Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cerebral arteriovenous malformations (AVMs) are high-flow, high-pressure vascular anomalies that cause neurologic morbidity, resulting in seizures, ischemic or steal symptoms, or hemorrhage.
Cerebral AVMs are classified on the basis of treatment risk, including nidus size, location within eloquent or noneloquent tissue, and pattern of venous drainage (superficial versus deep).
Complete obliteration of AVMs is necessary to reduce the risk of bleeding and to mitigate the risk of seizures. The medical literature lacks strong evidence that partial treatment confers protection from hemorrhage. Treatment modalities for cerebral or spinal AVMs include surgical resection, radiosurgery, endovascular occlusion, or a combination of these approaches.
Cerebral dural arteriovenous fistulas (dAVFs) are a distinct entity of vascular malformation composed of direct connections between meningeal arteries and dural venous sinuses or leptomeningeal veins. Neurologic morbidity associated with dAVFs can result in tinnitus, ocular symptoms, venous hypertension with neurologic deficits, and intracranial hemorrhage.
Cerebral dAVFs are classified by their risk of causing venous hypertension or hemorrhage due to the presence or absence of cortical venous drainage, but they can also be influenced by the presence of venous ectasia and the initial modality of presentation.
Spinal dAVFs are the most common type of spinal arteriovenous shunt. They cause neurologic morbidity due to venous hypertension. Surgical disconnection is associated with exceedingly high obliteration rates and low morbidity. Select spinal dAVFs can be occluded endovascularly.
Arteriovenous malformations (AVMs) and dural arteriovenous fistulas (dAVFs) are the two most common types of arteriovenous shunts in the central nervous system. AVMs are a source of neurologic morbidity due to associated ischemic or steal symptoms, hemorrhage, seizures (if cranial), or venous hypertension (if spinal). AVMs are composed of pial or dural arterial vessels that tangle and coalesce into a nidus that shunts blood flow into leptomeningeal veins in the absence of a capillary network. In contrast, patients with cerebral dAVFs may present with pulsatile tinnitus, ocular symptoms, or neurologic deficits secondary to venous hypertension. Cerebral dAVFs are composed of meningeal arteries that shunt blood flow within the leaflets of the dura into a venous sinus or into the leptomeningeal veins. Patients with spinal dAVFs typically present with signs of spinal venous hypertension; these lesions are composed of radicular artery feeders that shunt within the leaflets of the dura into an intradural vein at the junction of the nerve root dura and the thecal sac.
Since the 1960s, the quest to understand these lesions has led to the elucidation of many concepts in vascular physiology, anatomy, and embryology. The management of both AVMs and dAVFs has also evolved considerably, with advancements in neuroimaging, surgical techniques, and neuroendovascular diagnostics and treatment procedures. Nevertheless, the natural history and unpredictable course of both types of vascular lesions continue to present a challenge for neurosurgeons and interventionalists, motivating multidisciplinary collaborative approaches for a better understanding of the disease process in the hope of improving overall management and outcomes.
The formation of a vascular malformation within the central nervous system is believed to be a dysplastic process. Four types of dysplastic vascular malformations can be found in the central nervous system: developmental venous anomalies, capillary telangiectasias, cavernous malformations, and AVMs and arteriovenous fistulas (AVFs). Developmental venous anomalies are venous structures that drain normal brain tissue through an abnormally straight venous channel that is usually deep and fed by a cluster of veins typically referred to as a caput medusa ( Fig. 20.1A ). Patients with developmental venous anomalies are almost never symptomatic and should not be treated. Capillary telangiectasias are small dysplastic capillary vessels that are believed to be precursor lesions to cavernous malformations. Cavernous malformations are low-pressure, berry-like vascular clusters of capillaries ( Fig. 20.1B ). They have a tendency to bleed and to grow slowly. Over time, they often provoke seizures or deficits from local tissue effects, which then necessitates their surgical removal.
