Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Computed tomographic angiography (CTA) is a relatively rapid, thin-section, volumetric, helical CT technique performed with a time-optimized bolus of contrast medium to enhance visualization of the cerebral circulation. This approach may be tailored to illustrate various segments of the circulation, from arterial segments to the venous system. The ongoing development of multidetector CT scanners has advanced CTA, with increasing numbers of detectors used to further improve image acquisition and visualization.
Helical CT scanner technology, providing uninterrupted volume data acquisition, can rapidly image the entire cerebral circulation from the neck to the vertex of the head within minutes. Typical CT parameters use a slice (collimated) thickness of 1–3 mm with a pitch of 1–2, which represents the ratio of the table speed per rotation and the total collimation. Data are acquired as a bolus of iodinated contrast medium traverses the vessels of interest. For CTA of the carotid and vertebral arteries in the neck, the helical volume extends from the aortic arch to the skull base. Typical acquisition parameters are 7.5 images per rotation of the x-ray tube, 2.5-mm slice thickness, and a reconstruction interval (distance between the centers of two consecutively reconstructed images) of 1.25 mm. For CTA of the circle of Willis and proximal cerebral arteries, the data acquisition extends from the skull base to the vertex of the head. Typical acquisition parameters for this higher spatial resolution scan are 3.75 images per rotation, 1.25-mm slice thickness, and an interval of 0.5 mm. A volume of contrast ranging from 100 to 150 mL is injected into a peripheral vein at a rate of 2–3 mL/sec and followed by a saline flush of 20–50 mL. Adequate enhancement of the arteries in the neck or head is obtained approximately 15–20 seconds after injection of the contrast, although this may vary somewhat in each case. Image acquisition uses automated detection of bolus arrival and subsequent triggering of data acquisition. The resulting axial source images are typically postprocessed for two-dimensional (2D) and three-dimensional (3D) visualization using one or more of several available techniques, including multiplanar reformatting, thin-slab maximum-intensity projection (MIP), and 3D volume rendering. More recent CT with 320 detector rows enables dynamic scanning, providing both high spatial and temporal resolution of the entire cerebrovasculature (four-dimensional [4D] CTA). The cervical vessels are imaged by acquisition of an additional helical CT scan analogous to 64-detector row CT. An increasing spectrum of clinical applications utilizing this advanced technique remains under investigation ( ).
Careful consideration must be made for performing contrast-enhanced CT studies in patients with renal impairment. Exposure to all contrast agents may result in acute renal failure, called contrast-induced nephropathy (CIN), which is typically reversible but may potentially result in adverse outcomes. The incidence of renal injury appears to be associated with increased osmolality of contrast agents, which have been steadily declining with the newer generations of nonionic agents. Due to the perceived risk of CIN, many centers require that pre-imaging serum creatinine levels be taken and extra caution used with patients who have a creatinine level above 1.5 gm/dL or an estimated glomerular filtration rate below 60 mL/min/1.73 m 2 . Treatment for this condition relies on prevention of this disorder, and agents such as N -acetylcysteine and intravenous (IV) saline and/or sodium bicarbonate may reduce the incidence of CIN. Avoidance of volume depletion and discontinuation of potential nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs or metformin, is often recommended for patients prior to the procedure. Patients who are on hemodialysis should undergo dialysis as soon as possible afterwards to reduce contrast exposure ( ). For patients who undergo CTA on an emergent basis and cannot take these precautionary steps, there is emerging evidence that the risk of CIN remains low. A 2017 systematic review evaluating ischemic stroke patients undergoing both CTA with CT perfusion (CTP) studies found that the overall rate of acute kidney injury (AKI) was 3% and the overall rate of hemodialysis was 0.07%. There was no difference in AKI among these patients with and without chronic kidney disease (odds ratio [OR] = 0.63; 95% confidence interval [CI] = 0.34–1.12). When adjusting for baseline creatinine, there was no difference in AKI between patients undergoing CTA/CTP and those who underwent noncontrast scans (OR = 0.34; 95% CI = 0.10–1.21). These findings suggest that the current contrast exposure involved with CTA may not be associated with a statistically significant increase in the risk of AKI in stroke patients, including those with known chronic renal impairment ( ).
Metallic implants, such as clips, coils, and stents, are generally safe for CT imaging, but it should be noted that they may lead to severe streaking artifacts, limiting image evaluation. These artifacts occur because the density of the metal is beyond the normal range of the processing software, resulting in incomplete attenuation profiles. Several processing methods for reducing the artifact signal are available, and operator-dependent techniques such as gantry angulation adjustments and use of thin sections to reduce partial volume artifacts may help decrease this signal distortion. Generally, knowledge of the composition of metallic implants may help in determining the potential severity of artifacts on CT. Cobalt aneurysm clips produce a lot more artifacts than titanium clips. For patients with stents, careful consideration must be made in evaluating stenosis, as these implants may lead to artificial lumen narrowing on CTA. The degree of artificial lumen narrowing decreases with increasing stent diameter. Lettau et al. evaluated patients with various types of stents and found that CTA may be superior to magnetic resonance angiography (MRA) at 1.5 tesla (1.5 T) for stainless steel and cobalt alloy carotid stents, whereas MRA at 3 T may be superior for nitinol carotid stents ( ). Data remain limited for patients undergoing intracranial stent placement, but, compared with digital subtraction angiography (DSA), inter-reader agreement for the presence of in-stent stenosis is noted to be inferior.
