins was associated with higher risk of VTE.
While a number of risk-assessment models have been developed to guide decision making and balance the risk of a VTE event with risk of bleeding, some of the most commonly used are the “3 bucket” model, the Caprini, Padua, Rogers, and Improve scores. In the “3 bucket” model ( Table 147.1 ), patient and procedural factors are used to categorize risk of VTE as low, moderate, or high risk, with recommended prophylaxis for each category.
| Low Risk : Observation status, expected length of stay <48 hours. Minor ambulatory surgery unless multiple strong risk factors. Medical patients ambulatory in hall and not moderate or high risk. Ambulatory cancer patients admitted for short chemotherapy infusion. | No prophylaxis; reassess periodically, ambulate. |
| Moderate Risk (most general medical/surgical patients): Most general, thoracic, open gynecologic, or urologic surgery patients. Active cancer or past VTE/known thrombophilia in medical patient with length of stay >48 hours. Medical patients with decrease in usual ambulation and VTE risk factors (myocardial infarction, stroke, congestive heart failure, pneumonia, active inflammation/infection, dehydration, age >65). | Unfractionated heparin or low-molecular-weight heparin prophylaxis. |
| High Risk: Hip or knee arthroplasty, hip fracture surgery, multiple major trauma, spinal cord injury or major neurosurgery, abdominal–pelvic surgery for cancer. | Mechanical and pharmacologic prophylaxis. |
The Caprini score was first described in 1991 and similarly categorizes patients at low, moderate, or high risk for VTE ( Fig. 147.1 ). A score of 0–1 is associated with <10% incidence of DVT and is considered low risk, with no specific prophylaxis indicated. A score of 2 is considered moderate risk and a score of 3–4 is considered high risk; those at moderate or high risk of DVT should receive mechanical and/or pharmacologic prophylaxis. A score of 5 or more is considered highest risk with a 40%–80% incidence of DVT without prophylaxis, and these patients may