Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
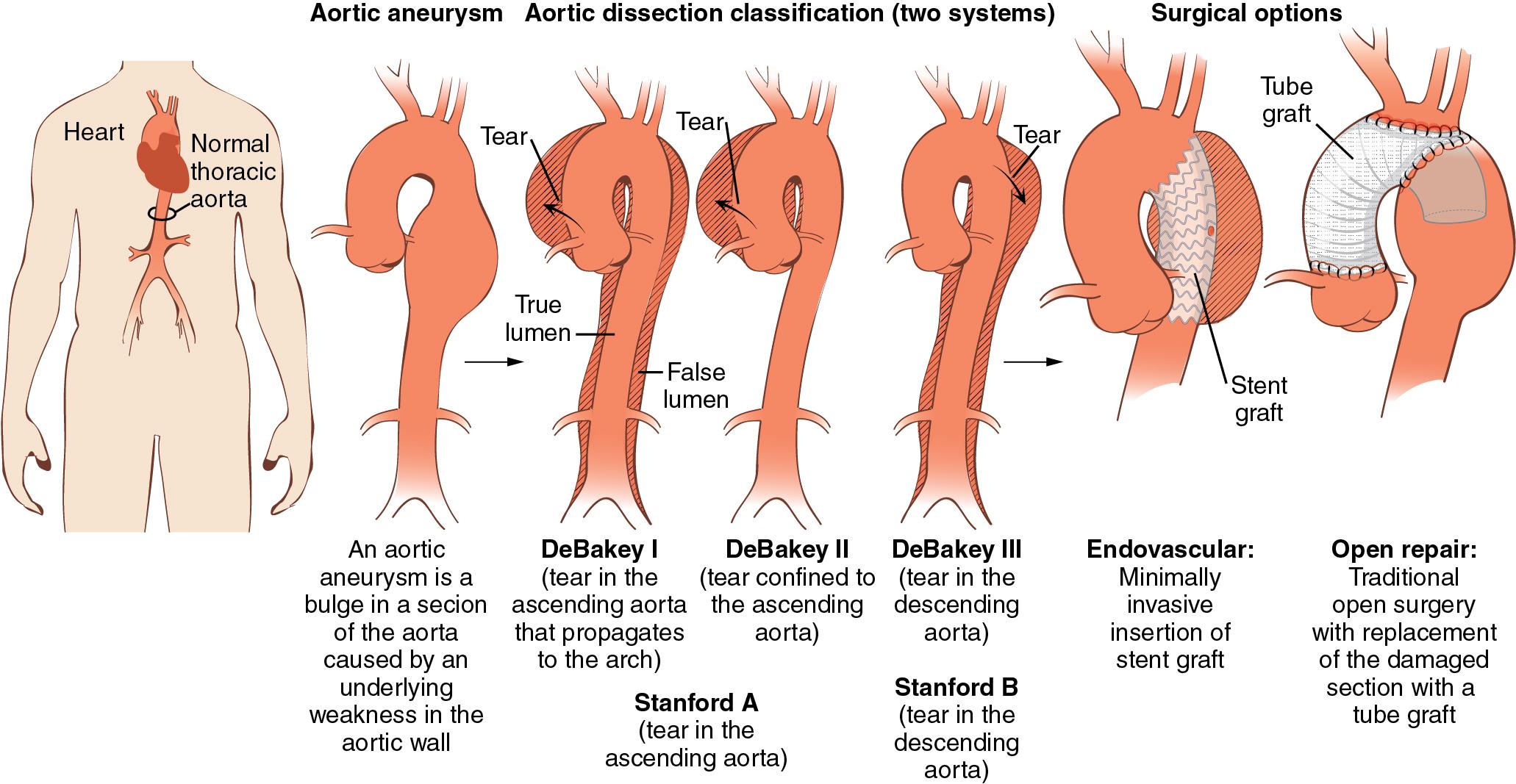
Aneurysms, dissections, and occlusive disease are the main pathologies that can affect arterial vessels. Whereas occlusive disease is more likely to occur in peripheral arteries, the aorta and its major branches are affected by two abnormalities that may be present simultaneously or occur at different stages of the same disease process—aneurysms (more common) and dissections ( Fig. 12.1 and Table 12.1 ). Although there can be some overlap, a clear distinction between these entities is critical, as approach and treatment may be very different. For example, an ascending aortic dissection is a catastrophic event that requires immediate surgical intervention and carries a mortality of 1%–2% per hour for the first 48 hours, with overall mortality between 27% and 58%. That is a very different clinical situation from aortic aneurysms that are primarily treated medically, with surgical intervention needed only when they reach a certain threshold diameter.
| Aortic Aneurysm | Aortic Dissection | |
|---|---|---|
| Definition | Dilatation of all three aortic layers | Blood entry into the media |
| False lumen | No | Yes |
| Predisposing factors | HTN, atherosclerosis, age, male, smoking, family history of aneurysm | HTN, atherosclerosis, preexisting aneurysm, inflammatory diseases, collagen diseases, family history of aortic dissection, aortic coarctation, bicuspid aortic valve, Turner syndrome, CABG, previous aortic valve replacement, cardiac catheterization, crack cocaine use, trauma |
| Symptoms | May be asymptomatic or present with pain mostly due to compression of adjacent structures or vessels | Severe sharp pain in the posterior chest or back pain |
| Diagnosis | CXR, echocardiography, CT, MRI, angiography | For patients in unstable condition, echocardiography; after patient’s condition is medically stabilized, imaging can include CT, CXR, aortography, MRI, echocardiography. |