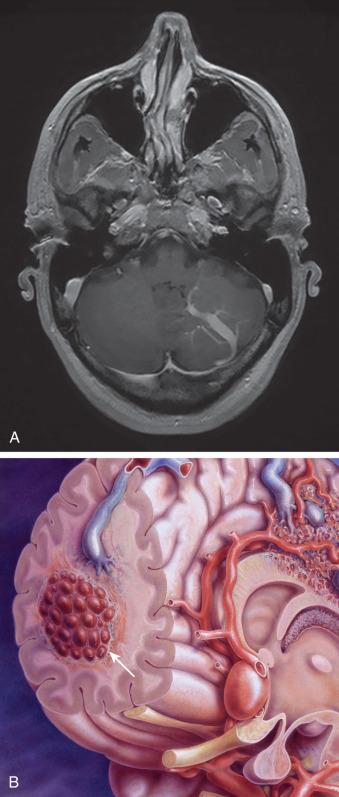
The last two categories of vascular malformations, AVMs and dAVFs, are the main focus of this chapter. The characteristic that separates AVMs and AVFs from other dysplastic vascular malformations is the presence of an arteriovenous shunt through which oxygenated blood passes directly from the arteries into the venous system without gas exchange in a capillary bed. Unlike other vascular malformations, these shunts are characterized by high blood flow and high-pressure flow.
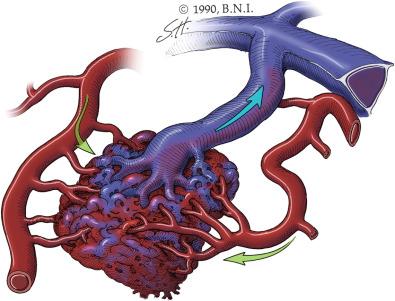
The three main types of AVMs found in the nervous system are plexiform, fistulous, or a combination of the two. The fistulous type (AVF) is present when an artery connects directly to a vein without a capillary bed in between. AVFs most commonly occur within the dural leaflets in the central nervous system as an acquired pathology termed a dAVF. Pial AVFs, often considered congenital lesions, are far less common and are typically discovered in younger patients. A plexiform malformation (AVM) is an artery that connects to a network of poorly differentiated, immature vessels (nidus) before reaching a vein ( Fig. 20.2 ).
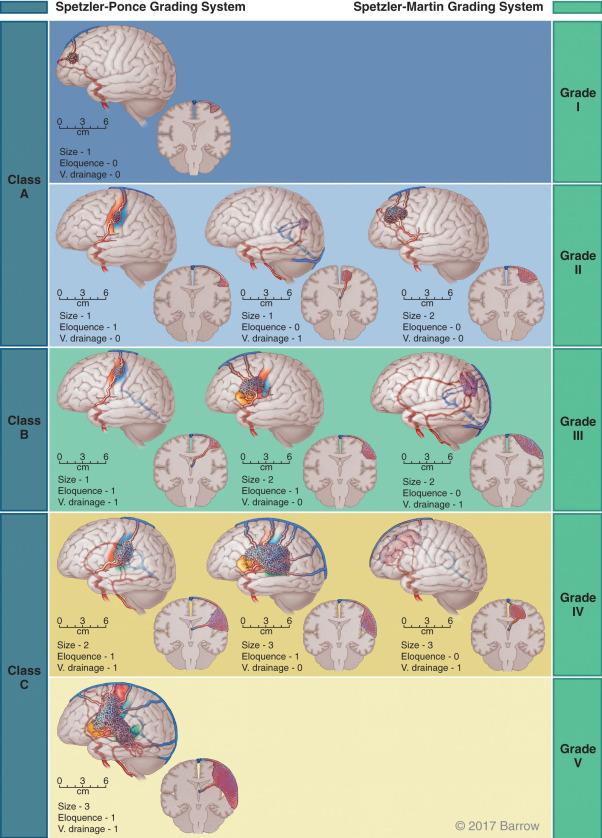
There are numerous classification schemes for AVMs and dAVFs. Although spinal arteriovenous shunt classifications have traditionally focused on the anatomy of the lesion itself, cerebral AVM classifications have historically aimed at helping both the patient and the physician understand the risk of various treatment modalities. Several classifications have been proposed with the same goal: accuracy of treatment and ease of applicability. The Spetzler-Martin grading system ( Fig. 20.3 ) is the most widely used and the most commonly cited classification for predicting the morbidity of surgical resection of an AVM. It is based on three simple variables: size of the malformation (1–3 points), the eloquence of the location (1 point, if in an eloquent location), and the presence of deep venous drainage (1 point, if present). The sum of all three variables provides the final grade, which ranges from I to V. Patients with a grade I or II AVM tolerate resection with acceptable morbidity, whereas those with a grade IV or V AVM may be considered for conservative management because of the high anticipated risk of surgical morbidity. It is important to emphasize that this classification scheme refers to surgical risk and is based on the experience of a highly experienced neurosurgical team; external validity may thus be limited. In addition, other factors such as perforator supply and diffuse nidal angioarchitecture are associated with increased surgical risk, whereas younger patient age and AVM hemorrhage are associated with lower surgical morbidity. These factors are not considered in the original Spetzler-Martin grading scheme; however, the Lawton supplementary scale incorporates some of these factors.
