Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In simple terms, the cardiovascular system consists of a sophisticated pump (i.e., the heart) and a remarkable array of tubes (i.e., the blood and lymphatic vessels). Arteries and arterioles (the efferent blood vessels in relation to the heart) deliver oxygen, nutrients, paracrine hormones, blood and immune cells, and many other products to the capillaries, small-caliber, thin-walled vascular tubes. These substances are then transported through the capillary wall into the extravascular tissues, where they participate in critical physiological processes. In turn, waste products are transported from the extravascular space back into the blood capillaries and returned by the venules and veins (the afferent vessels) to the heart. Alternatively, approximately 10% of the fluid returned to the heart courses via the lymphatic system to the large veins. To develop normally, the embryo requires the delivery of nutrients and removal of waste products beginning early in development, and, indeed, the cardiovascular system functions early during morphogenesis.
The fields of vascular embryology and angiogenesis have been revolutionized through experimentation with model organisms. In particular, this chapter focuses on key studies using common vascular developmental models, including the mouse, zebrafish, chick, and chick-quail transplants, each of which has its advantages. Among mammals, the most powerful genetic-engineering tools and the greatest breadth of mutants are readily available in the mouse. Furthermore, the mouse is a good model of many aspects of human vascular development, and, in particular, the vasculature of the mouse retina is a powerful model as it develops postnatally and is visible externally. The zebrafish is a transparent organism that develops rapidly with a well-described pattern of cardiovascular morphogenesis and sophisticated genetic manipulations that are readily available. The chick egg is large with a yolk sac vasculature that is easily visualized and develops rapidly. And finally, the coupling of chick-quail transplants with species-specific antibodies allows for cell tracing experiments. The combination of studies with these powerful model systems, as well as others, has yielded key insights into human vascular embryology and angiogenesis.
Although blood vessels are composed of three tissue layers, the vast majority of the vascular-development literature has focused on the morphogenesis of the intima, or inner, layer. This intima consists of a single layer of flat endothelial cells (ECs) that line the vessel lumen and are elongated in the direction of flow. Moving radially outward, the next layer is the media consisting of layers of circumferentially oriented vascular smooth muscle cells (VSMCs) and extracellular matrix (ECM) components, including elastin and collagen. In smaller vessels, such as capillaries, the mural cells consist of pericytes instead of VSMCs. Finally, the outermost layer of the vessel wall is the adventitia, a collection of loose connective tissue, fibroblasts, macrophages, cells expressing stem-cell markers, and small vessels, known as the vasa vasorum, that perfuse the cells of larger arteries.
This chapter summarizes many of the key molecular and cellular processes and underlying signals in the morphogenesis of the different layers of the blood vessel wall and of the circulatory system in general. Specifically, for intimal development, it concentrates on early EC patterning, specification and differentiation, lumen formation, branching, metabolism, and lymphatic vessel morphogenesis. In the second section, the development of the tunica media is divided into subsections examining the components of the media, VSMC origins, smooth muscle cell (SMC) differentiation, and patterning of the developing VSMC layers and the ECM. Finally, the chapter concludes with a succinct summary of morphogenesis of the adventitia, adventitial stem cells, and macrophages. Understanding these fundamental vascular developmental processes is important from a pathophysiological and therapeutic standpoint because many diseases almost certainly involve the recapitulation of developmental programs. For instance, in many vascular disorders, mature VSMCs dedifferentiate and exhibit increased rates of proliferation, migration, and ECM synthesis through a process termed phenotypic switching .
Development begins with the fertilization of the ovum by the sperm. Chromosomes of the ovum and sperm fuse, and then a mitotic period ensues. The early 16- to 32-cell embryo, or morula , consists of a sphere of cells with an inner core termed the inner cell mass . The first segregation of the inner cell mass generates the hypoblast and epiblast . The hypoblast gives rise to the extraembryonic yolk sac and the epiblast to the amnion and the three germ layers of the embryo, known as the endoderm , mesoderm , and ectoderm . The epiblast is divided into these layers in the process of gastrulation when many of the embryonic epiblast cells invaginate through the cranial-caudal primitive streak and become the mesoderm and endoderm, while the cells that remain in the embryonic epiblast become the ectoderm. Most of the cardiovascular system derives from the mesoderm, including the initial ECs, which are first observed during gastrulation. A notable exception to the mesodermal origin is the SMCs of the aortic arch and cranial vessels, which instead derive from the neural crest cells of the ectoderm.
