Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The hypersensitivity syndromes are a group of disorders mediated by immunologic or hypersensitivity reactions to foreign proteins such as food or drugs or to infectious agents, immunizations, and malignancies. Although diagnosis of these disorders is often relatively easy, difficulties commonly arise in determining the underlying cause. The most common hypersensitivity reactions are drug reactions, and the skin is a common target. The overall incidence of drug reactions in hospitalized children is 9.5%, and in outpatient children it is 2.5%, with reactivity to antibiotics occurring most often. However, up to 10% of parents report suspected hypersensitivity to at least one drug, most often β-lactam antibiotics.
The various types of drug reactions are summarized in Table 20.1 . Urticarial and exanthematous reactions are seen most often. , Some of these are life threatening and have been linked through the term s evere c utaneous a dverse r eactions (SCARs) : the Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) spectrum, drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis, and generalized bullous fixed-drug eruptions. Other hypersensitivity disorders are lichenoid drug eruptions ( Fig. 20.1 ) (see Chapter 4 ), nonbullous fixed drug eruptions, acneiform eruptions (see Chapter 8 ), pseudoporphyria (see Chapter 19 ), drug-induced vasculitis (see Chapter 21 ), and drug-induced lupus (see Chapter 22 ). Some children experience multiple antibiotic sensitivity, most commonly to penicillins, sulfonamides, cephalosporins, and macrolides. In these children the manifestations of drug sensitivity can be different; for example, a child may show an exanthematous response to amoxicillin, DRESS after phenytoin, and erythroderma after ingestion of sulfamethoxazole within a period of a few months. The general approach to a child with suspected drug reaction is outlined in Box 20.1 .
| Eruption | Key Drugs | Lesional Pattern | Mucosal Changes |
| Urticaria | Penicillins, cephalosporins, sulfonamides, minocycline, aspirin/NSAIDs, antiepileptics, monoclonal antibodies, radiocontrast media | Pruritic erythematous wheals | None |
| Angioedema | Aspirin/NSAIDs, ACE inhibitors | Swelling of subcutaneous and deep dermal tissues | May be present |
| Serum sickness–like reaction | Cephalosporins, penicillins, minocycline, sulfonamides, macrolides, rifampin, ciprofloxacin, griseofulvin, itraconazole, bupropion, fluoxetine, rituximab, omalizumab, H1N1 vaccine | Urticarial or erythema multiforme–like | None |
| Exanthematous | Penicillins, sulfonamides, cephalosporins, antiepileptics | Erythematous macules and/or papules | None |
| Drug hypersensitivity syndrome | Sulfonamides, phenytoin, phenobarbital, carbamazepine, lamotrigine, amoxicillin, allopurinol, sulfonamides, dapsone, minocycline, aspirin, vancomycin, azithromycin, abacavir, nevirapine, Chinese medicine | Edema (especially periorbital); erythematous macules and/or papules; sometimes vesicles or bullae | May be present |
| Lichenoid * | Captopril, enalapril, labetalol, nifedipine, propranolol, gold salts, hydrochlorothiazide, furosemide, spironolactone, hydroxychloroquine, ketoconazole, penicillamine, griseofulvin, tetracycline, carbamazepine, phenytoin, NSAIDs, hydroxyurea, imatinib, dapsone, sulfasalazine, allopurinol, iodides and radiocontrast media, IFN-γ, omeprazole, penicillamine, TNF inhibitors, sildenafil, leflunomide, human growth hormone | Discrete flat-topped reddish-purple papules and plaques | May be present |
| Fixed drug | Sulfonamides, ibuprofen, acetaminophen, salicylates, tetracyclines, pseudoephedrine, loratadine, teicoplanin, metronidazole, macrolides, barbiturates, lamotrigine, potassium iodide, quinine, phenolphthalein, foods and food flavorings (especially tartrazine) | Solitary to few erythematous, hyperpigmented plaques | Unusual |
| Pustular (AGEP) | β-Lactam antibiotics, cephalosporins, macrolides, clindamycin, terbinafine, paroxetine, hydroxychloroquine, contrast agents | Generalized small pustules and papules | None |
| Acneiform † | Corticosteroids, androgens, lithium, iodides, phenytoin, isoniazid, methotrexate | Follicular-based inflammatory papules and pustules predominate | None |
| Pseudoporphyria ‡ | NSAIDs, COX-2 inhibitors, tetracyclines, furosemide | Photodistributed blistering and skin fragility | None |
| Vasculitis § | Penicillins, NSAIDs, sulfonamides, cephalosporins | Purpuric papules, especially on the lower extremities; urticaria, hemorrhagic bullae, digital necrosis, pustules, ulcers | Rarely |
| Stevens–Johnson syndrome/toxic epidermal necrolysis | Sulfonamides, antiepileptics (especially phenytoin, carbamazepine, and lamotrigine), NSAIDs, penicillins, acetaminophen, allopurinol, dapsone, barbiturates | Target lesions, bullae, epidermal necrosis with detachment | Present |
| Drug-induced lupus ‖ | Minocycline, procainamide, hydralazine, isoniazid, penicillamine | Urticarial, vasculitic, erythematous | Rare |
* See Chapter 4 .
† See Chapter 8 .
‡ See Chapter 19 .
§ See Chapter 21 .
‖ See Chapter 22 .
Historical features
Drugs taken, date of initiation, and duration
Date of onset of eruption
Response to removal and rechallenge
Clinical features
Lesional morphology
Number of lesions and distribution
Involvement of mucous membranes
Associated features: pruritus, fever, lymphadenopathy, visceral involvement
Laboratory testing
Eosinophilia
Specific IgE levels
Prick and intradermal tests for late-phase reactions (β-lactam allergy)
Provocation testing
Allergy may be defined as a specific acquired alteration in the capacity of an individual to react to an antigen. Mediated by circulating or cellular antibodies, allergic reactions may be classified as immunoglobulin (Ig) E–mediated hypersensitivity (type I), cytotoxic (type II), Arthus-type toxic immune complex reactions (type III), and delayed-type hypersensitivity (type IV). Type I / anaphylactic reactions include local and systemic manifestations of the interaction between antigen and tissue cells previously sensitized with skin-sensitizing reaginic antibody, usually IgE. This interaction of antigen and antibody results in the release of pharmacologically active substances that produce urticaria, angioedema, anaphylaxis, hay fever, and asthma. Type II / cytotoxic reactions include those reactions initiated by antibody interacting with an antigenic component of the cell surface. The antigen may be a natural component of the cell or an unrelated antigen that has become associated with the cell. Examples include pemphigus (see Chapter 13 ), hemolytic disease of the newborn, transfusion reactions, hemolytic anemia, leukopenia, or thrombocytopenia caused by the reaction of antibody with drugs attached to blood cell surfaces. Type III / toxic immune complex reactions (Arthus-type reactions) are associated with the deposition of immune microprecipitates in or around blood vessels, which results in tissue damage through activation of complement or toxic products from leukocytes attracted to the areas. Examples include serum sickness, vasculitis, glomerulonephritis, and local Arthus reactions after injection of antigens into the skin, subcutaneous tissue, or muscle. Type IV / delayed-type hypersensitivity is the result of the interaction between antigen and specifically sensitized lymphocytic cells, which results in a mononuclear cell infiltration and the elaboration of toxic lymphoid cell products. Examples include tuberculin and other skin test reactions, allergic contact dermatitis (see Chapter 3 ), lichenoid drug eruptions (see Fig. 20.1 and Chapter 4 ), fixed drug eruptions, TEN, and graft-versus-host-type reactions (see Chapter 25 ). Patch testing may be useful in determining the cause of allergic contact dermatitis (see Chapter 3 ).
Urticaria, a systemic disease with cutaneous manifestations, occurs at some time in the life of about 15% to 20% of all persons. It is characterized by the appearance of transient well-circumscribed skin lesions that present as erythematous, intensely pruritic elevated swellings (wheals) of the skin or mucous membranes. More than 80% of cases of new-onset urticaria resolve in 2 weeks and more than 95% of new-onset cases within 3 months. Urticaria may be spontaneous or inducible (e.g., contact urticaria and physical urticarias from cold, pressure, heat, sun, water, exercise/stress, stroking, and vibrations). Taking a thorough history is important in understanding potential triggers, subtype of urticaria, and choice of therapy ( Box 20.2 ), and a physical examination will confirm the presence of urticaria and help to define its subtype. Several simple measures are available to track urticaria/angioedema activity, such as the Urticaria Activity Score, Angioedema Activity Score, and Urticaria Control Test ; none of these are currently validated in children.
Duration of disease
Frequency, duration of episodes, distribution, shape and size of wheals
Association with angioedema
Triggering factors—exercise, physical agents (e.g., cold, heat, pressure, sun, water), foods, OTC and prescription medications, infection, stress
Relationship to travel, menstrual cycle, time of day, hobbies
Other medical history—allergies, infections, autoimmune disease, etc.
Associated noncutaneous manifestations—fever, joint pain, abdominal symptoms
Family history
Response to previous and current therapies (including duration and dose)
Past evaluations and results
Urticaria usually represents a type I (or immediate) hypersensitivity reaction in which the protein or its metabolite binds to IgE on the surface of cutaneous mast cells, leading to activation, degranulation, and release of vasoactive mediators such as histamine, leukotrienes, and prostaglandins. Examples of immunologically mediated urticaria are reactions to latex and reactions to peanuts (see later). Urticaria may also result from nonimmunologic triggering of mast cell release, such as from radiocontrast media, aspirin, many types of nonsteroidal antiinflammatory drugs (NSAIDs), or opiates.
Typical lesions of urticaria have an edematous center with a halo of erythema ( Fig. 20.2 ). They vary from pinpoint-sized papules to large lesions several centimeters in diameter. Central clearing, peripheral extension, and coalescence of individual lesions result in a clinical picture of oval, annular, or sometimes bizarre serpiginous configurations ( Figs. 20.3 and 20.4 ). When annular or serpiginous patterns of urticaria occur, they can be confused with erythema multiforme (EM) or serum sickness–like reactions, and the designation urticaria multiforme has been used ( Fig. 20.5 ). ,
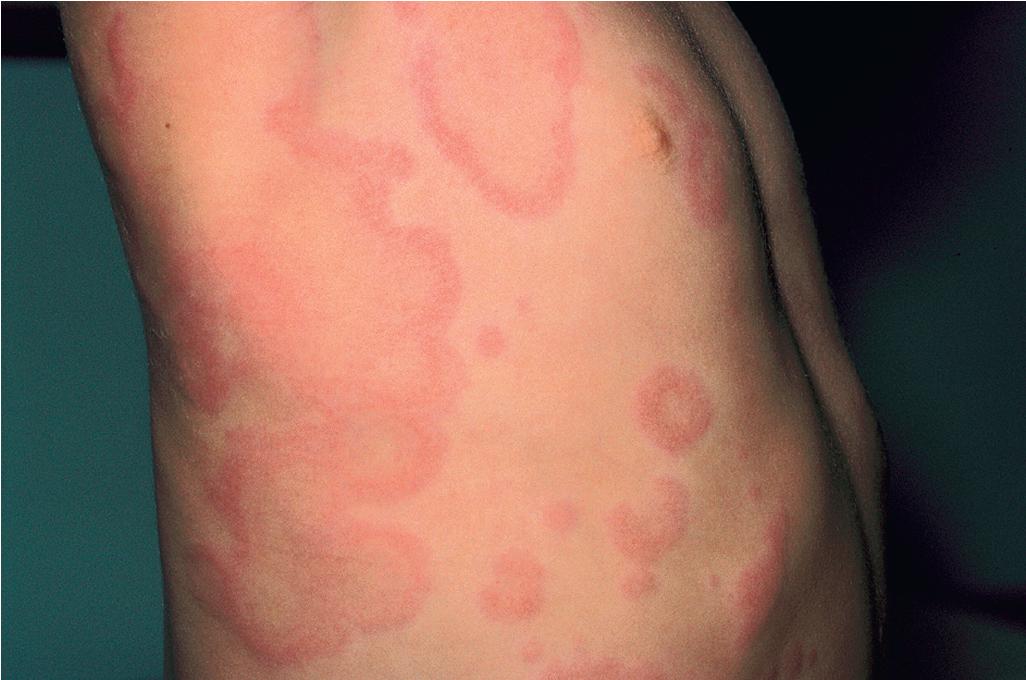
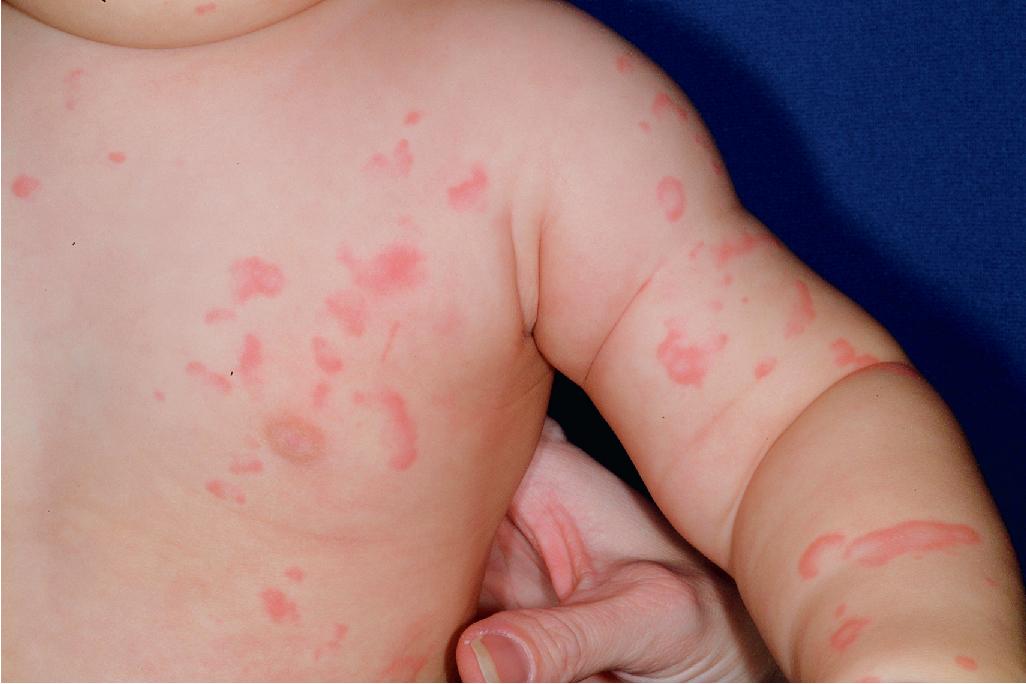
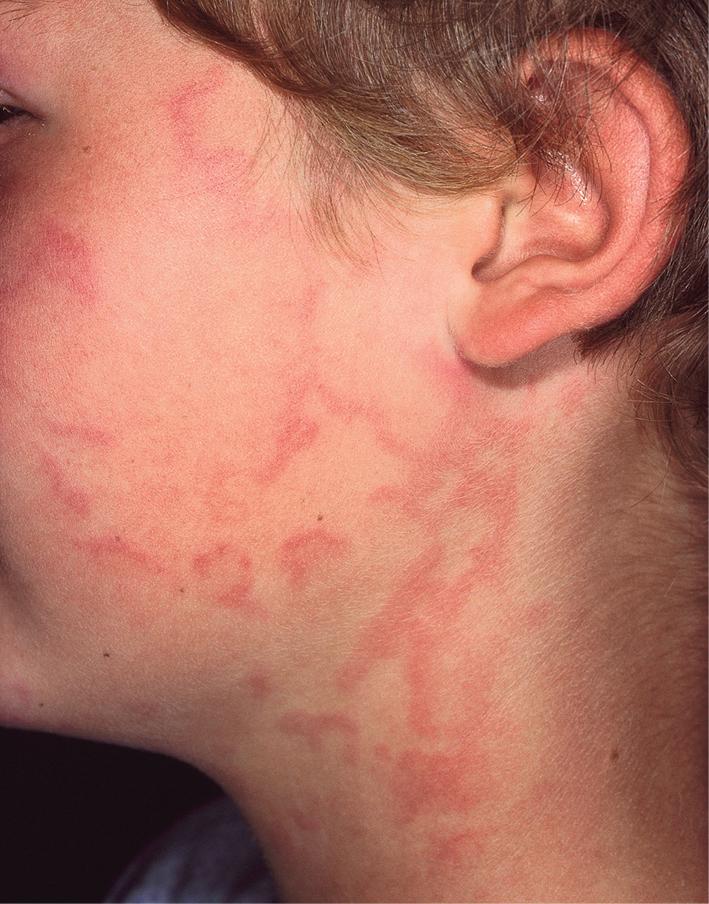
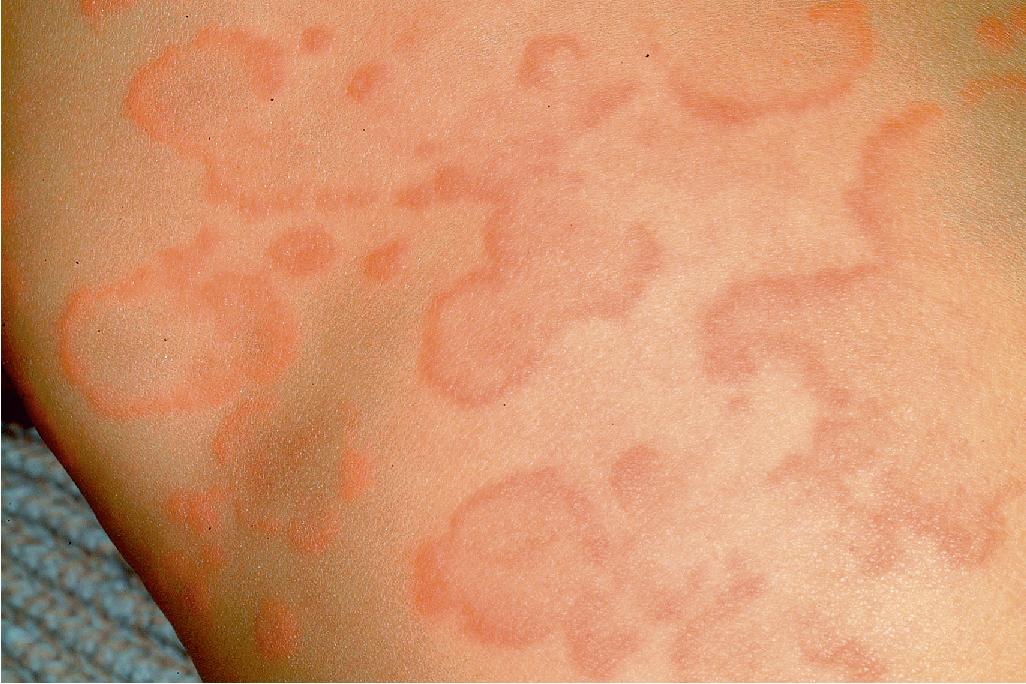
Urticaria-like lesions may be part of a variety of other disorders, particularly maculopapular cutaneous mastocytosis/urticaria pigmentosa (see Chapter 8 ); bullous pemphigoid (prebullous stage; see Chapter 13 ); urticarial vasculitis (individual lesions persisting beyond 24 hours); Henoch–Schönlein purpura and acute hemorrhagic edema of infancy (see Chapter 21 ); systemic-onset juvenile idiopathic arthritis and systemic lupus erythematosus (see Chapter 22 ); autoinflammatory disorders, particularly the cryopyrin-associated periodic syndromes and Schnitzler’s syndrome (especially with fever; see Chapter 25 ); and other hypersensitivity disorders (Wells’ syndrome, hypereosinophilic syndrome). ,
Urticaria may be localized to one small area or may become generalized ( Fig. 20.6 ). Subcutaneous extension may result in giant wheals. In infants and young children, swelling of the distal extremities with acrocyanosis may be a prominent feature of the urticarial reaction. Occasionally, particularly in infants and young children, bullae may form in the center of the wheal, usually on the legs and buttocks. Individual wheals rarely persist longer than 12 to 24 hours. Parents can draw a circle around a few lesions to verify clearance of each individual lesion by approximately 24 hours or less. Those lasting longer than 24 to 36 hours are probably not true urticaria and may represent another vascular pattern such as urticarial vasculitis (see Chapter 21 ) or EM.