| Management | Elective surgical repair, whether thoracic or abdominal, for diameter >6 cm or rapidly enlarging aneurysms with >10-mm growth over 6 mo for thoracic and diameter of >5.5 cm or >5-mm increase for abdominal; endovascular repair recommended owing to better patient outcomes, especially in patients at high risk, although no randomized trial data exist. | Type A dissection: Acute surgical emergency; as accurate diagnosis is made, patient will require acute medical management to decrease blood pressure and aortic wall stress. Type B dissection: If uncomplicated, medical management can be pursued. |
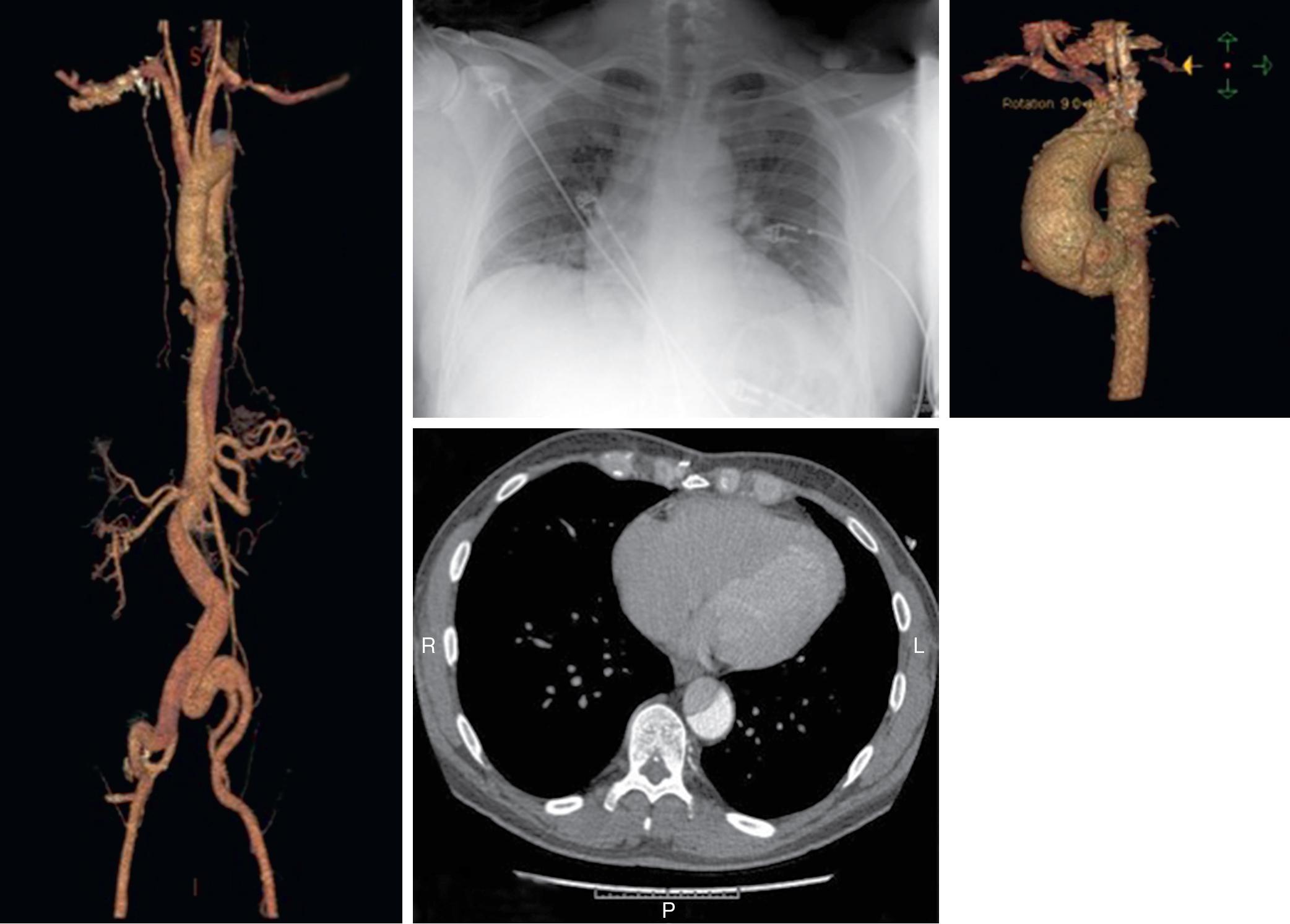
An aneurysm is a dilatation of all three layers of an artery, which appears ballooned in a certain region, such as the ascending or descending portions of the thoracic or abdominal aorta. The most common definition is a 50% increase in diameter compared with normal, or greater than 3 cm in diameter. Arterial diameter depends on age, gender, and body habitus. Aneurysms may occasionally produce symptoms because of compression of surrounding structures, but rupture with exsanguination is the most dreaded complication since only about 25% of patients who experience rupture of an abdominal aortic aneurysm survive ( Fig. 12.2 ).
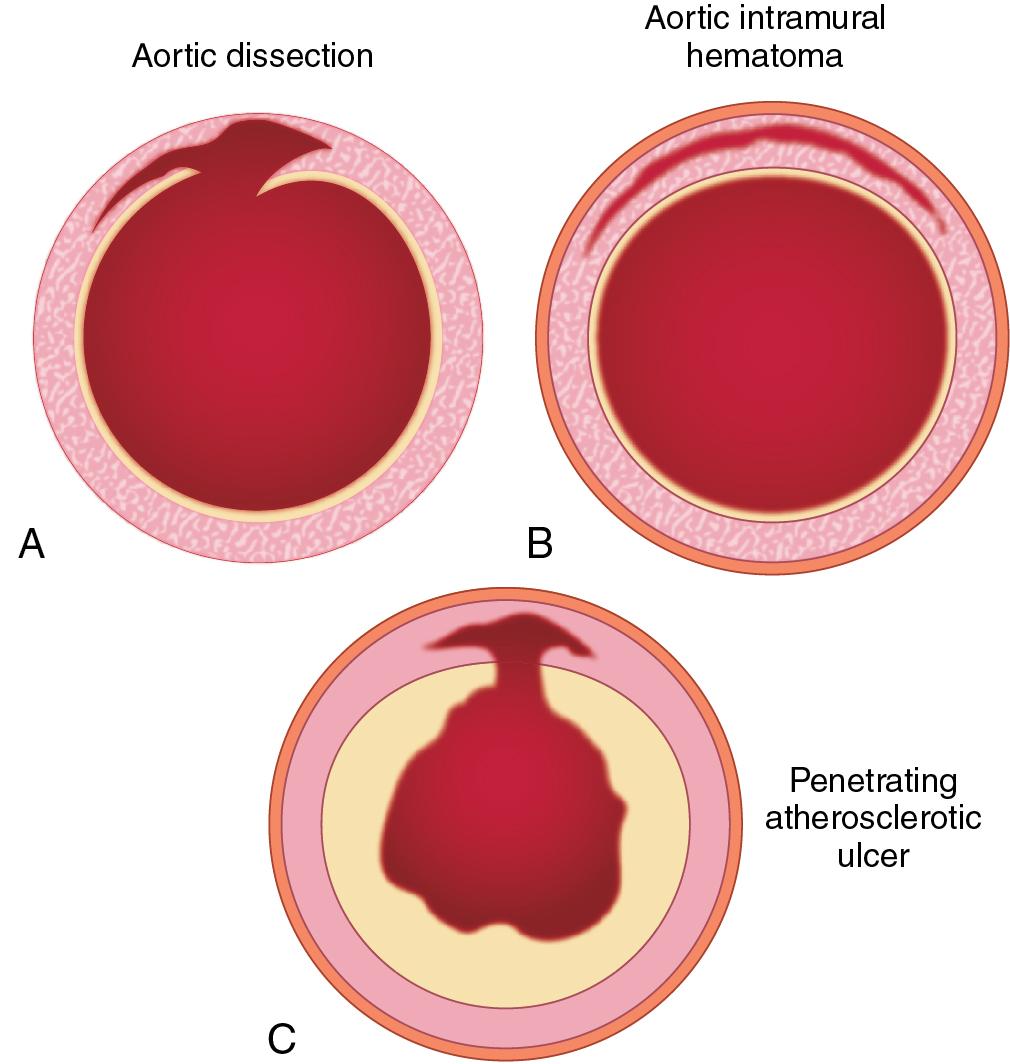
Dissection of an artery occurs when blood enters the medial layer. The media of large arteries is made up of organized lamellar units that decrease in number with distance from the heart. The initiating event of an aortic dissection is a tear in the intima. Blood surges through the intimal tear into an extraluminal channel called the false lumen. Blood in the false lumen can reenter the true lumen anywhere along the course of the dissection. The origins of aortic branch arteries arising from the area involved in the dissection may be compromised and the aortic valve rendered incompetent. This sequence of events occurs over minutes to hours. A delay in diagnosis or treatment can be fatal ( Fig. 12.3 ).
