Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The adult nulliparous uterus is a hollow, pear-shaped organ that weighs 40–80 g and measures 7–8 cm along its longest axis. It is divided into the cervix (discussed in Chapter 32 ) and the corpus . The portion of the corpus cephalad to a line connecting the insertion of the fallopian tubes is the fundus . The two lateral regions of the fundus associated with the intramural portion of the fallopian tubes are referred to as the cornua . The portion of the corpus that connects with the cervix is called the isthmus or lower uterine segment .
The uterine cavity has a triangular shape and a length of approximately 6 cm. It is lined by the endometrial mucosa, which constitutes the inner layer (endometrium) of the organ. It is surrounded by a thick muscular layer (myometrium) and a serosal covering, the latter extending to the point of peritoneal reflection. Two useful clues to distinguish the anterior from the posterior side of a hysterectomy specimen at time of gross examination are: (1) the fact that the point of peritoneal reflection is lower on the posterior side; and (2) the fact that the uterine insertion of the fallopian tube is posterior to the insertion of the round ligament.
The uterine lymph vessels drain to a rich network of lymph nodes, the main groups being parametrial and paracervical; internal (hypogastric), external, and common iliac; periaortic; and inguinal.
The endometrial mucosa is made up of glands and stroma. It is divided into a deeply seated basal layer and a superficial functional layer. The basal layer is the equivalent of the reserve cell layer of other epithelia and is responsible for the regeneration of the endometrium following menstruation. It is made up of weakly proliferative glands and spindled stroma. The functional layer is subdivided into two strata, the compactum (toward the surface) and the spongiosum (close to the basalis). The stroma is mainly composed of endometrial stromal cells (whose appearance changes considerably during the menstrual cycle, see later section) and vessels (of which the spiral arterioles are the most distinctive). Also present are stromal granulocytes. The immunohistochemical profile of endometrial stromal cells is “CD10 dominant” in relation to CD34, whereas the reverse is true for the endocervical stroma. The phenotypic profile of the endometrial glands will be discussed in connection with the various pathologic conditions that affect that tissue ; suffice to warn the reader that two markers associated with breast and lung/thyroid—that is, mammaglobin and thyroid transcription factor-1 (TTF-1), respectively—can be expressed by endometrial cells.
During childbearing age, the normal endometrium undergoes a series of sequential changes in the course of the ovulatory cycle that prepare it to receive the ovum ( Fig. 33.1 ). If the ovum is not fertilized, the proliferative endometrium is cast off by menstruation, and the cycle repeats itself. A normal endometrial cycle is associated with changes in both endometrial glands and stroma that allow the pathologist to diagnose microscopically the phase of the menstrual cycle. In a series of classic articles, Noyes and colleagues. set forth specific criteria by which a relatively accurate dating of the endometrium was made possible ( Fig. 33.2 ). At one time it was common for pathologists to be asked to “date” endometrial biopsy specimens (i.e. assess the development of the endometrial glands and stroma, relative to the time of ovulation) that were taken for investigation of infertility, but such requests have dramatically decreased over the past decade; nevertheless all surgical pathologists should be familiar with the normal cyclical changes in the endometrium.
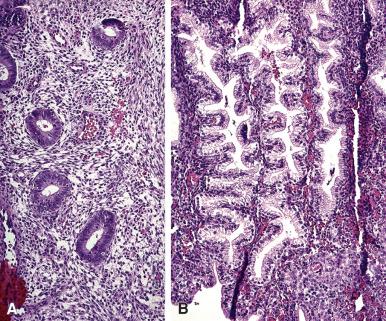
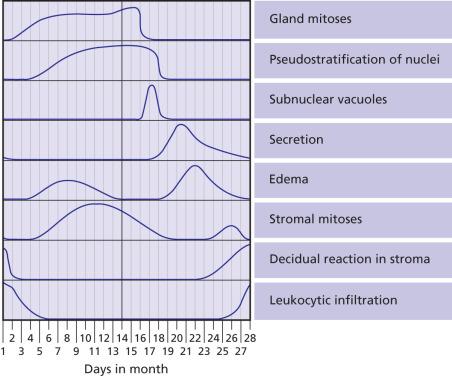
In general, the changes are quite uniform throughout the functional endometrium. The surface epithelium is less responsive to the hormonal influences than the glandular epithelium. For subnuclear vacuolation to be regarded as evidence of ovulation, it should be present in at least 50% of the functional glands present in the section, as patchy development of subnuclear vacuoles is a common finding in endometrium subject to prolonged unopposed estrogenic stimulation (so-called disordered proliferative endometrium). Neutrophils, which are seen in large numbers in areas of tissue degradation (beginning on day 26) are very rare during the other days of the cycle; they should be distinguished from the already mentioned stromal granulocytes, which do not stain for chloroacetate esterase.
The cellular condensation with nuclear crowding, squamoid appearance, and focal cytoplasmic eosinophilia seen in menstrual specimens should not be confused with a malignant or other pathologic process. The possibility of overdiagnosis is even greater in the rare instances in which the menstrual endometrium is seen within the lumen of blood vessels. It should be noted that endometrial tissue can also be found in myometrial vessels independently from menstruation.
The basal layer of the endometrium is not subject to the influence of progesterone. Similarly, the mucosa of the lower uterine segment responds only sluggishly to the hormonal stimulation and can appear different from sampled functionalis. This mucosa gradually merges with that of the endocervix; the hybrid endometrial–endocervical appearance of both glands and stroma allows its recognition in endometrial sampling (either biopsy or dilation and curettage [D&C] specimens).
The reappearance of glandular secretion and stromal edema once predecidual reaction is established—leading to the simultaneous presence of these three features in an endometrial specimen—is evidence that a fertilized ovum has implanted. An exaggerated expression of this pregnancy-related phenomenon is the Arias-Stella reaction , as originally described by the Peruvian pathologist Javier Arias-Stella. In this condition, secretory or proliferative changes in the endometrial glands are accompanied by prominent nuclear changes, manifested by hyperchromasia and marked enlargement ( Fig. 33.3 ). Normal and abnormal mitoses may also be present. These changes are almost always focal and may also occur in the cervix, endocervical polyps, adenomyosis, and endometriosis. They are more often seen in postabortion curettings (present in 20%–70% of cases and persisting for weeks), but they are also seen in normal orthotopic or ectopic pregnancies, hydatidiform mole, choriocarcinoma, and (very rarely) following the administration of exogenous hormones. In hydatidiform mole and choriocarcinoma, the nuclei of the endometrial cells may attain gigantic sizes. The most important differential diagnosis is with serous and clear cell types of endometrial adenocarcinoma. Immunostains for Ki-67 and p53 are helpful in this regard, their being usually low and wild type–pattern, respectively, in the Arias-Stella reaction.
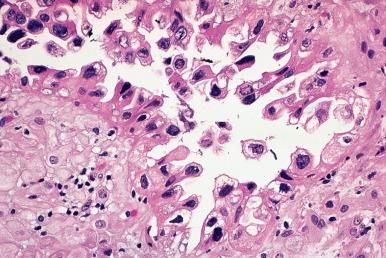
Another interesting pregnancy-related endometrial alteration (often associated with the Arias-Stella reaction) is the focal appearance of optically clear nuclei in the glandular cells, simulating viral inclusions; these are due to the replacement of normal chromatin by a fine filamentous network that is immunoreactive for biotin.
