Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fractures and dislocations of the upper extremity can range from benign problems requiring minimal intervention to life- or limb-threatening emergencies. Treatment considerations include injury pattern, mechanism, status of the soft tissues, associated neurologic or vascular injury, and other bodily injuries. We will first discuss key issues in the decision-making process in treatment of upper extremity injuries, then focus on specific injuries and their treatment.
An associated open soft tissue injury adds an element of urgency to the treatment of upper extremity fractures. A fracture is classified as “open” if the fracture or fracture hematoma communicates with the environment via a wound in the soft tissues. This can be caused by the bone protruding through the skin from “inside out” or from a penetrating mechanism causing an injury from the “outside in.” Regardless, the implication is that environmental contamination can increase the incidence of both infection and fracture healing complications. If there is a wound in the same limb segment as the fracture, the fracture should be considered open until proved otherwise. The Gustilo-Anderson classification system for open fractures ( Table 1 ) is the most frequently used system to classify these injuries, with increasing risk of infection correlated directly with higher type.
| Type I | Type II | Type III-A | Type III-B | Type III-C | |
|---|---|---|---|---|---|
| Wound size | <1 cm | 1–10 cm | >10 cm or extensive periosteal stripping | >10 cm or extensive periosteal stripping | >10 cm or extensive periosteal stripping |
| Soft tissue | Adequate soft tissue for primary coverage, or need for STSG | Wound requires myofascial flap coverage | Associated with arterial injury requiring repair | ||
| Antibiotic recommendations * |
|
|
|||
| Special considerations: | |||||
|
|||||
* Garner MR, Sethuraman SA, Schade MA, Boateng H. Antibiotic prophylaxis in open fractures: evidence, evolving issues, and recommendations. J Am Acad Orthop Surg . 28(8):309-315, 2020.
Treatment of open fractures begins with prompt antibiotic therapy, tetanus prophylaxis, and urgent irrigation and debridement of the fracture in the operating room.
Time to initiation of antibiotic administration has been shown to be the single most important factor driving outcomes in open fractures. Studies show increased infection rate when antibiotics are delayed for more than 3 hours from time of injury. The American Academy of Orthopaedic Surgeons guidelines for open fracture management recommends antibiotic initiation as soon as possible after injury and continuation for 3 days (type I and II fractures) or 5 days (type III). It is important to initiate antibiotic therapy as soon as possible after identification of an open fracture. Antibiotic recommendations by open fracture type can be found in Table 1 .
While the need for thorough surgical debridement of the open fracture site is indicated, the timing of such is often debated and is driven predominantly by the level of contamination found in the wound bed. Although some centers still take patients with open fractures of all types for urgent debridement within 6 to 8 hours, many centers with a dedicated orthopedic trauma room have found no increase in infection rate with longer delays to surgical debridement when the full resources of the center are more readily available. True life and limb emergencies, such as vascular injury, compartment syndrome, and severe crush injury, are exceptions to this and should be taken to the operating room as soon as the patient is adequately stabilized.
During debridement, the skin and fascia are extended to allow for the fracture bones to be delivered and adequately inspected and cleaned. It is important to remove all external contamination and devitalized skin, subcutaneous tissue, muscle, and bone. Tissue that has not yet declared its viability may be left in situ, and the patient should be returned to the operating room at regular intervals until all devitalized tissues have been adequately excised. Preliminary or definitive stabilization of the fracture and continuation of appropriate antibiotic therapy should follow.
Dislocations occur whenever articular surfaces are no longer in contact and also require urgent assessment, diagnosis, and treatment. Joint dislocations are considered emergencies because the risk of neurovascular compromise or progressive worsening of a neurovascular deficit increases with the amount of time the dislocation is present. Prolonged dislocation also increases the chance of osteonecrosis, although this is more commonly a concern in the lower extremity. Osteonecrosis is believed to be due to possible interruption of capsular blood supply from the increased capsular tension caused by the dislocation.
Diagnosis of a joint dislocation is often made from the history and physical examination. Acute dislocation is usually very painful, with muscle spasm and limited motion. The limb will usually be held in a somewhat fixed position characteristic of the specific dislocation. For example, an anterior shoulder dislocation will leave the arm in external rotation and slight abduction. Internal rotation and adduction are usually very limited. Loss of the normal contour of the joint can be seen with the humeral head palpable anteriorly and a sulcus sign evident posteriorly beneath the acromion.