The incidence of descending thoracic aneurysms is 5.9 to 10.4 per 100,000 person-years, and rupture occurs at a rate of 3.5 per 100,000 person-years. Although it is commonly accepted that the threshold for repair is a diameter of 6 cm or larger, one must be aware of the possibility of synchronous aneurysms involving the ascending aorta or arch, which occur in approximately 10% of patients. Dissection of the aorta can originate anywhere along the length of the aorta, but the most common points of origin are in the thorax, in the ascending aorta just above the aortic valve, and just distal to the origin of the left subclavian artery near the insertion of the ligamentum arteriosum.
The most frequently implicated factors in the development of aortic aneurysmal disease are hypertension, atherosclerosis, older age, male sex, family history of aneurysmal disease, and smoking. Causes of aortic dissection are deceleration injuries resulting from blunt trauma and use of crack cocaine, and iatrogenic dissection may occur secondary to aortic cannulation, including cardiac catheterization, cross clamping, aortic manipulation, or arterial incision for surgical procedures such as aortic valve replacement, bypass grafting, or aneurysm operations. Systemic hypertension is a factor that can be implicated in both genetic and nongenetic causes. Aortic dissection is more common in men, but there is also an association with pregnancy. Approximately half of all aortic dissections in women younger than age 40 occur during pregnancy, usually in the third trimester.
Causes of aneurysmal diseases are degenerative due to breakdown of the collagen and elastin in the aortic wall, infectious, and genetic. Thoracic aortic aneurysms and dissections associated with known genetic syndromes are well described. These include both conditions affecting large arteries, such as the aorta, and those involving the microvasculature. Four major inherited disorders are known to affect major arteries: Marfan syndrome, Ehlers-Danlos syndrome, bicuspid aortic valve, and nonsyndromic familial aortic dissection. Although it was once believed that mutant connective tissue proteins corrupted proteins from the normal allele (dominant negative effect) in combination with normal wear and tear, it is now known that matrix proteins, in addition to showing specific mechanical properties, have important roles in the homeostasis of the smooth muscle cells that produce them. Matrix proteins play a key metabolic function because of their ability to sequester and store bioactive molecules and participate in their precisely controlled activation and release. In the inherited disorders associated with aortic dissection, loss of this function (biochemical rather than mechanical) is thought to alter smooth muscle cell homeostasis. The end result is a change in matrix metabolism that causes structural weakness in the aorta.
Marfan syndrome is one of the most prevalent hereditary connective tissue disorders. Its inheritance pattern is autosomal dominant. Marfan syndrome is caused by mutations in the fibrillin-1 gene. Fibrillin is an important connective tissue protein in the capsule of the ocular lens, arteries, lung, skin, and dura mater. Fibrillin mutations can result in disease manifestations in each of these tissues. Because fibrillin is an integral part of elastin, recognition of the mutations in fibrillin led to the assumption that the clinical manifestations of Marfan syndrome in the aorta were secondary to an inherent weakness of the aortic wall exacerbated by aging. However, histologic studies of the aortas of Marfan syndrome patients also demonstrate abnormalities in matrix metabolism that can result in matrix destruction.
Although the genetics of thoracic aortic aneurysm disease in patients with Marfan syndrome are well documented, less is known about familial patterns of aneurysm occurrence not associated with any particular collagen or vascular disease. Up to 19% of people with thoracic aortic aneurysm and dissection do not have syndromes traditionally considered to predispose them to aortic disease. However, these individuals often have several relatives with thoracic aortic aneurysm disease, which suggests a strong genetic predisposition.
Bicuspid aortic valve is the most common congenital anomaly resulting in aortic dilation/dissection. It occurs in 1% of the general population. Histologic studies show elastin degradation in the aorta just above the aortic valve. Echocardiography shows that aortic root dilatation is common even in younger patients with bicuspid aortic valve. Bicuspid aortic valve clusters in families and is found in approximately 9% of first-degree relatives of affected individuals.
Nonsyndromic familial aortic dissection and aneurysm is found in approximately 20% of patients referred for repair of thoracic aneurysm or dissection. Affected families do not meet the clinical criteria for Marfan syndrome and do not have biochemical abnormalities in type III collagen, as in Ehlers-Danlos syndrome. In most of these families the inheritance pattern appears to be dominant with variable penetrance. At least three chromosomal regions have so far been mapped in families with nonsyndromic thoracic aortic aneurysm disease. The specific biochemical abnormalities predisposing to thoracic aortic aneurysm disease remain to be identified.
Abdominal aortic aneurysms have traditionally been viewed as resulting from atherosclerosis. This atherosclerosis involves several highly interrelated processes, including lipid disturbances, platelet activation, thrombosis, endothelial dysfunction, inflammation, oxidative stress, vascular smooth muscle cell activation, altered matrix metabolism, remodeling, and genetic factors. Atherosclerosis represents a response to vessel wall injury caused by processes such as infection, inflammation, increased protease activity within the arterial wall, genetically regulated defects in collagen and fibrillin, and mechanical factors. A familial component has also been identified, because 12% to 19% of first-degree relatives (usually men) of a patient with an abdominal aortic aneurysm will develop an aneurysm. Specific genetic markers and biochemical changes that produce this pathologic condition remain to be elucidated.
Factors that disrupt the normal integrity of the aortic wall or significant increases in shear tension may induce the occurrence of dissections. Examples of conditions associated with aortic dissection are hypertension, genetically triggered aortic disease (see earlier), bicuspid aortic valve, tetralogy of Fallot, atherosclerosis, penetrating atherosclerotic ulcer, trauma, intraaortic balloon pump, aortic/vascular surgery, coronary artery bypass graft, giant cell arteritis, aortitis, syphilis, pregnancy, and weightlifting.