Exceptionally, an “idiopathic” decidual reaction is seen in the postmenopausal endometrium in the absence of an exogenous or endogenous source of progesterone excess.
After the menopause the endometrium becomes inactive (simple glands lined by metabolically inactive, non-mitotic cuboidal epithelial cells and associated stroma) or atrophic (strips of inactive surface epithelial cells with no or minimal stroma). These glands may become cystically dilated (cystic atrophy), but the lining cells have an atrophic appearance, in contrast to the proliferative columnar cells lining cystic glands in endometrial hyperplasia without atypia. Although a distinction can be made between inactive and atrophic endometrium histologically, based on the presence or absence of endometrial stroma, it is not clinically relevant. Endometrial biopsy for postmenopausal bleeding is one of the most common specimens encountered in practice, with most such specimens showing the expected inactive/atrophic endometrium. In most series, approximately 5%–15% of the cases of postmenopausal bleeding are due to endometrial carcinoma and a similar proportion to endometrial polyps. Degenerative and other changes in the endometrial blood vessels have been suggested as a possible etiology in the majority of cases where no significant pathology is identified (i.e. there is only the expected inactive/atrophic endometrium).
The myometrium has a rich network of thick-walled vessels in its midportion, a landmark that can be recognized grossly. A peculiar vascular architecture sometimes encountered in the myometrium is that of arteries apparently free-floating within cleft-like spaces, which have been interpreted as venous channels.
Exogenous administration of estrogen preparations exposes the cycling or postmenopausal endometrium to a potent stimulus. Endometrial hyperplasia is a frequent development in women taking unopposed estrogen, and this can progress to atypical hyperplasia and adenocarcinoma. The large majority of tumors arising secondary to unopposed estrogen are well differentiated and superficial and are associated with an excellent prognosis.
Owing to the widespread use of progestational agents for therapeutic and contraceptive purposes, endometrial changes secondary to progestational effects are commonly encountered in practice. The effects of exogenous progestins are seen primarily in the stroma, which predominates and shows striking pseudodecidual changes ( Fig. 33.4 ). In contrast to the prominent stromal development, the glands are small, widely separated by stroma, and atrophic appearing.
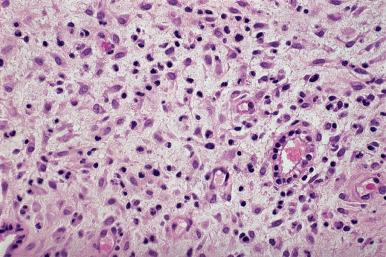
In endometrium from patients taking oral contraceptive pills, the effects in the endometrium reflect the progesterone predominance and variably show the features described previously, that is, stromal predominance, with pseudodecidual changes, and small atrophic glands. On very rare occasions, focal glandular changes of the Arias-Stella type may appear in patients taking contraceptive pills. In most cases, discontinuation of the hormones results in a restoration of a normal endometrial pattern in a matter of weeks.
Patients receiving hormone replacement therapy for postmenopausal symptoms will typically be on low-dose combination (estrogen and progestin) therapy; the goal is to relieve symptoms but to not have stimulation of the endometrium. Thus, the endometrium should appear inactive or atrophic. If a patient on postmenopausal hormone replacement therapy presents with bleeding and the biopsy shows proliferative changes, this reflects excessive estrogenic stimulation of the endometrium; if the dosage is not altered, continued bleeding is likely.
Endometrial changes secondary to treatment of endometrial hyperplasia with high-dose progestin therapy will be covered later, in the section on endometrial hyperplasia.
The Mirena coil (levonorgestrel ), increasingly used for the treatment of menorrhagia/endometrial hyperplasia, can induce a large variety of alterations in the endometrial mucosa, including pseudodecidualization, mucinous changes, ulceration, inflammatory infiltrates, stromal hyaline nodules, superficial micropapillary changes, infarcted decidua, dystrophic calcification, and others.
Progesterone receptor modulators used in the management of endometriosis and uterine leiomyomas most commonly cause only minimal and inconsistent changes in the endometrial mucosa but can cause cystic dilated glands that raise the differential diagnosis of endometrial hyperplasia. These changes can be distinguished from hyperplasia by the appearance of the epithelial cells lining the cysts, which appear inactive and may contain abortive subnuclear vacuoles. Abnormal stromal vessels are commonly seen.
Tamoxifen is a synthetic anti-estrogen used in the treatment and prophylaxis of breast carcinoma. Not only does it have an anti-estrogenic effect on the endometrium when competing with ovarian estrogen secretion, it also has a paradoxical estrogenic effect in the absence of ovarian estrogen secretion. It is associated with an increased frequency of proliferative endometrial lesions, including hyperplasias, polyps, and malignant tumors, but the latter are not seen often enough to justify routine endometrial biopsies in this population. None of the changes associated with tamoxifen are pathognomonic and, accordingly, biopsies taken from women receiving tamoxifen therapy are diagnosed using terminology and diagnostic criteria identical to those used for patients not on tamoxifen. With respect to endometrial carcinomas developing in patients on tamoxifen, in two reported series a significant number of the cases were high-grade tumors associated with a poor prognosis, but in others there was a great predominance of low-grade endometrioid adenocarcinomas, and the former studies may reflect some selection bias.
Acute endometritis is usually seen in association with abortion, the postpartum state, or instrumentation. Gonococcal endometritis is rarely seen by the pathologist because of its very transient nature. Before making a diagnosis of acute endometritis, the pathologist should remember that neutrophils are normally present in the endometrium on days 26, 27, and 28.
Chronic endometritis , characterized by an infiltrate of lymphocytes and plasma cells (often with an additional minor component of eosinophils ), may follow pregnancy or abortion, be the result of an intrauterine device (IUD), be associated with a submucosal leiomyoma, or be accompanied by mucopurulent cervicitis and/or pelvic inflammatory disease (PID). The presence of chronic endometritis is associated with infertility/recurrent implantation failure. There, is some evidence that antibiotic therapy in these patients can result in improved outcomes in subsequent in vitro fertilization cycles, thus it is an important and clinically actionable diagnosis. The most common symptoms are vaginal bleeding and pelvic pain, but most patients are asymptomatic. It should be emphasized that lymphoid follicles, with or without germinal centers, are a normal occurrence in the functional layers of the endometrial mucosa and, therefore, should not be considered as evidence of chronic endometritis. Therefore, identification of plasma cells constitutes the most important criterion for the diagnosis of chronic endometritis, whether by conventional criteria or—as suggested by some—by immunostaining the sections for immunoglobulins, CD38, VS38, or syndecan-1 ( Fig. 33.5 ). However, it should be kept in mind that scattered plasma cells can be found in the absence of endometritis and the demonstration of rare plasma cells with immunostains should not lead to overdiagnosis of endometritis. Clearly there remains important work to be done in refining the diagnostic criteria for chronic endometritis. The possibility of inflammation should be suspected—and plasma cells searched for—whenever there is an abnormal cyclic pattern, a focal mononuclear infiltrate, inflammatory cells in the glandular lumina, dense stroma, a stellate stromal pattern of proliferation, or foci of necrosis or calcification ( Fig. 33.6 ). Glandular alterations commonly accompany the inflammatory reaction. The presence of neutrophils in the endometrial surface is a good predictor of PID, especially if combined with plasma cells in the endometrial stroma.