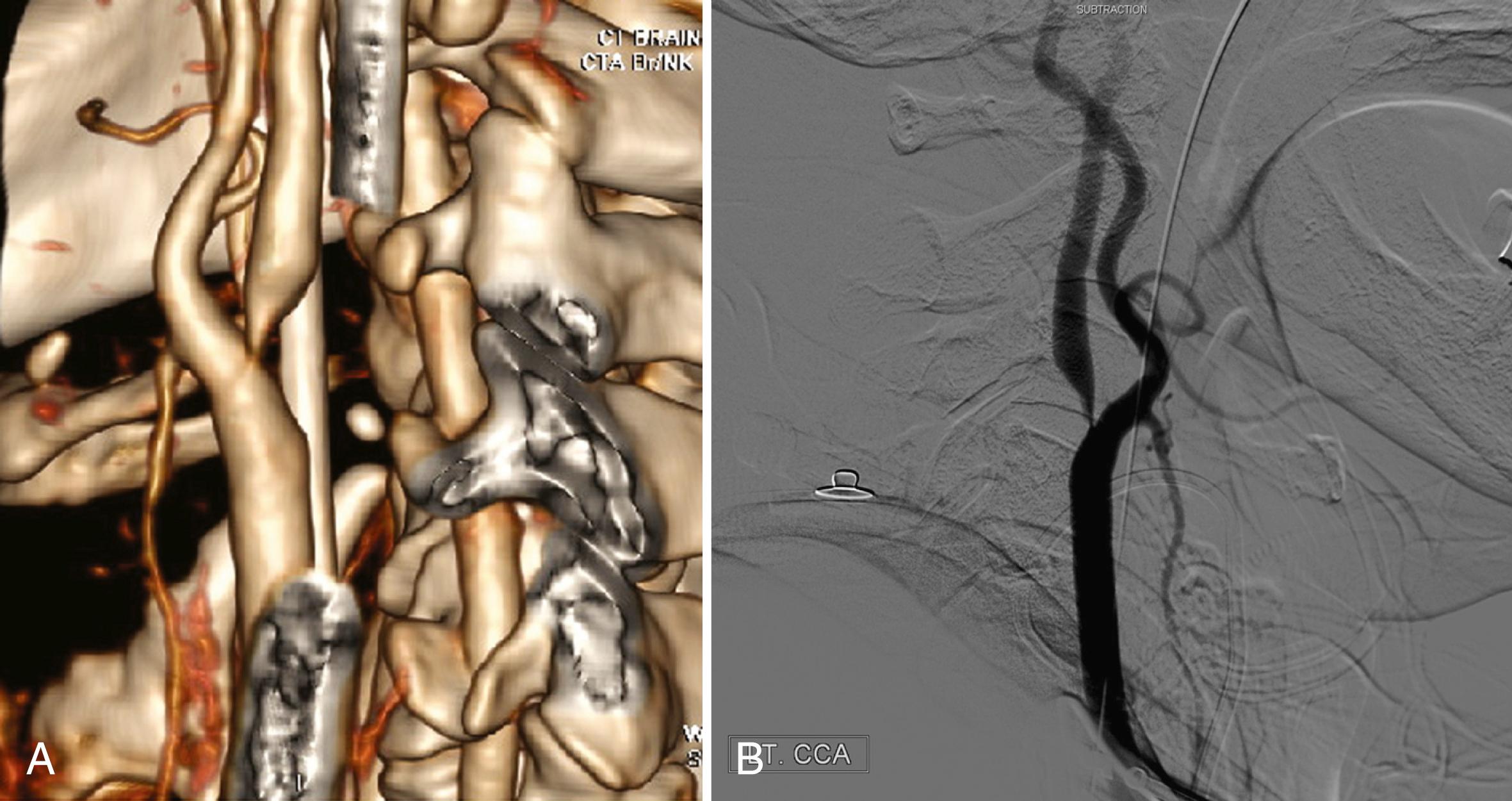
In evaluating occlusive disease of the extracranial carotid artery, CTA complements DSA and serves as an alternative to MRA ( Fig. 41.1 ). In the grading of carotid stenosis using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria, found that the rate of agreement between 3D CTA and DSA was 95%. In addition, CTA was significantly correlated with DSA in depicting the length of the stenotic segment. In reference to DSA, multiple studies have demonstrated a sensitivity of 77%–100% and a specificity of 95%–100% for CTA in detecting severe (70%–99%) stenosis ( ). Data for moderate (50%–69%) stenoses remain less reliable ( ). For detection of a complete occlusion, the sensitivity and specificity have been found to be 97% and 99%, respectively ( ). evaluated the use of multidetector CTA and carotid ultrasound in comparison to surgical observation for evaluating ulceration, which is a severe complication of carotid plaques. CTA was found to be superior, with 93.75% sensitivity and 98.59% specificity compared with carotid ultrasound, which demonstrated 37.5% sensitivity and 91.5% specificity. Furthermore, another study found that plaque ulceration on CTA had a high sensitivity (80.0%–91.4%) and specificity (92.3%–93.0%) for the prediction of intraplaque hemorrhage, an important marker of atherosclerotic disease progression, as defined on magnetic resonance imaging (MRI; ).
Fibromuscular dysplasia (FMD), which often involves a unique pattern of stenoses in the cervical vessels, may be detected by CTA, although no large studies have evaluated the sensitivity and specificity for detection. This disorder, which characteristically demonstrates a string-of-beads pattern of vascular irregularity on angiography, has been reliably demonstrated on carotid artery evaluations from case reports. This may potentially reduce the need for more invasive angiographic imaging in the future, although further studies in this area are required ( ).
Currently, either CTA or MRA is used to evaluate suspected carotid occlusive disease, with the choice of method determined by clinical conditions (e.g., pacemaker), accessibility of CT and MR scanners, and additional imaging capabilities (CT or MR perfusion brain imaging). In contrast to occlusions due to atherosclerosis or dissection, the absence of opacification on CTA may be seen during pseudo-occlusion. This phenomenon may occur due to sluggish or stagnant flow in the patent artery produced by a distal intracranial occlusion. Retrospective studies on patients who underwent mechanical thrombectomy have demonstrated a sensitivity ranging from 82% to 96% and a specificity ranging from 70% to 86% for the detection of pseudo-occlusions on CTA compared with DSA. The presence of an intracranial internal carotid artery (ICA) bifurcation (carotid-T) occlusion was more frequently associated with pseudo-occlusions rather than true occlusions. ( ) Clinicians should be acquainted with this potential finding as it may impact planning for acute endovascular stroke therapy.
Dissections of the cervicocephalic arteries, including the carotid and vertebral arteries, remain an important cause of ischemic strokes in young adults. CTA findings include demonstration of a narrowed eccentric arterial lumen in the presence of a thickened vessel wall, with occasional detection of a dissecting aneurysm. In subacute and chronic dissection, CTA has been shown to detect a reduction in the thickness of the arterial wall, recanalization of the arterial lumen, and reduction in the size or resolution of the dissecting aneurysm. Compared with DSA, CTA of the anterior and posterior circulations (pc) has been found to have a sensitivity of 51%–100% and a specificity of 67%–100% ( ). CTA is likely superior to MRI in evaluating aneurysms of the distal cervical ICA, a common site of dissection, because MRI findings are often complicated by the presence of flow-related artifacts. CTA depiction of dissections at the level of the skull base may be complicated in some cases because of beam hardening and other artifacts that obscure dissection findings, including similarities in the densities of the temporal and sphenoid bones with the dissected vessel.
CTA is a reliable alternative to MRA for evaluating arterial occlusive disease near the circle of Willis in patients with symptoms of acute stroke ( Fig. 41.2 ). The rapid imaging time has resulted in a significant escalation in the use of this modality during acute strokes ( ). A large database of acute stroke patients across multiple community and academic hospitals in Los Angeles and Orange counties found that the proportion of ischemic stroke patients undergoing CTA steadily increased from 4% in 2005 to 26% in 2012 ( ). CTA shows clinically relevant occlusions of major cerebral arteries and enhancement caused by collateral flow distal to the site of occlusion. Several published studies have noted sensitivities ranging from 92% to 100% and specificities of 82%–100% for the detection of intracranial vessel occlusion. ( ). have suggested that CTA has a higher sensitivity when directly compared with 3D time-of-flight MRA (TOF-MRA), with sensitivities of 100% and 87%, respectively.