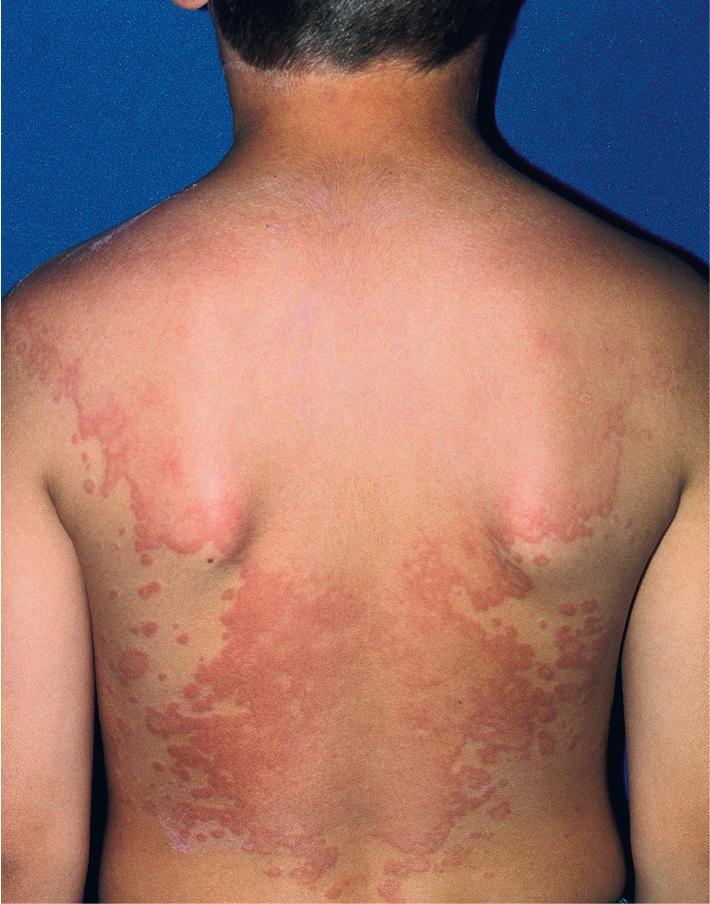
Urticaria of less than 6 weeks’ duration is considered acute. In 80% of children, acute urticaria is the result of infection, often viral, whereas in adolescents food and drugs play a more significant role. Among foods, dairy products play a more common role in younger children, in contrast to nuts, seafood, berries, and grains in older children. Streptococcal infection, mycoplasma, histoplasmosis, and coccidioidomycosis in endemic areas are also well-known triggers, although almost every infectious agent has been associated with urticaria.
Overall, up to 10% of episodes of urticaria are related to drugs, and these are usually acute in nature. The penicillins are the most common drugs to trigger urticaria, but only 10% to 20% of individuals who report allergy to penicillin are really allergic when skin prick testing to penicilloyl-poly-lysine and then oral drug challenge (if negative) is performed. Other drugs most likely to induce an urticarial drug reaction are cephalosporins, sulfonamides, tetracyclines (especially minocycline), antiepileptics, and monoclonal antibodies, including omalizumab. Although cross-intolerant reactions are more common than IgE-mediated reactions with NSAIDs, selective immediate reactions to NSAIDs are becoming increasingly recognized with the widespread use of these medications in children (especially ibuprofen). In vitro testing (immunoassays and basophil activation testing) can detect specific IgE antibodies for a limited number of drugs and complement prick testing but should be performed within 1 year after the occurrence of a reaction. Anaphylactoid reactions (anaphylaxis without identifiable specific IgE antibodies) most commonly result from ingestion of acetylsalicylic acid. Nonimmunologically mediated urticaria may result from radiocontrast media and NSAIDs. Psychologic stress may exacerbate (but not cause) urticaria.
Urticaria that recurs often and lasts longer than 6 weeks is termed chronic. Chronic urticaria occurs in 0.1% to 0.3% of children and is much more common in adults ; it may be present continuously or nearly every day (chronic continuous urticaria) or as attacks separated by symptom-free periods of up to several weeks (chronic recurrent or intermittent urticaria). Diagnosis and treatment of a patient with chronic urticaria demands a complete history and physical examination, appropriate laboratory evaluation, and an awareness of possible underlying disease.
Among children with chronic spontaneous (versus inducible) urticaria, 30% to 50% have autoimmune (also called autoreactive ) urticaria, caused by IgE antibodies to autoallergens (type I autoimmunity) or IgG autoantibodies recognizing the high-affinity IgE receptor (FcεRIα) or, less commonly, IgE itself (type II autoimmunity). Enzyme-linked immunosorbent assay and immunodot tests are in development to measure anti-FcεRIα antibodies directly. , Autoantibodies induce mast cell and basophil degranulation, so patients can be screened using the autologous serum skin test (ASST) or the in vitro basophil activation test (BAT). The ASST is nonspecific; evaluates serum histamine-releasing factors in general, not histamine-releasing autoantibodies; and is associated with a risk of infection. In the in vitro BAT, the demonstration of CD63 on the basophil surface by flow cytometry is a marker of histamine release , and distinguishes autoimmune from physical urticarias; it can also predict respond to cyclosporine versus omalizumab. , Patients may have autoimmune thyroid disease and, less commonly, celiac disease, type 1 diabetes, juvenile idiopathic arthritis, and systemic lupus erythematosus ( Fig. 20.7 ). Further investigation should be based on the known underlying causes, history, and examination and could include complete blood cell count, erythrocyte sedimentation rate, antinuclear antibody, CH50, free T4, thyroid-stimulating hormone, antithyroglobulin and antimicrosomal antibodies, and stool examination for parasites.
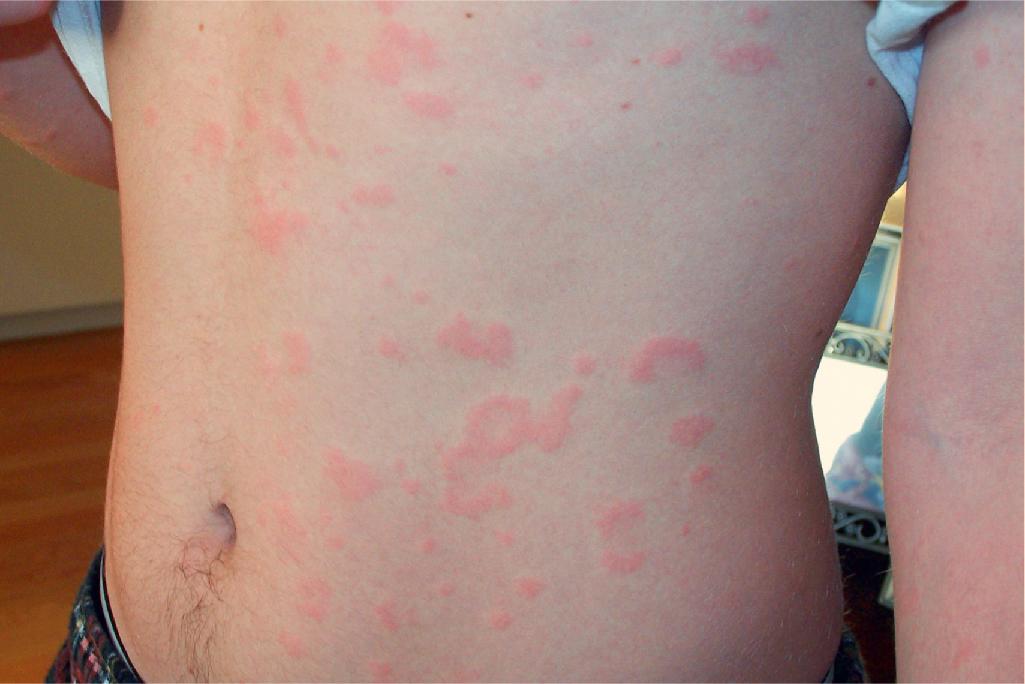
Reactions from food additives are unusual (overall in 9%), but elimination of an offending food or food additive leads to remission in 24 hours. Urticaria from food additives, artificial flavoring, or preservatives is not IgE mediated but rather is a pseudoallergic reaction and is often delayed (4 to 12 hours after ingestion). Food avoidance correlates better with a positive history of food reactivity than with positive prick testing. Among less common possible triggers of chronic urticaria are physical causes, drugs (especially aspirin and NSAIDs), immunizations, insect bites, inhalant or contact allergens, and infections (parasites, Helicobacter pylori , Chlamydia, dental abscesses, sinus infections, low-grade urinary tract infection, and otitis).
In pediatric studies the rate of resolution of chronic urticaria is about 10% annually, with remission rates at 1, 3, and 5 years after the onset of chronic urticaria range from 16% to 18%, 39% to 54%, and 50% to 68%, respectively, regardless of whether the chronic urticaria is autoimmune. , In general, being female and older than 10 years of age are poorer prognostic signs. Time to remission is linked to disease activity, with high activity scores associated with longer time to remission. In one study, chronic urticaria in children with positive BAT results (CD63 level > 1.8) was twice as likely to resolve after 1 year versus in those with a negative BAT result, suggesting that type 2 autoimmune urticaria (driven by IgG autoantibodies) resolves in children more quickly than type 1 autoimmune urticaria (driven by IgE autoantibodies).
Effective treatment of urticaria depends on identification of the causal factor and its elimination whenever possible. First-line treatment in children is administration of a nonsedating histamine (H1) blocker such as cetirizine, loratadine, desloratadine, fexofenadine, levocetirizine, ebastine, rupatadine, or bilastine. , Dose escalation may then be required up to twofold to fourfold those used for management of allergic rhinitis, especially in children with chronic urticaria. , For chronic spontaneous urticaria, antihistamines are effective in about 50% of children. Addition of hydroxyzine, diphenhydramine, or another sedating H1 blocker at night may be considered but should not replace nonsedating antihistamines. Sedating antihistamines are best administered about 1 hour before bedtime to minimize daytime drowsiness. Paradoxical hyperactivity and irritability, which requires discontinuation of the medication, has occasionally been described in some infants and children with the administration of H1 blockers, especially diphenhydramine. Doxepin, a tricyclic antidepressant with H1- and H2-antagonistic effects, has also been used for the treatment of chronic urticaria but may lead to intolerable sleepiness. Approximately 80% of patients respond to antihistamines. Concomitant administration of an H2 blocker (such as famotidine or cimetidine) tends to be of limited value, but a leukotriene receptor antagonist (e.g., montelukast) can be considered if antihistamines are insufficient. Antihistamine administration should be continued for a few weeks after clearance of the urticaria with gradual tapering of the dosage to determine whether they are still needed for control. Avoidance of aspirin and NSAIDs may be helpful because these medications may exacerbate chronic urticaria.
Monthly administration of subcutaneous omalizumab (150 to 300 mg for adolescents, given every 4 weeks) is effective in 65% to 80% of those who do not respond to antihistamine. Omalizumab is a monoclonal antibody that binds free serum IgE, inhibits IgE receptor activation, blocks antireceptor antibodies, and ultimately leads to unresponsiveness of cutaneous mast cells and basophils. Omalizumab has also been reported to be effective for physical urticarias, including cholinergic, cold, solar (see Chapter 19 ), heat, and delayed pressure types. Cyclosporine has a success rate of about 70% and is recommended as third-line care, given the potential side effects affecting blood pressure and renal function. Cyclosporine inhibits both T cells and histamine release from basophils and mast cells.
Dapsone, methotrexate, mycophenolate mofetil, cyclophosphamide, hydroxychloroquine, sulfasalazine, intravenous immunoglobulin (IVIG), and plasmapheresis have all been used for resistant chronic urticaria but have less evidence than omalizumab and cyclosporine. , The subcutaneous administration of 0.1 to 0.5 mL of epinephrine 1:1000 is often effective for patients with angioedema and severe urticaria. Although often effective in patients with severe or persistent urticaria, administration of systemic corticosteroids should be reserved for acute reactivity in recalcitrant patients and should be limited to 10 days because of the many potential side effects.