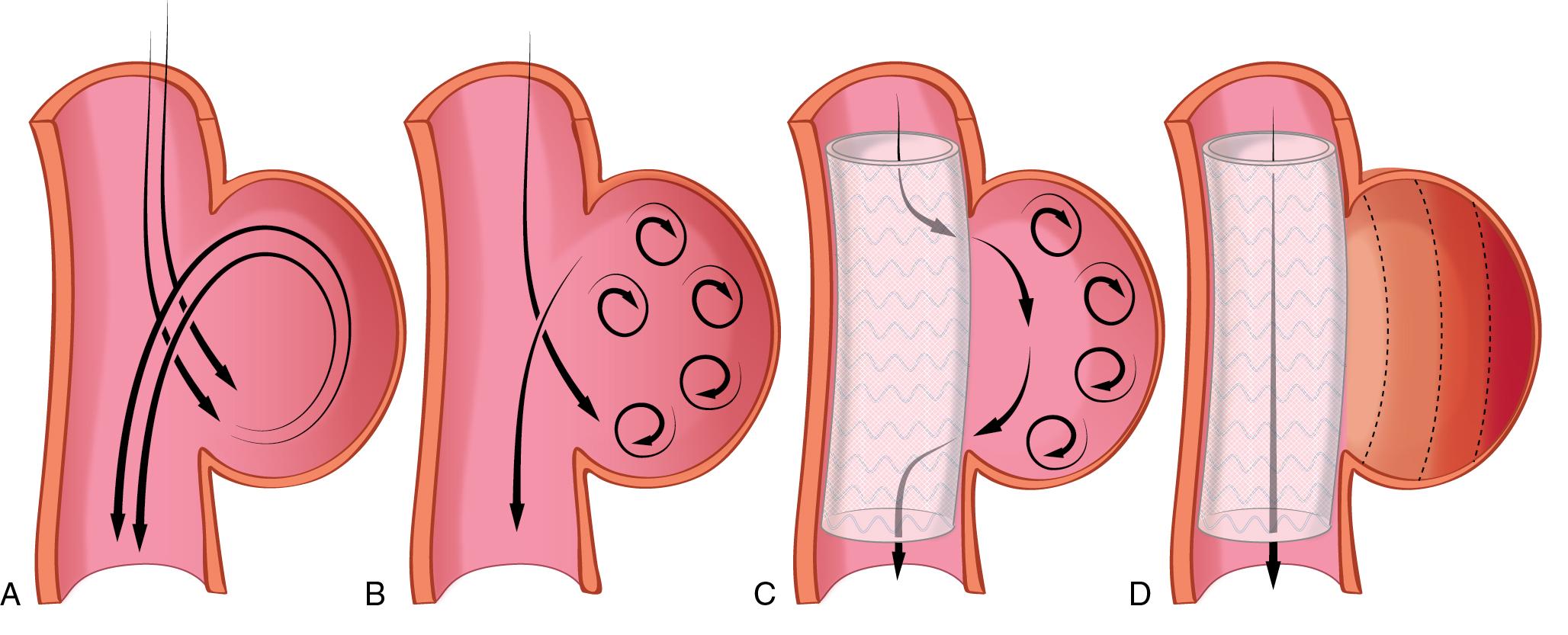
Aortic aneurysms can be classified morphologically as either fusiform or saccular. In fusiform aneurysm there is a uniform dilatation involving the entire circumference of the aortic wall, whereas a saccular aneurysm is an eccentric dilatation of the aorta that communicates with the main lumen by a variably sized neck. Aneurysms can also be classified based on the pathologic features of the aortic wall (e.g., atherosclerosis or cystic medial necrosis).
Arteriosclerosis is the primary lesion associated with aneurysms in the infrarenal abdominal aorta, thoracoabdominal aorta, and descending thoracic aorta. Aneurysms affecting the ascending aorta are primarily the result of lesions that cause degeneration of the aortic media, a pathologic process termed cystic medial necrosis.
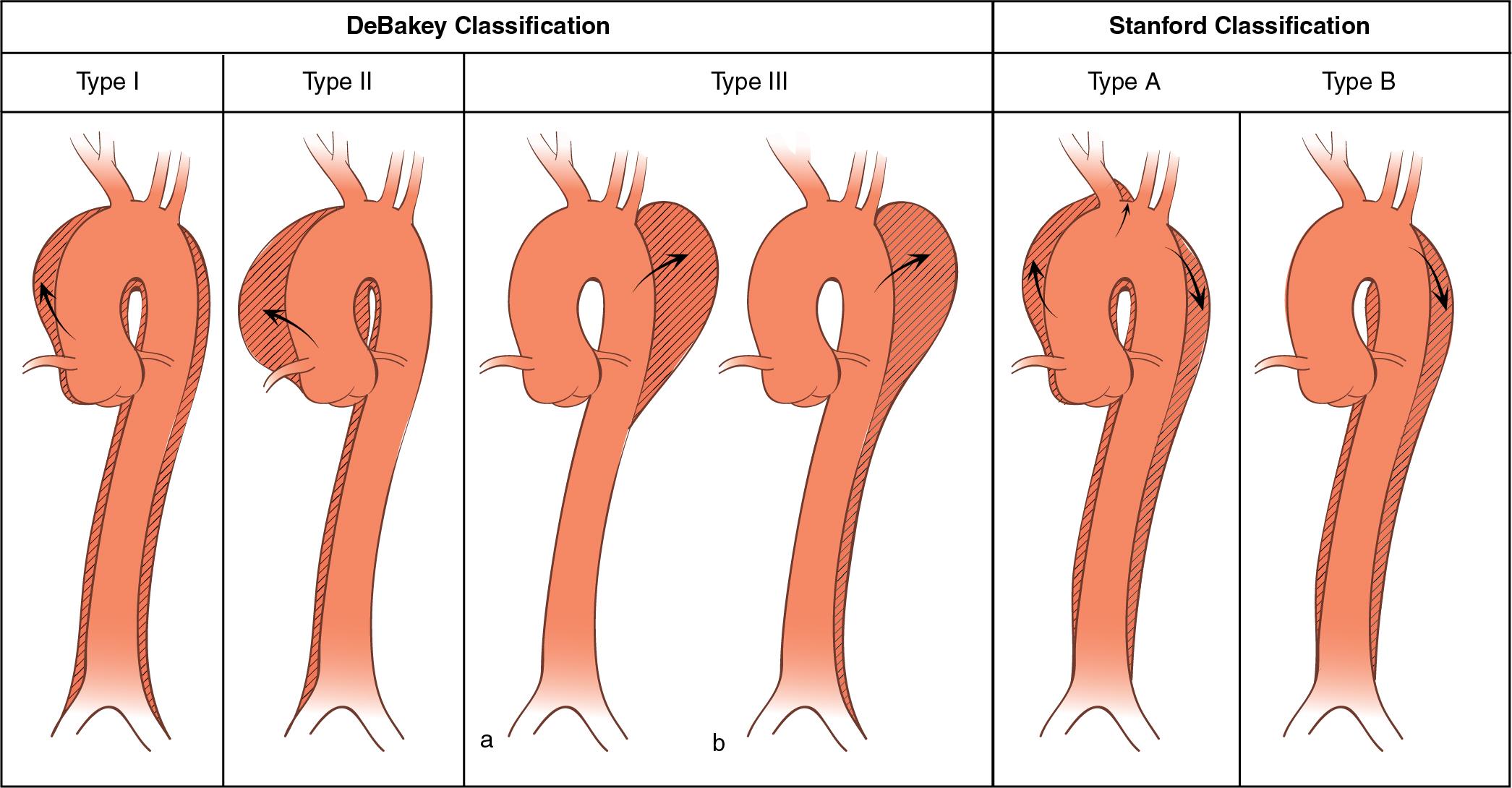
Aneurysms of the thoracoabdominal aorta may also be classified according to their anatomic location. Two classifications widely used for aortic dissection are the DeBakey and Stanford classifications ( Fig. 12.4 ; also see Fig. 12.1 ). The DeBakey classification includes types I to III. In type I, the intimal tear originates in the ascending aorta, and the dissection involves the ascending aorta, arch, and variable lengths of the descending thoracic and abdominal aorta. In DeBakey type II, the dissection is confined to the ascending aorta. In type III, the dissection is confined to the descending thoracic aorta (type IIIa) or extends into the abdominal aorta and iliac arteries (type IIIb). The Stanford classification describes thoracic aneurysms as type A or B. Type A includes all cases in which the ascending aorta is involved by the dissection, with or without involvement of the arch or descending aorta. Type B includes all cases in which the ascending aorta is not involved.