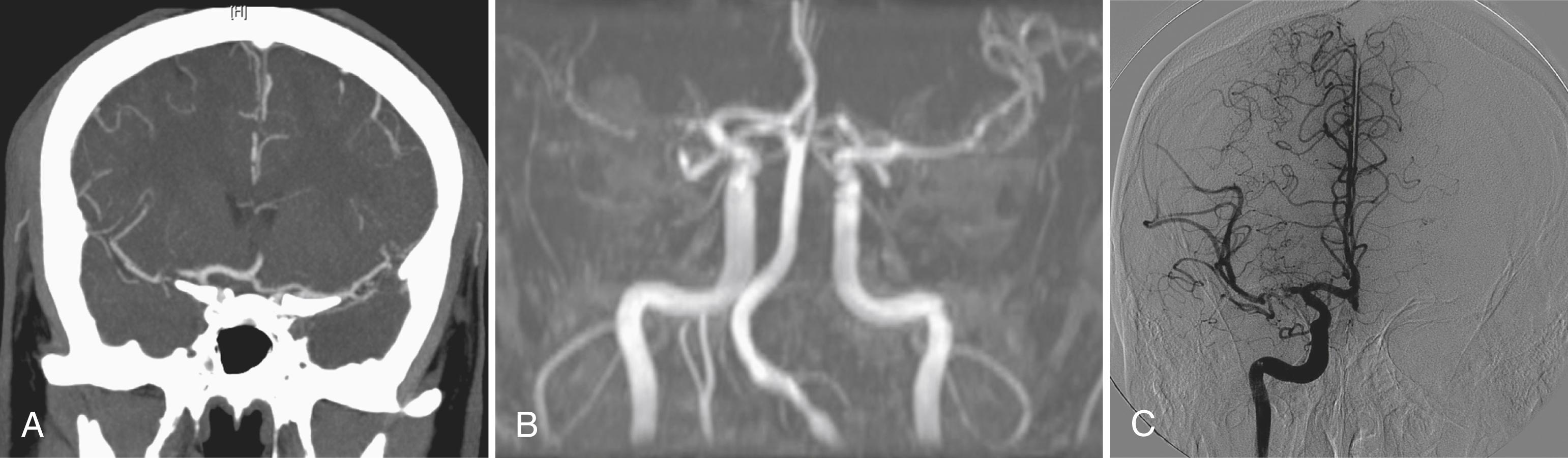
CTA source images (CTA-SI) may be used to provide an estimate of perfusion by taking advantage of the contrast enhancement in the brain vasculature that occurs during a CTA, possibly making it unnecessary to perform a separate CTP study with a second contrast bolus. In normal perfused tissue, contrast dye fills the brain microvasculature and appears as increased signal intensity on the CTA-SI. In ischemic brain regions with poor collateral flow, contrast does not readily fill the brain microvasculature. Thus, these regions demonstrate low attenuation. The hypoattenuation seen on CTA-SI correlates with abnormality on diffusion-weighted MRI (DWI), and they have been found to be more sensitive than noncontrast CT scans in the detection of early brain infarction ( ). The sensitivity of CTA-SI and DWI when directly compared has been found to be similar in detecting ischemic regions, but DWI is better at demonstrating smaller infarcts and those in the brainstem and posterior fossa. Such findings may be useful for patients with symptoms of acute infarction who cannot undergo MRI ( ).
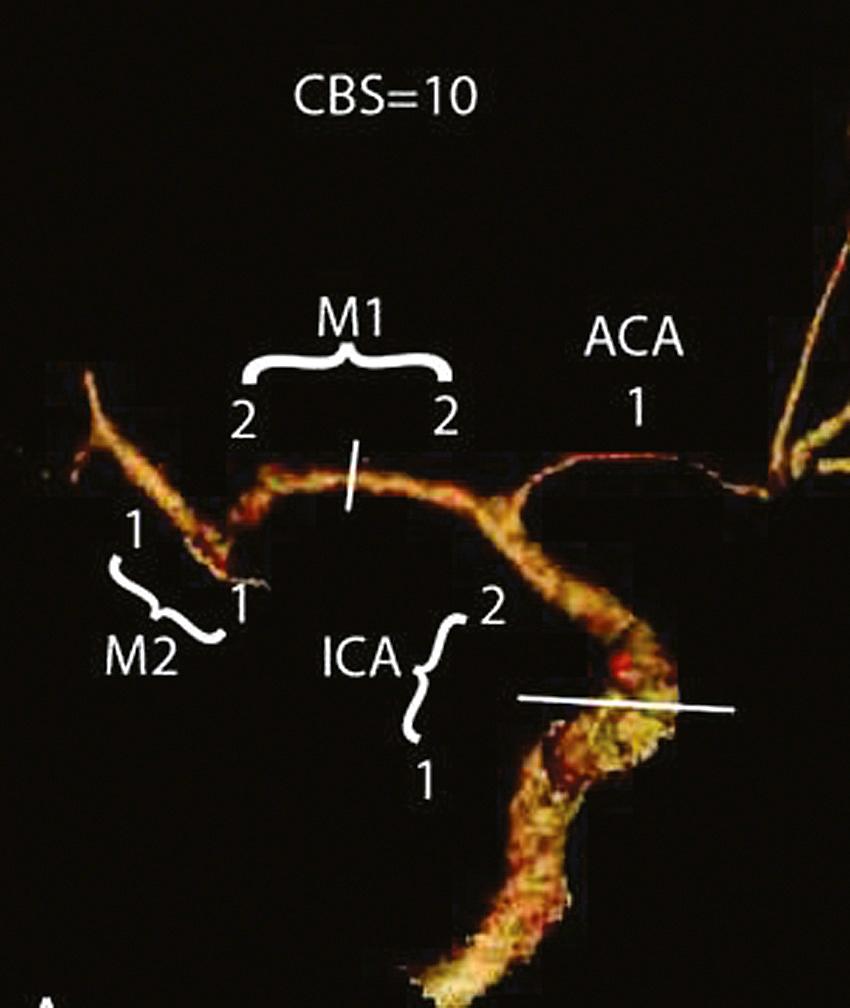
In addition to anatomical pathology and perfusion status, CTA imaging may potentially be used for prognostication in patients undergoing acute stroke intervention. The 10-point Clot Burden Score (CBS) was devised as a semiquantitative analysis of CTA to help determine prognosis in acute stroke ( Fig. 41.3 ). The CBS subtracts 1 or 2 points each for absent contrast opacification on CTA in the infraclinoid ICA (1), supraclinoid ICA (2), proximal M1 segment (2), distal M1 segment (2), M2 branches (1 each), and A1 segment (1). The CBS applies only to the symptomatic hemisphere. A CBS below 10 was associated with reduced odds of independent functional outcome (OR 0.09 for a CBS of 5 or less; OR 0.22 for CBS 6–7; OR 0.48 for CBS 8–9; all vs. CBS 10). The quantification of intracranial thrombus extent with the CBS predicts functional outcome, final infarct size, and parenchymal hematoma risk acutely ( ). An increased CBS is correlated with good clinical outcomes with a sensitivity of 58% and a specificity of 77% ( ). This scoring system requires external validation and may be useful for patient stratification in future stroke trials.