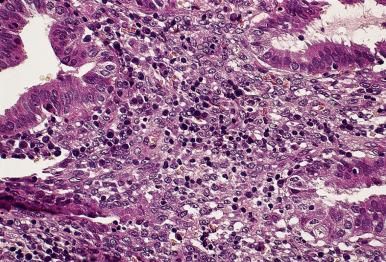
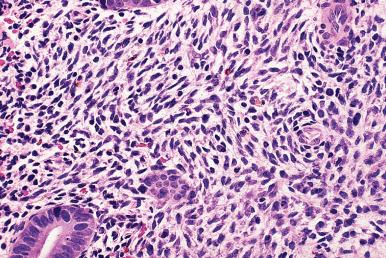
The myometrium is usually spared in most types of endometritis (sarcoidosis excluded, see later) unless the inflammation is very severe.
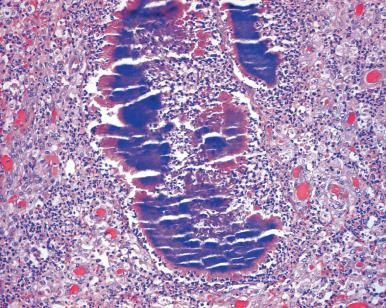
IUDs inserted for the purpose of contraception result in many biologic changes. The most common change is focal or extensive chronic endometritis, which may be accompanied by necrosis and squamous metaplasia. On occasion, the inflammation spreads through the fallopian tubes to produce PID and sometimes tubo-ovarian abscesses. One of the agents involved in the inflammatory process is Actinomyces ( Fig. 33.7 ). The organisms can be detected in microscopic sections or in cytology preparations, but care should be exercised in distinguishing them from pseudoactinomycotic radiate granules; the latter lack central branching filaments and diphtheroid forms. The changes that can develop following the insertion of the Mirena coil were described in the previous section.
Pyometra refers to the accumulation of pus within the endometrial cavity. It is the consequence of the combined effect of obstruction and infection. Whiteley and Hamlett reviewed 35 cases in postmenopausal patients. Only five were secondary to carcinoma; the remaining 30 were the result of benign cervical stricture originating from senile atresia, surgery, or cauterization.
Hematometra is the accumulation of blood within the endometrial cavity, usually as a result of cervical occlusion. This may lead to the disappearance of the endometrial mucosa and its replacement by sheets of lipid-containing histiocytic cells, a process known as histiocytic or xanthogranulomatous endometritis when diffuse and as nodular histiocytic hyperplasia when localized. Sometimes the histiocytes are found to contain a yellowish-brown cytoplasmic pigment, in which case the term ceroid-containing histiocytic granuloma has been used.
Endometrial tuberculosis is rare in the United States but still common in other parts of the world. Menstrual disturbances are common. The microscopic diagnosis is based on the demonstration of acid-fast bacilli in tubercles or culture. The presence of plasma cells and leukocytes probably results from secondary infection. Tubercles may be missed unless multiple levels of curettings are examined. Since the granulomas tend to concentrate in the superficial functional layers of the endometrium, it is recommended that the biopsy be taken during the late secretory phase, and tissue should be submitted for culture as well as histopathologic examination.
Chlamydial infection has been identified through the immunohistochemical demonstration of chlamydial antigens in endometrial epithelial cells or by polymerase chain reaction (PCR). These cases are associated with severe acute and/or chronic inflammation. Plasma cells tend to be very numerous; as a matter of fact, it has been proposed that the presence of these cells is linked to Chlamydia infection.
Viral infection of the endometrium is probably more common than generally suspected. Cytomegalovirus endometritis and diffuse papillomatosis (condyloma) have both been documented ; the former can have a granulomatous quality.
Coccidioidomycosis of the uterus has been reported in the United States in a few cases as a localized infection; it probably results from a clinically inapparent and completely resolved primary lung infection.
Postoperative granulomas of the endometrium have been described following endometrial ablation procedures; they may be thought of as the female counterpart of those seen in the prostate or bladder following transurethral resection (TUR).
Malakoplakia of the endometrium has been reported, sometimes in a recurrent fashion and occasionally in association with endometrial adenocarcinoma.
Sarcoidosis of the uterus occurs, but it remains a diagnosis of exclusion ( Fig. 33.8 ). In contrast to tuberculosis, the granulomatous reaction usually spreads to the myometrium. Granulomatous endometritis can also follow hysteroscopic resection of the endometrium.
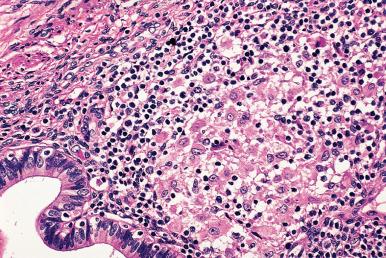
Giant cell arteritis may involve the uterus and other genital organs of elderly women, either as an isolated finding (i.e. no systemic symptoms and normal acute phase reactants) or as part of a generalized process. The former event is more frequent than the latter, but sometimes female genital tract disease is the first manifestation of systemic periarteritis nodosa. Over 90% of patients with isolated vasculitis of the myometrium have a nongranulomatous histology.
The endometrial glands and stroma are subject to a variety of metaplastic changes, many of them hormonally induced. They are often accompanied by hyperplastic changes in the endometrial glands but can occur independently from them and, therefore, need to be evaluated separately. They should not be regarded by themselves as evidence for the existence of a neoplastic process, but the presence of squamous metaplasia and mucinous metaplasia, in particular, should heighten awareness of the possibility of coexistent hyperplasia. Papillary syncytial and ciliated cell metaplasia are much less likely to be associated with hyperplasia. The endometrial metaplasias include:
Squamous (morular) metaplasia . This is most commonly encountered in hyperplastic endometrium but can be seen in association with leiomyoma or uterine polyps. Frank keratinization is very rare (ichthyosis uteri) . A more common finding is the presence of nonkeratinizing squamoid cells occurring either diffusely (adenoacanthosis) or in the form of berry-like aggregates (morules or morular metaplasia; Fig. 33.9 ). Most are seen in premenopausal women, in those receiving exogenous hormones, or in association with polycystic ovary syndrome (PCOS). This change is distinguished from well-differentiated endometrial adenocarcinoma with squamous metaplasia because of the benign appearance of the glandular elements, without glandular crowding or atypia. The morules represent a functionally inert tissue, in the sense of being devoid of sex hormone receptors and having an extremely low proliferation rate. Surprisingly, they are immunoreactive for the intestinal transcription factor CDX2.
