Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Over the past decade there have been huge advances in transfemoral (TF) transcatheter aortic valve implantation (TAVI), including changes to both the prosthesis and delivery systems. This evolution has resulted in lower profile delivery systems thus enabling TF TAVI to be performed completely percutaneously. Whereas previously access was achieved predominantly via the femoral artery using a surgical cutdown technique, the use of a vascular closure device has now become standard practice. Percutaneous TF TAVI has been proven to be safer with improved efficacy over alternative access routes. , Benefits include early mobilization within 24 hours of the procedure and shorter length of hospital stay. However, vascular closure is not completely without risk and the vast majority of TAVI-related complications are vascular, attributable to unsuccessful vascular closure. This chapter reviews the advantages of fully percutaneous access and the different commercially available percutaneous closure devices used in TF TAVI and a stepwise approach to closure device delivery.
Highlight favorable vessel characteristics for percutaneous closure
Understand good access techniques that facilitate successful vascular closure
Utility of collagen and suture-based closure devices and their delivery
Understanding advantages and disadvantages of a surgical cutdown technique
Comparison of percutaneous closure devices and success rates
Tips for successful vascular closure
TF TAVI is the least invasive form of access and is superior to other access routes with regard to clinical outcomes.
The vast majority of TAVI procedures are performed transfemorally using a completely percutaneous approach.
Alternative access routes are reserved for use in the small proportion of patients (<20%) in whom femoral access is precluded and are discussed in further detail in Chapter 7 .
Vascular closure is performed using percutaneous vascular closure devices (VCDs) to achieve hemostasis.
The majority of vascular complications associated with TAVI arise in patients with tortuous and heavily calcified iliofemoral vessels. Heavy calcification of the common femoral artery can lead to failure of percutaneous closure devices and the potential for vascular complications.
Desirable characteristics for percutaneous access and closure include reasonable caliber leg vessels (>5 mm in diameter) without significant calcification or tortuosity.
To facilitate patient selection, imaging of the iliofemoral arterial tree should be performed, ideally using a combination of computed tomography (CT) and invasive iliofemoral angiography. CT imaging of the vasculature is essential to confirm a vessel diameter of >5 mm and to assess the extent of calcification (circumferential, anterior wall of the common femoral artery) and the degree of tortuosity, thus establishing suitability for percutaneous access and closure.
VCDs should also be avoided or used with caution in patients with abdominal obesity; increasing distance from the puncture site to the common femoral artery can complicate both percutaneous access and closure and should be avoided when greater than 9.5 cm.
Where vasculature prohibits percutaneous access, alternative access routes and closure methods should be considered.
Good access is key to achieving good closure.
To optimize the chance of successful closure using a VCD, arterial access should be performed using ultrasound guidance.
An ideal puncture site can be identified on ultrasound by an experienced operator. The puncture should be performed below the inguinal ligament to reduce the risk of retroperitoneal bleeding or hematoma but proximal to the bifurcation of the common femoral artery.
Ultrasound should be used longitudinally and in the transverse plane to ensure the puncture is directed in the center of the vessel and in an area free of calcium ( Fig. 11.1 ).