Prompt radiographic assessment is important, such that associated fractures are recognized prior to any attempt at reduction. Failure to do so may result in further displacement of fractures and significantly worsen the prognosis of the injury.
Dislocations and fractures with neurologic or vascular compromise should be reduced as quickly as possible in order to reduce the potential for irreversible injury to the affected structures. Following reduction, a repeat examination is required to determine if the neurovascular compromise has been relieved.
Fractures associated with gunshot wounds deserve special mention because they are difficult to classify as either open or closed. Their status as an open or closed injury is determined by whether the injury was inflicted by a high- or low-velocity weapon. According to the Wound Ballistics Manual of the Office of the Surgeon General, muzzle velocity greater than 2500 feet/second constitutes “high velocity.” This is important because the kinetic energy of the bullet varies directly with the square of its velocity and only linearly with its mass. Shotgun wounds, however, are generally considered high energy despite a lower muzzle velocity because of the high level of energy imparted by the blast.
The majority of fractures caused by low-velocity weapons can be given antibiotics but otherwise be treated as “closed” fractures, with little to no increased risk of infection. Many may be treated to completion with closed methods, such as functional bracing or casting. If one chooses open management, fracture comminution is often found to be more extensive than can be appreciated on plain radiographs.
In contrast, high-velocity gunshot wound fractures are best treated as “open” injuries. As the high-velocity missile passes through the limb, it not only causes significant fracture comminution but also carries with it a shock wave that passes through the soft tissue and creates a cavitary lesion with severe muscular and neurovascular injury. In this situation, a prompt debridement is necessary to remove devitalized bone and muscle, and additional serial debridements may be required until all tissues have declared themselves as viable or not.
There may also be associated neurologic deficits due to the “blast effect” of the initial injury. When due to a low-velocity gunshot wound, these are usually due to the percussive wave produced by the bullet, which leads to a temporary neuropraxia without laceration of the nerve. These injuries do not warrant immediate exploration as most will resolve with observation alone. If persistent neurologic deficits occur, an electromyogram may be indicated between 3 and 6 weeks to assess the nerve for evidence of recovery. High-velocity gunshot wounds should be treated more aggressively. The cavitary lesion may be so large that the nerve may be disrupted and may even have a segmental defect. Because the cavitary wound itself requires prompt debridement, nerves that are not functioning based on physical examination should be explored simultaneously. If lacerated, the nerve should be repaired, if possible. However, in most cases, the extent of the nerve injury from the blast cannot be determined at the time of the initial exploration, and the patient may require a delayed exploration to determine the size of the nerve defect and to perform a reconstruction by staged nerve grafting.
In the upper extremity, compartment syndrome most commonly occurs in the forearm. Volkmann first described permanent contracture of the forearm flexors as a result of ischemia in 1881, believing that the pathophysiology was arterial insufficiency and venous stasis resulting from a tight cast or bandage. For nearly the next hundred years, attention primarily focused on the “arterial” problem, with some improvements in clinical results noted when compartments were opened to explore for arterial injury and perform repair. In the 1970s and 1980s, a better understanding of the pathophysiology of compartment syndrome emerged.
Any condition that results in increased tissue pressure within a closed compartment will obstruct venous flow. If the problem continues unabated, pressure will rise until arteriolar pressure is exceeded, at which point flow through the capillary bed ceases. This typically occurs when the pressure within the compartment is within 30 mm Hg of the diastolic blood pressure. If elevated compartment pressures persist, the muscle within the compartment will become ischemic, necrose, and eventually contract.
Signs of compartment syndrome include marked pain on passive digital motion, a tense or swollen forearm, and either paresthesia or reduced sensation in the hand. Intracompartmental pressure measurements may be helpful in deciding borderline cases or when the patient’s condition precludes participation in examination (i.e., intubated or altered patient), but in general, the diagnosis is made clinically. Once the diagnosis of acute compartment syndrome is established, an emergent forearm fasciotomy is required to release the pressure in order to prevent muscle necrosis and subsequent late contracture of the fingers due to contracture of the necrotic flexor muscles in the forearm.
Gunshot wounds to the forearm require special attention even if there is no associated fracture due to a high risk for development of forearm compartment syndrome. Patients should be monitored for at least 8 to 12 hours for clinical evidence of increased pressure within the forearm from arterial injury causing bleeding into the relatively confined spaces of the forearm compartments.