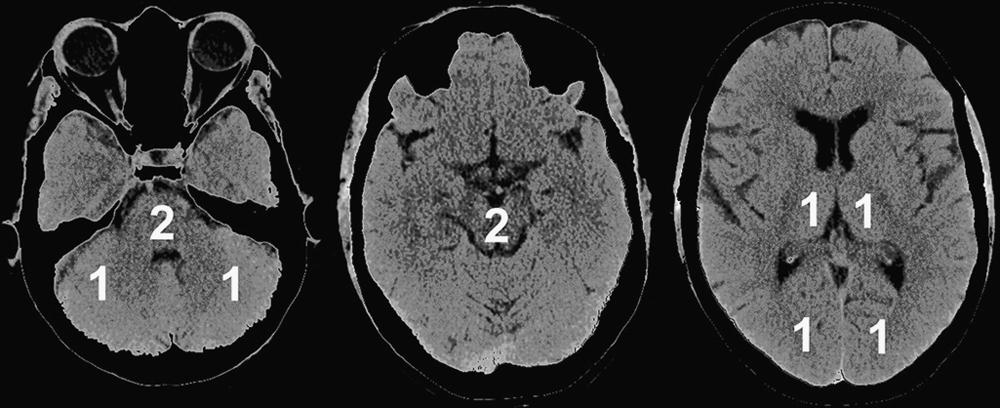
The Alberta Stroke Program Early CT Score (ASPECTS) is a 10-point analysis of the topographic CT scan score used in patients with middle cerebral artery (MCA) stroke ( Fig. 41.4 and Box 41.1 ). Segmental assessment of MCA territory is made, and 1 point is removed from the initial score of 10 if there is evidence of infarction in the following regions: putamen, internal capsule, insular cortex, anterior MCA cortex, MCA cortex lateral to insular ribbon, posterior MCA cortex, anterior MCA territory immediately superior to M1, lateral MCA territory immediately superior to M2, and posterior MCA territory immediately superior to M3. An ASPECTS score of 7 or less predicts a worse functional outcome at 3 months and symptomatic hemorrhage. The ASPECTS scoring system can be similarly applied to CTA-SI and, compared with noncontrast CT, has been found to be more reliable in predicting the final infarct size, particularly in early time windows ( ). evaluated acute stroke patients receiving IV thrombolysis and/or thrombectomy and found that CTA-SI-ASPECTS was a reliable predictor of a poor clinical outcome despite successful revascularization with a sensitivity of 35% and a specificity 97% (positive predictive value [PPV] 86%; negative predictive value [NPV] 7%). Subsequent studies on patients undergoing endovascular treatment have demonstrated that CTA SI-ASPECTS correlates with follow-up MR DWI better than noncontrast CT ASPECTS and was better able to predict favorable functional outcomes ( ). This scoring method may serve to reliably predict futile recanalization and remains a valuable tool for treatment decisions regarding the indication of revascularization therapies.
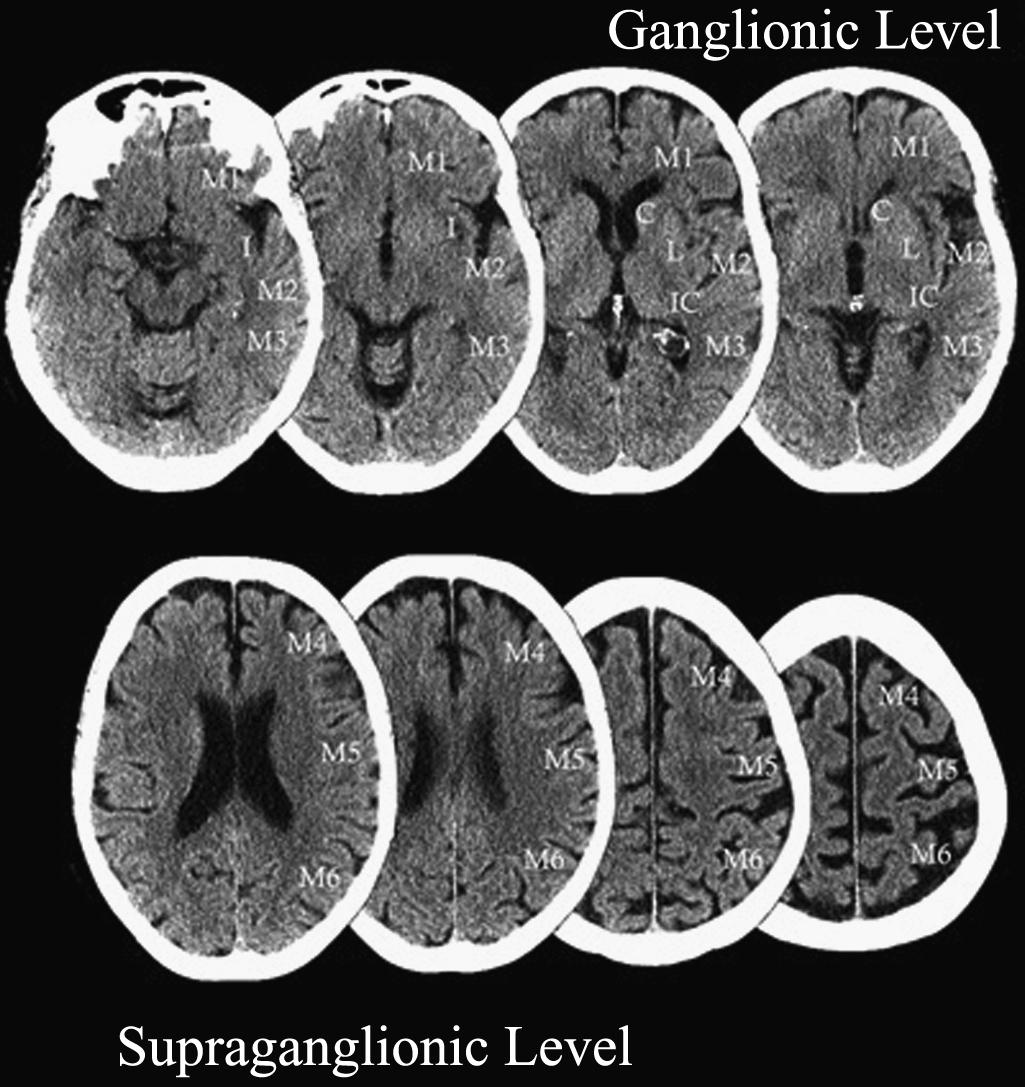
∗ This is a 10-point quantitative scoring system for patients with acute MCA-territory strokes. Segmental assessment of MCA territory is made, and 1 point is removed from the initial score of 10 if there is evidence of infarction in that region.