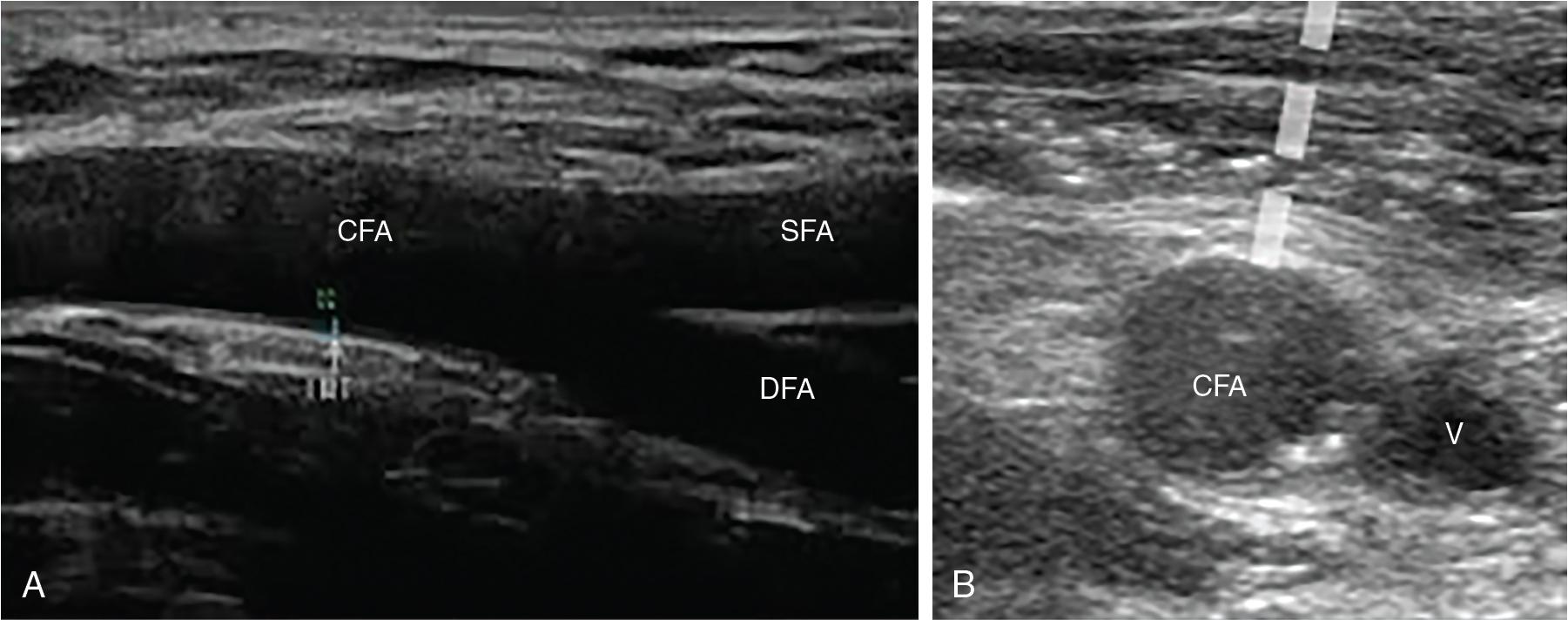
Fluoroscopy using crossover femoral angiography can also be used as an adjunct, and is useful in identifying the bifurcation of the common femoral artery relative to the femoral head and also iliofemoral tortuosity.
Current delivery systems have a minimum sheath size of 14 French and manual pressure alone is often insufficient in achieving prompt hemostasis. The use of percutaneous closure devices is therefore essential to achieving safe closure.