In contrast to an acute compartment syndrome, a compartment syndrome that was missed, or diagnosed late, or a “crush” syndrome should not be routinely released. If an extremity is severely crushed, muscle necrosis occurs immediately, with pressure rising later as a response to, rather than a cause of, the muscle damage. Once muscle necrosis has already occurred, fasciotomy does nothing to prevent it, and it does expose the necrotic muscle to outside contaminants, thus leading to an increased risk of infection and a possible need for amputation.
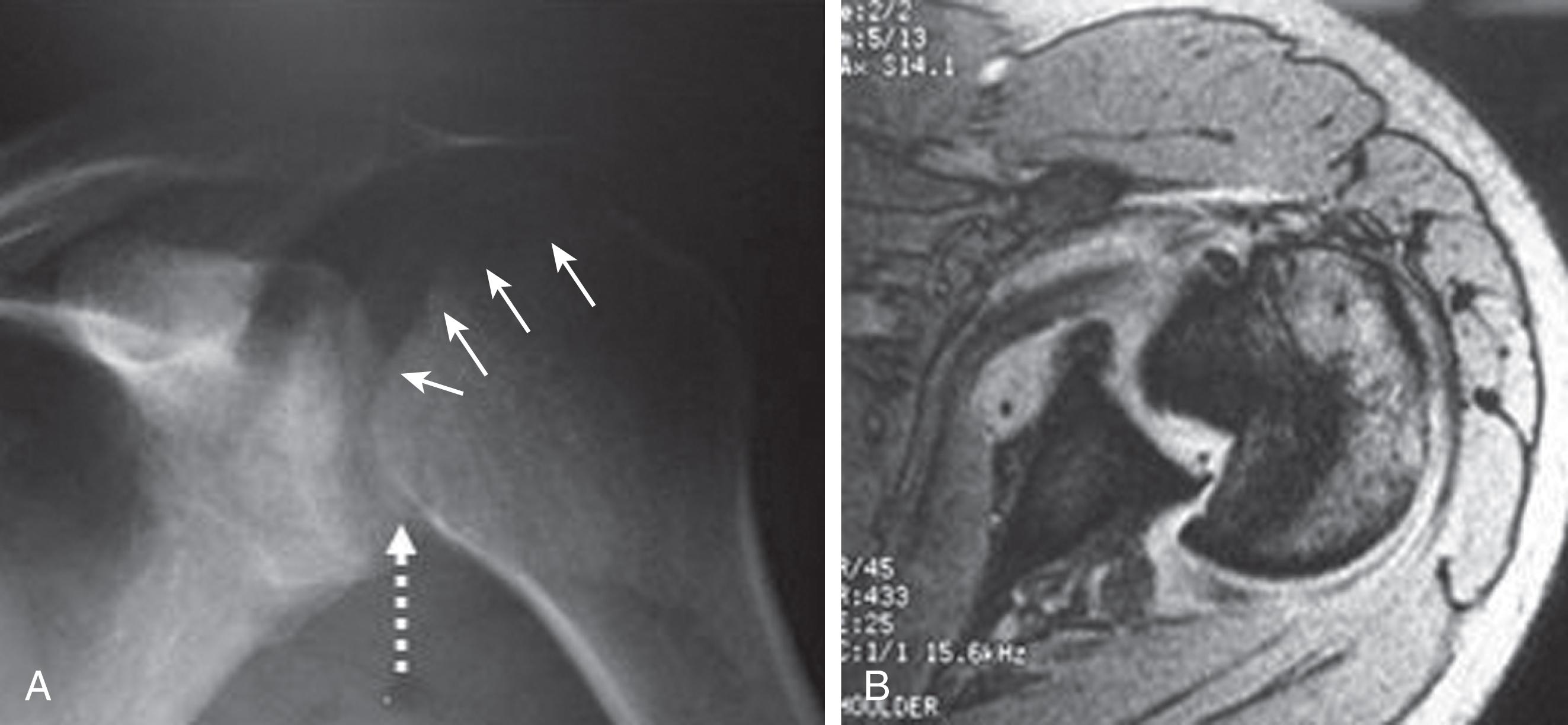
For fractures and dislocations, proper radiographic evaluation is essential before making any decisions regarding treatment of the injury. Ideally, two orthogonal views of the injured extremity should be obtained along with radiographs of the joints adjacent to the injured bone. Injuries to the shoulder girdle should have a minimum of three radiographic views in a standard trauma series. This series includes a true anteroposterior (AP) view (also known as a “Grashey”), a scapular Y view, and an axillary view. Subtle injuries can be missed if adequate radiographs are not obtained. Figure 1 shows a missed posterior dislocation that was not properly diagnosed until a magnetic resonance imaging study was obtained 6 months after the initial injury.