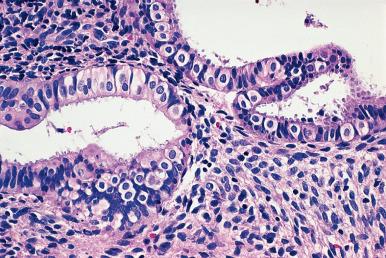
Ciliated cell (tubal) metaplasia . Scattered ciliated cells are normally present in the endometrial mucosa; when markedly increased in number, the appearance resembles that of a fallopian tube, and the term listed earlier is used ( Fig. 33.10 ). Ciliated cell metaplasia is common in atrophic endometrium, to the point where it need not be commented on this setting. Occasionally the condition is associated with atypical nuclear features (“atypical tubal metaplasia”), but even under these circumstances it does not seem to constitute a factor of significant risk for the development of endometrial adenocarcinoma.
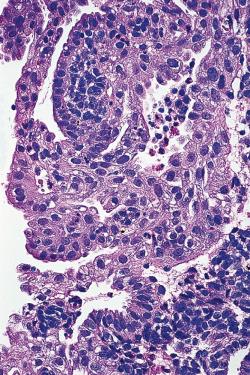
Papillary syncytial metaplasia (papillary syncytial change). This alteration is characterized by the presence of syncytial to papillary aggregates of eosinophilic cells along the surface epithelium ( Fig. 33.11 ). It is often seen in association with endometrial breakdown.
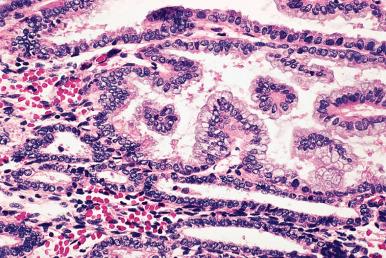
Mucinous metaplasia . In this condition, the endometrial mucosa reverts to a pattern morphologically, histochemically, and ultrastructurally similar to that of the endocervical mucosa ( Fig. 33.12 ). It is particularly common in endometrial polyps. The main differential diagnosis is with normal cervix or atypical mucinous proliferation of the endometrium/mucinous variant of endometrial adenocarcinoma. With regards to the differential diagnosis with normal cervix, mucinous metaplasia can be recognized by its involvement of endometrial tissue (i.e. association with endometrial stromal cells and non-metaplastic endometrial glandular epithelium), and by the absence of reserve cells. Mucinous metaplasia should be focal, bland, and without glandular architectural features; in their presence a diagnosis of atypical mucinous glandular proliferation (discussed later) should be entertained.
Eosinophilic (oxyphilic; oncocytic) metaplasia . This is characterized by cells with abundant eosinophilic cytoplasm. It is distinguished from atypical endometrial hyperplasia and the exceptionally rare oncocytic carcinoma by the absence of glandular crowding and atypical nuclear features.
Hobnail and clear cell metaplasia . In these closely related metaplastic changes, the epithelium is clear, with tall cells having apically located nuclei ( Fig. 33.13 ). The differential diagnosis is with clear cell (mesonephroid) adenocarcinoma.
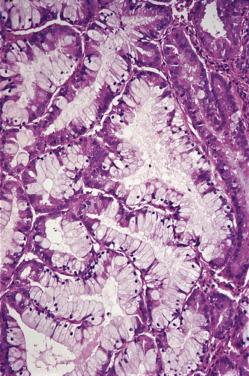
Arias-Stella reaction. See under Normal anatomy.
Stromal metaplasia . This includes the formation within the endometrial stroma of islands of smooth muscle, cartilage, and bone. It should be noted that in most instances the presence of cartilage or bone is the result of retained fetal parts.
Adenomyosis refers to the presence of islands of endometrial glands and stroma deep within the myometrium, whereas endometriosis is the term employed for the occurrence of endometrial tissue outside the uterus. These two disorders are usually regarded as closely related, but their microscopic appearance is somewhat different. In most cases, adenomyosis is made up of the nonfunctional (basal) layer of the endometrium and is frequently connected with the mucosa. Endometriosis, on the other hand, is composed of the functional layers of the endometrium. As such, it may go through proliferative, secretory, and menstrual stages similar to those of its orthotopic counterpart. However, studies using conventional morphologic techniques, immunohistochemistry, and cell proliferation markers have shown that the endometriotic lesions are consistently more proliferative than the normally located endometrium, both during childbearing years and in postmenopause. Adenomyosis is rare in postmenopausal women, except for the tamoxifen-associated cases, which tend to show stromal fibrosis, glandular dilation, and various metaplastic changes.
Many pathogenetic theories have been proposed over the years for endometriosis: origin from congenital müllerian or wolffian rests, implantation of endometrium, lymphatic or hematogenous spread, and serosal metaplasia. The finding of clonality, both within and a single endometriotic focus, and across multiple foci from the same patient, as well as the demonstration of oncogenic mutations in the epithelium of endometriosis, lend support to the implantation theory but it should be noted that more than one pathogenetic mechanism may be involved.
Both endometriosis and adenomyosis may result in pelvic pain, characteristically associated with the menstrual period. Between 30% and 40% of women with endometriosis are infertile, the exact mechanism being still obscure. Exceptionally, adenomyosis may lead to rupture at the time of pregnancy.
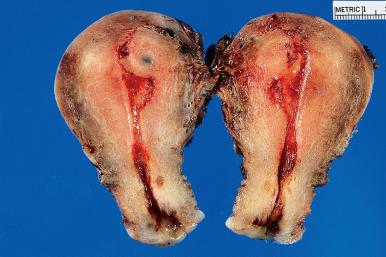
Adenomyosis results grossly in an enlarged and globular uterus because of the myometrial hypertrophy that regularly accompanies it. This enlargement is often asymmetrical. The diagnosis may be suspected on cut section in the presence of depressed small cystic lesions in obvious but ill-defined bulging zones of muscle hypertrophy ( Fig. 33.14 ). Adenomyosis may form nodules, grossly, in which case the designation adenomyoma or adenomyotic nodule can be used. Leiomyomas in utero with adenomyosis may themselves be involved by the process; distinction between leiomyoma with adenomyosis and adenomyoma/adenomyotic nodule is subjective but is made based on the endometrial glands and stromal being only at the periphery of the nodule in the former while they are present throughout the latter.
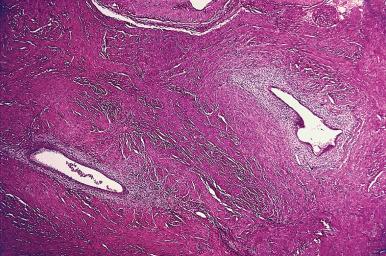
Microscopically, the diagnosis of adenomyosis depends on the thresholds used by the individual pathologist, some of which are very liberal indeed. The interface between endometrium and myometrium is normally an irregular one, without interposition of a submucosal layer; invaginations of the endometrial basal layer into the superficial portions of the myometrium should be regarded as a normal finding. By convention, the diagnosis of adenomyosis should be reserved for those cases in which endometrial glands and stroma are seen in the myometrium at a distance of at least one low-power field from the endometrial–myometrial junction ( Fig. 33.15 ).