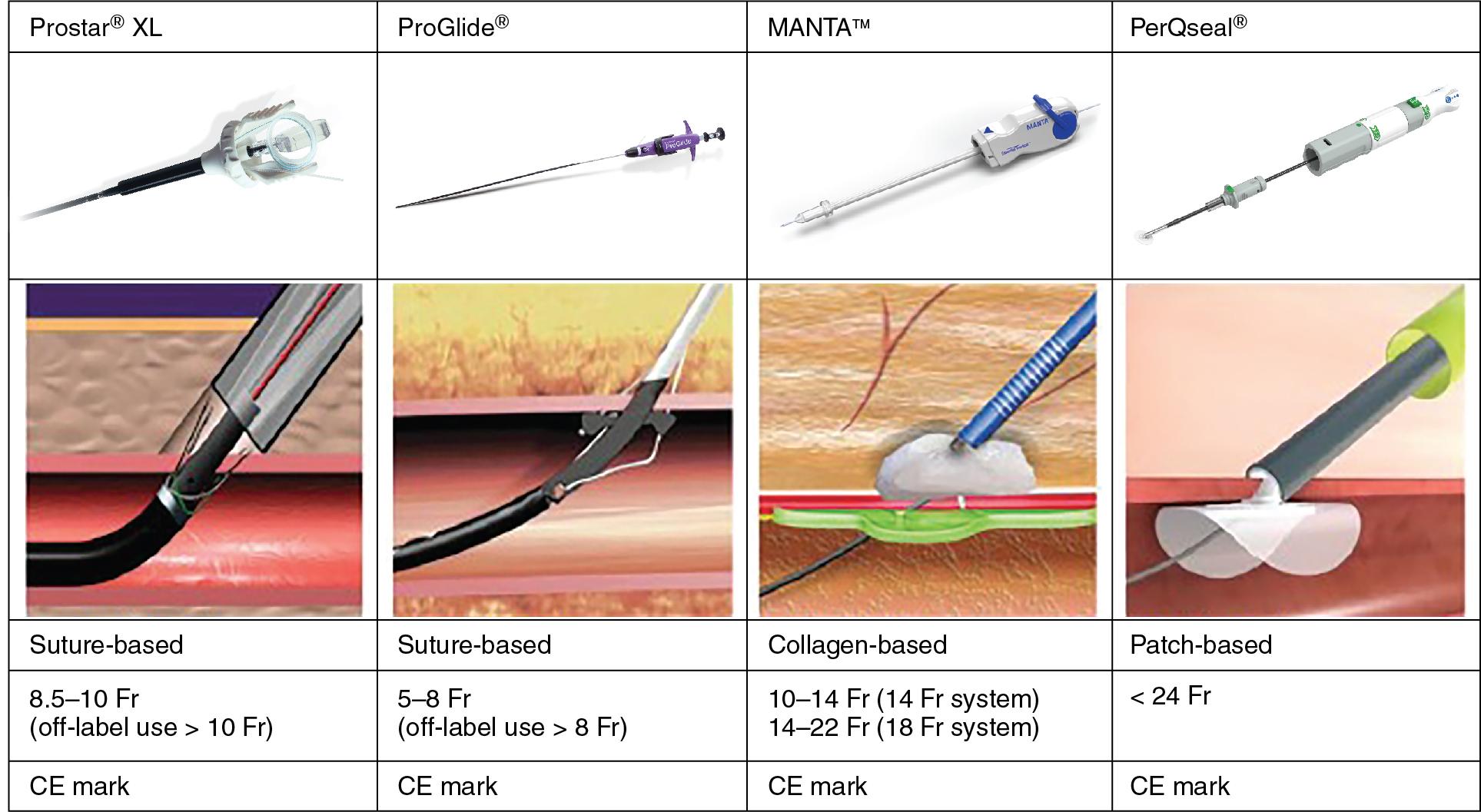
There are a wide range of commercially available Conformité Européenne (CE)–marked devices ( Fig. 11.2 ).
The two most established and widely used VCDs, Perclose ProGlide and Prostar XL (Abbot Vascular), are both suture-based closure devices.
The MANTA system (Teleflex, Wayne, PA) is a novel collagen-based device similar to the Angio-Seal (Terumo, Tokyo).
Another less frequently used closure device is the PerQseal from Vivasure Medical (Galway, Ireland), which features a bioabsorbable intravascular patch.
The MANTA VCD (Teleflex) is a percutaneous collagen-based closure device that became available for use in clinical practice in 2016.
It is similar to the Angio-Seal (Terumo) system used in femoral angiography but designed to close a much larger puncture site.
It is available in two sizes: the 14-French MANTA is designed to close femoral puncture sites from 10- to 14-French devices or sheaths, and the 18-French system is designed to close femoral puncture sites from 15- to 20-French devices. It has less frequently been used for closure of axillary punctures after TAVI.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here