The sternoclavicular joint has the least bony stability of any major joint in the body. Virtually all of its integrity comes from the surrounding ligaments. Sternoclavicular dislocation is one of the rarest dislocations, representing perhaps 3% of shoulder girdle injuries, but is common enough that most major trauma centers will see one or two a year. This injury is typically the result of a high-energy mechanism, such as a motor vehicle accident or a sports injury.
The true ratio of anterior-to-posterior dislocations is unknown, because most reports in the literature concern the more rare posterior type, but anterior dislocations are more common in clinical practice. Anterior dislocations typically present as a deformity with a palpable bump directly over the sternoclavicular joint. Posterior dislocations can present with dyspnea or dysphagia or tachypnea and stridor that is worse when supine. Patients may present with paresthesias in the affected upper extremity or venous congestion or a diminished pulse when compared with the contralateral side.
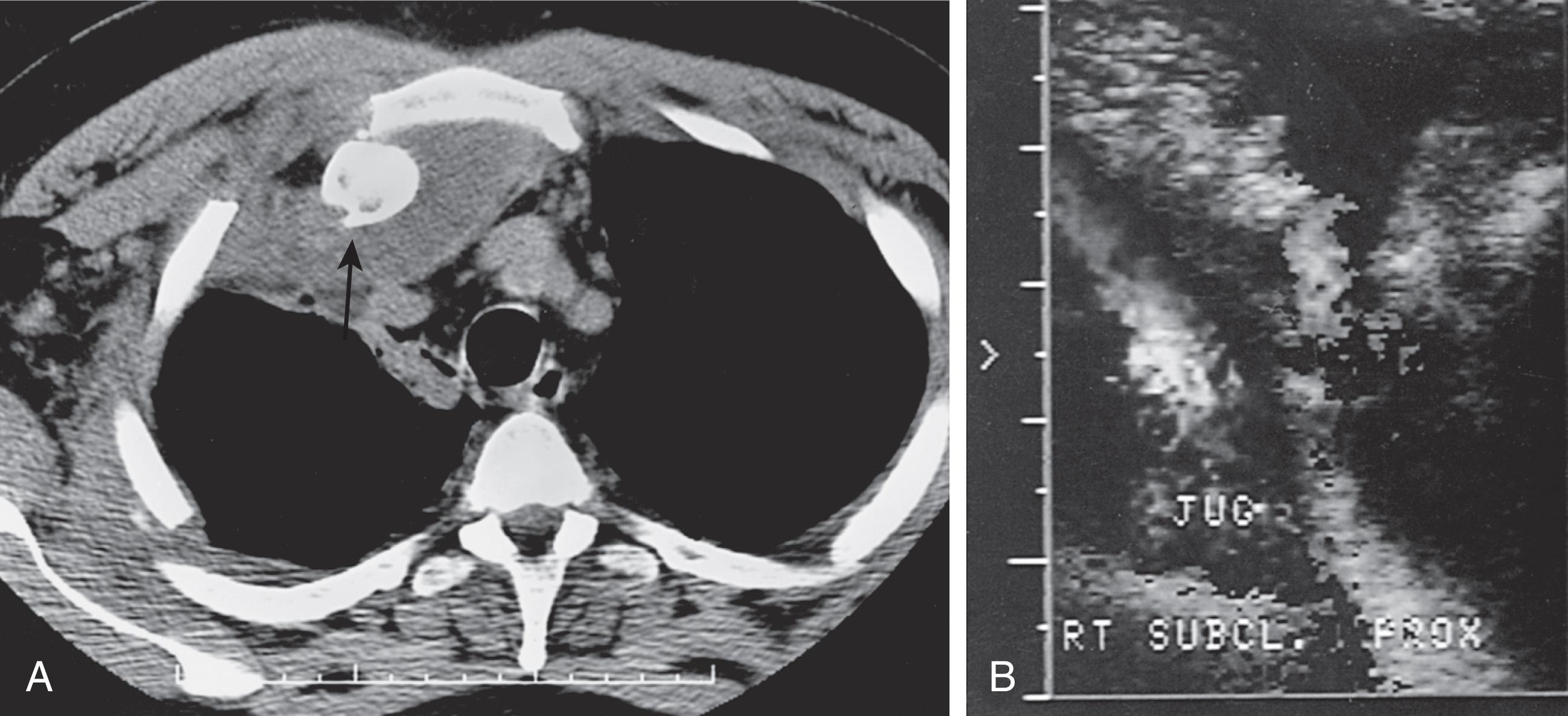
Imaging of these injuries is difficult because the sternoclavicular joint overlies the spine and ribs on standard radiographs. A “serendipity” view, an AP chest film centered on the top of the sternum and aimed 45 degrees cephalic, will occasionally show the dislocation, but a computed tomography (CT) scan will demonstrate the injury most clearly ( Fig. 2 ).