Urticaria may occur from skin exposure to antigen rather than through ingestion or inhalation ( Fig. 20.8 ). In this case the lesions tend to be localized to the site of contact, although rarely satellite lesions or even more generalized involvement has occurred. Infants and children with IgE-mediated food allergy may also have contact urticaria at sites of cutaneous exposure, such as on the face. In one study in which a drop of cow’s milk was applied by the physician to the subject’s healthy-appearing cheek (“finger test”), 45% of children with IgE-mediated cow’s milk allergy developed a wheal and flare reaction within 20 minutes at the site of contact (37% of those without a history of atopic dermatitis and 71% of those with atopic dermatitis [AD]). No child with non-IgE cow’s milk allergy (i.e., with gastrointestinal symptoms, even with AD) or healthy controls developed contact urticaria.
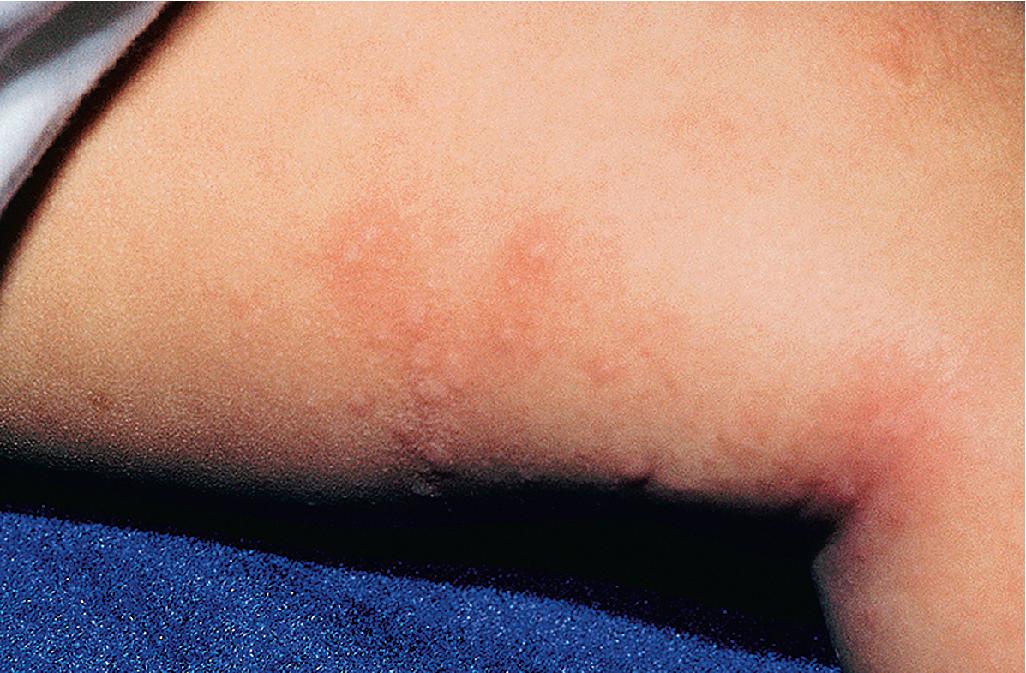
Exposure to certain jellyfish, corals, caterpillars, moths, and chemicals can lead to contact urticaria through pharmacologic means. Immunologic reactions can also result in contact urticaria, as often occurs with exposure to fish or latex. Localized small wheals have also been described after contact with the spines of pet hedgehogs. Aquagenic urticaria is a variant of contact urticaria in which small urticarial papules arise at follicles after exposure to water.
The physical urticarias are a group of disorders in which wheals occur in response to various physical stimuli. These include dermographism and pressure, cholinergic, aquagenic, solar (see Chapter 19 ), and cold urticaria. Dermographism and cholinergic urticaria are quite common; cold urticaria is less common, and other patterns of physical urticaria are relatively rare. Challenge testing allows the diagnosis to be confirmed.
Dermographism (dermatographism) is manifested by a sharply localized edematous or wheal reaction with a surrounding zone of erythema that occurs precisely at the site and within seconds of firm stroking of the skin ( Fig. 20.9 ). The wheal tends to be maximal in size and intensity at about 6 minutes after onset and persists for approximately 15 minutes. This common phenomenon, known as the triple response of Lewis, is often seen in infants, occurs in about 50% of children, and is noted in only about 1% of adolescents or adults. Nonsedating antihistamines and avoidance of triggers usually controls dermographism, but some patients require cyclosporine or narrowband UVB.
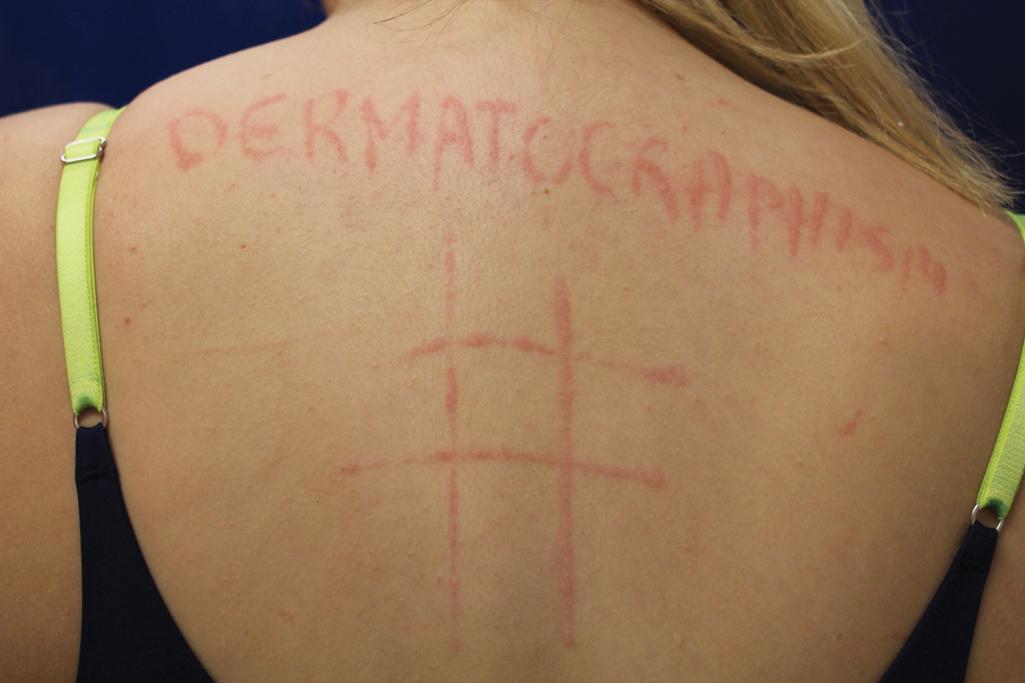
Pressure urticaria is a variant of dermographism, characterized by the development of hives or a deeper swelling simulating angioedema after local pressure, such as from clothing or jewelry, or weight bearing. The reaction is often painful rather than pruritic and may occur immediately after the pressure or, more commonly, after a 4- to 6-hour delay (“delayed pressure urticaria”). Because of this delay in appearance, patients often fail to appreciate the cause of the disorder. The palms and soles are most commonly involved. Patients respond poorly to antihistamine therapy; some patients have responded to the leukotriene antagonist montelukast.
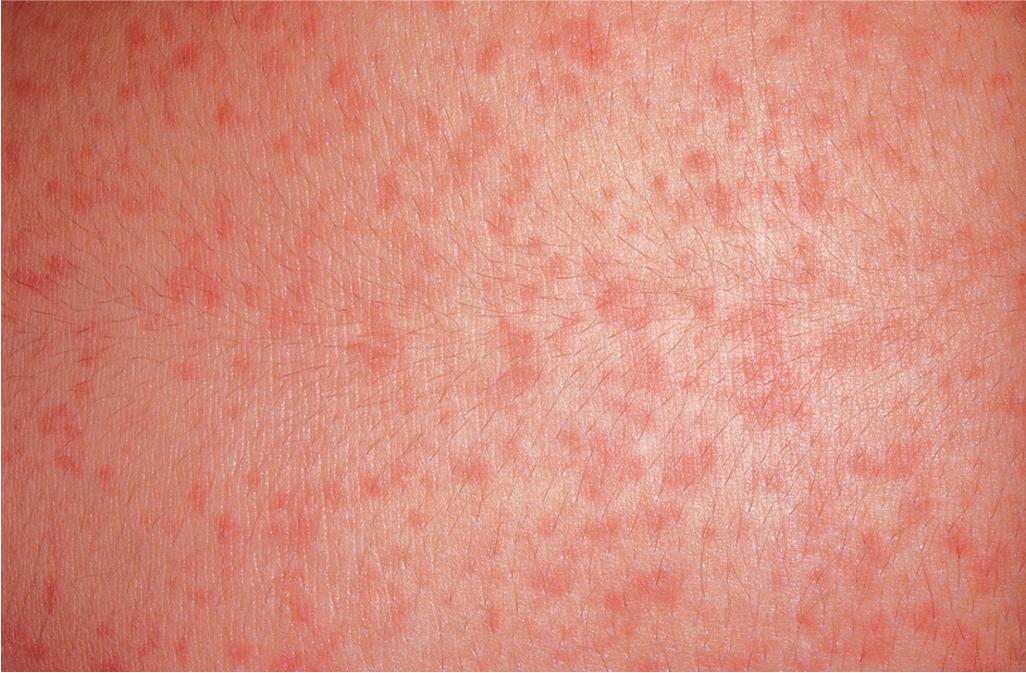
Cholinergic urticaria (micropapular urticaria), which occurs in 5% to 7% of individuals with urticaria, is a very distinctive type. , It usually starts in adolescence and is associated with heat, exertion, or emotional stress. Cholinergic urticaria is characterized by a generalized eruption, especially on the trunk and arms, which consists of discrete, tiny wheals, 1 to 3 mm in diameter, with or without a surrounding area of erythema ( Fig. 20.10 ). The wheals first appear within minutes after sweating begins and occasionally are accompanied by exercise-induced bronchospasm, headache, gastrointestinal discomfort, and faintness. The duration of the eruption varies from 30 minutes to several hours; after an episode, a refractory period of about 24 hours ensues. A subset of patients with cholinergic urticaria have hypohidrosis or anhidrosis.
Acetylcholine is thought to participate in the pathomechanism of wheal formation, but immediate type skin responses to one’s own sweat have been described. Once cholinergic urticaria occurs, the condition may recur for periods of months to years and then tends toward spontaneous improvement and resolution. Provocation tests for the diagnosis of cholinergic urticaria should be performed by increasing the body temperature through exercise (treadmill or stationary bicycle) or the use of a hot bath (42 o C for 15 minutes). Treatment consists of H1 nonsedating antihistamines, which may need to be increased up to fourfold. Approximately 50% respond to this regimen. Addition of H2 blockade and/or montelukast may be helpful. Some patients have responded to omalizumab. Other studies have demonstrated the efficacy of anticholinergic agents, the addition of propranolol (β2-adrenergic blocker), and treatment and injection with botulinum toxin. Potential precipitating factors and avoidance of heat, excessive exertion, and excitement that leads to sweating should be avoided whenever feasible.
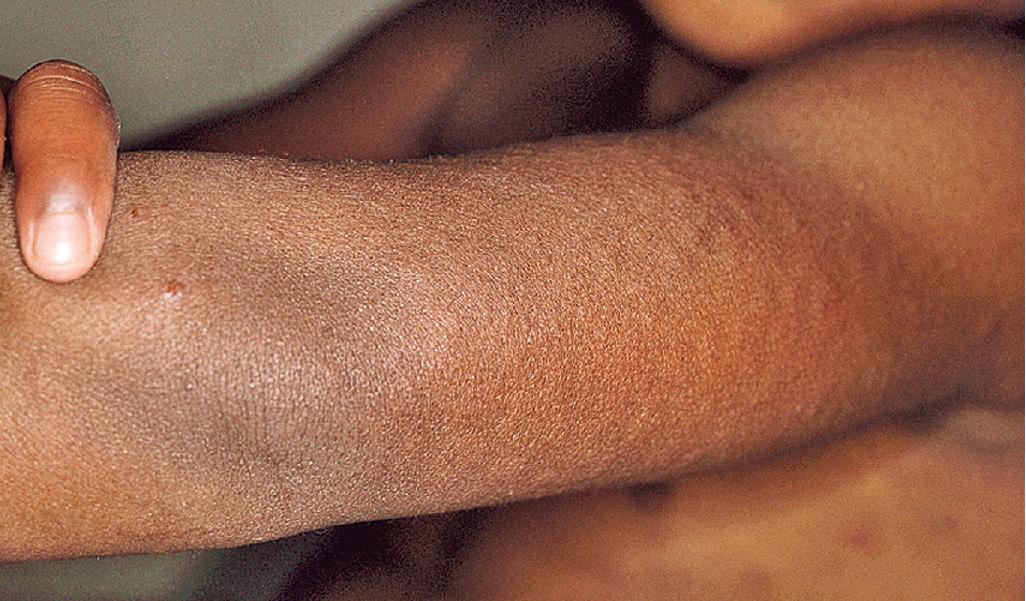
Aquagenic urticaria, a disorder that resembles cholinergic urticaria, occurs most often in adolescence and is characterized by small, intensely pruritic, perifollicular papular wheals with surrounding erythema ( Fig. 20.11 ). The palms and soles are spared. The disorder is precipitated by contact with water or perspiration (irrespective of temperature). Exercise and other cholinergic factors do not precipitate this disorder. Patients can drink water without adverse reaction. Salt-dependent aquagenic urticaria is a subtype in which the children react primarily or exclusively when there is exposure to saltwater. Antihistamines often improve reactivity but do not totally suppress it.
Cold urticaria accounts for approximately 3% of chronic urticaria and has been documented in infants as young as 6 months. The urticarial reaction develops within a few minutes or hours after exposure to cold air or water, often on rewarming. It is classified as localized (type 1; 67% of patients), generalized (type 2; 14%), or with angioedema or anaphylaxis (type 3; 19%). Type 3 reactions usually occur with water immersion (swimming) but have also been reported with ingestion of cold food or beverages or exposure to cold air. In highly sensitive individuals the urticarial reaction may be associated with respiratory or cardiovascular compromise, hypotension and, on occasion, syncope, loss of consciousness, and drowning. Respiratory signs such as nasal stuffiness, cough, and dyspnea and oral or gastrointestinal symptoms such as swelling of the lips, swelling of the oral mucous membranes, dysphagia, and abdominal cramps may occur. Patients who experience oropharyngeal reactions to cool liquids or foods (especially lip swelling) are at increased risk for the development of systemic reactions.
Cold urticaria in children and adolescents is usually acquired, occurs more often in girls, and often appears suddenly. Anaphylaxis has been described in up to 30% at presentation. Once symptoms develop, they are generally short-lived and recurrences usually disappear after a few months or years. Secondary forms of cold urticaria may also be associated with cold hemolysin and cold agglutinin syndromes. These forms, generally seen in adults, cause Raynaud phenomenon, acrocyanosis, and cutaneous ulcers. Some cases of cold urticaria manifested by itching, erythema, purpura, atypical Raynaud phenomenon, and ulceration are the result of cryoglobulins (see Chapter 21 ).
Cold urticaria can rarely be a manifestation of familial cold autoinflammatory syndrome (FCAS), an autosomal dominant disorder resulting from mutations in CIAS1/NLRP3 (see Chapter 25 ). It is characterized by an urticarial or papular eruption, fever, chills, arthralgia, and sometimes headache, malaise, muscle tenderness, and significant leukocytosis. Patients more commonly complain of burning or stinging than of pruritus. Although the tendency to familial cold urticaria generally persists for life, the severity may decrease with advancing age. It generally develops after a latent period of several hours, and once it develops, it may persist for up to 48 hours. Familial atypical cold urticaria (FACU) is an autosomal dominant disorder with onset during childhood in which the urticaria and/or angioedema develop shortly after cold exposure (not delayed). Cold-stimulation testing is negative (no wheal), and in contrast with FCAS, patients do not have fever, chills, or joint complaints. PLCG2 (phospholipase C gamma 2)–associated antibody deficiency and immune dysregulation (PLAID) is an autosomal dominant immunodeficiency syndrome associated with evaporative cold urticaria. , The cold urticaria presents within the first year in all patients and is characterized as pruritic blotchy macules but not true wheals; ice cube testing is negative. Patients may develop ulcerative lesions of the nasal tip, and occasionally fingers and toes, during the neonatal period that heal by infancy. They tend to develop granulomatous dermatitis, which may be widespread. Recurrent sinopulmonary infections, common variable immunodeficiency (CVID), and autoantibodies (especially antinuclear and antithyroid antibodies) are commonly associated.