Caudate
Putamen
Internal capsule
Insular cortex
M1—Anterior MCA cortex
M2—MCA cortex lateral to insular ribbon
M3—Posterior MCA cortex
M4—Anterior MCA territory immediately superior to M1
M5—Lateral MCA territory immediately superior to M2
M6—Posterior MCA territory immediately superior to M3
sought to determine whether CTA-SI ASPECTS could be combined with the CBS system for improved prognostication. A 10-point ASPECTS score based on CTA-SI and the 10-point CBS were combined to form a 20-point score for patients presenting acutely with stroke who received thrombolysis treatment. For patients with a combined score of 10 or less, only 4% were functionally independent, and mortality was 50%. In contrast, 57% of patients with scores of 10 or greater were functionally independent, and mortality was 10%. Additionally, parenchymal hematoma rates were 30% versus 8%, respectively. A similar semiquantitative scoring system for CTA-SI was devised for patients presenting with acute basilar artery occlusion and termed the pc-ASPECTS ( Fig. 41.5 ). This 10-point scoring system subtracts 1 or 2 points each for areas of hypoattenuation in the left or right thalamus, cerebellum, or posterior cerebral artery (PCA) territory, respectively (1 point), or any part of the midbrain or pons (2 points). Median follow-up pc-ASPECTS was lower in patients with a CTA-SI pc-ASPECTS less than 8 than in patients with a CTA-SI pc-ASPECTS of 8 or higher, respectively. Hemorrhagic transformation rates were 27.3% versus 9.5%, respectively, for patients who received thrombolysis. The results indicate that such analysis can predict a larger final infarct extent in patients with basilar artery occlusion. Larger prospective trials are required for validation, but the systematic acute evaluation of CTA along with CTA-SI may potentially be used to help guide future stroke treatments ( ).
Whole-brain dynamic time-resolved CTA or 4D CTA is a novel technique capable of generating time-resolved cerebral angiograms from skull base to vertex. This modality offers additional hemodynamic information on leptomeningeal collateral status as well as the extent of any retrograde flow. Unlike a conventional cerebral angiogram, this technique also visualizes simultaneous pial arterial filling in all vascular territories ( ). Due to the increased sensitivity for collateral flow, 4D CTA has been shown to more closely outline intracranial thrombi than conventional single-phase CTA, which may potentially assist neurointerventional treatment planning, and prognostication ( ).
Significant advancements have recently been made in the treatment of acute ischemic stroke. An increasing proportion of acutely presenting stroke patients are receiving successful recanalization with mechanical thrombectomy devices. However, treatment is often time dependent, requiring an ideal imaging selection tool that is able to accurately detect salvageable brain rapidly with widespread availability. Cerebrovascular collateral status at baseline has been found to be an important determinant of future clinical outcomes among patients with acute ischemic stroke undergoing mechanical thrombectomy. CTA is increasingly recognized as a valid tool for assessing collateral flow and predicting clinical outcomes in these patients. A higher rate of patients with good collaterals on CTA have improved functional outcomes compared with those with poor collaterals ( ). One study found that the evaluation of collateral flow was noted to be consistent by both CTA and conventional angiography and remains the strongest predictor of clinical outcome ( ).
Multiple methods have been ascribed for the assessment of collateralization on CTA in acute ischemic stroke patients, but there is no clear consensus yet on the optimal scoring system. The most common methods utilize the presence and extent of leptomeningeal vascular enhancement in the symptomatic hemisphere relative to the asymptomatic side. One such scoring system adapted from conventional angiography is the modified American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) system that assigns a score of 0 for nonexistent or barely visible pial collaterals on the ischemic site during any point in time, 1 for partial collateralization of the ischemic site until the late venous phase, 2 for partial collateralization of the ischemic site before the venous phase, 3 for complete collateralization of the ischemic site by the late venous phase, and 4 for complete collateralization of the ischemic site before the venous phase. A higher modified ASITN/SIR score is associated with better collateral flow on the symptomatic side. In comparing multiple CTA-based collateral scores with CTP scans among patients with emergent large vessel occlusions, one study found that modified ASITN/SIR collateral scores demonstrated good correlation with early infarct core (rho = −0.696, P < .001) and mismatch ratio (rho = 0.609, P < .001; ).
Multiphase CT angiography (mCTA) is an imaging technique that can be helpful in identifying patients that will benefit from mechanical thrombectomy by quickly evaluating the degree and extent of pial arterial filling in the whole brain in a time-resolved manner. Imaging of the brain vasculature from the skull base to the vertex is attained in three phases after contrast material injection. The first phase is composed of angiography from the aortic arch to the vertex on a multidetector CT scanner. Imaging acquisition during this phase is timed to occur during the peak arterial phase in the healthy brain tissue and is triggered by bolus monitoring. Imaging acquisition during the second and third phases occurs from the skull base to the vertex in the equilibrium/peak venous and late venous phases in the healthy brain. The two additional phases of mCTA use no additional contrast material. One collateral scoring system adapted for mCTA is the ASPECTS on collaterals system, which includes a six-point scale that also assigns a higher value for better collateral flow in the ischemic region. A score of 0 is assigned if no vessels are visible in any phase. A score of 1 indicates that only a few vessels are visible in the ischemic territory on any phase. A score of 2 is given if there is a two-phase delay associated with reduced prominence and extent of peripheral vascular filling or if there is a single-phase delay associated with ischemic regions with no vascular enhancement. A score of 3 is assigned for a delay on two phases or a single-phase delay associated with significantly reduced number of vessels in the ischemic territory. A score of 4 indicates that contrast delay is present on one phase, but the prominence and extent of peripheral vascular enhancement remains unchanged. A score of 5 is given if there is no delay in enhancement of pial vessels on all phases. The six-point scale can be further trichotomized into a descriptive categorization of collaterals as being poor (0–1), intermediate (2–3) or good (4–5).