Many patients with thoracic aortic aneurysms are asymptomatic at the time of presentation, and the aneurysm is detected during testing for other disorders. Symptoms resulting from thoracic aneurysm typically reflect impingement of the aneurysm on adjacent structures. Hoarseness results from stretching of the left recurrent laryngeal nerve. Stridor is due to compression of the trachea. Dysphagia is due to compression of the esophagus. Dyspnea results from compression of the lungs. Plethora and edema result from compression of the superior vena cava. Patients with ascending aortic aneurysms associated with dilatation of the aortic valve annulus may have signs of aortic regurgitation and congestive heart failure.
Acute, severe, sharp pain in the anterior chest, neck, or between the shoulder blades is the typical presenting symptom of thoracic aortic dissection. The pain may migrate as the dissection advances along the aorta. Patients with aortic dissection often appear as if they are in shock (vasoconstricted), yet the systemic blood pressure may be quite elevated. Patients who have severe hypotension or even shock at presentation have a worse prognosis. Hypotension at presentation is more common with proximal dissections. Other symptoms and signs of acute aortic dissection, such as diminution or absence of peripheral pulses, reflect occlusion of branches of the aorta and may be followed by inadequate treatment because of falsely low blood pressure measurements. Neurologic complications of aortic dissection may include stroke caused by occlusion of a carotid artery, ischemic peripheral neuropathy associated with ischemia of an arm or a leg, and paraparesis or paraplegia caused by impairment of the blood supply to the spinal cord. Myocardial infarction (MI) may reflect occlusion of a coronary artery. Gastrointestinal ischemia may occur. Renal artery obstruction is manifested by an increase in serum creatinine concentration. Retrograde dissection into the sinus of Valsalva with distortion of aortic valve, aortic insufficiency, and even rupture into the pericardial space leading to cardiac tamponade is a major cause of death. Approximately 90% of patients with acute dissection of the ascending aorta who are not treated surgically die within 3 months.
Abdominal aortic aneurysms are usually detected as asymptomatic pulsatile abdominal masses.
Widening of the mediastinum on chest radiograph may be diagnostic of a thoracic aortic aneurysm. However, enlargement of the ascending aorta may be confined to the retrosternal area, so the aortic silhouette can appear normal. Computed tomography (CT) and magnetic resonance imaging (MRI) can be used to diagnose thoracic aortic disease, but in acute aortic dissection the diagnosis is most rapidly and safely made using echocardiography with color Doppler imaging ( Fig. 12.5 ). Although transthoracic echocardiography (TTE) is the mainstay in evaluation of the heart, including evaluation for complications of dissection like aortic insufficiency, pericardial effusions, and impaired regional left ventricular function, it is of somewhat limited value in assessment of the distal ascending, transverse, and descending aorta. Transesophageal echocardiography (TEE), on the other hand, plays an essential role in diagnosing aortic dissection because it is both highly sensitive and specific (98% and 95%, respectively), has the advantage of using portable equipment, and can be performed as a single study, especially in patients in unstable condition. Angiography of the aorta may be required for patients undergoing elective surgery on the thoracic aorta so that the complete extent of the dissection and the location of all compromised aortic branches can be defined.
Abdominal ultrasonography is a very sensitive test for the detection of abdominal aortic aneurysms. CT is also very sensitive and is more accurate than ultrasonography in estimating aneurysm size.
Improvements in CT technology, such as the advent of helical CT and CT angiography, have increased the role of CT imaging in the evaluation and treatment of abdominal aortic aneurysms. Helical CT provides excellent three-dimensional anatomic detail and is particularly useful for evaluating the feasibility of endovascular stent graft repair of the aneurysm. In addition to radiation exposure, a disadvantage of CT scanning includes the utilization of a contrast substance with potential for worsening renal function.
MRI is useful for accurate measurement of aneurysm size and evaluation of relevant vascular anatomy without the need for the use of ionizing radiation or contrast medium.
Medical management of an aortic aneurysm focuses on decreasing its expansion rate and thus potentially avoiding its evolution toward dissection and/or rupture. Careful management of blood pressure, hyperlipidemia, and smoking cessation are essential. Avoidance of strenuous exercise, stimulants such as cocaine, and overall stress are important aspects of long-term care of these patients. The most commonly used agents are β blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers, as well as statins for lipid control. In addition, patients with known aneurysms should be followed at regular intervals to evaluate for possible continued expansion of their aneurysm and subsequent need for surgical intervention.
Because myocardial ischemia or infarction, respiratory failure, renal failure, and stroke are the principal causes of morbidity and mortality associated with surgery of the thoracic aorta, preoperative assessment of the function of the corresponding organ systems is needed. Assessment for the presence of myocardial ischemia, previous MI, valvular dysfunction, and heart failure is important in performing risk stratification and planning maneuvers for risk reduction. A preoperative percutaneous coronary intervention or coronary artery bypass grafting may be indicated in some patients with ischemic heart disease.
Preoperative evaluation of cardiac function might include exercise or pharmacologic stress testing with or without echocardiography or radionuclide imaging. Severe reductions in vital capacity and FEV 1 (forced expiratory volume in the first second of expiration), as well as abnormal renal function, may mitigate against abdominal aortic aneurysm resection or significantly increase the risk of elective aneurysm repair.
Cigarette smoking and the presence of chronic obstructive pulmonary disease (COPD) are important predictors of respiratory failure after thoracic aortic surgery. Spirometric tests of lung function and arterial blood gas analysis may better define this risk. Reversible airway obstruction and pulmonary infection should be treated with bronchodilators, antibiotics, and chest physiotherapy. Smoking cessation is highly desirable.
The presence of preoperative renal dysfunction is the single most important predictor of the development of acute renal failure after surgery on the thoracic aorta. Preoperative hydration and avoidance of hypovolemia, hypotension, low cardiac output, and nephrotoxic drugs during the perioperative period are important to decrease the likelihood of postoperative renal failure.