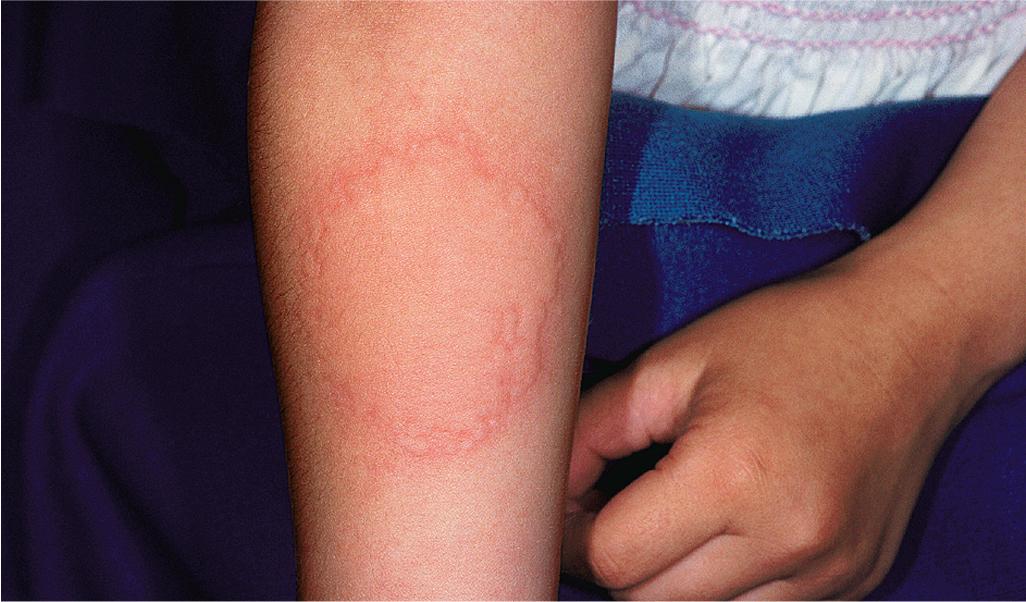
The diagnosis of cold urticaria requires a careful history and investigation for other possible causal factors. The diagnosis may be confirmed by reproducing signs through local application of an ice cube for periods of 2 to 10 minutes with observation after removal for at least 10 minutes. The best areas for this testing are the face, neck, and particularly the arms ( Fig. 20.12 ). Results are positive, however, only in 70% of those tested. Although a positive test result is associated with an increased risk of anaphylaxis, in one study 12% of patients with anaphylaxis had a negative cold stimulation test. Some patients fail to respond to ice but do respond to cold water or generalized cooling of the body. Critical temperature threshold testing shows an inverse relationship with disease severity and activity, which can be helpful for assessing both the severity and the impact of therapy. Cold urticaria should be distinguished from cold-induced cholinergic urticaria, in which cholinergic urticaria occurs while exercising in the cold and is not induced by ice cube testing. Immediate ice cube tests also tend to be negative with FCAS, FACU, and PLAID syndromes.
Underlying disorders are rare in children. Cryoglobulinemia, cryofibrinogenemia, and cold agglutinins have all been reported, particularly in adults, and can reflect an underlying diagnosis of essential mixed cryoglobulinemia, hepatitis, autoimmune disease, or lymphoma. Cold urticaria can also be associated with infectious disorders (toxoplasmosis, Epstein–Barr virus [EBV], H. pylori, hepatitis C, and human immunodeficiency virus [HIV]) and autoimmune disease (particularly lupus erythematosus or celiac disease).
Patients with cold urticaria, and particularly with severe or widespread urticarial reactions, should carry epinephrine and be forewarned of the risk of drowning after loss of consciousness when swimming or bathing in cold water. The treatment of cold urticaria is aided by oral administration of antihistamines, particularly nonsedating H1 blockers, although the sedating antihistamine cyproheptadine is also particularly helpful for cold urticaria, and leukotriene receptor antagonists have been used adjunctively. Increases in doses of nonsedating antihistamine to four times the standard dosages have shown additional benefit (versus standard dosing) without increased adverse events. For those patients who are unresponsive to systemic antihistamines, treatment with omalizumab appears to be helpful in almost 50%, suggesting a role for IgE. Desensitization to cold is an alternative that is now rarely performed; an extremity is gradually cooled in cold water for 5 to 10 minutes per day with a gradual increase in the time of exposure and decrease of the temperature over a period of weeks or months. This treatment is not regularly effective and must be done cautiously to minimize the risk of systemic reaction. Patients with cold urticaria as a manifestation of an autoinflammatory syndrome often require interleukin (IL)–1 blockers (e.g., anakinra) for response. ,
Anaphylaxis is a life-threatening, immediate hypersensitivity reaction to the administration of an antigen that has previously produced a specific sensitization. It is characterized within minutes to 1 hour after injection or ingestion of antigen by pruritus of the palms, soles, and scalp, urticaria, and/or angioedema with systemic signs (weakness, dyspnea, hypotension, and circulatory collapse). Mucosal involvement of the airway leads to acute respiratory distress, and swelling of the gastrointestinal mucosa can result in abdominal pain, vomiting, or diarrhea. The incidence in children and adolescents is 10.5 episodes per 100,000 person-years, and most children have a personal history of atopy. Anaphylaxis can occur during infancy, although the risk peaks during early childhood and decreases thereafter. A biphasic reaction may occur, with recurrence usually within 8 hours of the initial episode. Anaphylactic reactions most often occur after ingestion of foods, , especially peanuts, cow’s milk, and hen’s eggs in children (versus wheat and shellfish in adults), or in response to drugs or insect venom. , Anaphylactic reactions to latex and peanuts are particularly troublesome, because they are so ubiquitous in the environment. Having asthma as well as IgE-mediated food allergy increases the risk of developing food-induced anaphylaxis. Exercise-induced anaphylaxis may also occur and is commonly food dependent. Two subtypes have been described: nonspecific food-dependent exercise-induced anaphylaxis, in which filling the stomach before exercise is responsible, regardless of the kind of food ingested, and specific food-dependent exercise-induced anaphylaxis, an IgE-mediated food allergy in which anaphylaxis only occurs in combination with exercise. Several types of foods have been described to trigger exercise-induced anaphylaxis, but wheat appears to be most common.
Latex allergy is a reaction to a peptide in natural rubber and occurs most commonly in children with spina bifida, presumably as a result of the frequent exposure to catheters and latex gloves. The use of latex-free or latex-safe equipment has markedly lowered the risk to children with spina bifida. , Atopic children also show an increased risk of latex allergy, with up to 4% showing prick test reactivity. Type I hypersensitivity reactions to latex may range from contact urticaria to life-threatening anaphylaxis. Severe anaphylactic shock without cutaneous erythema or urticaria has been described. Type I reactions to latex differ from the type IV contact dermatitis reaction to antigens in rubber products (see Chapter 3 ). Children with type I hypersensitivity reactions often experience lip swelling after sucking on a pacifier or blowing up a balloon ( Table 20.2 ). Aerosolized powder from latex gloves and inhalation may lead to runny nose, sneezing, itchy, swollen eyes, and wheezing in susceptible individuals. Approximately half of persons who react to latex show reactivity to certain foods, resulting in oral mucosal pruritus, urticaria, and wheezing; bananas, kiwis, avocados, and chestnuts contain proteins that cross-react with latex and are most commonly associated.
| Household | Medical |
| Balloons | Adhesive tape |
| Balls (basketball, Koosh, squash, tennis) | Bandages |
| Cell phone cases | Blood pressure cuffs |
| Computer mouse pads | Bulb syringes |
| Condoms | Catheters |
| Disposable diapers/rubber pants | Dental devices |
| Erasers | Electrode pads |
| Gloves for dishwashing | Face masks and their straps |
| Pacifiers, nipples | Gloves |
| Rubber bands | Injection ports |
| Rubber cement | Rubber syringe stoppers |
| Rubber raincoats and rubber boots | Stethoscope tubing |
| Shoe soles | Tourniquets |
| Socks with elastic | Vial stoppers |
| Sports equipment | Wound drains |
| Swimming masks and goggles | |
| Toys | |
| Underwear |
Skin prick testing is most likely to yield positive responses to either latex or cross-reacting foods. Serum latex-specific IgE by ImmunoCAP can also be used to detect type I reactivity to latex, but the high rate of false positives should be taken into account when making a diagnosis of latex allergy in patients with pollen allergy (30%), especially in those sensitized to more than one pollen species. Avoidance of latex and related triggers is critical, and nonlatex substitutes such as Mylar balloons and vinyl or neoprene gloves are generally available. Desensitization is the only effective way to resolve latex allergy; percutaneous and sublingual approaches appear to be safer than subcutaneous administration.
Nut-induced anaphylaxis is a risk in the 1.1% of individuals who are allergic to peanuts and tree nuts , and has resulted in 50 to 100 deaths annually in the United States. Screening tests such as prick tests and radioallergosorbent tests (RASTs) are suggestive, but only food challenge confirms reactivity. Avoidance of peanuts is extremely difficult, and there has been no means to protect against accidental ingestion, which often occurs despite reassurance from servers. Allergic reactions through kissing are well documented, particularly in adolescents. In one study, 13% of subjects still had detectable levels of peanut 1 hour after ingestion of 2 tablespoons of peanut butter on a sandwich.
Although prenatal and postnatal avoidance of peanuts was encouraged for at-risk individuals in the past, current practices include early exposure to peanut to cause tolerance. , In a landmark trial of 500 infants at risk for peanut allergy, infants who avoided peanuts had a much higher risk of developing allergy than those who were given small numbers of peanuts regularly, regardless of whether they were sensitized to peanuts at baseline. , Guidelines recommend that infants with severe atopic dermatitis, egg allergy, or both be evaluated by specific IgE (sIgE) measurement and/or skin prick testing (SPT) and oral food challenge, if necessary) ; if peanut sIgE is less than 0.35 and/or SPT yields a 0- to 2-mm reaction, peanut can be introduced as early as 4 to 6 months of age (recommended is 6 to 7 g of peanut protein per week, divided into at least three feedings weekly; it should be introduced before 11 months of age). If the SPT measures 3 to 7 mm, peanut can be introduced in a supervised office setting or oral food challenge can be performed. For those with sIgE 0.35 or greater and/or SPT 8 mm or larger, referral to a specialist for continued evaluation and management is recommended. Infants with mild to moderate atopic dermatitis do not have to undergo this testing but should have peanut introduction as early as 6 months, whereas those without atopic dermatitis or food allergy can have peanut introduction that is age appropriate and according to family preferences.
For those with early evidence of peanut sensitization and allergy, immunotherapy has shown promise. Early oral immunotherapy (E-OIT), given for a median of 29 months in children 9 to 36 months of age with peanut allergy, led to sustained unresponsiveness to peanuts in oral food challenge at 4 weeks after stopping the E-OIT in 78% of those of those tested. Other oral immunotherapy studies have similarly shown benefit in most children.
Omalizumab substantially increases the threshold of sensitivity to peanuts and may protect against unintended ingestion. Oral immunotherapy with gradual increases in exposure to peanuts can decrease basophil activation, titrated skin prick tests, and peanut-specific IgE levels within 6 months.
The treatment of anaphylaxis consists of the intravenous administration of antihistamines such as diphenhydramine (1.25 mg/kg), subcutaneous epinephrine (the adult-dose epinephrine autoinjector contains 0.3 mg of epinephrine as 0.3 mL of epinephrine 1:1000, and the pediatric-dose autoinjector contains 0.15 mg of epinephrine as 0.3 mL of epinephrine 1:2000) for grade 2 anaphylaxis or higher, and sometimes intravenous corticosteroid. , Epinephrine is the only medication shown to be lifesaving when administered promptly, but it is underused. Patients should have easy access to at least two doses of an epinephrine autoinjector, with thorough training regarding correct use of a given device and an emergency action plan. In addition to EpiPen, Auvi-Q is an autoinjector available that provides audio and visual cues regarding patient use and was found favorable to the traditional autoinjector for method of instruction, preference to carry, and device size.
Because of the delay in onset of effectiveness, oral medications are not indicated. If hypotension is present, intravenous fluids should be initiated to permit the rapid administration of plasma volume expanders, fluids, and electrolytes. Once shock is overcome and oral fluids are well tolerated, oral corticosteroids may be initiated. Anyone at risk for anaphylaxis should wear a medical alert bracelet and carry epinephrine in case of emergency. Management of anaphylaxis in schools has recently received attention, especially because of its distinct challenges and the need to be able to urgently administer epinephrine.
The term angioedema (also called giant urticaria or Quincke edema ) describes swelling of the eyelids, hands, feet, genitalia, the lips, tongue, airway, and gastrointestinal tract. , A 2018 classification included angioedema within the spectrum of urticaria. Angioedema results from vasodilation, increased tissue permeability, and subsequent interstitial edema. These changes can occur because of mast cell or basophil degranulation with release of vasoactive molecules (histamine, leukotrienes, prostaglandin D2, platelet-activating factor) as an IgE-mediated type I hypersensitivity reaction or through direct cell degranulation. Alternatively, these changes can occur because of upregulated kinin pathway activation (increases in bradykinin) with loss of vascular endothelial cadherin in endothelial tight junctions and water movement to the extracellular space.
When it occurs in association with wheals, angioedema is a feature of urticaria. Fifty percent of patients with angioedema also show urticaria, and 10% of infants and children with urticaria show at least mild angioedema. Angioedema may also be a component of anaphylactic reactions. Angioedema with urticaria and/or pruritus is typically seen with IgE-mediated reactions, although urticaria is absent in 10% to 15% of patients with angioedema from an allergic cause. As with urticaria, chronic angioedema in children is often idiopathic and triggered by bacterial or viral infection. Most cases of idiopathic acquired angioedema are histamine responsive (histaminergic; termed idiopathic histaminergic acquired angioedema [IH-AAE]), and medications (especially aspirin and NSAIDs), allergens, and physical agents have also been implicated. Some patients with chronic angioedema may have IgG antibodies directed against the IgE receptor or IgE itself (autoimmune angioedema).
In one retrospective study, NSAIDs were the trigger for angioedema in 21% of 16 to 21 year olds (versus only 2% in preschool years), reminding of the need to inquire about use of over-the-counter products, including intermittently. In another study, 6% of children with angioedema unrelated to urticaria found NSAID use as the trigger, and atopy increases the risk. More than 80% of hypersensitive children may cross-react on challenge with another NSAID.
Angioedema without urticaria has been classified by the European Academy of Allergy and Clinical Immunology into four acquired and three autosomal dominant hereditary forms. Two of the three acquired forms (induced by angiotensin-converting enzyme inhibitors and a nongenetic form of C1-INH deficiency) usually occur in adults.
Diagnostic evaluation should involve acquiring history of potential triggers (foods, recent infection, over-the-counter and prescription medications, exercise, response to heat and pressure, bites or stings, allergens, stress). Asking about occurrence of angioedema in the family, whether associated with wheals or pruritus, and response to antihistamine/steroid intervention is also important in considering hereditary angioneurotic edema. In most cases of angioedema, allergy testing and laboratory evaluation are not needed. The lip swelling of more chronic angioedema can be confused with the enlarged lips seen in children with inflammatory bowel disease, especially Crohn’s disease, or that of granulomatous cheilitis (see Chapter 25 ). In addition, allergic contact dermatitis involving the face is sometimes mistaken for acute facial angioedema, although the associated dermatitis distinguishes the conditions. “Factitious” angioedema in children in association with psychiatric disease has also been described.
Treatment of acute angioedema is focused on elimination of triggers (if possible) and administration of nonsedating H1 antihistamines, which can be prescribed at up to four times standard dosing with minimal side effects. Next-step management is the addition of sedating first-generation antihistamines, H2 antihistamines, and leukotriene inhibitors. Immunomodulators are only considered if the child fails to respond to at least twice-daily dosing with nonsedating antihistamine plus a sedating antihistamine at night. Cyclosporine and omalizumab are most commonly used in children (especially as young as 9 and 12 years old, respectively). Alternatives are dapsone, methotrexate, sulfasalazine, and colchicine, but experience has largely been in adults. Systemic steroids are not used for chronic management.