Although ECs are thought to derive exclusively from mesodermal origins, the other germ layers may play an important role in regulating the differentiation of the mesodermal cells to an EC fate. In a classic study of quail-chick intracelomic grafts, host ECs invaded limb bud grafts, whereas in internal organ grafts, EC precursors derived from the graft itself. Thus the authors hypothesized that the endoderm (i.e., from internal organ grafts) stimulates the emergence of ECs from associated mesoderm, whereas the ectoderm (i.e., from the limb bud grafts) may have an inhibitory influence. Yet, the endoderm does not appear to be absolutely required for the initial formation of EC precursors.
The initial primitive vascular system is formed prior to the first cardiac contraction, and the early development of ECs involves the interplay of multiple signaling pathways. This early vasculature develops through vasculogenesis, a two-step process in which mesodermal cells differentiate into angioblasts in situ, which, in turn, subsequently coalesce into blood vessels. Early in this process, many EC progenitors apparently pass through a bipotential hemangioblast stage in which they can give rise to endothelial or hematopoietic cells. Fibroblast growth factor (FGF) 2 and bone morphogenetic protein (BMP) 4 signaling are required for mesoderm specification and its differentiation toward endothelial and hematopoietic cell fates. In addition, Indian hedgehog is secreted by the yolk sac visceral endoderm during vasculogenesis and promotes the differentiation of posterior epiblast cells into both endothelial and hematopoietic cells. The visceral endoderm also secretes vascular endothelial growth factor (VEGF), which is widely implicated in EC biology. The ligand VEGF-A signals predominantly through receptor VEGFR2, and Vegfr2 -null embryos lack blood vessel islands and vasculogenesis and die in utero. Importantly, most genes that specify an EC fate contain binding sites for the E-twenty-six (ETS) family of transcription factors, and ETS variant 2 regulates the differentiation of mesodermal progenitors toward an EC fate.
Following the formation of the initial vascular plexus, more capillaries are generated through sprouting and nonsprouting angiogenesis, and the vascular system is refined through pruning and regression. In the most well studied form of angiogenesis, existing blood vessels sprout new vessels, usually into areas of low perfusion, through a process involving proteolytic degradation of surrounding ECM, EC proliferation and migration, lumen formation, and EC maturation. Nonsprouting angiogenesis is often initiated by EC proliferation, which results in lumen widening. The lumen then splits through transcapillary ECM pillars or fusion and splitting of capillaries to generate more vessels. In addition, the developing vascular tree is fine-tuned by the pruning of small vessels. Although not involved in the construction of the initial vascular plan, flow is an important factor in shaping the maturation of the vascular system, determining which vessels mature and which regress. For instance, unperfused vessels will regress.
Classically, it was thought that arterial and venous blood vessel identity was established as a result of oxygenation and hemodynamic factors, such as blood pressure, shear stress, and the direction of flow. However, over the past two decades, it has become increasingly evident that arterial-specific and venous-specific markers are segregated to the proper vessels quite early in the program of vascular morphogenesis. For instance, ephrinB2, a transmembrane ligand, and one of its receptors, the EphB4 tyrosine kinase, are expressed in the mouse embryo in an arterial-specific and relatively venous-specific manner, respectively, prior to the onset of angiogenesis ( Fig. 1.1 ). EphrinB2 and EphB4 are each required for normal angiogenesis of both arteries and veins. However, in mice homozygous for a tau-lacZ knock-in into the ephrinB2 or EphB4 locus (which renders the mouse null for the gene of interest), lacZ staining is restricted to arteries or veins, respectively. This result indicates that neither of these signaling partners is required for the arterial and venous specification of ECs.