Duplex imaging of the carotid arteries or angiography of the brachiocephalic and intracranial arteries may be performed preoperatively in patients with a history of stroke or transient ischemic attacks (TIAs). Patients with severe stenosis of one or both common or internal carotid arteries could be considered for carotid endarterectomy before elective surgery on the thoracic aorta.
As noted earlier, the mainstay of treatment for aortic aneurysm is medical management, so thoracic aortic aneurysm repair is an elective procedure, and surgery is contemplated only when aneurysm size exceeds a diameter of 5.5 cm. This size limit may be decreased somewhat for patients with a significant family history, a previous diagnosis of any of the hereditable diseases that affect blood vessels, or an aneurysm growth rate of 10 mm or more per year. A number of important technical advances have decreased the risk of surgery on the thoracic aorta, including the use of adjuncts such as distal aortic perfusion, profound hypothermia with circulatory arrest, monitoring of evoked potentials in the brain and spinal cord, and cerebrospinal fluid (CSF) drainage, as well as the rapid increase in endovascular procedures for aortic repairs.
The recommendation for surgery for abdominal aortic aneurysms larger than 5.5 cm in diameter is based on clinical studies indicating that the risk of rupture within a 5-year period is 25% to 41% for aneurysms larger than 5 cm. Smaller aneurysms are less likely to rupture, but patients with aneurysms less than 5 cm in diameter should be followed with serial ultrasonography. However, these recommendations are only guidelines. Each patient must be evaluated for the presence of risk factors for accelerated aneurysm growth and rupture, such as tobacco use and family history. If the abdominal aortic aneurysm expands by more than 0.6 to 0.8 cm per year, repair is usually recommended. Surgical risk and overall health are also part of the evaluation to determine the timing of aneurysm repair. Endovascular aneurysm repair is a valid alternative to surgical repair.
The classic triad of hypotension, back pain, and a pulsatile abdominal mass is present in only about half of patients who have a ruptured abdominal aortic aneurysm. Renal colic, diverticulitis, and gastrointestinal hemorrhage may be confused with a ruptured abdominal aortic aneurysm.
Most abdominal aortic aneurysms rupture into the left retroperitoneum. Although hypovolemic shock may be present, exsanguination may be prevented by clotting and the tamponade effect of the retroperitoneum. Euvolemic resuscitation may be deferred until the aortic rupture is surgically controlled in the operating room, because euvolemic resuscitation and the resultant increase in blood pressure without surgical control of bleeding may lead to loss of retroperitoneal tamponade, further bleeding, hypotension, and death.
Patients in unstable condition who have a suspected ruptured abdominal aortic aneurysm require immediate operation and control of the proximal aorta without preoperative confirmatory testing or optimal volume resuscitation.
Ascending and aortic arch dissection requires emergent or urgent surgery; however, descending thoracic aortic dissection is rarely treated with urgent surgery.
The International Registry of Acute Aortic Dissection is a consortium of 21 large referral centers around the world. This registry’s data have shown the in-hospital mortality rate of patients with ascending aortic dissection is approximately 27% in those who undergo timely and successful surgery. This is in contrast to an in-hospital mortality rate of 56% in those treated medically. Other independent predictors of in-hospital death include older age, visceral ischemia, hypotension, renal failure, cardiac tamponade, coma, and pulse deficits.
Long-term survival rates (i.e., survival at 1–3 years after hospital discharge) are 90% to 96% in the surgically treated group and 69% to 89% in those treated medically who survive initial hospitalization. Thus aggressive medical treatment and imaging surveillance of patients who for various reasons are unable to undergo surgery appear prudent.
All patients with acute dissection involving the ascending aorta should be considered candidates for surgery. The most commonly performed procedures are replacement of the ascending aorta and aortic valve with a composite graft (Dacron graft containing a prosthetic valve) or replacement of the ascending aorta and resuspension of the aortic valve. In the last decade it appears that more centers perform valve-sparing surgical procedures that allow for reimplantation of the aortic valve.
In patients with acute aortic arch dissection, resection of the aortic arch (i.e., the segment of aorta that extends from the origin of the innominate artery to the origin of the left subclavian artery) is indicated. Surgery on the aortic arch requires cardiopulmonary bypass, profound hypothermia, and a period of circulatory arrest. With current techniques, a period of circulatory arrest of 30 to 40 minutes at a body temperature of 15°C to 18°C can be tolerated by most patients. Focal and diffuse neurologic deficits are the major complications associated with replacement of the aortic arch. These occur in 3% to 18% of patients, and it appears that selective antegrade cerebral perfusion decreases but does not completely eliminate the morbidity and mortality associated with this procedure.
Patients with an acute but uncomplicated type B aortic dissection who have normal hemodynamics, no periaortic hematoma, and no branch vessel involvement at presentation can be treated with medical therapy. Such therapy consists of (1) intraarterial monitoring of systemic blood pressure and urinary output and (2) administration of drugs to control blood pressure and the force of left ventricular contraction. Short-acting β blockers such as esmolol and nitroprusside or nicardipine, and more recently clevidipine, are commonly used for this purpose. This patient population has an in-hospital mortality rate of 10%. Long-term survival rate with medical therapy only is approximately 60% to 80% at 4 to 5 years and 40% to 50% at 10 years.
Surgery is indicated for patients with type B aortic dissection who have signs of impending rupture (persistent pain, hypotension, left-sided hemothorax); ischemia of the legs, abdominal viscera, or spinal cord; and/or renal failure. Surgical treatment of distal aortic dissection is associated with a 29% in-hospital mortality rate.
Classically the ascending aorta and aortic arch are approached via median sternotomy and require cardiopulmonary bypass. The descending thoracic aorta is repaired through a thoracotomy incision, and abdominal aneurysms are repaired via laparotomy. Endovascular repairs require groin incisions and have only minimal scars.
Surgical resection of thoracic aortic aneurysms can be associated with a number of serious, even life-threatening complications. There is the risk of spinal cord ischemia (anterior spinal artery syndrome) with resulting paraparesis or paraplegia. Cross clamping and unclamping the aorta introduces the potential for adverse hemodynamic responses such as myocardial ischemia and heart failure. Hypothermia, an important neuroprotective maneuver, can be responsible for the development of coagulopathy. Renal insufficiency or renal failure occurs in up to 30% of patients. Approximately 6% of patients will require hemodialysis. Pulmonary complications are common; the incidence of respiratory failure approaches 50%. Cardiac complications are the leading cause of mortality.
Cross clamping the thoracic aorta can result in ischemic damage to the spinal cord (see Fig. 12.2 ). The frequency of spinal cord injury ranges from 0.2% after elective infrarenal abdominal aortic aneurysm repair to 8% in elective thoracic aortic aneurysm repair to 40% in the setting of acute aortic dissection or rupture involving the descending thoracic aorta. Manifestations of anterior spinal artery syndrome include flaccid paralysis of the lower extremities and bowel and bladder dysfunction. Sensation and proprioception are spared.