Several grading schemes have been proposed to facilitate predicting the success of radiosurgical treatment of cerebral AVMs; they account for AVM size and location. The radiosurgery-based AVM scale also factors patient age into the equation: (0.1) × (volume in milliliters) + (0.02) × (age in years) + 0.3 if the location is in the basal ganglia, thalamus, or brainstem. AVM scores of less than 1 were associated with obliteration and no radiosurgical morbidity in 89% of cases. Similar to the Spetzler-Martin grading scale, the Virginia Radiosurgery AVM Scale assigns points for volume (0 points if less than 2 mL, 1 point if 2–4 mL, 2 points if more than 4 mL) and eloquence (1 point). An additional point is added for prior AVM hemorrhage. An AVM grade of 0 or 1 was associated with a morbidity-free AVM obliteration rate of 83% and 79%, respectively.
Cranial dAVF grading schemes help predict natural history and are based on the original classification scheme by Djindjian. Type I dAVFs drain into a venous sinus and do not pose a risk of venous hypertension or intracranial hemorrhage. Type II dAVFs drain into a venous sinus with reflux into cortical veins; thus they pose a risk of intracranial hemorrhage. Type III dAVFs drain directly into cortical veins, and type IV dAVFs drain into cortical veins with associated ectasia. Borden and Shucart exclude the type IV dAVF and attempt to extrapolate Djindjian's scheme to spinal arteriovenous shunts; although the Borden and Shucart scheme is not used for spinal lesions, some use it instead of Djindjian's today. Cognard and colleagues elaborated slightly on Djindjian's scheme ( Table 20.1 ).
| Djindjian | Borden-Shucart | Cognard |
|---|---|---|
| Type I: Drainage into sinus or meningeal vein | Type 1: Venous drainage directly into dural venous sinus or meningeal vein | Type I: Venous drainage into dural venous sinus with antegrade flow |
| Type II: Initial drainage into sinus with reflux into other sinuses or cortical veins | Type 2: Venous drainage into dural venous sinus with CVR | Type IIa: Venous drainage into dural venous sinus with retrograde flow |
| Type III: Initial drainage into cortical veins | Type 3: Venous drainage directly into subarachnoid veins (CVR only) | Type IIb: Venous drainage into dural venous sinus with antegrade flow and CVR |
| Type IV: Initial drainage into cortical veins with giant venous pouch | Type IIa+b: Venous drainage into dural venous sinus with retrograde flow and CVR | |
| Type III: Venous drainage directly into subarachnoid veins (CVR only) | ||
| Type IV: Type III with venous ectasias of the draining subarachnoid veins | ||
| Type V: Direct drainage into spinal perimedullary veins |
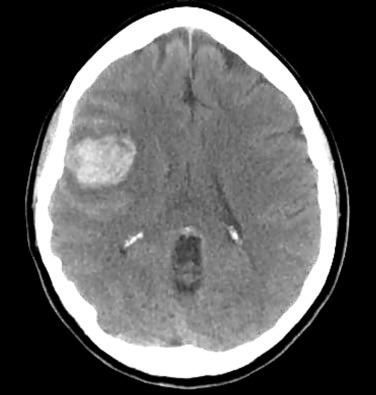