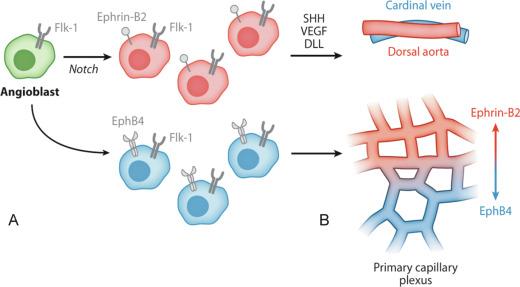
Furthermore, even before initial ephrinB2 and EphB4 expression and prior to the first heartbeat, Notch pathway members, delta C and gridlock, mark presumptive ECs in the zebrafish. The zebrafish gene deltaC is a homologue of the Notch ligand gene Delta , and gridlock (grl) encodes a basic helix-loop-helix protein that is a member of the Hairy-related transcription factor family and is downstream of Notch. The lateral plate mesoderm (LPM) contains artery and vein precursors, and prior to vessel formation, the grl gene is expressed as two bilateral stripes in the LPM. Subsequently, gridlock expression is limited to the trunk artery (dorsal aorta) and excluded from the trunk vein (cardinal vein). Lineage-tracking experiments of the zebrafish LPM suggest that by the 7- to 12-somite stage, an individual angioblast is destined to contribute in a mutually exclusive fashion to the arterial or venous system.
In addition to being an early marker of arterial ECs, the Notch pathway is a key component of a signaling cascade that regulates arterial EC fate (see Fig. 1.1 ). In zebrafish, downregulating the Notch pathway through genetic means or injection of mRNA encoding a dominant-negative Suppressor of Hairless, a known intermediary in the Notch pathway, results in reduced ephrinB2 expression with loss of regions of the dorsal aorta. Reciprocally, contiguous regions of the cardinal vein expand and EphB4 expression increases. By contrast, activation of the Notch pathway results in reduced expression of flt4, a marker of venous cell identity, without an effect on arterial marker expression or dorsal aorta size, suggesting that Notch is not sufficient to induce arterial EC fate. Further studies demonstrated that Sonic hedgehog (SHH) is upstream of VEGF, and VEGF-A binds to VEGFR2 and its coreceptor neuropilin 1 (NRP1), leading to activation of Notch signaling. The specific expression of NRP1 and NRP2 in arteries and veins, respectively, is established prior to blood flow. Importantly, the fate of venous ECs is mediated primarily by the chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII). Deletion of the gene encoding COUP-TFII in ECs leads to veins expressing NRP1 and Notch signaling molecules. Taken together, these results suggest that the SHH-VEGF-Notch axis is necessary for arterial EC differentiation and that venous identity is not simply a default pathway in the absence of Notch signaling but is actively maintained by COUP-TFII.
A study of the origins of the coronary vascular endothelium highlights the plasticity of ECs during early mouse development. This study suggests that ECs sprout from the sinus venosus, the structure which returns blood to the embryonic heart, and dedifferentiate as they migrate over and through the myocardium. ECs that invade the myocardium differentiate into the coronary arterial and capillary ECs, whereas those that remain on surface of the heart will redifferentiate into the coronary veins.
Tubular structures are essential for diverse physiological processes, and the proper construction of these tubes is critical. Tube morphogenesis requires the coordinated migration and growth of cells that comprise the tubes, and the intricate modulation of the biology of these cells invariably uses sensors that detect external stimuli. This information is then integrated and translated into a biological response. Important examples of such biological sensors include the growth cones of neurons and the terminal cells of the Drosophila tracheal system. Both of these sensors have long dynamic filopodia that sense and respond to external guidance cues and are critical in determining the ultimate pattern of their respective tubular structures.
Similarly, endothelial tip cells are located at the end of angiogenic sprouts and are polarized with long filopodia that play both a sensory and motor role ( Fig. 1.2 ). In a classic study published approximately 40 years ago, Ausprunk and Folkman reported that on the day after V2 carcinoma implantation into the rabbit cornea, ECs of the host limbal vessels display surface projections that resemble “regenerating ECs,” consistent with what is now classified as tip cell filopodia. Tip cells are highly migratory leading cells, are rarely proliferative, and are enriched in platelet-derived growth factor (PDGF)-B, VEGFR-2, the Notch ligand delta-like ligand 4 (DLL4), and apelin. Proximal to tip cells are stalk cells which also express VEGFR-2 but, unlike tip cells, are highly proliferative (see Fig. 1.2 ). During the initiation of sprouting angiogenesis, endothelial tip cells develop initial projections prior to stalk cell proliferation.