Hereditary angioneurotic edema (HAE; hereditary angioedema), which occurs in approximately 1 in 50,000 to 1 in 10,000 persons, is characterized by recurrent episodes of nonpruritic, nonerythematous edema of the subcutaneous tissue, particularly the hands, feet, and face, and of the gastrointestinal and/or upper respiratory tracts. , Most commonly, HAE results from a mutation in SERPING1, leading to haploinsufficiency of C1 inhibitor (C1-INH; type 1 HAE), the serine protease that inhibits proteases involved in the bradykinin pathway. Interestingly, levels are usually 5% to 30% of normal, despite having one normal allele. In about 15% of cases there is a mutation in SERPING1 (usually a specific missense mutation in exon 8) that leads to C1-INH dysfunction (type 2 HAE). Deficiency or dysfunction of C1-INH leads to transient episodes of increased vascular permeability with increased cleavage of C4 by C1, depleting C4 levels. The major factor responsible for edema formation is now known to be bradykinin, which can induce leakage of postcapillary venules. Bradykinin is produced when high-molecular-weight kininogen is cleaved by plasma kallikrein, the activity of which is controlled by C1-INH. Less often, C1-INH function is normal (type 3 HAE). In most of these cases, heterozygous mutations are found in F12, encoding factor XII, a coagulation factor. The clinical features of deficiency of C1-INH and deficiency of factor XII HAE) are similar, and laboratory testing is required to distinguish these disorders. More recently, families with type 3 HAE have been found with a mutation in PLG , encoding plasminogen, and an additional family with type 3 HAE had a mutation in ANGPT1, encoding angiopoietin-1 protein, which maintains permeability of the vascular endothelial barrier.
The earliest symptoms of HAE often begin in infancy or early childhood, with 50% of cases manifesting before the age of 10 years. The frequency and severity of attacks are typically exacerbated during adolescence and subside in the fifth decade. Onset during the first decade is associated with greater severity. Based on survey data, prodromes have been described in 82.5% to 95.7% of patients surveyed, most commonly involving the skin or gastrointestinal tract and hours to days before the onset of angioedema; fewer than 10% reported were rarely or never able to predict an attack. Affected individuals are prone to sudden attacks of circumscribed subcutaneous edema (angioedema). In children the extremities (especially upper) and face are most often affected, and swelling may be severe enough to cause remarkable disfiguration of the affected parts. Areas of swelling may migrate, but the condition generally subsides 24 to 72 hours after onset. Several weeks of remission generally follow attacks. The skin and mucosal lesions may appear spontaneously or may be precipitated by minor trauma, especially dental work or surgery, strenuous exercise, infection (especially of the airway), menses, pregnancy, administration of oral contraceptives or angiotensin-converting enzyme inhibitors, extremes of temperature, or emotional disturbances. There is no pitting, discoloration, pain, or itching associated with the edema. Urticaria is not associated, but up to 50% of affected children show a nonpruritic serpiginous or reticulated eruption that resembles erythema marginatum. The erythema marginatum–like eruption may even be present in the neonatal period and should prompt evaluation of HAE if appropriate.
Gastrointestinal involvement is the presenting sign in 40% to 80% of affected children. The recurrent colic and severe abdominal pain can simulate the acute abdominal pain of appendicitis. Nausea, vomiting, and diarrhea are additional complaints. Abdominal ultrasound may demonstrate edematous swelling of the intestinal wall and free peritoneal fluid. Involvement of the mucous membranes of the hypopharynx and larynx, although seen less often, may be particularly devastating; up to 25% of patients experience asphyxia, which is the leading cause of death and usually occurs during the third decade of life.
Diagnosis of HAE can be confirmed by the finding (at least twice and separated by 1 to 3 months) of reduced levels of C4 or C1-INH (quantitative and/or functional), or both. For those with C1-INH deficiency, levels tend to be 10% to 40% of normal, reflecting the haploinsufficiency.
Of note, C4 and C1-INH levels do not tend to reach adult levels until 1 to 3 years of age, necessitating genetic testing in infants. If levels of C4 are reduced in the presence of normal levels of C1-INH protein, confirmation must rely on demonstration of a functional lack of C1-INH by chromogenic or immunoenzymatic assay.
Antihistamines and corticosteroids have not been effective in the management of HAE patients. Epinephrine is beneficial in the control of swelling in few patients. Tracheostomy commonly is lifesaving in patients with laryngeal obstruction. Management is classified as treating the acute attack (on-demand therapy), short-term prophylaxis (preprocedural), and long-term prophylaxis. Treatment for on-demand therapy (which the most common need for pediatric patients) is focused on either replacement of C1-INH or inhibiting the synthesis or activity of bradykinin. ,
Several forms of plasma-derived human C1-INH concentrate are now approved by the US Food and Drug Administration (FDA) for intravenous pediatric use for acute abdominal, facial, and laryngeal episodes. The median time to onset of relief in one pediatric study was 25 minutes and to complete resolution was 8 hours. These include Berinert (all ages; 20 units/kg), Cinryze (6 to 11 years old, 500 U; adolescents, 1000 U), and Ruconest (older than 13 years of age; 50 IU/kg for those weighing more than 84 kg). An alternative, subcutaneous approach for acute episodes is with ecallantide, a plasma kallikrein inhibitor (FDA approved for patients older than 12 years) ; ecallantide is associated with a 3% risk of anaphylaxis, so it must be administered by a healthcare provider. Icatibant, an inhibitor of the bradykinin receptor B2, is approved for pediatric patients in Europe (30 mg subcutaneously) but not yet by the FDA.
In general, attacks tend to be less frequent and less severe in children, and most will not require long-term prophylaxis. However, prophylactic C1-INH administration with dosage escalation may be considered for children with more than two attacks per month, the need for acute treatment more than once annually, or more than one episode of severe abdominal pain in a year. ,
When used prophylactically, the concentrate reduced the median monthly attack rate by almost eightfold. Plasma-derived C1-INH can be given every 3 to 4 days or as needed. Lanadelumab (Takhzyro; 300 mg every 2 weeks), a monoclonal antibody inhibitor of kallikrein, was approved by the FDA for long-term prophylaxis in patients 12 years and older. , Oral small molecular inhibitors of kallikrein are being studied with promising results. Certain forms of plasma-derived C1-INH (specifically Berinert and Ruconest) are also first-line therapies for preprocedural prophylaxis. For prophylaxis (but not acute attacks), a subcutaneous form of C1-INH (Haegarda) is now FDA approved for patients 12 years and older; in trials, subjects received twice-weekly dosing of 40 or 60 IU/kg, and both groups experienced fewer HAE attacks compared with placebo-treated patients. Fresh frozen plasma (FFP; 1 to 2 units per patient) has also been administered prophylactically before dental, medical, or surgical procedures, but controlled studies are lacking in children, and the risk of allosensitization and anaphylaxis is greater than with C1-INH. In the past, antifibrinolytics (especially tranexamic acid, given orally 25 mg/kg per day) and anabolic androgens (especially danazol) were used for prophylaxis, but given their poor efficacy and toxicity (especially androgens and hepatic toxicity), they are rarely used in affected children.
Antifibrinolytics (ε-aminocaproic acid and tranexamic acid) are less efficacious than other approaches and are now rarely used for prophylaxis. Attenuated androgens such as danazol are only appropriate for short-term prophylactic therapy, such as before dental procedures, because of their potential side effects.
As prevention, children and adolescents with HAE should not participate in contact sports, and toddlers should not attend daycare before kindergarten to minimize the risk of infection that could trigger attacks. Even the eruption of teeth and minor mechanical trauma can induce edema formation. If menstruation worsens HAE symptoms, long-term prophylactic doses can be increased, but oral contraceptives are triggers of the angioedema and should be avoided. Although use of angiotensin-converting enzyme inhibitor is unusual in children, these medications can exacerbate the signs of angioedema in affected individuals and should be avoided.
Scombroid fish poisoning (scombrotoxism) is a clinical syndrome that results from the ingestion of spoiled fish of the Scombridae family (tuna, mackerel, and bonito) and fish such as bluefish, mahi-mahi, amberjack, herring, sardines, and anchovies. , The disorder, thought to be related to high levels of histamine and saurine produced when these fish are improperly refrigerated, is characterized by pruritus; a diffuse erythema of the face and upper body, somewhat resembling a sunburn; and, at times, giant hive-like lesions that develop within minutes to hours after ingestion of the toxic fish. Bacteria in the fish express a decarboxylase that converts histidine to histamine, which resists cooking and freezing. Symptoms resemble a histamine reaction and commonly include a hot, burning sensation rather than pruritus. The conjunctivae are often markedly injected, and many patients have a severe throbbing headache, tachycardia, palpitations, nausea, vomiting, abdominal cramps, diarrhea, dryness and a burning sensation or peppery taste in the mouth, urticaria, angioneurotic edema, oral blistering, hypotension, blurred vision, and asthma-like symptoms. Although superficially resembling an allergic reaction, patients with scombroid fish poisoning can be reassured that they do not have fish allergy and that scombroidosis will not occur when fish are handled properly. Symptoms are generally self-limiting and even if untreated tend to resolve within 8 to 10 hours. Treatment is supportive, and most patients treated with oral antihistamines become asymptomatic within 2 or 3 hours.
In contrast to scombroid fish poisoning, ciguatera fish poisoning is a common disorder endemic throughout the Caribbean and Indo-Pacific islands but has been reported in the United States. It is caused by the ingestion of ciguatoxin, which originates from a dinoflagellate (Gambierdiscus toxicus). Present in certain fish such as the red snapper, amberjack, and sturgeon, ingestion of affected fish (whether cooked, raw, or frozen) may result in this disorder. Clinical symptoms, which usually appear within 12 hours but sometimes within minutes after ingestion of ciguatoxin, include abdominal pain, cramping, diarrhea, nausea, vomiting, paresthesias, pain or burning when cold water is touched, arthralgia, myalgia, and, in severe cases, hypotension, shock, respiratory depression, paralysis, coma, and death. The duration of illness averages 8.5 days but may be prolonged. Treatment consists of supportive measures, and intravenous mannitol has been found to be extremely effective, lessening the neurologic and muscular dysfunction of affected patients within minutes of administration.
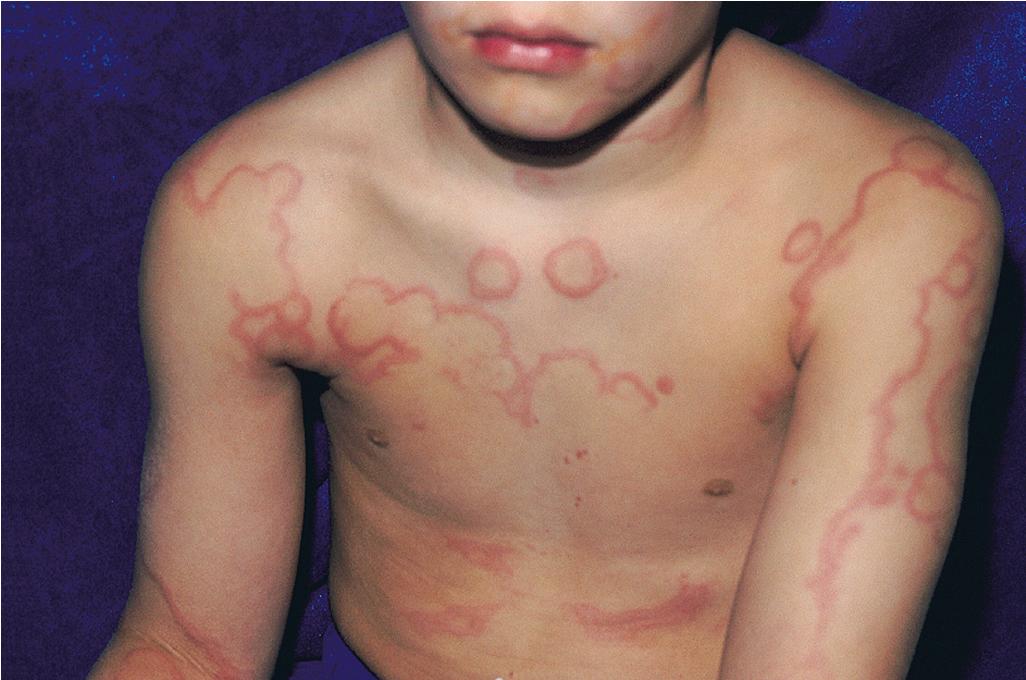
Serum sickness is an allergic reaction characterized by a cutaneous eruption (morbilliform, urticarial, or purpuric), , malaise, fever, lymphadenopathy splenomegaly, proteinuria, and arthralgias. The skin rash (90%), arthritis (52%), and fever (41%) occur most often. The syndrome, originally noted and most commonly seen after the administration of antiserum of horse or rabbit origin, is now rarely encountered. However, serum sickness–like reactions (SSLRs) occasionally occur in children, especially 1 to 3 weeks after exposure to cefaclor, which has been described in approximately 0.2% of treated children. , Penicillins (especially amoxicillin), tetracyclines, cefprozil, sulfonamides, macrolides, ciprofloxacin, rifampin, griseofulvin, itraconazole, fluoxetine, bupropion, rituximab, , omalizumab , and influenza vaccine have also been implicated as triggers of SSLR. SSLR more closely resembles urticaria (or urticaria multiforme) than true serum sickness ( Figs. 20.13 and 20.14 ), and lesions commonly show large areas of lilac or violaceous discoloration, especially centrally ( Fig. 20.15 ). This classic observation has led to use of the term purple urticaria to describe the skin lesions seen in SSLR. Noncutaneous features most commonly include fever, malaise, lymphadenopathy, and arthralgias with periarticular swelling, particularly symmetrically involving the knees and metacarpophalangeal joints ( Fig. 20.16 ). True arthritis is uncommon and seen significantly less often than in patients with true serum sickness. Affected children may also demonstrate facial edema, eosinophilia, headache, myalgia, and gastrointestinal symptoms, but renal and neurologic disease is rarely associated. Histologic evaluation of biopsy section may reveal vasculitis associated with cutaneous deposition of immune complexes, but hypocomplementemia and circulating immune complexes, as seen in true serum sickness, are absent.