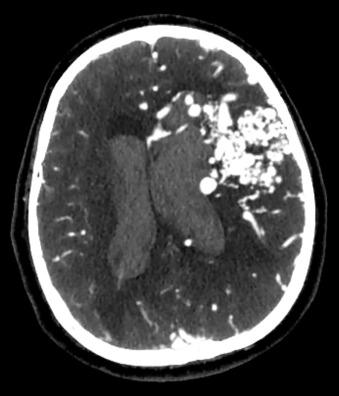
Noncontrast computed tomography (CT) of the head should be the first imaging modality in any case of suspected intracranial hemorrhage ( Fig. 20.4 ). Although not useful for ruling out the presence or absence of an AVM, noncontrast CT can demonstrate certain AVMs that produce calcium deposits, which aids the diagnostic process. Contrast-enhanced CT can demonstrate serpiginous vascular enhancement that is distinctively typical of an AVM. In the case of a dAVF, abnormally enlarged dural sinuses or draining cerebral veins can be seen after contrast administration.
CT angiography (CTA) is helpful for recognizing brain AVMs ( Fig. 20.5 ). CTA allows calculation of the volume of an AVM nidus, identifies associated vessels, and aids in quantifying any embolic material present within the AVM. In addition, CTA is useful for characterization and stereotactic localization of the AVM before surgical resection or radiosurgical treatment. In an AVF, the presence of asymmetrically visible and enlarged arterial feeding vessels has high sensitivity and specificity for the diagnosis of dAVFs. CTA can therefore be used as a screening tool in patients with suspected fistulas. In one study of 125 cerebral AVMs, the sensitivity of CTA in detecting AVMs was 90%, whereas that for magnetic resonance angiography was 74%. The sensitivity of CTA increased to 96% for unruptured AVMs and to 100% for AVMs at least 3 cm in size. The study emphasized the significantly greater sensitivity of CTA in detecting AVM-associated aneurysms compared to magnetic resonance imaging (MRI).
MRI is a useful tool in the diagnosis and management of AVMs ( Fig. 20.6A ). It can define complex structures such as the AVM nidus while also localizing adjacent eloquent structures, which is helpful for treatment planning. On T2-weighted MRI, hypointense signals are indicative of flow voids that represent the various feeding arteries, draining veins, or vessels of the nidus itself. Peripheral to the nidus, hypointense signals on T2-weighted MRI and gradient echo MRI can also show hemosiderin deposition, which indicates subclinical hemorrhage. Magnetic resonance angiography and magnetic resonance venography may be helpful in delineating the presence of flow in main vessels to and from the nidus. These imaging techniques are noninvasive methods for determining the progress of obliteration after radiosurgical or embolic therapy. For dAVFs, MRI may show the presence of flow voids on the surface of the brain or in the soft tissues. If the vascular lesion is large enough, magnetic resonance angiography may show an enlarged sinus and possible feeding and draining vessels. T2-weighted MRI is helpful in visualizing lesions with associated venous ectasia.