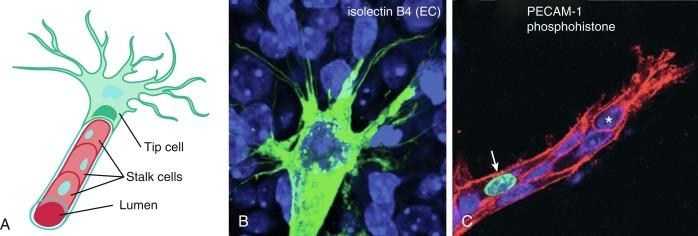
The mouse retina model has been widely used in studies of angiogenesis and is an excellent model for studying different aspects of blood vessel development: the retinal vasculature is visible externally and develops postnatally through a stereotyped sequence of well-described steps. In addition, at most time points, the retina simultaneously includes sprouting at the vascular front and remodeling at the core. The VEGF pathway is critical for guiding angiogenic sprouts, and in the retina, the expression of the ligand VEGF-A is limited to astrocytes with the highest levels at the leading edge of the front of the extending EC plexus, suggesting that the astrocytes lay down a road map for the ECs to follow. VEGF-A signals through VEGFR-2 on tip and stalk cells. Interestingly, the proper distribution of VEGF-A is required for tip cell filopodia extension and tip cell migration, whereas the absolute concentration, but not the gradient, of VEGF-A appears to be critical for stalk cell proliferation.
VEGF induces DLL4 expression, and Notch pathway-mediated lateral inhibition is critical for assigning ECs in sprouting angiogenesis to tip and stalk positions ( Fig. 1.3 ). DLL4 is specifically expressed in arterial and capillary ECs, and in development, DLL4 is enriched in tip cells, whereas Notch activity is greatest in stalk cells. Attenuation of Notch activity through genetic (i.e., Dll4 (+/−) ) or pharmacological (i.e., γ-secretase inhibitors) approaches results in increased capillary sprouting and branching, filopodia formation, and tip cell marker expression.
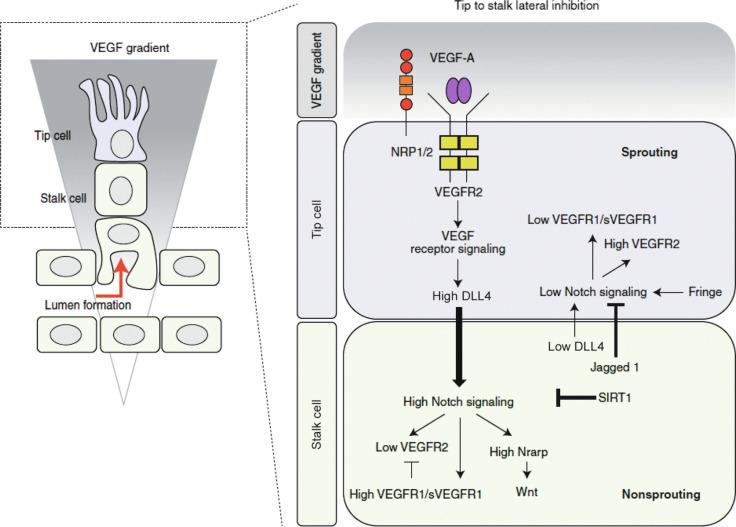
In addition to DLL4, Jagged1 is another Notch ligand that is involved in regulating tip and stalk cell fate in a related but contrasting manner. Stalk cells express higher levels of Jagged1, and Jagged1 levels are inversely proportional to the amount of EC sprouting. Interestingly, Jagged1 antagonizes DLL4-Notch signaling, reducing overall EC Notch activity. Thus high stalk expression of Jagged1 enhances the differential Notch activity in stalk (high activity) versus tip (low activity) cells by competitively inhibiting against DLL4-mediated Notch activity in adjacent tip cells.