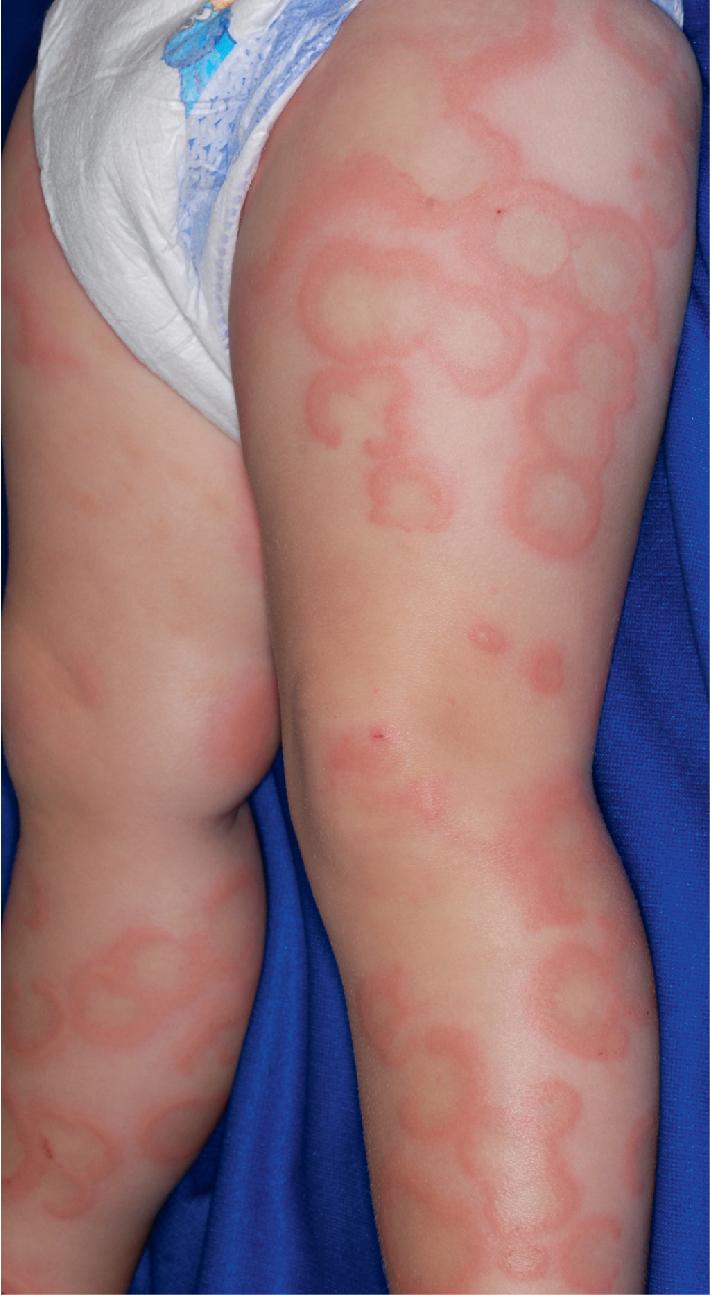
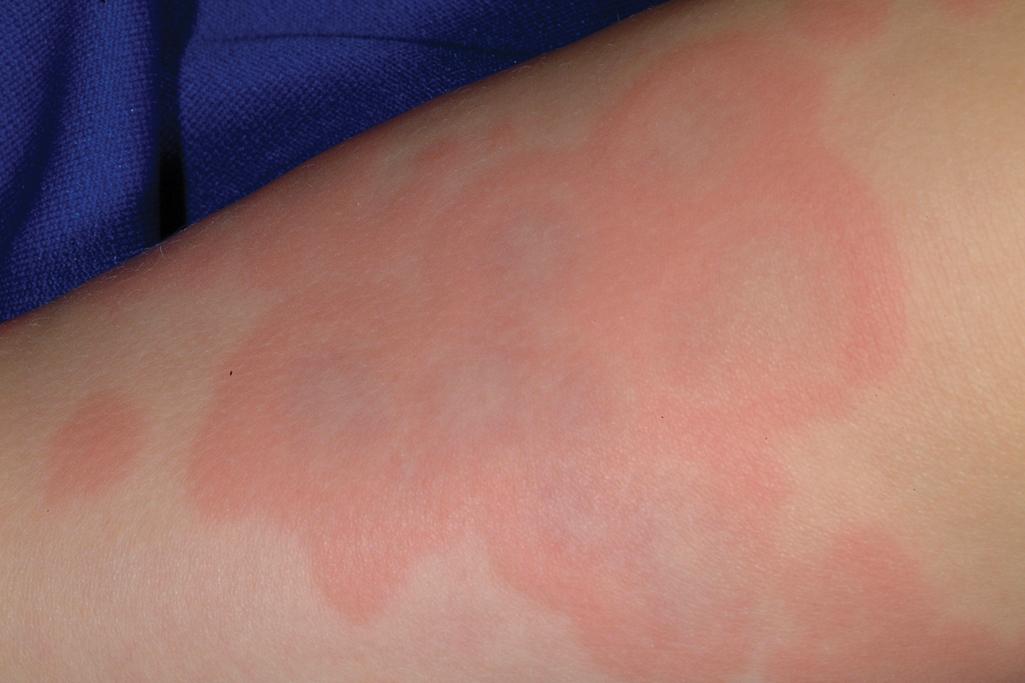
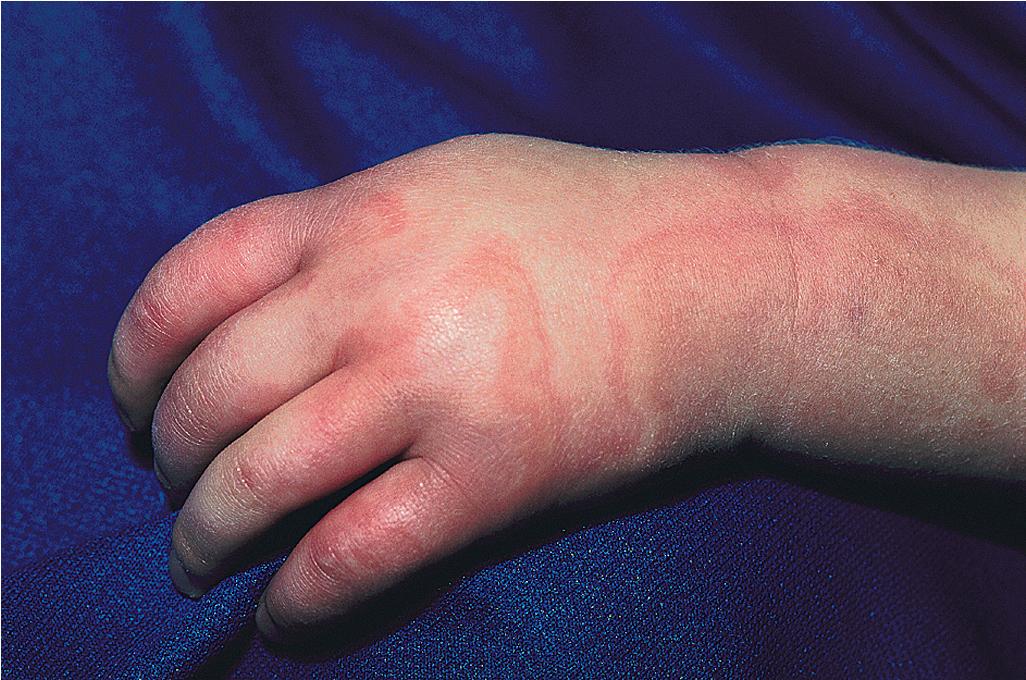
SSLR generally is a self-limiting disease that subsides within 2 to 3 weeks after discontinuation of the causative agent. In the case of cefaclor, biotransformation of the parent drug, genetic defects in metabolism of reactive intermediates, and increased in vitro lymphocyte cytotoxicity have been thought to participate. SSLR has been described as well in association with other drugs, infection with hepatitis B or C, and immunization against hepatitis B, rabies, and tetanus toxoid. Treatment with antihistamines, NSAIDs, and, if severe, a short course of systemic corticosteroids helps alleviate symptoms. In general, the risk of cross-reaction among β-lactam antibiotics is low, and patients who have reacted to cefaclor or cefprozil will usually tolerate other cephalosporins.
The most common drug eruptions are morbilliform or exanthematous eruptions, characterized by erythematous macules or papules ( Figs. 20.17 and 20.18 ). The risk of this type of eruption is increased by viral infection, such as the near 100% incidence of an exanthematous reaction in patients taking penicillin who have EBV infection, or the increased risk of drug reactions in patients taking sulfonamides who have HIV infection.
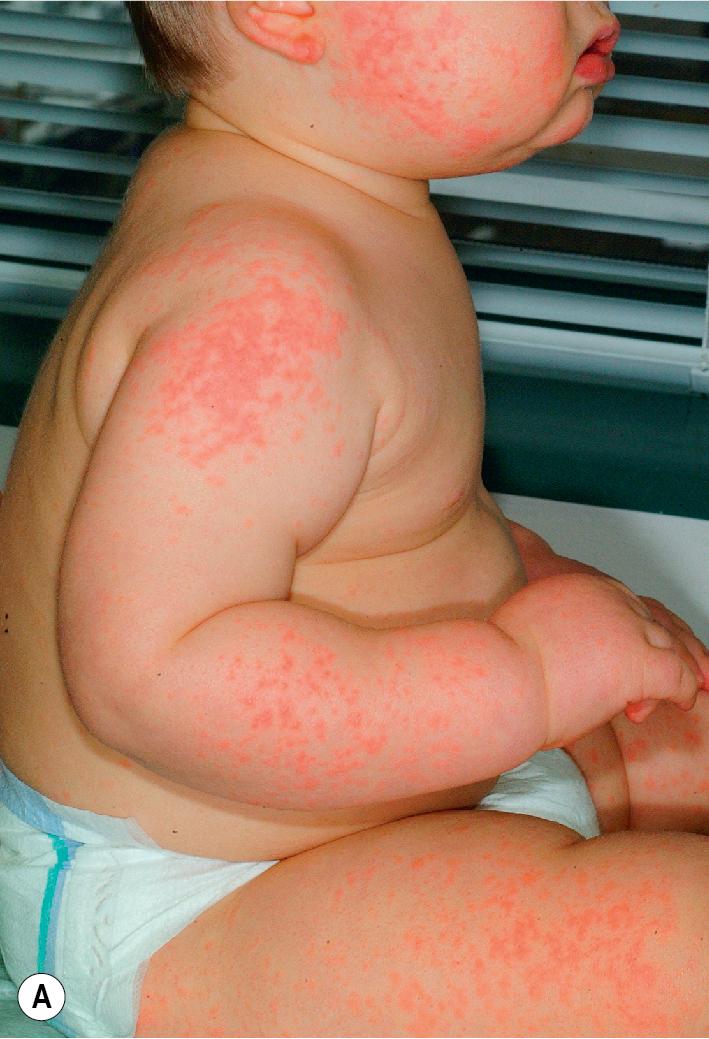
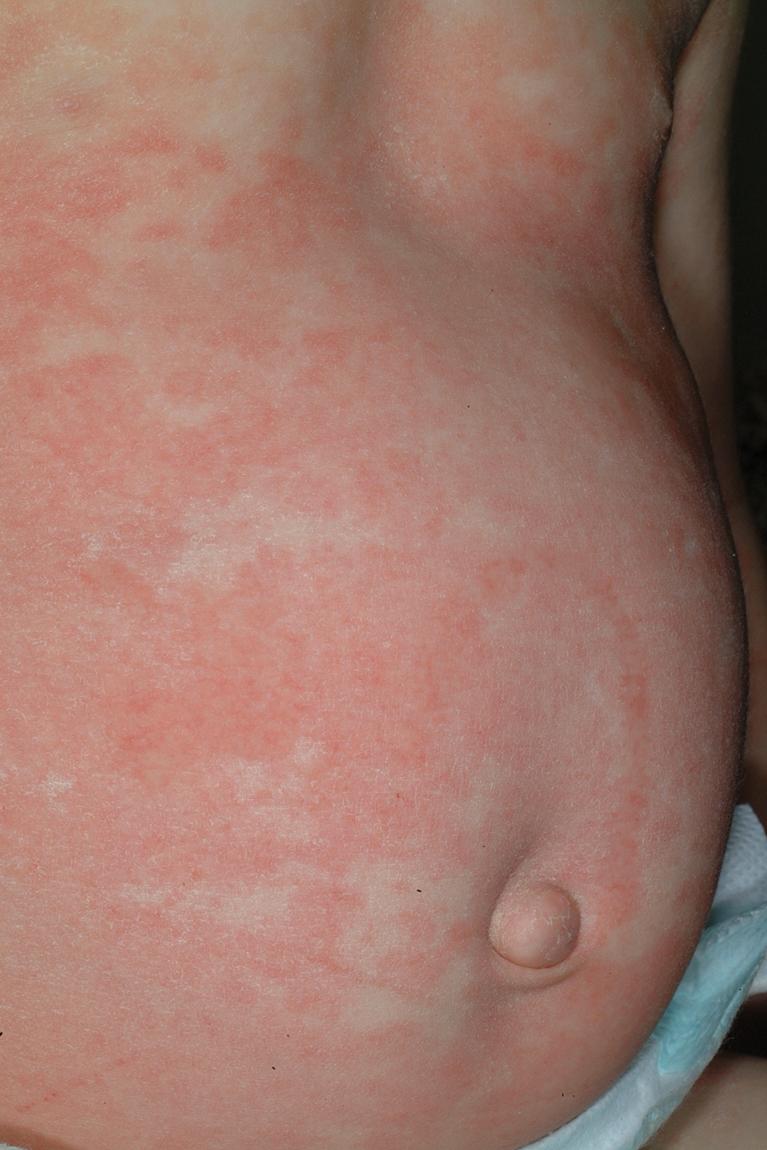
Most commonly, an exanthematous eruption begins 7 to 14 days after initiation of a new medication and sometimes after drug discontinuation. If an individual is rechallenged after an initial course of taking a medication, the reaction may develop within a few days. Most commonly, the eruption is symmetric and begins initially on the trunk before becoming generalized. Mucous membranes are usually spared, but palms and soles are often involved. Patients may experience varying degrees of associated pruritus, and some have low-grade fever. Not uncommonly the eruption turns more of a brownish-red color in 7 to 14 days and may desquamate.
Penicillins, sulfonamides, cephalosporins, and antiepileptics are the most likely categories of drugs to cause exanthematous reactions. Viral exanthems tend to be indistinguishable from exanthematous drug eruptions and more commonly occur in pediatric patients. Eosinophilia favors a diagnosis of drug reaction; biopsy is generally not helpful. More severe drug reactions may be heralded by facial edema or marked eosinophilia, as in systemic hypersensitivity syndrome, or by mucous membrane lesions and dusky skin, as in SJS or toxic epidermal necrosis.
Treatment is largely supportive. The decision to discontinue a drug must be made based on the need for continuing the medication. Many patients will show clearance of the eruption despite continuation of medication (e.g., when an infant with otitis is treated with amoxicillin). However, patients may show progression to erythroderma. Desensitization has been used for reactions to sulfonamides in patients with HIV infection. Given the many nonallergic causes of morbilliform exanthematous reactions from use of β-lactams, oral drug provocation testing (with exposure to increasing amounts of the antibiotic) under direct physician supervision should be considered to exclude true allergy, especially for older children and adults with maculopapular and nonimmediate urticarial exanthems.
DRESS syndrome, which is also called drug hypersensitivity syndrome (DHS), presents as an exanthematous drug reaction in association with fever, facial swelling, conjunctivitis, and often internal organ involvement that leads to mortality in 10% of patients. The clinical manifestations of DRESS syndrome typically begin 1 to 6 weeks after initiation of a responsible medication.
Fever and malaise often appear first and may be seen in association with cervical lymphadenopathy and pharyngitis. The cutaneous eruption occurs in approximately 75% of patients. It often starts on the face with edema, especially periorbital, then erythema and pruritus ( Fig. 20.19 ). The erythema then spreads caudally. The exanthematous eruption raises concern about SJS or TEN; the lack of mucosal involvement in DRESS can be a useful distinguishing feature.
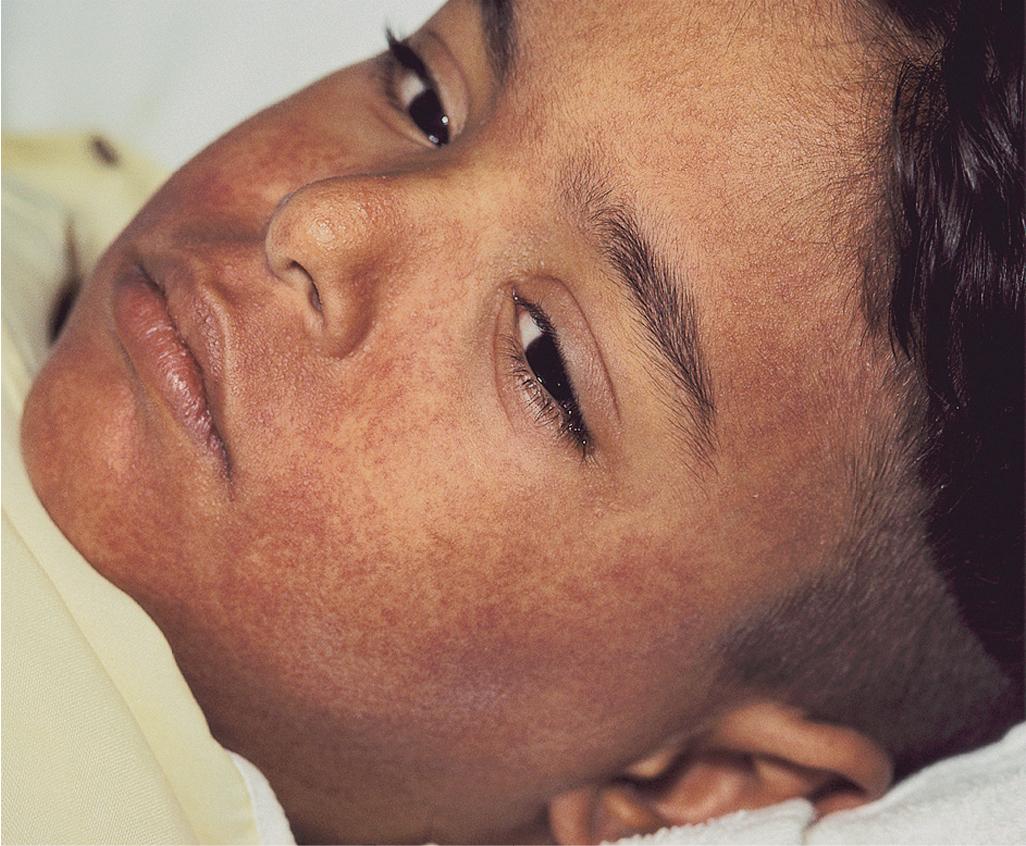
Of visceral sites of involvement, the liver is most common (about 50%), and the hepatitis may be fulminant, leading to death. Lymphadenopathy is often seen, and patients may complain of joint pain. Inflammation of the kidney, central nervous system, heart, and lungs has often been described. Thyroiditis occurs in a subset of patients and may not be noted until 2 to 3 months after onset. Atypical lymphocytosis and eosinophilia are seen in most affected individuals early in the course.