Microscopically, the endometrium of adenomyosis usually has a proliferative appearance, consistent with its basal layer nature. When the normally located endometrium is in the secretory phase, this is also true for one-fourth of the foci of adenomyosis. These foci can be involved by any of the diseases affecting the orthotopic endometrium, including hyperplasia and adenocarcinoma. It is important to recognize this phenomenon, lest a case of in situ or superficial endometrial adenocarcinoma associated with similar changes in the foci of adenomyosis be misinterpreted as a deeply invasive malignancy. Involvement of adenomyosis by endometrial adenocarcinoma does not seem to affect outcome.
Vascular involvement may be present and should not be overinterpreted as a sign of malignancy.
Some small islands of adenomyosis are made up predominantly of endometrial stroma ( stromal adenomyosis , incomplete adenomyosis or adenomyosis with sparse glands ; Fig. 33.16 ) ; however, any sizable intramyometrial focus composed entirely of endometrial stroma is likely to represent an endometrial stromal sarcoma.
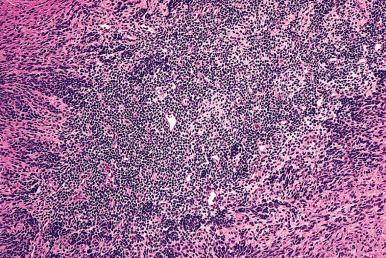
Endometriosis , which is thought to occur in 1%–7% of women in the United States, is a major cause of pelvic pain and subfertility. It can be located in the cervix, vagina, vulva, rectovaginal septum, ovary, fallopian tubes, uterine ligaments, appendix, small and large bowel, bladder and ureters, pelvic peritoneum, hernia sacs, lymph nodes, kidney, and skin, and even within skeletal muscles, peripheral nerves, pleura, lung, and nasal cavity ( Figs. 33.17 and 33.18 ). The specific features as they pertain to the various sites are discussed in the respective chapters. Spontaneous cutaneous endometriosis is limited to the umbilicus and inguinal area. In other locations, such as the lower abdominal wall, it practically always arises in surgical scars (particularly those from cesarean sections).
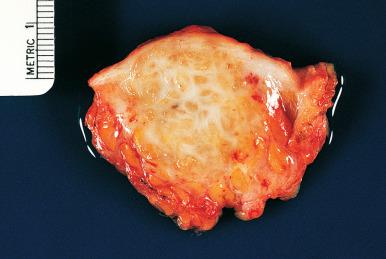
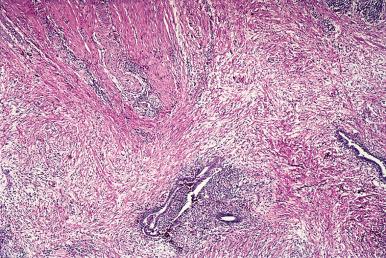
Grossly, endometriosis appears as bluish cystic nodules, often surrounded by fibrosis. Exceptionally, it may present as multiple polypoid masses grossly simulating a neoplastic process ( polypoid endometriosis or endometriotic polyposis ). These masses can be located in the colon, ovary, uterine serosa, ureter, fallopian tubes, and several other sites.
Microscopically, endometrial glands and stroma are seen, often embedded in a dense fibrous mass exhibiting signs of fresh and old hemorrhage. The stromal component of endometriosis can undergo smooth muscle metaplasia. Along those lines, it is still being argued whether the nodular lesion resembling a miniature uterus, which can be found in several places within the peritoneal cavity (ovary, small bowel, etc.), is a peculiar variant of endometriosis with smooth muscle metaplasia of the stroma or a malformation of the müllerian ducts.
Other morphologic variations in the theme of endometriosis, some of which can be of confusing interpretation, include the presence of stromal micronodules, stromal elastosis, prominent myxoid changes, perineurial invasion, and regeneration of the surrounding skeletal muscle. Endometriotic foci can undergo prominent mucinous metaplasia, a change that can result in a mistaken diagnosis of well-differentiated mucinous adenocarcinomas. Such change has been interpreted as a metaplasia toward endocervical-type epithelium and designated as endocervicosis ( Fig. 33.19 ).
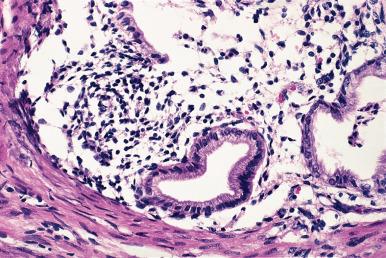
Although well-documented cases of endometriosis of lymph nodes exist, most cases so designated are made up of glands lined by ciliated or nonciliated cuboidal epithelium unaccompanied by stroma, limited to the capsule or cortical area of the node ; as such, they are more reminiscent of fallopian tube than endometrial epithelium and are better designated as endosalpingiosis . They have been found in approximately 14% of nodes in females but practically never in males.
Endosalpingiosis is particularly frequent in the pelvic peritoneum in connection with low-grade ovarian serous tumors. It can also present in the form of a florid cystic mass and involve the uterine wall in a transmural fashion.
The combination of endometriosis with endocervicosis and/or endosalpingiosis is sometimes referred to as müllerianosis.
Endometriosis, like adenomyosis, is subject to any of the metaplastic, hyperplastic, and atypical changes that may supervene in the orthotopic endometrium. More importantly, it may undergo malignant transformation. The most common forms are endometrioid and clear cell carcinoma, but endometrial stromal sarcoma, carcinosarcoma, and adenosarcoma have also been reported. Benign and borderline tumors of either serous or endometrioid nature can also develop, both from conventional endometriosis and from endosalpingiosis. The most common site for endometriosis-related neoplasms is within endometriotic cysts of the ovary, with pelvic peritoneum, rectovaginal septum, intestinal wall, and other sites being much less common.
The treatment of endometriosis may be hormonal or surgical depending on the circumstances. It is believed that the laparoscopic ablation of minimal and mild endometriosis enhances fecundity in infertile women.
Bleeding not associated with an organic cause in women of childbearing age belongs to the large and somewhat nebulous category known as dysfunctional uterine bleeding . Examination of specimens obtained on D&C or endometrial biopsy from patients with dysfunctional uterine bleeding is done primarily to exclude neoplasia/preneoplastic changes, or other pathology that may be causing the abnormal bleeding, such as an endometrial polyp. A wide range of changes can be seen in the endometrium that differ from normal proliferative or secretory endometrium, but attempts by pathologists to classify these changes into categories that can guide treatment have been largely unsuccessful. On a personal note, it was a disheartening experience as a senior surgical pathologist to talk to a group of younger gynecologists and find out how little clinical value there was in my carefully crafted reports describing minor morphologic changes in the endometrium associated with dysfunctional uterine bleeding! What follows is a description of some of the histologic abnormalities that may be encountered in endometria sampled for dysfunctional uterine bleeding, but the suggested diagnostic terminology is provided in the upcoming section on “Curettage and biopsy.”