Chronic anterior dislocation (>3 weeks old) may be treated with a sling for comfort, with typical return to unrestricted activity by 3 months. Because of the proximity of the mediastinum and its great vessels to the sternoclavicular joint, acute (<3 weeks old) anterior or posterior dislocations are typically treated with closed reduction in the operating room under general anesthesia, with the general surgery trauma, thoracic, or vascular surgery team on notice nearby in case an open procedure is to be performed.
Although most anterior dislocations are unstable after closed reduction, we still recommend an attempt to reduce the dislocation closed. Occasionally, the clavicle remains reduced, but if it does not, one usually accepts the deformity, because an anteriorly dislocated sternoclavicular joint typically becomes asymptomatic, and the deformity is less of a problem than the potential complications of operative fixation.
In contrast to anterior dislocations, the complications of an unreduced posterior dislocation are numerous: thoracic outlet syndrome, vascular compromise inclusive of blunt subclavian arterial and venous injuries including thrombosis of these vessels, and erosion of the medial clavicle into any of the vital mediastinal structures that lie posterior to the sternoclavicular joint. Closed reduction for acute posterior sternoclavicular dislocation can usually be obtained and is generally stable. Often, general anesthesia is necessary. However, when a posterior dislocation is irreducible or the reduction is unstable, an open reduction should be performed. Depending on the exact pathoanatomy, either open reduction with ligament repair or medial clavicle resection and ligament reconstruction may be performed.
The clavicle is one of the most commonly fractured bones, representing 4% of all fractures and over a third of all fractures in the shoulder region. Fractures in the medial third are quite rare; fractures of the middle third represent nearly 70%, and fractures in the lateral third account for 20% to 30% of all clavicle fractures. Despite a long-standing and widely held belief that virtually all clavicle shaft fractures do well without surgery, recent data demonstrate that this is often not the case.
Initial studies on the natural history of clavicle fractures demonstrated union rates of greater than 99% with nonoperative management and nonunion rates of 5% to 10% or higher for clavicle fractures treated with surgery. Missing from these studies was information on the age of the patients, the severity of injury, and the nature of the fractures selected for surgery. More recent studies focusing on the natural history of clavicle fractures in adults have demonstrated nonunion rates of 4% to 6% for all fractures, with nonunion rates as high as 15% when the fractures are significantly displaced. Shortening of a fractured clavicle by greater than 2 cm has proved to be particularly problematic.
A recent published systematic review of 2144 previously reported clavicle fractures has confirmed a nonunion rate of 15% for displaced fractures and a relative reduction of 86% in the risk for nonunion when displaced fractures are treated operatively. Finally, a multicenter randomized comparative trial of nonoperative treatment versus plate fixation demonstrated faster, easier, and more complete recovery in the surgical group, with both doctors and patients rating the surgical results significantly better at all-time points, including the final result. Complications in the surgical group were primarily related to the hardware (plate) used.
Current teaching holds that surgical repair is indicated for shortening more than 20 mm, open injury or threatened skin, neurovascular compromise, scapulothoracic dissociation, and displaced pathologic fracture. Accepted relative indications are displacement more than 20 mm, floating shoulder, polytrauma, expected prolonged recumbency, a patient unable to tolerate immobilization, bilateral fractures, and ipsilateral upper extremity fracture. Complete displacement (no cortical contact between the main fragments on any radiograph view) is also a relative indication in an informed patient willing to accept the risk of surgery in order to obtain a faster recovery.