benefit from extended prophylaxis up to a month following surgery. However, these are historically derived rates which are from nonprophylaxed patients, and overestimate the actual VTE incidence. A meta-analysis published in 2017 pooled 13 studies including 7590 patients and found patients with higher Caprini scores were significantly more likely to have VTE events. There was no association between Caprini score and postoperative bleeding.
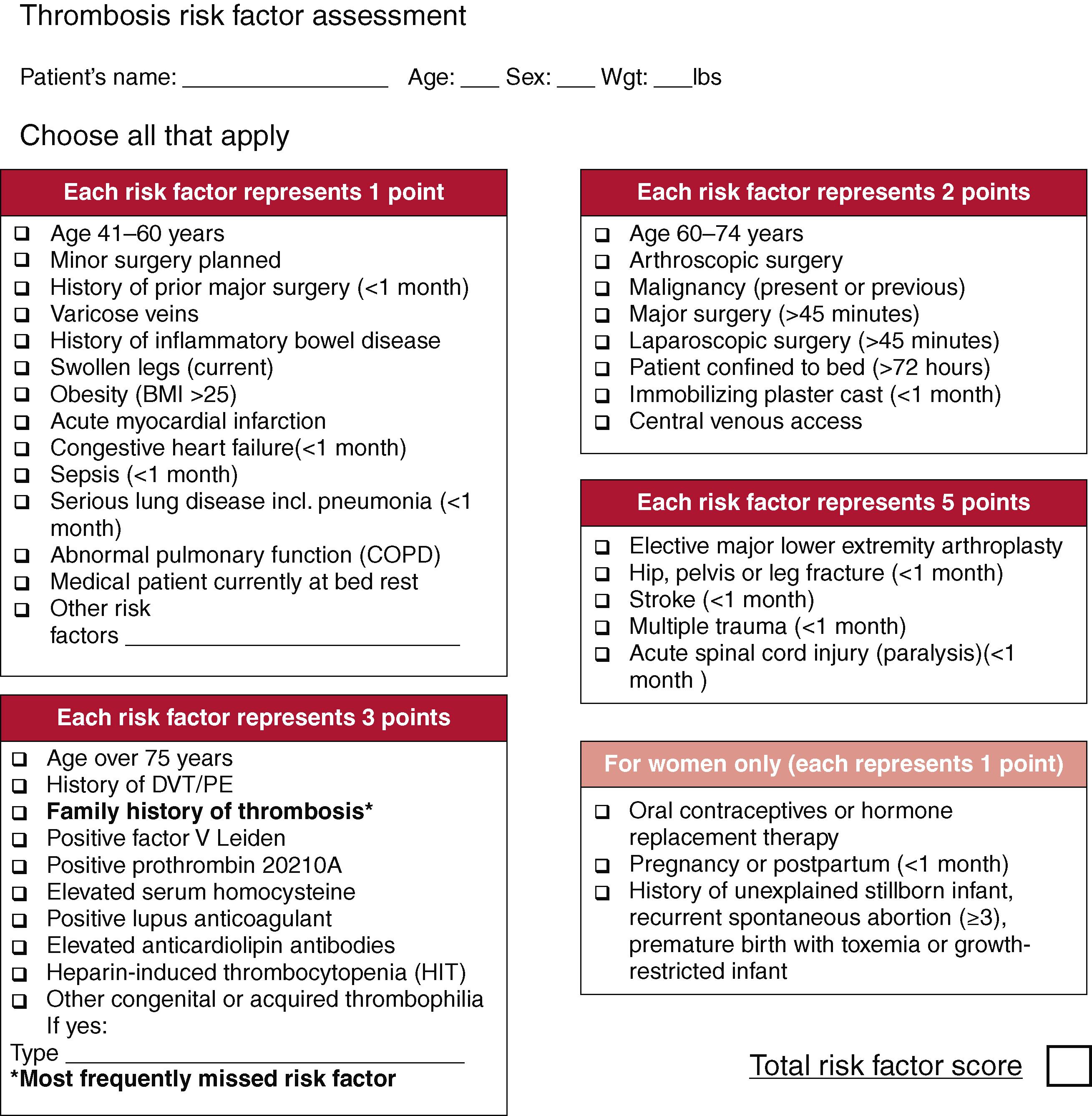
Risk stratification of all hospitalized patients will help determine who are considered moderate or high risk for VTE and should be treated with mechanical and/or pharmacologic prophylaxis to decrease VTE incidence.
Mechanical methods of VTE prophylaxis include graduated elastic compression stockings, intermittent pneumatic compression of the legs, foot compression devices, and inferior vena cava filters.
Graduated elastic compression stockings reduce the cross-sectional area of the veins and increase the velocity of venous blood flow. While compression stockings are made with a variety of levels of compression, those used for DVT prophylaxis have optimal flow augmentation with a pressure of 18 mm Hg at the ankle decreasing to 8 mm Hg at the thigh.
A Cochrane review of randomized controlled trials of compression stockings for DVT prophylaxis following surgery first published in 2000 and updated in 2010 included 18 trials. They found that while compression stockings alone decreased the incidence of DVT (13% of those with stockings developed DVT compared to 26% without stockings), compression stockings used with another method of DVT prophylaxis were more effective than stockings alone (4% of those with stockings and another prophylaxis developed DVT compared to 16% with the other method alone). Two other meta-analyses studying compression stockings in patients undergoing surgery found a 68% reduction in the incidence of DVT with compression stockings and the combination of pharmacologic prophylaxis with compression stockings is more effective than stockings alone.