DRESS is most commonly caused by trimethoprim-sulfamethoxazole and the aromatic anticonvulsant agents, although viral infection has also been linked to DRESS. Sulfonamides are metabolized by acetylation to a nontoxic metabolite for renal excretion; slow acetylation has been associated with an increased risk of developing DRESS. Only aromatic amine forms of sulfonamides require this mechanism (e.g., these reactions do not occur with sulfonylureas and furosemide). Aromatic antiepileptics are metabolized by the cytochrome P450 system, and hereditary alterations in metabolism may lead to the accumulation of toxic arene oxide metabolites. Because these medications cross-react, affected individuals should avoid phenobarbital, phenytoin, carbamazepine, oxcarbazepine, and lamotrigine. Alternative antiepileptics include valproic acid, benzodiazepines, gabapentin, zonisamide, topiramate, levetiracetam, and vigabatrin. Other drugs that less commonly cause this syndrome are minocycline, amoxicillin, dapsone, vancomycin, , abacavir, nevirapine, aspirin, allopurinol, and azithromycin, as well as traditional Chinese medicine. Reactions to doxycycline have not been described in patients who develop DRESS in response to minocycline. Clinical signs may vary dependent on the causative drug. For example, lamotrigine rarely causes eosinophilia, abacavir leads to primarily respiratory and gastrointestinal manifestations without eosinophilia or hepatitis, and allopurinol reactions often involve the kidney. ,
The eruption of DRESS syndrome persists for weeks to months after medication withdrawal. After an initial period of improvement, the cutaneous and visceral manifestations of DRESS may flare 3 to 4 weeks after onset. Lymphocyte transformation tests may be useful in confirming the triggering medication but should be performed 5 to 8 weeks after onset in patients with DRESS. Challenge with the offending drug after initial reaction leads to reactivation of the fever and erythroderma within hours after initiation.
A role for concurrent herpes family viral infections has been emphasized in adult DRESS, particularly with reactivation of EBV and human herpesvirus (HHV) type 6. , A pediatric study found concomitant evidence of HHV-6 infection in 50% of the children and in association with greater pulmonary involvement (50%) and longer duration of fever and hospital stay.
Patients with suspected DRESS should have a variety of laboratory tests to consider visceral involvement. These include complete blood cell count, hepatic transaminases, serum creatinine level, urinalysis, and thyroid testing (which should be repeated after 2 to 3 months). Although topical corticosteroids and antihistamines may quell the pruritus in mild cases, patients with visceral involvement (especially for hepatitis, myocarditis, and interstitial pneumonitis) should be treated with systemic corticosteroids (1 to 2 mg/kg per day) for a few weeks, with gradual taper thereafter. Systemic corticosteroid treatment tends to shorten the hospital stay and reduce febrile days. Anecdotal cases of successful treatment of DRESS with IVIG have been described. Counseling of family members is important because first-degree relatives of individuals with DRESS have a higher risk of developing these drug reactions.
Stevens–Johnson syndrome and toxic epidermal necrolysis are now considered to be variants of the same hypersensitivity disorder. The incidence of these conditions is 6.3 to 7.5 per 100,000 children per year. In a study of 708 patients, approximately 18% of those with SJS and TEN were children. Classification has been based on the degree of epidermal detachment. Epidermal detachment of less than 10% of the total body surface area is considered SJS (53 per 100,000 children); more than 30%, TEN (4 per million); and between 10% and 30%, a transitional SJS-TEN overlap condition (16 per million). In another study, 85% of pediatric cases had SJS, whereas 6% had SJS-TEN overlap and 9% had TEN. Patients may appear to have SJS initially and typical TEN with disease progression ( Table 20.3 ). At one time, SJS was lumped with EM (see Erythema Multiforme section and Table 20.3 ), which is now considered to be a distinct entity, primarily resulting from hypersensitivity to herpes simplex infection. Reactive infectious mucocutaneous or mucosal-predominant eruption (RIME), which includes mycoplasma-induced rash and mucositis (MIRM) and Chlamydia pneumoniae –induced rash with mucositis (CIRM), has also been separated from SJS (see Reactive Infectious Mucocutaneous or Mucosal-Predominant Eruption section and Table 20.3 ). Similarly, TEN was once confused with staphylococcal scalded skin syndrome (SSSS), a more superficial and crusted disorder in which the superficial blistering is caused by an exfoliatin produced by Staphylococcus aureus (see Chapter 14 ).
| Erythema Multiforme | RIME | SJS | SJS-TEN | TEN | |
| Lesional morphology | Fixed typical or atypical target lesions | Vesiculobullous; no typical papular targets | Targetoid lesions, dusky red macules, bullae | Targetoid lesions, dusky red macules, bullae | Targetoid lesions, dusky erythematous macules and plaques; detachment of epidermis |
| Localization of skin lesions | Acral distribution is characteristic; can be widespread | May be no skin involvement or limited | May be scattered and isolated; may be confluent, especially on the trunk and face | May be scattered and isolated; often confluent | Usually extensive involvement with widespread confluence |
| Involved skin | <2% | <10% | <10% | 10% to 30% | >30% |
| Biopsy features | Individual apoptotic keratinocytes, but not large sheets of epidermal necrosis | Interface dermatitis with dyskeratosis | More interface dermatitis | Significant interface dermatitis + necrolysis | Predominantly necrolysis |
| Mucosal changes | Tends to be milder | Predominant erosive mucositis | Prominent | Prominent | May be less than in SJS |
| Systemic involvement | Minimal or absent | Often present (cough, fever, malaise, arthralgias) | Often present | Always present | Always present |
| Most common underlying cause | Typically HSV | Most often Mycoplasma; Chlamydia has been associated | Often drug-induced. Sometimes Mycoplasma | Usually drug | Most often drug; can be a feature of graft-vs.-host disease |
The SJS-TEN spectrum of SCARs is driven by cytotoxic T cells and features high levels of TNF, perforin/granzyme B, Fas/Fas ligand, and granulysin, which promote keratinocyte apoptosis. Microribonucleic acid (miR)–18a-5p, which targets an apoptosis gene (BCL2L10), has been identified as a serum biomarker that can distinguish early TEN from other drug eruptions Patients carrying HLA-B*15:02 , which is common in the Asian population, are at strongly increased risk for carbamazepine-induced SJS/TEN, and this population should have human leukocyte antigens (HLAs) screened before initiating antiepileptic drugs. , Other HLA antigens are being recognized in non-Asian populations. Lymphocyte transformation tests have helped to identify the causative agents in some affected children, but in contrast to those for children with DRESS syndrome, they need to be performed during the first week of the disorder. The risk of developing SJS and TEN is significantly increased in patients with HIV infection and who have a decreased capacity to detoxify reactive intermediate drug metabolites (e.g., slow acetylators or individuals with defects in epoxide hydrolase–mediated detoxification).
The underlying triggers of SJS and TEN are similar, although drugs cause the majority of cases overall and in almost all cases of TEN (see Table 20.1). In children, SJS is less commonly caused by medication than in adults, and underlying mycoplasma or herpetic infection should be considered. Although more than 200 medications have been implicated as potential triggers of SJS-TEN, sulfonamides, penicillins, phenobarbital, carbamazepine, and lamotrigine have been most strongly associated with SJS-TEN. An algorithm for assessing drug causality in epidermal necrolysis (ALDEN) has been proposed and correlates well with case-control analysis results.
Most patients show evidence of SJS or TEN 7 to 21 days after the first drug exposure. Occurrence is almost always within the first 8 weeks of drug use and rarely within the first few days of drug administration. Children with an earlier onset (mean 2 to 3 days) have been previously exposed to the drug or a cross-reacting analogue. Aromatic antiepileptics (phenobarbital, phenytoin, carbamazepine, lamotrigine) tend to cross-react and cannot be substituted for each other. Drugs with longer half-lives are associated with a higher incidence than those related but with shorter half-lives. NSAIDs, including ibuprofen, and acetaminophen may also be triggers and possibly exacerbants with other drugs and are best avoided during hospitalization for SJS-TEN. Other infections, , neoplasia, autoimmune disorders, and vaccination have also been implicated, particularly herpes simplex virus, which is more typically linked to EM.
Older children and adults are most likely to develop this spectrum of disorders, but the condition has been described in young infants and neonates. High fever, pronounced constitutional symptoms, and varying degrees of generalized targetoid lesions, bullae, epidermal detachment, and mucosal erosions (of at least two sites) are characteristic (see Table 20.3 ). Patients not uncommonly show a 1- to 14-day prodromal period before the abrupt eruption of the typical features. During this prodromal period, affected children may show fever, malaise, headache, cough, coryza, sore throat, vomiting, diarrhea, chest pain, myalgia, and arthralgias.
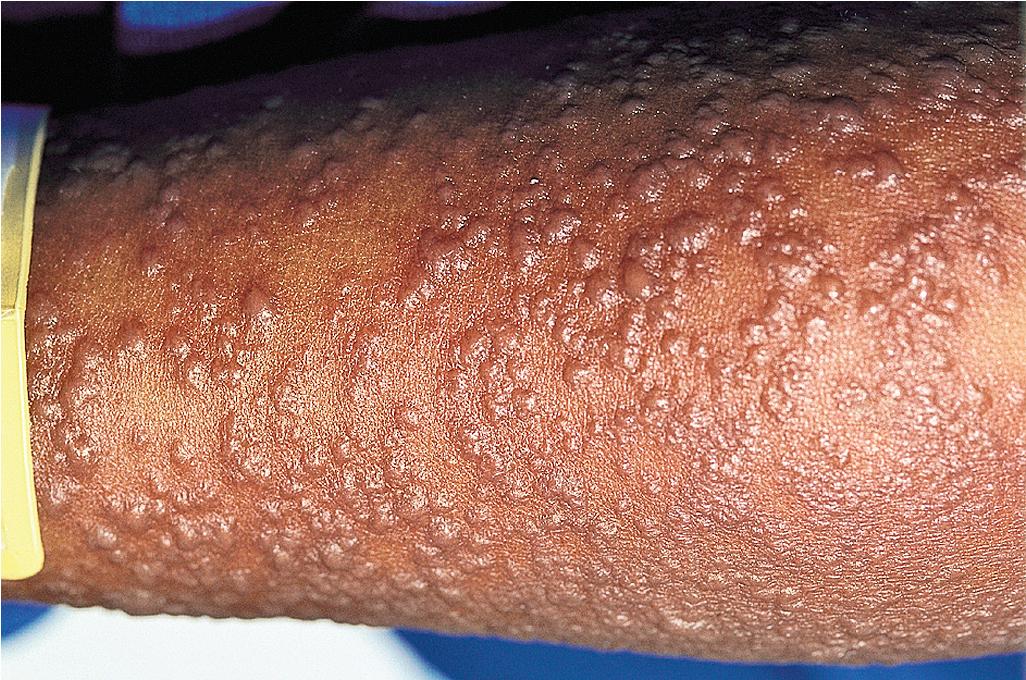
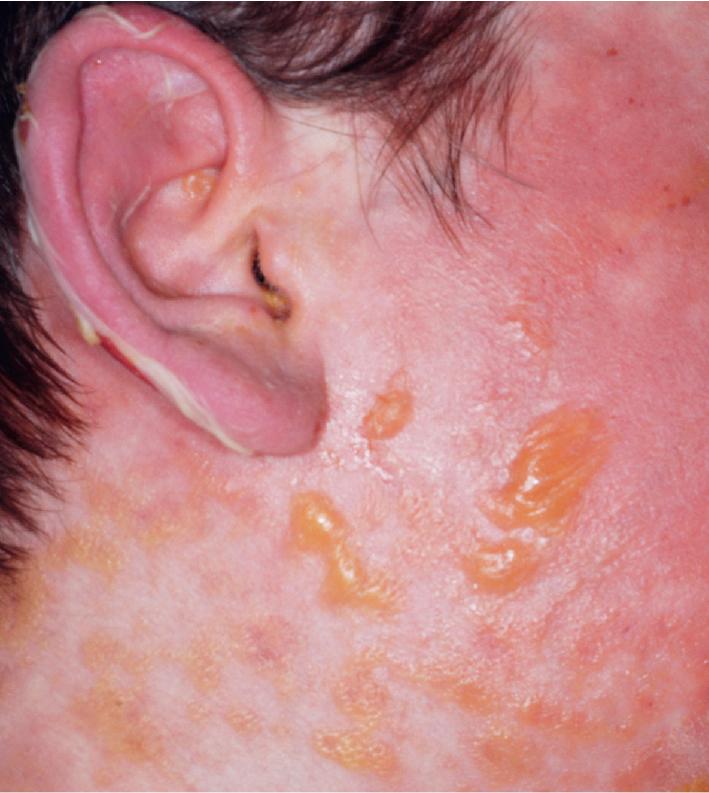
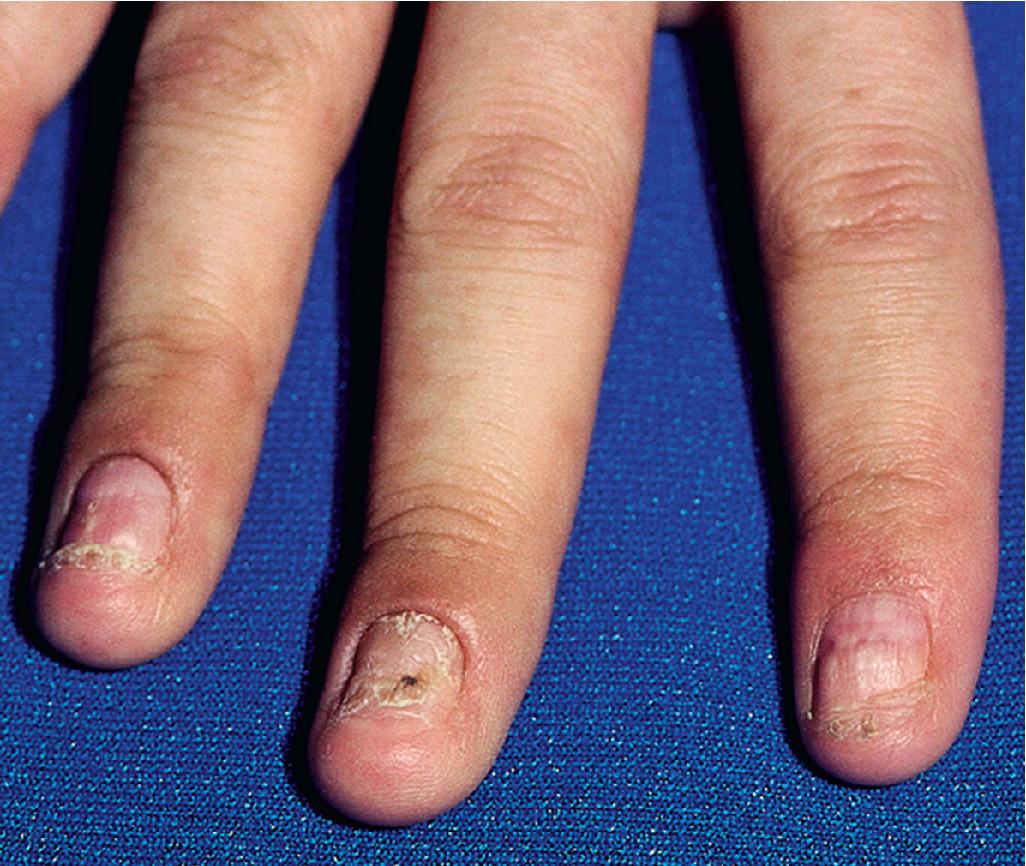
Cutaneous involvement most often appears initially on the face and upper trunk. Palms and soles are commonly involved ( Figs. 20.20 and 20.21 ). Erythematous and purpuric macules may develop flaccid gray discoloration or bullae ( Figs. 20.22 and 20.23 ), become confluent, and sometimes detach, leaving a raw, denuded base ( Figs. 20.24 and 20.25 ). Some of the macular lesions may show a dusky center, which gives a target-like (targetoid) appearance; however, the characteristic concentric rings of EM are absent. Some patients show limited targetoid lesions and little detachment (more typical of SJS), and others show extensive detachment (more typical of TEN). The early morbilliform eruption of TEN may be localized but more often is an extensive, painful erythroderma. After exerting light mechanical pressure with a finger to an area of erythema, the epidermis in patients with either SJS or TEN becomes wrinkled and peels off like wet tissue paper (see Fig. 20.25 ), the characteristic Nikolsky sign. The Nikolsky sign may be seen in patients without clinical evidence of epidermal detachment and can be a useful diagnostic tool. In addition to SJS and TEN, however, the Nikolsky sign may be seen in a variety of other bullous disorders (especially pemphigus, epidermolysis bullosa [see Chapter 13 ], and the SSSS [see Chapter 14 ]). Histopathologic examination of affected skin demonstrates necrosis of the lower epidermal cells with a sparse mononuclear cell infiltrate and, in the case of TEN, extensive necrosis with a subepidermal split. The epidermal necrosis correlates with the dusky blue coloration of lesions. The nails become dystrophic because of nailbed inflammation ( Fig. 20.26 ).