The spinal cord is supplied by one anterior spinal artery and two posterior spinal arteries. The anterior spinal artery begins at the fusion of branches of both vertebral arteries and relies on reinforcement of its blood supply by six to eight radicular arteries, the largest and most important of which is the great radicular artery of Adamkiewicz. Multiple levels of the spinal cord do not receive feeding radicular branches, which leaves watershed areas that are particularly susceptible to ischemic injury. These areas are in jeopardy during aortic occlusion or hypotension ( Fig. 12.6 ). Damage can also result from surgical resection of the artery of Adamkiewicz (because the origin is unknown) or exclusion of the origin of the artery by the cross clamp. In this situation, not only is the anterior spinal artery blood flow reduced directly, but the potential for collateral blood flow to the spinal cord is also reduced because aortic pressure distal to the cross clamp is very low.
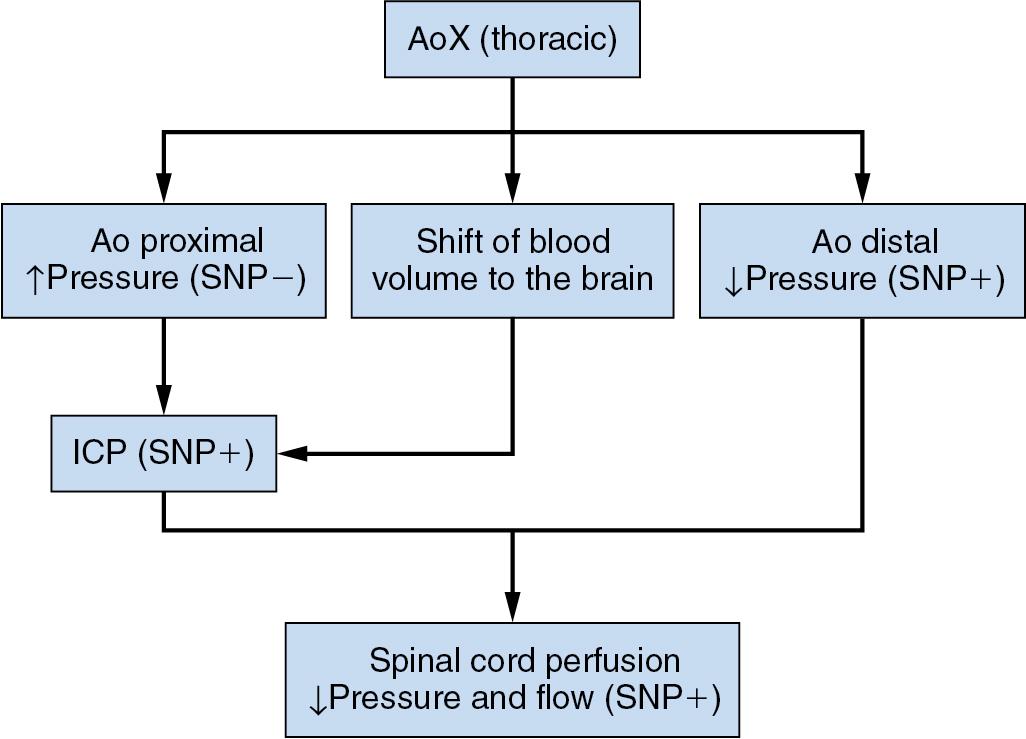
The risk of paraplegia during thoracic aortic surgery is determined by the interaction of four factors: (1) the decrease in spinal cord blood flow, (2) the rate of neuronal metabolism, (3) postischemia reperfusion injury, and (4) blood flow after reperfusion. The duration of aortic cross clamping is critical in determining the risk of paraplegia. A brief period of thoracic aortic cross clamping (<30 minutes) is usually tolerated. If cross clamp time is more than 30 minutes, the risk of spinal cord ischemia is significant, and use of techniques for spinal cord protection is indicated. These include partial circulatory assistance (left atrium to femoral artery bypass), reimplantation of critical intercostal arteries when possible, CSF drainage, maintenance of proximal hypertension during cross clamping, reduction of spinal cord metabolism by moderate hypothermia (30°–32°C), including spinal cooling, avoidance of hyperglycemia, and the use of mannitol, corticosteroids, and/or calcium channel blockers.
There is debate regarding the incidence of spinal cord ischemia after endovascular repair. Although some studies report an incidence similar to open aortic surgery, others show a lower rate with endovascular repair. Nevertheless, the incidence seems to be directly correlated with the severity of aortic disease. The theoretical reason is that although the respective vessel may be taken out of circulation, with endovascular repair (as opposed to open repair) there is no dissection of other vessels that may represent important collateral flow, which ensures perfusion of the spinal cord.
Thoracic aortic cross clamping and unclamping are associated with severe hemodynamic and homeostatic disturbances in virtually all organ systems because of the decrease in blood flow distal to the aortic clamp and the substantial increase in blood flow above the level of aortic occlusion. There is a substantial increase in systemic blood pressure and systemic vascular resistance (SVR), with no significant change in heart rate. A reduction in cardiac output usually accompanies these changes. Systemic hypertension is attributed to increased impedance to aortic outflow (increased afterload). In addition, there is blood volume redistribution caused by collapse and constriction of the venous vasculature distal to the aortic cross clamp. An increase in preload results. Evidence of this blood volume redistribution can be seen as an increase in filling pressures (central venous pressure, pulmonary capillary occlusion pressure, left ventricular end-diastolic pressure). Substantial differences in the hemodynamic response to aortic cross clamping can be seen at different levels of clamping: thoracic, supraceliac, and infrarenal. Changes in mean arterial pressure, end-diastolic and end-systolic left ventricular area and ejection fraction, and wall motion abnormalities may be assessed by TEE or pulmonary artery catheterization and are minimal during infrarenal aortic cross clamping but dramatic during intrathoracic aortic cross clamping. Some of these differences result in part from different patterns of blood volume redistribution. Preload may not increase if the aorta is clamped distal to the celiac artery because the blood volume from the distal venous vasculature may be redistributed into the splanchnic circulation. For the increase in afterload and preload to be tolerated, an increase in myocardial contractility and an autoregulatory increase in coronary blood flow are required. If coronary blood flow and myocardial contractility cannot increase, left ventricular dysfunction is likely. Indeed, echocardiography often indicates abnormal wall motion of the left ventricle during aortic cross clamping, which suggests the presence of myocardial ischemia. Hemodynamic responses to aortic cross clamping are blunted in patients with aortoiliac occlusive disease.
Pharmacologic interventions intended to offset the hemodynamic effects of aortic cross clamping, especially clamping of the thoracic aorta, are related to the effects of the administered drug on arterial and/or venous capacitance. For example, vasodilators such as nicardipine, nitroprusside, and nitroglycerin often reduce the clamp-induced decrease in cardiac output and ejection fraction. The most plausible explanation for this effect is a drug-induced decrease in SVR and afterload, and increased venous capacitance.