Studies of compression stockings in hospitalized patients who have not undergone surgery are more inconclusive. A study of patients with stroke who were treated with compression stockings identified no decreased risk of VTE, but a higher risk of skin complications associated with stockings (5.1% vs. 1.3%). Another study of patients with stroke compared thigh-high compression stockings to knee-high compression stockings and did find a lower incidence of VTE with thigh-high stockings, however this group also had skin complications in 3.9% of patients. In a study of orthopedic patients, 56% of patients with thigh-high compression stockings developed constriction bands from folding or rolling of stockings. Given that patients report more discomfort with thigh-high compression stockings with a relatively high rate of skin complications, including pressure bands, tears, and skin necrosis, enthusiasm for compression stockings as DVT prophylaxis has waned. Approximately 2% of patients in a surgical intensive care unit were noted to have pressure injury related to compression stockings. Notably, compression stockings should not be used for patients with severe leg edema, dermatitis, or those with peripheral arterial disease.
Intermittent pneumatic compression (IPC) devices consist of inflatable boots or sleeves wrapped around the legs and secured by Velcro, that are connected to an electrical compressor that intermittently insufflates air to a preselected pressure. The sleeves can be applied to the calf alone, calf and thigh, foot and calf, or whole limb. Some types of IPC devices inflate the sleeves sequentially from distal to proximal to increase venous flow and are called sequential compression devices (SCDs). In addition to flow augmentation, IPC stimulates fibrinolytic activity through the release of endothelial tissue-type plasminogen activator (t-PA), prostacyclin, von Willebrand factor, and tissue factor pathway inhibitor.
IPC devices are generally considered the most effective mechanical prophylaxis method in preventing postoperative DVT. In nonorthopedic surgery, the American College of Chest Physicians (ACCP) estimates a reduction in symptomatic VTE from 60 to 30 per 1000 as a result of using IPC in high-risk patients, and recommends use of IPC alongside pharmacologic prophylaxis or alone, in case of contraindication for pharmacologic methods. In orthopedic surgery patients who are generally at higher risk of VTE postoperatively, ACCP guidelines recommend IPC in addition to pharmacologic prophylaxis. A systematic review of the literature that analyzed 19 trials including 2225 patients concluded that IPC as monotherapy reduced DVT from 23% in patients without IPC to 10% in those receiving IPC ( P < 0.0001). Similarly, a meta-analysis of 15 studies with 2270 patients who underwent different types of surgery showed that, in comparison with no prophylaxis, IPC reduced the risk of DVT by 60% ( P < 0.001).
For patients with contraindications to pharmacologic prophylaxis including active bleeding or high risk of bleeding, IPC can be used alone. The ENDORSE study revealed that 9% of surgical patients considered at high VTE risk had some contraindication to receive pharmacologic prophylaxis. However, IPC may not decrease PE incidence: a meta-analysis of nine studies including 3347 neurosurgery and orthopedic surgery patients found lower incidence of DVT with IPC devices, however no difference in PE. Similarly, in the CLOTS 3 trial of patients immobilized following acute stroke, while IPC reduced the risk of DVT at 30 days after randomization, there was no difference in the secondary outcome of imaging- or autopsy-confirmed PE at 30 days after randomization.
Risks of IPC are similar to compression stockings with a small risk of skin injury, and thus use of IPC should be avoided in patients with dermatitis, severe edema, or peripheral arterial disease. While presence of DVT is a theoretical contraindication for IPC due to the concept that thrombus could be pushed to embolize, evidence for this is lacking. Patient discomfort and lack of reapplication after ambulation are common reasons for noncompliance.
Foot compression devices or foot pumps are inelastic slippers or boots with an air bladder in the area of the sole of the foot which rapidly inflates to a pressure up to 200 mm Hg over 3 seconds every 20 seconds. This plantar compression increases venous outflow and reduces stasis in the legs, similar to IPC. Although there is evidence that foot pumps reduce DVT compared to no prophylaxis, pharmacologic prophylaxis has better results. , Foot pumps could represent an alternative in trauma patients in whom anticoagulants are contraindicated and leg IPC cannot be used because of the presence of casts, wounds, or external fixators.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here