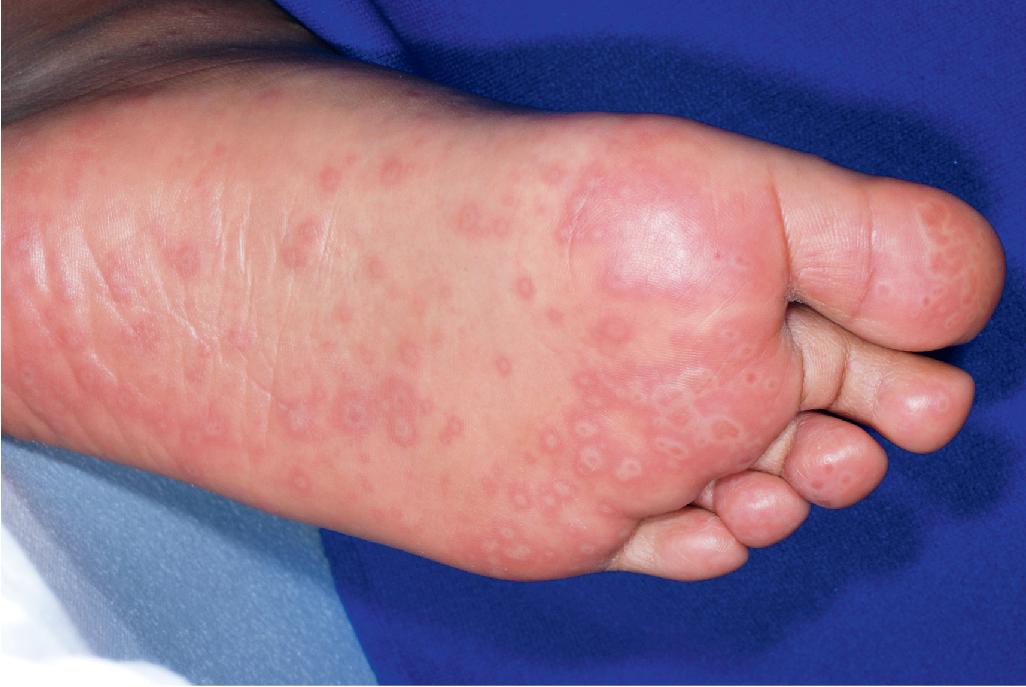
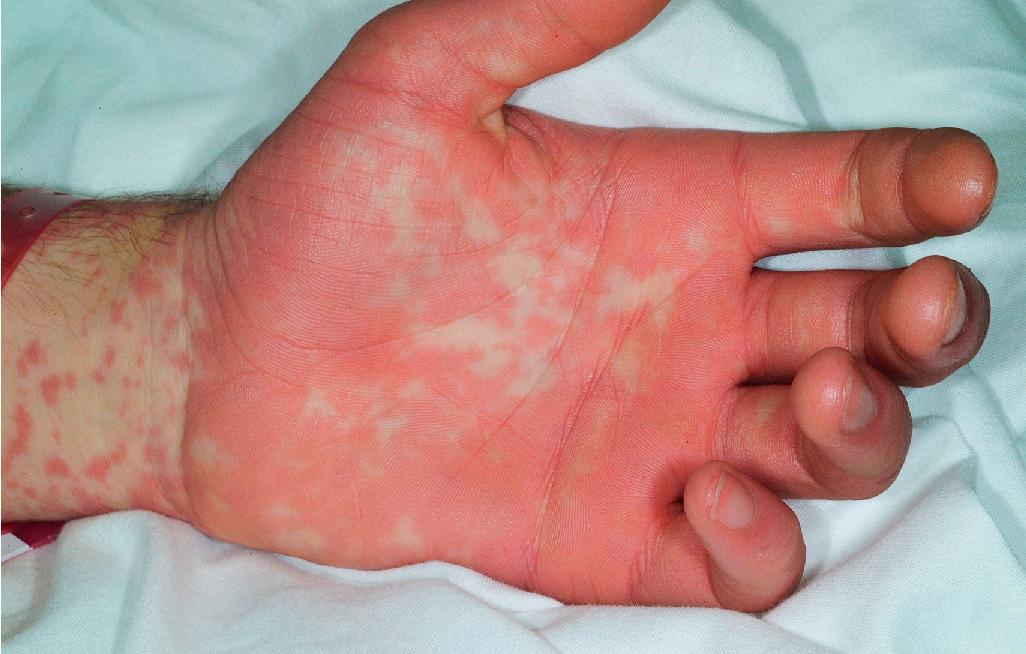
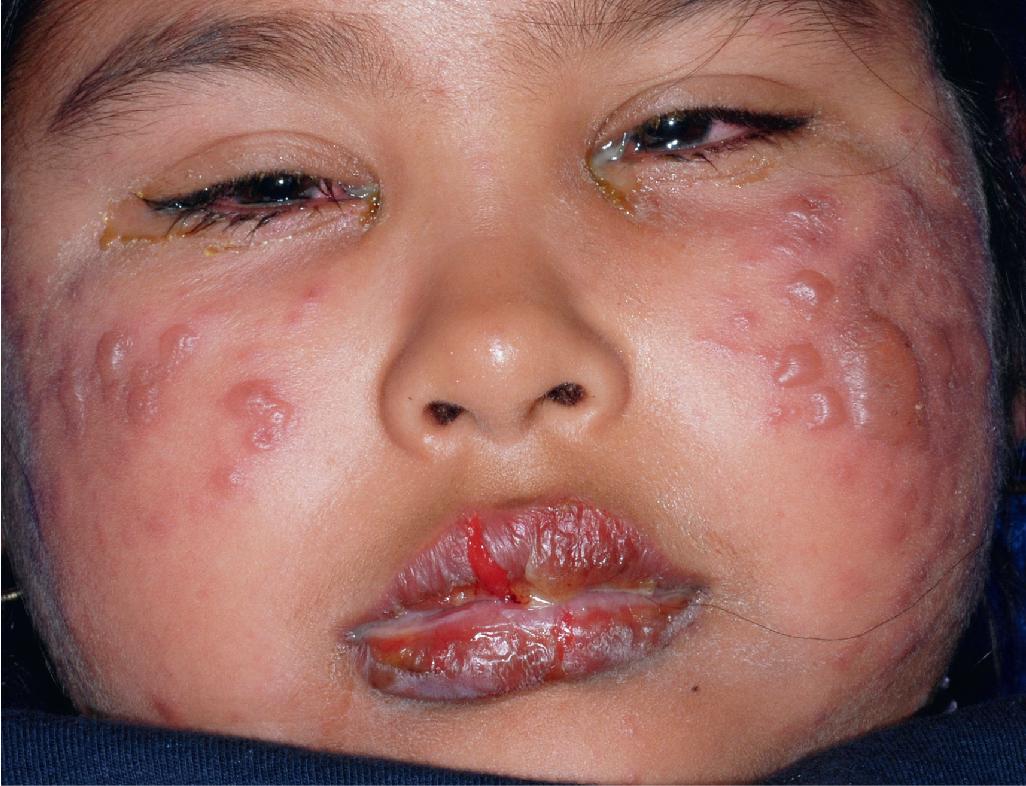
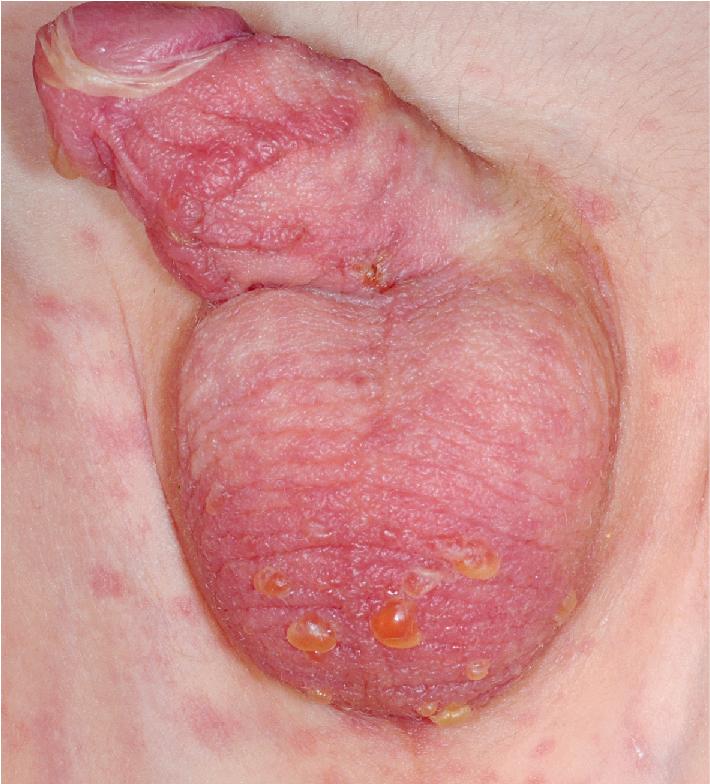
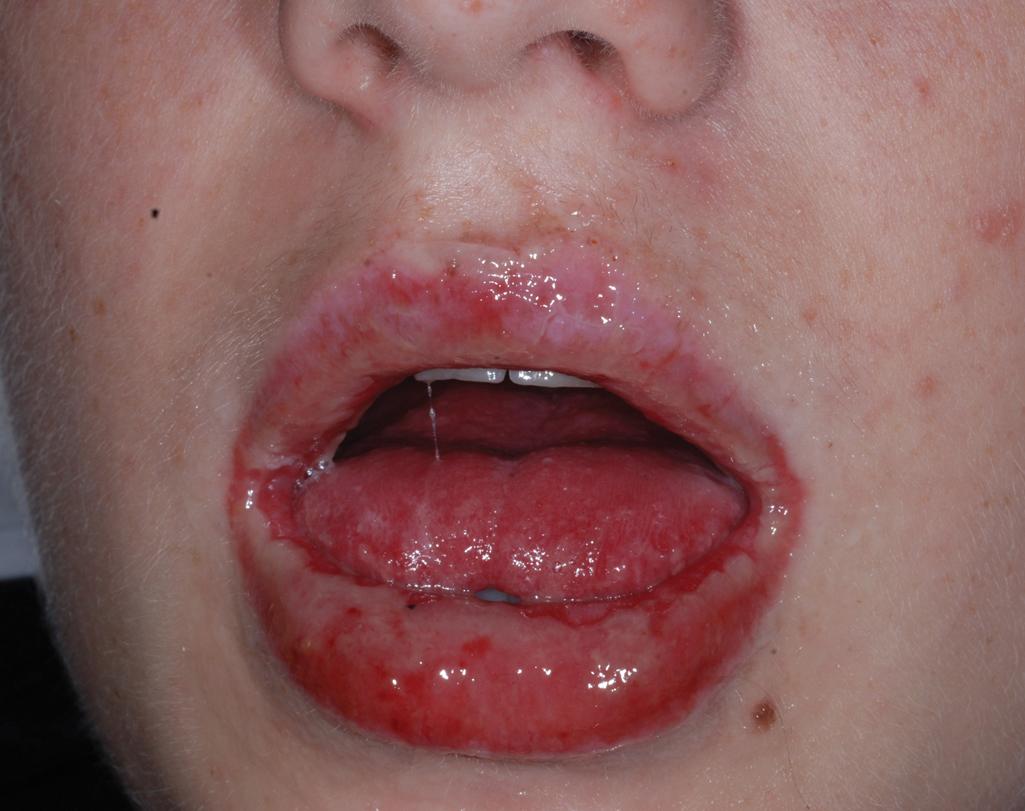
Extensive mucosal involvement is more typical of SJS than TEN. The mucosal manifestations of SJS tend to occur 1 to 2 days before cutaneous manifestations. The mucous membranes of the lips, tongue, buccal mucosae, eyes, nose, genitalia, and rectum may show extensive bullae with grayish white membranes, characteristic hemorrhagic crusts, and painful superficial erosions and ulcerations ( Figs. 20.27 and 20.28 ). Uncommonly, the esophageal and respiratory epithelial mucosae are affected. By definition, two or more mucosal surfaces are involved. The oral mucosa is always affected, resulting in inability to drink or eat and leading to a risk of dehydration. Genital area lesions lead to painful micturition and defecation. Although lesions of the oral mucosae tend to heal without scarring, strictures or stenosis of esophageal, vaginal, urethral, and anal mucosae have been described as sequelae.
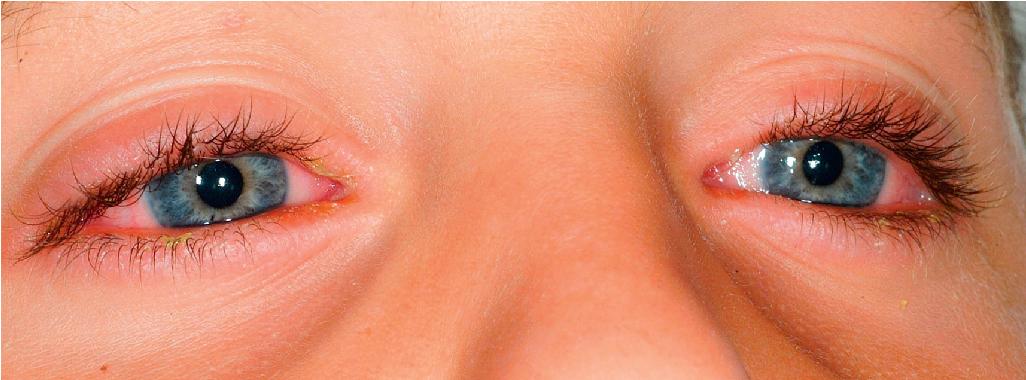
Severe purulent conjunctivitis with photophobia is the typical ocular manifestation (see Fig. 20.28 ). Corneal ulcerations, keratitis, uveitis, and panophthalmitis may also occur. Sequelae occur in 40% of patients and may be grave, with a possibility of keratoconjunctivitis sicca, corneal ulceration or neovascularization, trichiasis, symblepharon, and partial or even complete blindness. The severity of the acute ophthalmologic manifestations does not correlate with long-term complications. Pulmonary involvement may occur as an extension from the oropharynx and tracheobronchial tree or may be the result of pneumonitis associated with an initiating viral infection or secondary infection. In extreme cases, renal involvement with hematuria, nephritis, and in some cases progressive renal failure may result. Esophageal or tracheal ulceration, pyoderma, lymphadenopathy, hepatosplenomegaly with elevated transaminase levels, myocarditis, arthritis and arthralgias, and/or septicemia may also complicate the disorder ( Box 20.3 ).
Fever
Dehydration
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here