It is important, however, to recognize that perfusion pressures distal to the aortic cross clamp are decreased and are directly dependent on proximal aortic pressure (i.e., the pressure above the level of aortic clamping). Blood flow to tissues distal to aortic occlusion (kidneys, liver, spinal cord) occurs through collateral vessels or through a shunt. It decreases dramatically during aortic clamping. Blood flow to vital organs distal to the aortic clamp depends on perfusion pressure, not on cardiac output or intravascular volume.
Clinically, drugs and volume replacement must be adjusted to maintain distal aortic perfusion pressure even if that results in an increase in blood pressure proximal to the clamp. Strategies for myocardial preservation during and after aortic cross clamping include decreasing afterload and normalizing preload, coronary blood flow, and contractility. Modalities such as placement of temporary shunts, reimplantation of arteries supplying distal tissues (spinal cord), and hypothermia may influence the choice of drugs and end points of treatment.
Cross clamping of the thoracic aorta just distal to the left subclavian artery is associated with severe decreases (~90%) in spinal cord and renal blood flow, glomerular filtration rate, and urinary output. Infrarenal aortic cross clamping is associated with a large increase in renal vascular resistance and a decrease (~30%) in renal blood flow. Renal failure following aortic surgery is almost always due to acute tubular necrosis. Ischemia-reperfusion insults to the kidneys play a central role in the pathogenesis of this renal failure.
Cross clamping of the thoracic aorta is associated not only with a decrease in distal aortic/anterior spinal artery pressure but also with an increase in CSF pressure. Presumably, intracranial hypertension resulting from systemic hypertension above the clamp produces redistribution of blood volume and engorgement of the intracranial compartment (intracranial hypervolemia). This results in redistribution of CSF into the spinal fluid space and a decrease in the compliance of the spinal fluid space. CSF drainage may increase spinal cord blood flow and decrease the incidence of neurologic complications.
Pulmonary damage associated with aortic cross clamping and unclamping is reflected by an increase in pulmonary vascular resistance (particularly with unclamping of the aorta), an increase in pulmonary capillary membrane permeability, and development of pulmonary edema. The mechanisms involved may include pulmonary hypervolemia and the effects of various vasoactive mediators.
Aortic cross clamping is associated with formation and release of hormonal factors (caused by activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system) and other mediators (prostaglandins, oxygen free radicals, complement cascade). These mediators may aggravate or blunt the harmful effects of aortic cross clamping and unclamping. Overall, injury to the spinal cord, lungs, kidneys, and abdominal viscera is principally due to ischemia and subsequent reperfusion injury caused by the aortic cross clamp (local effects) and/or the release of mediators from ischemic and reperfused tissues (distant effects).
Unclamping of the thoracic aorta is associated with substantial decreases in SVR and systemic blood pressure. Cardiac output may increase, decrease, or remain unchanged. Left ventricular end-diastolic pressure decreases, and myocardial blood flow increases. Gradual release of the aortic clamp is recommended to allow time for volume replacement and to slow the washout of vasoactive and cardiodepressant mediators from ischemic tissues.
The principal causes of unclamping hypotension include (1) central hypovolemia caused by pooling of blood in reperfused tissues; (2) hypoxia-mediated vasodilation, which causes an increase in vascular capacitance in the tissues below the level of aortic clamping; and (3) accumulation of vasoactive and myocardial-depressant metabolites in these tissues. Vasodilation and hypotension may be further aggravated by the transient increase in carbon dioxide release and oxygen consumption in these tissues following unclamping. Correction of metabolic acidosis does not significantly influence the degree of hypotension following aortic unclamping.
Management of anesthesia in patients undergoing thoracic aortic aneurysm resection requires consideration of monitoring systemic blood pressure, neurologic function, and intravascular volume and planning the pharmacologic interventions and hemodynamic management that will be needed to control hypertension during the period of aortic cross clamping. Proper monitoring is more important than selection of anesthetic drugs in these patients.
Surgical repair of a thoracic aortic aneurysm requires aortic cross clamping just distal to the left subclavian artery or between the left subclavian artery and the left common carotid artery. Therefore blood pressure monitoring must be via an artery in the right arm, since occlusion of the aorta can prevent measurement of blood pressure in the left arm. Monitoring blood pressure both above (right radial artery) and below (femoral artery) the aneurysm is less commonly done but may be useful. This approach permits assessment of cerebral, renal, and spinal cord perfusion pressure during cross clamping.
Blood flow to tissues below the aortic cross clamp is dependent on perfusion pressure rather than on preload and cardiac output. Therefore during cross clamping of the thoracic aorta, proximal aortic pressures should be maintained as high as the heart can safely withstand unless other modalities (e.g., temporary shunts, hypothermia) are implemented. A common recommendation is to maintain mean arterial pressure near 100 mm Hg above the cross clamp and above 50 mm Hg in the areas distal to the cross clamp.
Use of vasodilators to treat hypertension above the level of the aortic cross clamp must be balanced against the likelihood of a decrease in perfusion pressure in the tissues below the clamp. Indeed, nitroprusside may decrease spinal cord perfusion pressure both by decreasing distal aortic pressure and by increasing CSF pressure as a result of cerebral vasodilation (see Fig. 12.6 ). It is prudent to limit the use of drugs that decrease proximal aortic pressure and cause cerebral vasodilation. Use of temporary shunts to bypass the occluded thoracic aorta (proximal aorta–to–femoral artery or left atrium–to–femoral artery shunts) may be considered when attempting to maintain renal and spinal cord perfusion. Partial cardiopulmonary bypass is another option to maintain distal aortic perfusion.
Somatosensory evoked potentials (SEPs) and electroencephalography (EEG) are monitoring methods for evaluating central nervous system viability during the period of aortic cross clamping. Unfortunately, intraoperative monitoring of SEPs is not completely reliable for detecting spinal cord ischemia during aortic surgery as it reflects dorsal column (sensory tract) function. Ischemic changes in anterior spinal cord function (motor tracts) are not detected. Monitoring of motor evoked potentials would indicate anterior spinal cord function but is impractical, since it prohibits use of neuromuscular blocking drugs. Spinal cooling with epidural instillation of iced saline during cross clamping in thoracic aneurysm surgery has been used successfully for many years in some institutions across the United States on the basis that lowering the spinal cord temperature directly will improve recovery of potentially poorly perfused tissues after reimplantation of patent critical intercostal vessels by the surgeon. Spinal drainage has been used to decrease pressure around the spinal cord and avoid ischemia in a confined space if the spinal cord dilates after adequate perfusion is reestablished. CSF pressure is also maintained at a value of less than 10 cm H 2 O in the days immediately after surgery for the same reason—namely, that an increase in pressure in the spinal canal may decrease perfusion to the spinal cord and impair motor function. This method is used successfully in both open surgical procedures and endovascular repairs of the aorta (see later). Another method that can be useful is atriofemoral bypass to maintain distal aortic perfusion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here