Mosaic analyses indicate that competition between cells (in this case for Notch activity) is critical in determining the division of labor in sprouting angiogenesis. Genetic mosaic analysis involves the mixing of at least two populations of genetically distinct cells frequently in the early embryo and, subsequently, comparing the contribution of each cell population to a specific structure or process. Notably, mosaic analysis usually is complementary to experiments with total knockouts and often can, in fact, be more informative as complete removal of a gene may impair interpretation by grossly distorting the tissue architecture or eliminating competition between cells that harbor differing levels of a gene product.
Experiments using mosaic analysis of Notch pathway mutants in a wild-type background indicate that the Notch pathway acts in a cell autonomous fashion to limit the number of tip cells. In comparison to wild-type ECs in the mouse retina, ECs that are genetically engineered to have reduced or no Notch1 receptor expression are enriched in the tip cell population. In addition, mosaic studies of Notch signaling components in the developing zebrafish intersegmental vessels (ISVs) are informative. The ISVs traverse between the somites from the dorsal aorta to the dorsal longitudinal anastomotic vessel (DLAV) and are widely used in investigation of blood vessel development. The ISV has been classified as consisting of three (or four) cells in distinct positions: a base cell that contributes to the dorsal aortic cell, a connector cell that courses through the somites, and the most dorsal cell that contributes to the DLAV. LPM angioblasts contribute to the ECs of all the trunk vasculature, including the dorsal aorta, posterior cardinal vein, ISVs, DLAV, and the subintestinal venous vessels. Precursors destined for the ISVs and DLAV initially migrate to the midline dorsal aorta and then between somites to their ultimate positions. Siekmann and Lawson generated mosaic zebrafish by transplanting into early wild-type embryos marked cells from embryos either lacking the key Notch signaling component recombining protein suppressor of hairless (Rbpsuh) or expressing an activated form of Notch. Interestingly, rbpsuh -deficient cells were excluded from the dorsal aorta and enriched in the DLAV position. In turn, transplanted cells harboring activated Notch mutations were excluded from the DLAV in mosaics and instead preferentially localized to the base cell and dorsal aorta positions.
Taken together, the findings indicate that in sprouting angiogenesis, ECs compete for the tip position through Notch-mediated lateral inhibition of neighboring cells (see Fig. 1.3 ). High levels of DLL4 and Jagged1 are expressed on tip and stalk cells, respectively, resulting in high relative Notch activity in stalk cells and thereby promoting distinct tip and stalk cell identity. Furthermore, in the developing retina, the expression of DLL4 is regulated by VEGF-A, which is secreted by astrocytes in response to hypoxia.
The pattern of many branched structures such as the vasculature is critical for function, and diverse branched structures use similar signaling pathways to generate their specific patterns. A number of well-studied systems, such as the Drosophila trachea, mammalian lung, ureteric bud (UB), and the vasculature, consist of hierarchical tubes, progressing from larger to smaller diameter, that transport important gas and/or fluid constituents. The molecular strategies underlying the morphogenesis of these patterns often includes receptor tyrosine kinase–mediated signaling, as well as fine-tuning with inhibitors of these signaling pathways.
Similar to its key role in Drosophila tracheogenesis, the FGF pathway is essential for determining branch patterning in the mammalian lung. In the mouse, the trachea and lung bronchi bud from the epithelium of the gut wall at approximately E9.5, and, subsequently, three distinct branching subroutines are repeated in various combinations to generate a highly stereotyped, complex treelike structure that facilitates gas exchange. In early embryogenesis, the visceral mesenchyme adjacent to the heart expresses FGF10, and FGF10 binds endodermal FGFR2b. Fgf10 null mice lack lungs and have a blind trachea, and similarly, Fgfr2b (−/−) mice form underdeveloped lungs that undergo apoptosis. As an additional level of regulation, the inhibitor of FGFR signaling, sprouty, is a key component of an FGF-induced negative feedback loop in the lung. In response to FGF10, FGFR2b induces sprouty2 tyrosine phosphorylation and activation, and active sprouty2 inhibits signaling downstream of FGFR2b. In addition, carefully regulated levels of the morphogens SHH and BMP4 modulate the branching of lung airways.