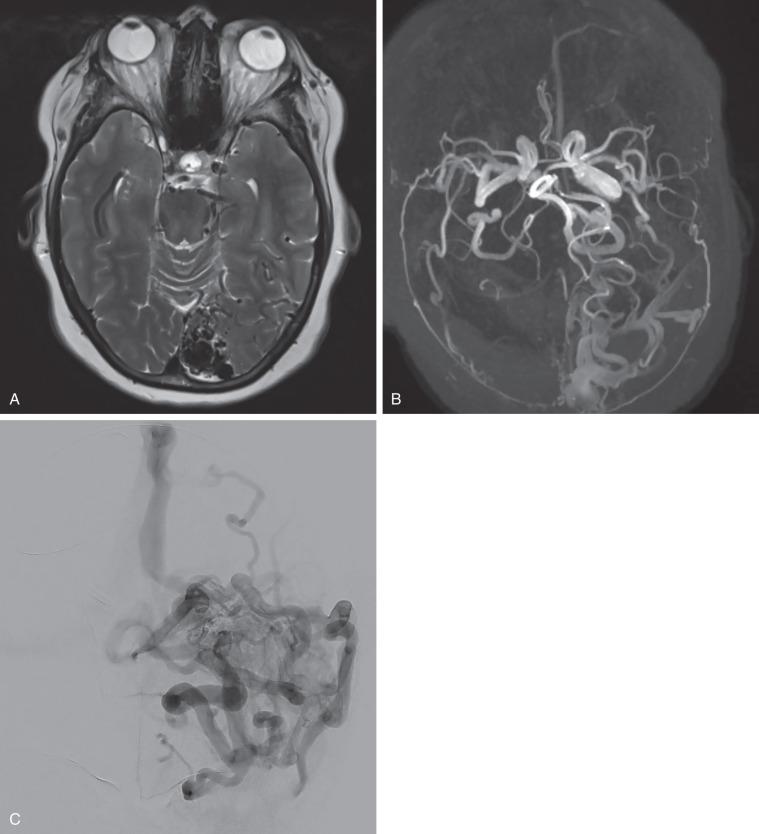
Digital subtraction angiography is the gold standard imaging technique for the diagnosis and angiographic evaluation of cerebral AVMs and dAVFs ( Fig. 20.6B–C ). The following factors should be identified, characterized, and evaluated on these angiograms: arterial supply (with attention to the presence of perforator supply); nidus location, size, and architecture (compact or diffuse); feeding artery and intranidal aneurysms; and drainage pattern (deep or superficial; outflow stenosis).
The arterial supply is visualized to define the anatomy of the AVM and to potentially identify pedicles for embolization if indicated. Early classification schemes focused on the number of arterial suppliers to the AVM, although modern schemes have not emphasized this angiographic factor. Periventricular AVMs have a particular proclivity to recruit a choroidal arterial supply, whereas surface AVMs may have a meningeal arterial supply. A middle meningeal artery supply is a particularly inviting pedicle for embolization when indicated. A careful examination for the presence of a perforator supply is critical for surgical planning. These pedicles can be a surgical nuisance; coagulation and operative control of these vessels are critical because the surgeon may have to continue following bleeding vessels that retract into deep, often eloquent, tissue. Embolization of these pedicles is a welcome adjunct, although embolization can be challenging and limited by the small size and tortuosity of the vessel.
Defining the nidus location is important for predicting both natural history and treatment risk for the AVM. Deep AVMs have a worse natural history than superficial lesions. Furthermore, the treatment of deep AVMs or AVMs in eloquent regions is associated with a greater risk and an often lower rate of complete obliteration with any treatment modality. Although modern studies have not shown a relationship between nidus size and natural history risk, larger AVMs are associated with greater risk and lower rates of complete obliteration for any treatment modality. Similarly, diffuse (as opposed to compact) nidus angioarchitecture is associated with greater risk and lower rates of complete obliteration for any treatment modality.
Feeding artery and intranidal aneurysms are important angiographic components to identify for both natural history evaluation and treatment planning. These “high-risk” features independently increase the prospective risk of intracranial hemorrhage. Although they can be addressed during intraoperative resection, they can also be preoperatively embolized or treated with embolization alone in the case of inoperative AVMs.
The drainage pattern of AVMs is an important factor for predicting both their natural history and their treatment risk. Exclusive, deep venous drainage is an independent risk factor for AVM hemorrhage. Although less substantiated by natural history studies, venous outflow stenosis or obstruction is also a risk factor. Deep venous drainage does not necessarily impact the risk of radiosurgery or embolization, but it has been identified as a risk factor for surgical resection.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here