The presence of fibrin clumps in the endometrial stroma (a finding not usually present in the normal menstrual endometrium), the identification of fragmented pieces with dense stromal cellularity (a process known as stromal crumbling ), and the presence of an increased number of apoptotic bodies at the base of the glands (so-called Benirschke granules) may all be seen. Another type of defect seen in the ovulatory group of bleeders is known as irregular shedding of the endometrium . The term refers to a regularly recurring menorrhagia in which the bleeding phase of the cycle requires 7 days or more for completion, without subsequent prolongation of the cycle. This is due to a lag in the shedding of the secretory endometrium, which is normally completed by the fourth day of menstruation. Tissue obtained five or more days after the onset of menstrual bleeding shows retained secretory endometrium in addition to fragmented menstrual and/or early proliferative endometrium.
The term membranous dysmenorrhea has been used for a rare condition characterized by the painful passage of an endometrial cast of the uterus during the first few days of menstruation. This cast has the microscopic appearance of decidua. The disease is thought to result from a hyperprogestational response and may be induced by the administration of high doses of progesterone.
Anovulatory cycles result in exuberant disordered proliferative endometrium , recognized by irregularity of glandular outlines, with more complexity of gland profiles than is encountered in normal proliferative phase. There is not sufficient glandular crowding to warrant a diagnosis of endometrial hyperplasia. The glandular epithelial cells lack atypia and show nuclear features similar to those of normal proliferative glands. There may be abortive subnuclear vacuoles and apoptotic bodies within glands. Anovulatory cycles are common in PCOS but also very common at puberty and in the perimenopausal period. Distinction between disordered proliferative endometrium and normal proliferative endometrium, on one hand, and endometrial hyperplasia without atypia on the other, is subjective. The general rule of diagnosing an abnormal state only when changes are clear-cut can be applied in this situation; only diagnose disordered proliferative endometrium when the architecture of the glands is too abnormal to fit into the spectrum of normal proliferative endometrium. Distinction between disordered proliferative endometrium and hyperplasia without atypia will be discussed next.
Endometrial hyperplasia is recognized by increased gland-to-stroma ratio and glandular architectural irregularity and complexity. There have been many classifications of endometrial hyperplasia over the years, but the one in current use is the one put forward by the World Health Organization (WHO) in 2014.
Endometrial hyperplasia without atypia occurs as a result of unopposed estrogenic stimulation of the endometrium and is on a continuum with disordered proliferative endometrium. There is glandular branching, irregularity, and crowding ( Fig. 33.20 ). The diagnostic threshold for diagnosis of endometrial hyperplasia without atypia, rather than disordered proliferative endometrium, is highly subjective, but at the point where glands predominate over stroma, a diagnosis of endometrial hyperplasia without atypia is appropriate. In the past, endometrial hyperplasia was subdivided into simple and complex hyperplasia, based on the degree of glandular architectural complexity; this is no longer done as it is not a clinically relevant subclassification. The risk of progression to carcinoma with endometrial hyperplasia without atypia is low (1%–3%). The chief differential diagnosis is with atypical hyperplasia, and this is based on the assessment of cytologic atypia; the criteria for recognition of atypia are presented in the next section.
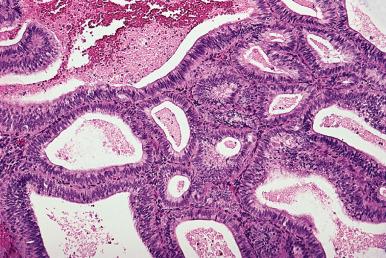
Atypical hyperplasia is the precursor lesion of low-grade endometrioid adenocarcinoma. It shows glandular crowding and irregularity in addition to cytologic atypia. The nuclear atypia is characterized by enlargement, rounding, pleomorphism, and presence of nucleoli. Quite apart from these descriptive terms, the presence of cytologic atypia is most reliably recognized by comparison of the cytologic features in the hyperplastic focus to those of proliferative endometrial glandular epithelial cells outside the area of hyperplasia ( Fig. 33.21 ). This approach to diagnosis of cytologic atypia, proposed by Mutter and colleagues, has enhanced the reproducibility of the diagnosis of atypical hyperplasia. It is exactly equivalent to the way we evaluate colonic biopsies for adenomatous change and, as is the case for colonic adenomas, the cytologic atypia within the atypical hyperplasia reflects a clonal proliferation of cells with some but not all the mutations necessary for transformation to carcinoma. One cautionary note is warranted on using this approach to diagnose cytologic atypia; if there is coexistence of proliferative endometrium and inactive endometrium, there are going to be differences between the nuclei in the two areas, but this does not reflect premalignant change. The recognition of atypia is based on cytologic differences between the endometrium in the hyperplastic focus and proliferative endometrium in non-hyperplastic endometrium.
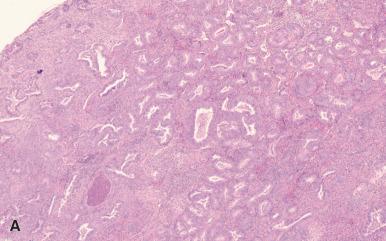
The presence of metaplastic changes complicates the interpretation of cytologic features, and we can offer no easy to solution to this vexing, recurrent problem: is this atypical hyperplasia or hyperplasia without atypia, but with metaplastic changes? This is further complicated by the frequent coexistence of metaplasia and atypical hyperplasia. As a practical guideline, the greater the degree of glandular crowding, the more likely it is that the cytologic changes are atypical.
Endometrioid intraepithelial neoplasia (EIN) is an alternative diagnostic term for atypical hyperplasia. The EIN terminology was proposed by Mutter, who developed the much improved diagnostic criteria for recognition of premalignant change in the endometrium. What has happened is that there has been universal adoption of the new criteria for diagnosis of endometrial premalignancy, but less uniform adoption of EIN terminology. Thus, at this time, atypical hyperplasia and EIN are completely synonymous diagnostic terms, reflecting the same diagnostic criteria.
The incidence of carcinoma in women with atypical hyperplasia has been in the neighborhood of 15% and has reached 30% in some series. A significant minority of women who have a diagnosis of atypical hyperplasia made on biopsy or curettings will have adenocarcinoma (almost invariably low grade and minimally or non-myoinvasive) diagnosed on the subsequent hysterectomy specimen.
The distinction between a case of atypical hyperplasia with severe architectural and cytologic atypia and a well-differentiated adenocarcinoma can be very difficult, largely because of the fact that endometrial hyperplasia and carcinoma represent different points in a disease continuum at the morphologic, ultrastructural, immunocytochemical, and molecular genetic levels. A careful reading of the landmark study by Longacre and colleagues is recommended. Although more than 20 years have passed since the publication of this paper, it remains a beautiful example of good study design, and the data produced continue to inform everyday practice. The presence of confluence of glands, such there are no longer small glands surrounded by stroma, or a papillary/villoglandular architecture are features supporting a diagnosis of well-differentiated adenocarcinoma. On biopsy or curettings it has been recommended that the diagnosis of “atypical hyperplasia, cannot exclude low-grade adenocarcinoma” be used for those cases where the features are intermediate between atypical hyperplasia and grade 1 endometrioid adenocarcinoma, and we endorse this view. We do not recommend the use of this terminology for hysterectomy specimens.