As with the lung, generation of the metanephric kidney requires signals conveyed through epithelial receptor tyrosine kinase. The metanephric mesenchyme secretes glial-derived neurotrophic factor (GDNF), which activates the receptor tyrosine kinase RET and its membrane-anchored coreceptor Gdnf family receptor α 1 (GFRα1), thereby inducing the UB to evaginate from the nephric duct. These components are required for UB branching, because UB outgrowth fails in mice null for Gdnf, Gfr α 1, or Ret . Furthermore, Ret is frequently mutated in humans with renal agenesis. In addition, FGFR2b is also highly expressed on UB epithelium and FGFR2b-mediated signaling regulates UB branching. FGF7 and FGF10 are expressed in mesenchymal tissue surrounding the UB, and FGFR2b binds with comparable affinity to these ligands. Similar to lung development, BMP4-mediated signaling modulates the branching of the renal system.
The most well-studied molecular determinants of vascular branching are the VEGF family of ligands (VEGF-A, -B, -C, and -D) and endothelial receptor tyrosine kinases (VEGFR1, 2, and 3). VEGF is a potent EC mitogen, motogen, and vascular permeability factor, and the level of VEGF is strictly regulated in development, because VEGF heterozygous mice die at approximately E11.5 with impaired angiogenesis and blood island formation. During embryogenesis, VEGFRs are expressed in proliferating ECs and the ligands in adjacent tissues. For instance, the secretion of VEGF by the ventricular neuroectoderm is thought to induce capillary ingrowth from the perineural vascular plexus. Mice null for Vegfr2 or Vegfr1 die at approximately E9.0 with Vegfr2 (−/−) mice lacking yolk-sac blood islands and vasculogenesis, 11 and Vegfr1 (−/−) mice displaying disorganized vascular channels and blood islands. Although VEGFR3 expression eventually restricts to lymphatic ECs, its broad vascular endothelial expression early in development is critical for embryonic morphogenesis. Indeed, Vegfr3 -null mice undergo vasculogenesis and angiogenesis; however, the lumens of large vessels are defective, resulting in pericardial effusion and cardiovascular failure by E9.5. Low oxygen levels induce vascular EC branching through hypoxia inducible factor-1 alpha–mediated expression of VEGFR2. VEGFR1 largely functions as a negative regulator of VEGF signaling by sequestering VEGF-A. The affinity of VEGFR1 for VEGF-A is higher than that of VEGFR2, and VEGFR1 kinase domain mutants are viable.
Although not as well studied as the role of the VEGF pathway in vessel branching, other signaling pathways, such as those mediated by FGF, Notch, and other guidance factors, are also likely to play important roles. For instance, EC-specific deletion of Fgfr1 on a global Fgfr3 -null background attenuates branching of the skin vasculature. In addition, transgenic FGF expression in myocardium augmented coronary artery branching and blood flow, whereas expression of a dominant-negative FGFR1 in retinal-pigmented epithelium reduced the density and branching of retinal vessels. The role of the Notch pathway is discussed earlier in the section of endothelial tip and stalk cells. Finally, the maturation of branches to a more stable state that is resistant to pruning is thought to largely be regulated by signaling pathways that modulate EC branch coverage by mural cells. Interestingly, two of the most important such pathways involve receptor tyrosine kinases such as the angiopoietin-Tie and the PDGF ligand-receptor pathways.
ECs at the tip of newly formed branches do not create lumens, but as the vasculature matures, formation of a lumen is an essential step in generating tubes that can transport products. Angioblasts initially migrate and coalesce to form a solid cord that is subsequently hollowed out to generate a lumen through a mechanism that is controversial. Approximately 100 years ago, researchers first suggested that vascular lumenization in the embryo occurs through an intracellular process involving vacuole formation. Seventy years later, Folkman and Haudenschild developed the first method for long-term culture of ECs, and bovine or human ECs cultured in the presence of tumor-conditioned medium were shown to form lumenized tubes. In this and similar in vitro approaches, an individual cell forms CDC42 + pinocytic vacuoles that coalesce, extend longitudinally, and then join the vacuole of neighboring ECs to progressively generate an extended lumen. Subsequently, a study using two-photon high-resolution time-lapse microscopy suggested that the lumens of zebrafish ISVs are generated through a similar mechanism of endothelial intracellular vacuole coalescence followed by intercellular vacuole fusion.