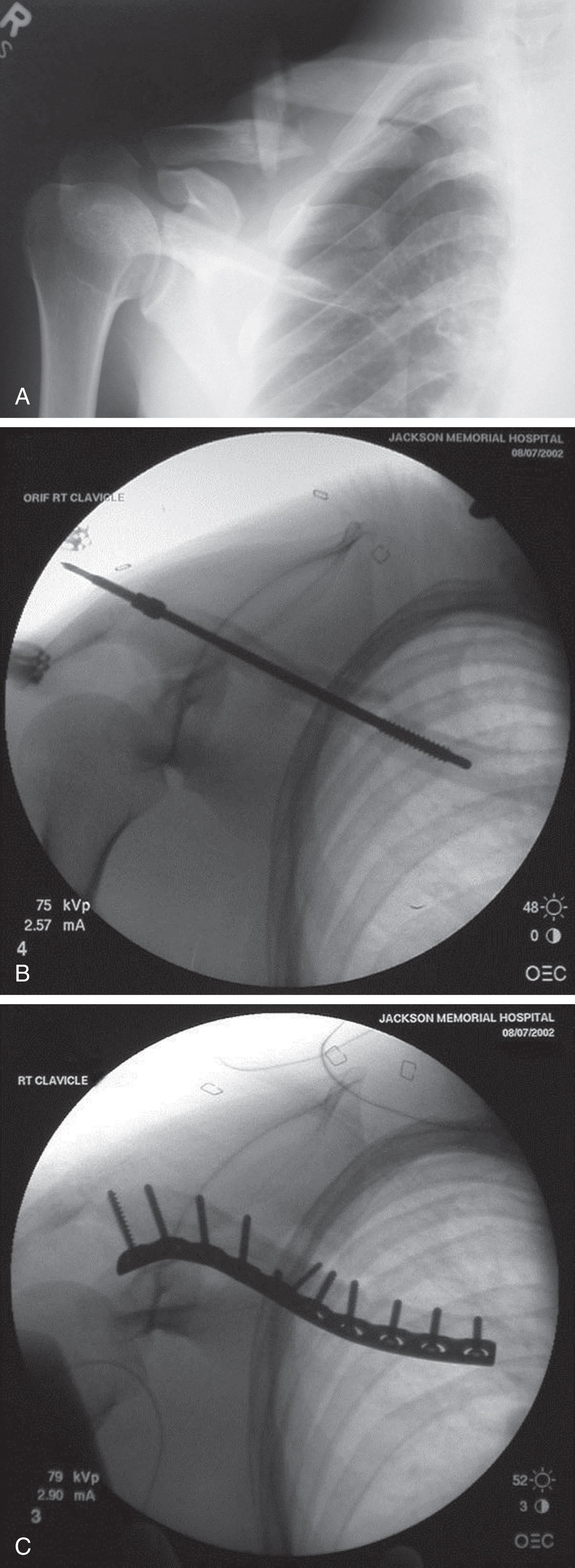
When patients with clavicle fractures are selected for nonoperative management, the current recommendation is for sling immobilization without an attempt at reduction of the fracture. When internal fixation is indicated, surgery may be performed with either a plate and screws or an intramedullary device ( Fig. 3 ). Although both techniques have their proponents and detractors, and recently multiple meta-analyses have been published to compare plate vs. intramedullary fixation for displaced mid-shaft clavicle fractures, no large study has demonstrated superiority of one technique over the other.
Fractures of the lateral, or distal, third of the clavicle are also fairly common, comprising approximately 20% to 30% of all clavicle fractures. The rate of nonunion in lateral third clavicle fractures has been reported to be greater than that of midshaft clavicle fractures, with some authors reporting a nonunion rate of greater than 20%. This had led some authors to recommend early surgery, although others have noted that many of these nonunions become asymptomatic with time, leading them to recommend nonoperative treatment for these fractures. Although fractures of the lateral clavicle have not been as thoroughly studied as fractures of the midshaft, recent reports of surgery performed using newer techniques demonstrate excellent healing and function with a low rate of complications. When operative fixation is chosen, plate and screw fixation is probably the most familiar method, although numerous other methods, such as screw fixation alone, may be used.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here