There is an increasing trend to treat atypical hyperplasia or even low-grade adenocarcinoma with high-dose progestin therapy, so as to avoid hysterectomy and preserve fertility. The interpretation of findings in follow-up biopsies is complicated by prominent progestational effects, including squamous and mucinous metaplasia of the epithelium and stromal decidual reaction. In our experience, there are three possible diagnostic results for these follow-up specimens: either 1. There is no evidence of residual hyperplasia, 2. There is residual hyperplasia with treatment effects (metaplasia and decidual change), or 3. There is residual hyperplasia that shows no treatment effects, and may even show more severe atypia or progression to frank carcinoma, when compared to the pretreatment biopsy.
Atypical mucinous glandular proliferation of the endometrium is considered to be a precursor of mucinous carcinoma. It is recognized based on glandular crowding and complexity with prominent mucinous metaplasia of the glandular lining cells, but these cells can show minimal cytologic atypia. We have previously acknowledged the difficulty in distinguishing between atypical hyperplasia and carcinoma, but it is even more difficult for mucinous proliferations. The likelihood of adenocarcinoma in the hysterectomy after a diagnosis of atypical mucinous glandular proliferation was 45% in a recent study. Atypical mucinous glandular proliferation of the endometrium should therefore be considered to be a premalignant lesion with significant chance of progression or coexistent carcinoma, with hysterectomy being the preferred treatment. Awareness of atypical mucinous proliferation of the endometrium is important as we have seen this premalignant change misdiagnosed as normal endocervical tissue.
Papillary proliferation of the endometrium (PPE) was first described by Lehman and Hart and is characterized by a prominent papillary architecture, with papillae covered by epithelial cells with minimal cytologic atypia ( Fig. 33.22 ). There is a variation from small foci of simple papillae with short nonbranching stalks to extensive complex papillae with elongated stalks and branches. The absence of significant nuclear atypia separates this from carcinoma. The lining epithelium consists of a single layer cells with pale eosinophilic or mucinous cytoplasm, sometimes ciliated. Papillary proliferation frequently involves an endometrial polyp. PPE has subsequently been subdivided into simple and complex forms, by Ip et al. Simple PPE is characterized by short, predominantly nonbranching stalks (occasional secondary branches may be seen) localized to 1 or 2 foci involving the surface of polyps, non-polypoid endometrium, or within cystically dilated glands, or involvement of <50% of a polyp. Complex PPE consists of complex papillae (short or long stalks with frequent secondary and complex branching) and/or diffuse involvement (three or more foci within a specimen, or involvement of >50% of polyp). Complex PPE, in particular, often coexists with conventional atypical hyperplasia or even carcinoma. The clinical significance of simple PPE is considered equivalent to that of hyperplasia without atypia, while complex PPE is equivalent to atypical hyperplasia, based on a limited number of reported cases.
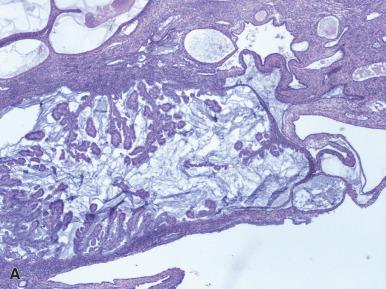
The term (atypical) secretory hyperplasia has been applied to a pattern of architectural abnormalities and cellular atypia within a secretory endometrium, with the atypical glands resembling those seen in the sixteenth to the seventeenth day of the normal cycle. This change should be distinguished from the Arias-Stella reaction and from hormonally treated conventional endometrial hyperplasia. The natural history of this condition is not well known, but involution appears to be relatively common when the background endometrium also shows secretory changes. Normal secretory endometrium appears relatively crowded, and it is best to be conservative in diagnosis of hyperplasia in the setting of secretory endometrium.
Tissue from the endometrial cavity taken for diagnostic purposes was traditionally obtained by dilation of the cervical canal and curettage of the endometrial cavity (D&C). Information about endocervical extension of an endometrial neoplasm can be obtained by performing a fractional curettage (i.e. a separate sampling from the endometrial and endocervical cavities during the same procedure). The endocervical specimen should be obtained first so as to minimize contamination from the endometrium. However, even when this precaution is taken, small isolated tumor fragments may be found in endocervical curetting specimens in cases where there is no infiltration of the cervix by tumor. Therefore, the presence of endocervical extension of a tumor can only be diagnosed if cancer and normal endocervical glands are seen in the same tissue fragment . Otherwise, simply record the presence of carcinoma in the material submitted from the endocervix and let the clinician decide whether this is significant on the basis of the findings at the time of curettage.
Endometrial biopsy has emerged as an alternative to D&C for the evaluation of the endometrium. It can be performed safely in the office (but causes significant discomfort and pathologists should not be cavalier in recommending resampling of the endometrium). Biopsy may also be carried out under hysteroscopic guidance. A meta-analysis study with the use of the Pipelle showed a detection rate for endometrial adenocarcinoma of 99.6% in postmenopausal women and 91% in the premenopausal group. Another issue is the accuracy of the grading of endometrial carcinoma done in a biopsy when compared with that seen in the hysterectomy specimen. In one study, the concordance was 45% for grade I, 63.3% for grade II, and 75.6% for grade III, the overall concordance being 64.5%. Even comparing the diagnosis of low-grade (grade I or II) versus high-grade (grade 3) carcinoma in biopsy/curettings versus hysterectomy, there remains distressing variation, but with some improvement (kappa—0.7). Histotype diagnosis (i.e. endometrioid, serous, clear cell, etc.) shows only moderate agreement between biopsy/curettings and hysterectomy (kappa—0.44).
A curious artifact that can be seen in hysteroscopically assessed tissue is pseudolipomatosis , a condition analogous to its more common counterpart in the gastrointestinal tract. It results from air or gas entering into the endometrial mucosa and simulating fatty infiltration.
An exceptionally rare occurrence is the failure to find tumor in a hysterectomy specimen following a diagnosis of adenocarcinoma in an endometrial biopsy. This distressing event, which has been referred to as vanishing endometrial carcinoma (in analogy to the same phenomenon as described in the prostate) is probably explained by the very minute size of the tumor, most or all of which has been removed by the biopsy procedure.
The language used in reporting on endometrial biopsies where there are only benign findings is highly variable from pathologist to pathologist, in contrast to the standardized language and formatting we use for cancer specimens. There remain pathologists who believe that they are being called upon to determine whether ovulation has occurred, even though this has not been an expectation for more than a decade, while other pathologists report in great morphologic detail on biopsies taken for infertility when their clinical colleagues only want reassurance that there is no polyp, endometritis, or hyperplasia/carcinoma. Please do discuss with clinicians their needs, so that you can better serve your patients by providing clinically relevant information in your surgical pathology reports. We do believe, however, that more uniform reporting of benign endometrial biopsy specimens will enhance the value of our reports, improving their comprehensibility. A guideline for uniform diagnostic categories to use in reporting endometrial biopsies has been proposed by the Canadian Gynecological Pathology Special Interest Group ( Box 33.1 ); when trialed in practice, more than 90% of cases could be reported using this template, in a range of practice scenarios.