More recently, however, a number of studies have called this intracellular vacuole coalescence model into question and, instead, support an alternate model in which the lumen is generated extracellularly. One such investigation 63 suggests that in contrast to what has been thought previously, ECs are not arranged serially along the longitudinal axis of the zebrafish ISV but, instead, overlap one another substantially; the circumference of an ISV at a given longitudinal position usually traverses multiple cells. If the lumen of a vessel were derived intracellularly in a unicellular tube, then the tube would be “seamless” (as in the terminal cells of the Drosophila airways) and have intercellular junctions only at the proximal and distal ends of the cells. However, in the 30-hour postfertilization zebrafish, the junctional proteins zona occludens 1 (ZO-1) and VE-cadherin are coexpressed, often in two medial “stripes” along the longitudinal axis of the ISV, suggesting that ECs align and overlap along extended regions of the ISV. Thus the lumen is exclusively an extracellular structure developmentally (i.e., between adjacent cells, and not within the cytoplasm of a single cell).
EC polarization is a prerequisite for lumen formation, and the Par3 complex, VE-cadherin, and microtubule dynamics play a critical role in establishing polarity. Endothelial-specific deletion of the gene encoding integrin β1 reduces levels of Par3 and leads to a multilayered endothelium with cuboidal-shaped ECs and frequent occlusion of mid-sized vascular lumens. VE-cadherin is a transmembrane EC-specific cell adhesion molecule that fosters homotypic interactions between neighboring ECs, and in vascular cords, VE-cadherin is distributed broadly in the apical membrane. VE-cadherin deletion is lethal in the embryonic mouse, because the development of VE-cadherin (−/−) embryonic vessels arrests at the cord stage and does not proceed to lumenization. Under normal conditions, during polarization, junctions form at the lateral regions of the apical membrane as VE-cadherin translocates to these regions, which also harbor ZO-1. VE-cadherin is required for the apical accumulation of de-adhesive molecules, such as the highly glycosylated podocalyxin/gp135, which likely contributes to lumen formation through cell-cell repulsion. In addition to anchoring neighboring ECs, VE-cadherin also is linked through β-catenin, plakogobin, and α-catenin to the F-actin cytoskeleton. In reparative angiogenesis of ischemic hindlimbs, the RAS homolog R-RAS activates AKT and stabilizes microtubules, augmenting lumen formation. VEGF-A treatment also activates AKT but, in contrast, does not induce microtubule stabilization or lumenogenesis. Mechanistically, R-RAS distributes activated AKT along microtubule fibers, all the way to the (+) end, whereas VEGF-A induces perinuclear localization of activated AKT.
Although establishing polarity of the ECs is a critical step, it is not sufficient to induce lumen formation. Indeed, in Vegfa (+/−) mice, ECs of the dorsal aorta polarize but this vessel does not develop a lumen. VEGF-A activates Rho-associated protein kinases (ROCKs), which induce nonmuscle myosin II light chain phosphorylation, thereby enhancing the recruitment of nonmuscle myosin to the apical membrane. Actomyosin complexes at the apical surface are thought to play an important role in pulling the apical membranes of neighboring cells apart, thus generating an extracellular lumen.
Another important component of the process of EC cord lumenization is the dynamic dissolution and formation of inter-EC junctions. EGFL7 is an EC-derived secreted protein that promotes EC motility and is required for tube formation. The knockdown of Egfl7 in zebrafish impairs angioblasts from dissolving their junctions, thus preventing them from separating, which is required for tube formation. Interestingly, the excessive cell-cell junctions in migratory angioblasts may explain the delayed migration of these cells in endodermless zebrafish.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here