Interrater reliability using this technique has been noted to be excellent among readers ( ). Furthermore, the interpretation of mCTA can easily be adopted as one study demonstrated a high interrater agreement between stroke neurology trainees and an experienced neuroradiologist ( ). When compared with single-phase CTA, mCTA has been shown to be superior in both interrater reliability as well as the ability to predict clinical outcomes in patients undergoing acute reperfusion therapy. Poor collaterals noted on mCTA have been shown to be an independent predictor of development of malignant MCA infarction ( ). The interpretation of collaterals on mCTA has been compared with standard CTP imaging, as described elsewhere. Investigators have demonstrated that mean Tmax, cerebral blood flow, and cerebral blood volume values on CTP correspond with different score categories on mCTA ( ). The use of mCTA may provide a viable alternative for CTP in the evaluation of acute ischemic stroke patients in centers where such modality is not available or where time constraints may deter performing additional imaging. In addition to collateral assessment, mCTA has also been found to be useful in better identifying distal vessel occlusions due to delayed distal opacification, termed the “delayed vessel sign.” When compared with single-phase CTA, the use of later phases may significantly improve the sensitivity and time to interpretation in identifying such occlusions ( ).
CTA offers a more readily available and less costly alternative to DSA in the evaluation of intracranial atherosclerotic disease (ICAD). The sensitivities for detection of intracranial stenoses range from 78% to 100%, with specificities of 82%–100% ( ). The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) study more recently evaluated CTA findings of intracranial atherosclerosis against DSA in a prospective blinded multicenter setting. Based on DSA stenosis defined as 50%–99%, the PPV of CTA was only 46.7% and the NPV was 73.0%. For DSA stenosis defined as 70%–99%, the PPV of CTA was 13.3% and the NPV was 83.8% ( ). CTA is considered to be superior to transcranial Doppler (TCD) ultrasound in detecting intracranial stenoses with a high false-negative rate noted for Doppler ultrasound ( ). Studies also suggest that CTA has a higher sensitivity when directly compared with 3D TOF-MRA. found that CTA had a sensitivity of 98%, while MRA had a sensitivity of 70% for detection of intracranial stenosis. Additionally, CTA may be superior to both MRA and DSA in detecting pc stenoses when slow or balanced flow states were present, possibly owing to a longer scan time, which allows for more contrast to pass through a critical stenosis. Although previous studies noted decreased accuracy with the presence of atheromatous calcifications, the sensitivity and specificity of CTA for stenosis quantification remains consistent when appropriate window and level adjustments are made to account for frequently associated blooming artifacts.
The diagnosis of cerebral venous thrombosis (CVT) was previously often made with conventional angiography and more recently by MRI techniques. Magnetic resonance venography (MRV) is commonly considered the most sensitive noninvasive test in diagnosing CVT. However, given the prolonged imaging time and often limited availability, CTA has been studied as a potential alternate means of detecting CVT. Spiral CT with acquisition during peak venous enhancement has been implemented with single-section systems but remains limited in spatial and temporal resolution. One study directly comparing CTV with MRV demonstrated a sensitivity and a specificity of 75%–100%, depending on the sinus or venous structure involved ( ). Multidetector-row CTA (MDCTA) offers higher spatial and temporal resolution, which allows for high-quality multiplanar and 3D reformatting. Two small studies found 100% specificity and sensitivity with MDCTA when compared with MRV. The venous sinuses could be identified in 99.2% and the cerebral veins in 87.6% of cases. MDCTA may be equivalent to MRV in visualizing cerebral sinuses, but further studies are needed to evaluate the diagnostic potential of MDCTA in specific types of CVT, such as cortical venous thrombosis, thrombosis of the cavernous sinus, and thrombosis of the deep cerebral veins. The advantages of MDCTA include the short examination duration and the possible simultaneous visualization of the cerebral arterial and venous systems with a single bolus of contrast. MDCTA visualizes thrombus via contrast-filling defects and remains less prone to flow artifacts. A potential problem with this technique lies in the fact that in the chronic state of a CVT, older organized thrombus may show enhancement after contrast administration and may not produce a filling defect, leading to a false-negative result. The addition of a noncontrast CT with the MDCTA is sometimes used to remove another potential to obtain false-negative results from the presence of a spontaneously hyperattenuated clot that could be mistaken for an enhanced sinus. This phenomenon is known as the cord sign and may be seen in 25%–56% of acute CVT cases ( ).
Patients presenting acutely with intracerebral hemorrhage (ICH) within the first few hours of symptom onset are known to be at increased risk for hematoma expansion. However, only a fraction of such patients arrive at a hospital within this time frame, so alternative means of identifying potential hemorrhage expansion have been sought because it is an important predictor of 30-day mortality. One such prognostic marker has been identified on CTA: the spot sign , defined as tiny, enhancing foci seen within hematomas, with or without clear contrast extravasation. The predicting hematoma growth and outcome in ICH using contrast bolus CT (PREDICT) investigation was a multicenter prospective study that validated the CTA spot sign as an independent predictor of hematoma expansion. The CTA spot sign demonstrated an excellent interrater agreement with a sensitivity of 51% and specificity of 85%, with a PPV of 61% and a NPV of 78% ( ). A more recent meta-analysis of 29 studies observed similar findings with a pooled sensitivity of 62% and a specificity of 88%. The spot sign was significantly associated with increased risk of hematoma expansion, a higher risk of in-hospital death, poor discharge outcomes, and increased 3-month mortality ( ).
Later image acquisition may improve detection for the spot sign, and this marker is seen more frequently in the venous phase compared with the arterial phase of CTA evaluation. noted that when a 90-second delayed CTA acquisition was added, the sensitivity increased from 55% to 64%. However, the presence of significant hematoma expansion and higher total hematoma enlargement were observed more frequently among spot sign–positive patients with earlier phases of image acquisition ( ). The timing of CTA evaluation relative to the onset of the patient’s symptoms also has a significant impact in the detection of this radiographic marker. One systematic review demonstrated that the frequency of the spot sign significantly decreased from 39% within 2 hours of onset to 13% beyond 8 hours. Additionally, there was a significant decrease in hematoma expansion in spot-positive patients as onset-to-CTA time increased with PPVs decreasing from 53% to 33% ( ).
DSA has been the standard imaging method for diagnosis and preoperative evaluation for patients with ruptured and unruptured cerebral aneurysms. However, DSA is invasive and subject to complications resulting from catheter manipulation. Thus, in patients at greater risk for cerebral aneurysms, the use of noninvasive techniques such as CTA to screen for aneurysms is particularly attractive.
The main disadvantages of CTA are radiation exposure, the use of iodinated contrast material, difficulty in detecting very small aneurysms, and imaging artifacts from endovascular coils in treated aneurysms. CTA has diagnostic limitations for determining the presence of a residual lumen and the size/location of the remnant neck of a treated aneurysm because of the streak artifacts caused by clips, coils, flow diverters, and other embolization-related devices.