Non-diagnostic sample (no endometrial tissue present)
Scant fragments of inactive endometrial surface epithelium and/or stroma; this is a suboptimal specimen for histopathological assessment and may not be representative *
* Fewer than 10 strips of epithelium in an endometrial biopsy or curettage specimen is suboptimal and is associated with decreased sensitivity and negative predictive value.
Inactive/atrophic endometrium
Proliferative endometrium
Normal proliferative
Weakly proliferative
Disordered proliferative
Secretory endometrium
Normal secretory
Date ___
Polypoid secretory endometrium
Irregular secretory
Menstrual endometrium
Endometrial polyp(s)
Endometrial hyperplasia without atypia
Chronic endometritis
Changes consistent with exogenous hormonal therapy
Progestin
Progesterone receptor selective agonist
Oral contraceptive
Products of conception
Chorionic villi
Chorionic plate
Chorionic membranes
Implantation site trophoblast
Exaggerated implantation site
Embryonic/fetal tissue
Pregnancy-related endometrial changes
Pregnancy-related endometrial changes only; no products of conception identified
Placental site nodule/remote implantation site
Adipose tissue, mesothelium—lined tissue or other extrauterine tissues present
A final comment is called for on the criteria for insufficiency in an endometrial biopsy or curetting. Remember that the expected finding in a postmenopausal woman is scant inactive or atrophic endometrium. The presence of strips of atrophic endometrium is normal in this clinical scenario and should not be considered insufficient for diagnosis, a diagnosis that should be reserved for instances where there is no endometrial tissue sampled. Sakhdari et al. have suggested that the presence of 10 or more endometrial strips constitutes an adequate sample, while fewer than 10 strips is suboptimal (negative predictive values in the group with an adequate sample was >99% versus approximately 80% in the group with a suboptimal sample). This finding should be validated independently, but it appears that there are three categories of samples: adequate, benign but suboptimal because of their scanty nature (<10 strips of endometrial epithelium), and insufficient for diagnosis (no endometrial tissue sampled).
Endometrial polyps protrude into the endometrial cavity and often exhibit changes secondary to vascular insufficiency or prolapse ( Fig. 33.23 ). They consist of epithelial and stromal components, and a key to recognition in fragmented specimens, e.g. endometrial curettings, is the altered cellularity of the stroma compared to normal endometrium, with the polyp stroma usually showing reduced cellularity with a more “fibrous” appearing and spindle cell stroma, compared to normal endometrial stroma. The glands usually show some degree of cystic change. They may be lined by an active pseudostratified epithelium containing mitotic figures or, in the postmenopausal patient, by a flat, inactive epithelium ( Fig. 33.24 ). The abnormal glandular architecture may raise the possibility of endometrial hyperplasia, but the absence of glandular crowding excludes the diagnosis of hyperplasia. Note that hyperplasia (without atypia or atypical) or carcinoma may be present in an endometrial polyp and are diagnosed using the same criteria as are used in the absence of a polyp.
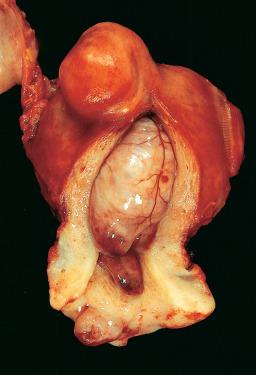
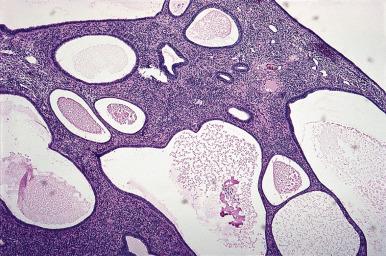
The glands and stroma of the polyp are unresponsive to progesterone stimulation and retain their integrity throughout the menstrual cycle. The frequent coexistence of endometrial polyps and endometrial hyperplasia points to a shared pathogenesis for these two lesions. The clinical significance and management of atypical hyperplasia are the same irrespective of whether it involves or is confined to an endometrial polyp.
Exceptionally, the stroma of endometrial polyps contains scattered atypical (bizarre) cells.
Endometrial polyps occur with increased frequency after tamoxifen exposure. These are characteristically multiple, large, and fibrotic and may exhibit stromal decidualization and mucinous metaplasia.
With the increasing use of hysteroscopy, we frequently receive endometrial biopsies of polypoid endometrium that microscopically is seen to be normal secretory endometrium. Unfortunately there is no uniform diagnosis used for this relatively common scenario, and proposed diagnoses include “polypoid secretory endometrium” or “secretory pseudopolyp.”
Endometrial polyps having smooth muscle fibers (not connected with blood vessel walls) in addition to the customary glands and stroma are designated as adenomyomatous polyps (polypoid adenomyomas) ( Fig. 33.25 ). In order to make this diagnosis, the smooth muscle should be integral to the polyp; benign endometrial polyps when they start to prolapse often draw smooth muscle into the polyp stalk, and this phenomenon does not warrant diagnosis as adenomyomatous polyp. They have a characteristic hard consistency and a grayish color.
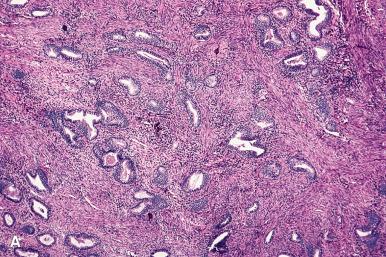
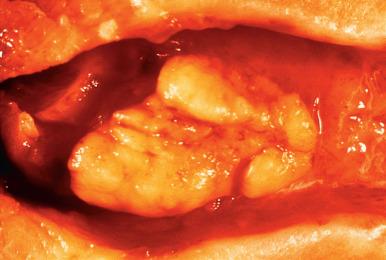
An important variation on the theme is the atypical polypoid adenomyoma . These tend to occur in premenopausal women (average age, 40 years) and present with abnormal uterine bleeding ( Fig. 33.26 ). Some are associated with Turner syndrome. Microscopically, they are identified by the fact that the glands occurring between the endometrial stroma and smooth muscle exhibit varying degrees of hyperplasia and atypia (often with squamous morular metaplasia), sometimes approaching the appearance of low-grade carcinoma ( Fig. 33.27 ). Recognition is important to avoid misdiagnosis as adenocarcinomas with myometrial invasion. The behavior reflects the degree of complexity and atypia of the epithelial component, which can range from atypia without hyperplasia, to atypical hyperplasia and, on occasion, frank carcinoma of low-grade endometrioid type.
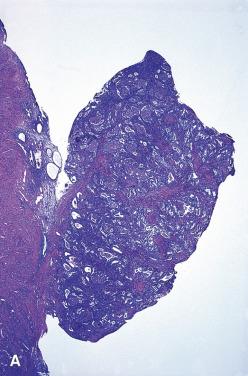
At the molecular level, atypical polypoid adenomyomas share several alterations with hyperplasias.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here