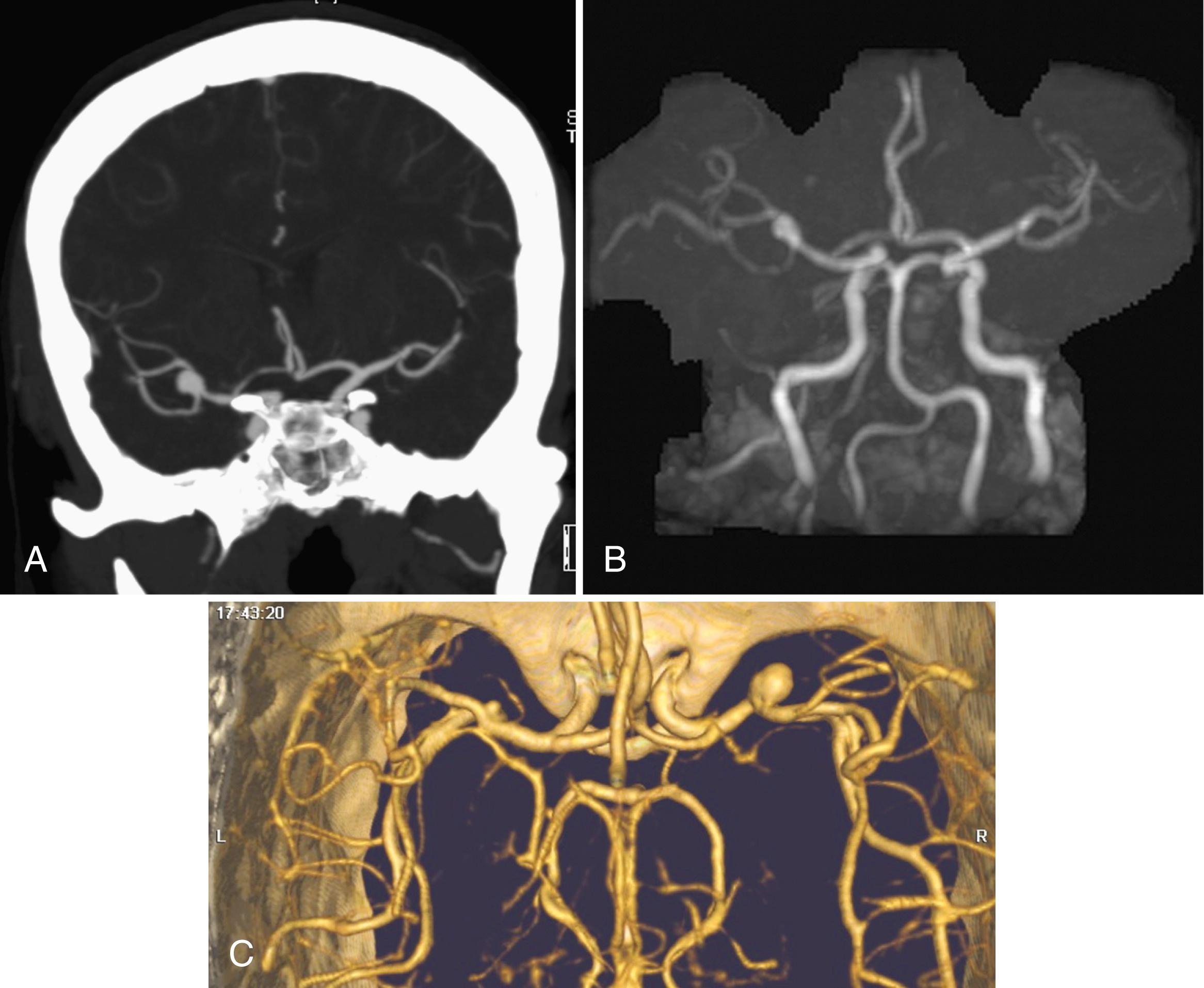
In general, the accuracy of CTA is felt to be at least equal if not superior to that of MRA ( Figs. 41.6 and 41.7 ) in most circumstances, and in some cases, its overall accuracy approaches that of DSA ( ). CTA can provide quantitative information, such as dome-to-neck ratios and aneurysm characterization, such as the presence of mural thrombi or calcium, branching pattern at the neck, and the incorporation of arterial segments in the aneurysm. The incorporation of 3D volume-rendered images in particular provided a surgically useful display of the aneurysm sac in relation to skull base structures (see Fig. 41.7 ). Additionally, 3D CTA may help identify cerebral veins, which generally display more anatomical variation than arteries. The presence of an unexpected vein or the lack of collateral drainage from a region drained by a vein that may need to be sacrificed during surgery can alter the approach to resection of an aneurysm. This anatomical information may permit more informed selection for a therapeutic procedure (surgery versus endovascular coiling) and in planning the treatment approach.
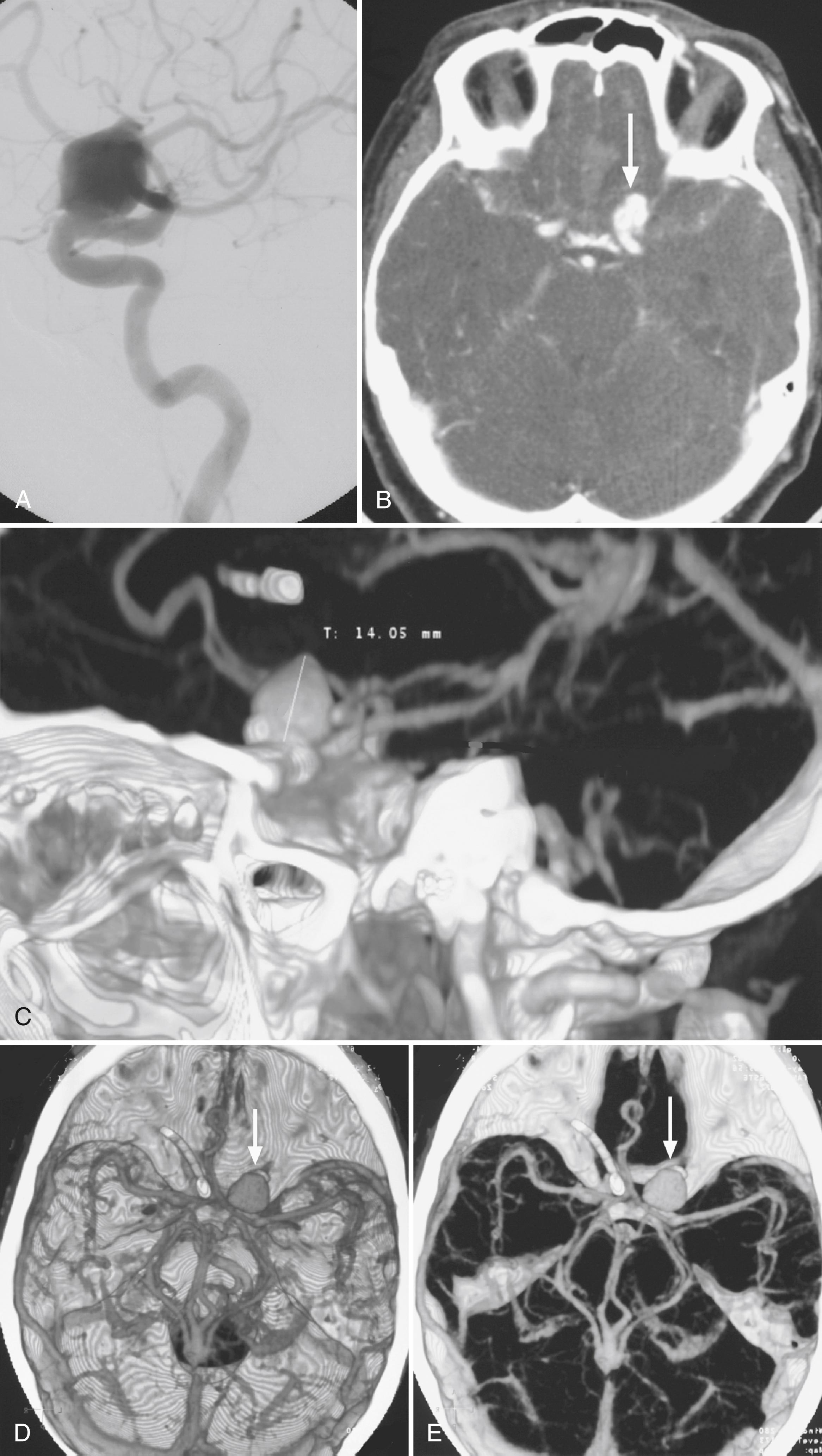
For the detection of cerebral aneurysms, a meta-analysis of eight studies demonstrated that CTA had a pooled sensitivity of 99% and a specificity of 94% on a per-patient basis. On a per-aneurysm basis, the pooled sensitivity was 96% and the specificity was 91% ( ). Interrater agreement has been noted to be high when evaluating aneurysm features, such as location, side, maximum diameter, and dome of the intracranial aneurysm. The level of agreement has been observed to be lower during assessment of neck diameter, presence of multiple aneurysms, and aneurysm morphology. The degree of interrater agreement increases with rater seniority emphasizing the importance of interpretation experience ( ). conducted a large single-center retrospective evaluation demonstrating a lower sensitivity of 57.6% for smaller aneurysms less than 5 mm and 45% for aneurysms originating from the ICA. The limited sensitivity of conventional CTA for the detection of very small aneurysms and aneurysms adjacent to the skull can be significantly improved by using subtracted CTA, which offers bone-free visualization. evaluated subtracted 320 detector-row volumetric CTA for the detection of small aneurysms less than 3 mm and found sensitivity, specificity, and accuracy were 96.9%, 99.2%, and 98.6%, respectively, on a per-aneurysm basis. In contrast to typical aneurysms located near the base of the brain, distal aneurysms such as mycotic and oncotic aneurysms may be more difficult to detect on CTA. One study found a lower sensitivity of 45.5% and a specificity of 90.0%, indicating DSA should be considered when strong clinical suspicion exists for such aneurysms ( ). For patients presenting with a nontraumatic subarachnoid hemorrhage and a negative CTA, causative vascular pathology has been identified with subsequent DSA in 9%–13% of cases ( ). However, in specific cases of perimesencephalic subarachnoid hemorrhage, which are rarely associated with a ruptured aneurysm, CTA has been noted to have a NPV as high as 100%, suggesting that follow-up DSA may not be warranted in these patients ( ).
Postoperative aneurysms typically require follow-up imaging to exclude the presence of residual aneurysm, new aneurysmal growth, or recanalization. For the detection of treated aneurysms, one meta-analysis found that CTA had a sensitivity and specificity of 92.6% and 99.3%, respectively, using multidetector CTA. Although DSA remains the gold standard, CTA may present a promising, cost-effective, noninvasive alternative for long-term evaluation ( ). However, noted that, in a tertiary center, CTA had limited accuracy, particularly with small aneurysms, with a 20.5% false-positive rate most often in the anterior communicating artery or basilar artery bifurcation regions. Additionally, they noted a 21.6% false-negative rate most commonly in the cavernous segment ICA and MCA regions. There is an increasing utilization of flow diversion and intrasaccular embolization devices for the treatment of intracranial aneurysms, which pose new challenges for follow-up evaluation with CTA due to increased metallic artifacts and limited visualization of aneurysmal contrast opacification. Several small studies have noted technical feasibility and reliability in using this modality for subsequent assessment, but further prospective investigation is warranted ( ).
A common sequela of aneurysmal subarachnoid hemorrhage is the development of cerebral vasospasm. TCDs are more commonly employed for surveillance, while DSA remains the gold standard for confirming this condition. However, CTA may offer high diagnostic accuracy as one meta-analysis demonstrated a pooled sensitivity of 79.6% and a specificity of 93.1% ( ). This modality may offer a sufficient alternate means of evaluation, especially for patients who may not have sufficient bone windows for ultrasound evaluation.
A cerebral arteriovenous malformation (AVM) requires DSA for accurate spatial and temporal assessment of blood flow to the feeding arteries, nidus, and draining veins. CTA has been found to have sensitivities of 87% and 96% for ruptured and unruptured AVMs, respectively. For large AVMs (>3 cm), the overall sensitivity was found to be 100%. Importantly, the sensitivity for identifying associated aneurysms was 88%, making this a useful adjunct imaging modality ( ). The use of 4D CTA allows for improved accuracy in the diagnosis and classification of shunting patterns using the Spetzler-Martin grading system for AVMs. Moreover, cross-sectional imaging and perfusion data obtained from this modality may assist in treatment planning ( ).
Limited data exist for CTA in the identification of a dural arteriovenous fistula (DAVF), but recent investigations have demonstrated that 4D CTA may correctly reveal the angioarchitecture and differentiate the various patterns of venous drainage. ( ). reported that 4D CTA may be used for the follow-up assessment of patients who underwent transarterial DAVF embolization. Despite differences in temporal and spatial resolutions, the intermodality agreement between 4D CTA and DSA has been found to be excellent in determining shunt location, identification of drainage veins, and fistula occlusion after treatment. This modality may offer a potentially feasible alternative for follow-up evaluation. A retrospective review of eight studies evaluating 4D CTA for the detection of both AVMs and DAVFs noted a pooled sensitivity of 77% with a specificity of 100%. The use of 4D CTA offers a practical, minimally invasive alternative for evaluating these cerebrovascular pathologies and may reduce the need for DSA, which carries a risk of important complications ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here