Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service. [Strohl 1I21RX002041].
This work is also supported by the National Institutes of Health, Stimulating Peripheral Activity to Relieve Conditions (SPARC) [Strohl 1U18EB021792] and will be available through the Material Sharing Policy of the program.
Dr. Damato was supported in part by a training award from the National Institute of Biomedical Imaging and Bioengineering, awarded through the SPARC program [3U18EB021792-01S1 (Strohl, PI)].
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the National Institute of Health.
Obstructive Sleep Apnea (OSA) afflicts approximately 3%–9% of women and 10%–17% of men between the ages of 30–70 years ( ). The disorder is characterized by recurrent episodes of decreased airway patency during sleep due to partial or complete collapse ( ), leading to a reduction of airflow (hypopnea) or absence of airflow (apnea). OSA severity is defined by the number of apneas and hypopneas occurring per hour of sleep (Apnea Hypopnea Index or AHI) with mild disease defined as 5–14 events/h, moderate disease as 15–29 events/h, and severe disease as 30 or more events per hour of sleep ( ). Left untreated, OSA induces repetitive bouts of hypoxemia with hypercapnia which subsequently evoke systemic inflammation, endothelial dysfunction, metabolic dysregulation, and sympathetic over-activation. These physiologic alterations increase risk for systemic hypertension, cardiovascular morbidity, glucose intolerance, and neurocognitive impairment ( ).
OSA occurs when neuromuscular control of a collapsible oropharynx and/or retropalatal pharynx (velopharynx) is insufficient to maintain functional patency during sleep, a time of decreased upper airway muscle tone. Anatomic traits such as retrognathia, hypopharynx, nasal septal deviation, or polyps negatively impact airflow and pressure relationships, thereby contributing to upper airway collapsibility and instability. Dysfunction in neurochemical control of the upper airway and/or chest wall musculature also contribute ( ). This has led to hypotheses proposing the collective influence of four distinct pathways promoting the pathophysiologic mechanism of OSA. These include: (1) sleep itself (specifically a low respiratory arousal threshold); (2) anatomy (small airway size and high pharyngeal compliance); (3) reduced neural activation and reflex responses of muscles contributing to upper airway patency (i.e., inadequate upper airway muscle drive); and (4) loop gain (controls on overshoot and undershoot of ventilatory response) ( ). Interventions targeting these contributing pathways provide opportunities to interrupt the intermittent, cyclic reductions, or cessations of airflow in sleep apnea.
First-line treatments primarily target the anatomical contribution to OSA. Continuous positive airway pressure (CPAP), delivered via nasal face mask, works by inflating and keeping open the collapsible portion of the upper airway. Secondarily, CPAP inhibits genioglossal muscle activity, reduces loop gain by lung inflation, and reduces sleep fragmentation by decreasing arousals triggered by apneic events ( ). Despite clinical effectiveness, adherence to therapy is often limited and attributed to mask discomfort, nasal and pharyngeal dryness, loss of partner intimacy, and claustrophobia ( ).
Alternatively, increased pharyngeal space can be achieved either with oral-appliance therapy or site-selective surgery on soft tissue or bony structures. Oral appliances, which can be customized to a person’s dentition, are designed to fit over the teeth and hold or advance the mandible and tongue forward. Although critical closing pressure of the airway is improved, clinical effectiveness of oral appliances is best in persons with mild to moderately severe OSA ( ). Furthermore, adherence is limited by patient complaints of continued snoring, dry mouth or excessive salivation, mouth or teeth discomfort, muscle tenderness, or temporomandibular joint irritation and inflammation ( ). Additionally, an effective oral appliance requires sufficient molar dentition in all four quadrants.
With the exclusion of nasal surgery for fixed obstruction, surgical modification of pharyngeal soft tissues or bony structures of the upper airway faces other challenges. Airway narrowing can occur in the velopharynx, the oropharynx, and/or the retrolingual pharynx (hypopharynx). This narrowing varies between persons, influenced by obesity as well as multiple skeletal dimensions such as width of the hard palate, length of the bony mandible, and orientations of the hyoid bone and maxilla ( ). With multiple potential sites of airway obstruction, surgical alternatives may provide inadequate relief for persons with moderate to severe disease ( ).
CPAP, oral appliance therapy, and surgery exert a therapeutic effect through structural enhancements of upper airway size and stability. In contrast, neuromodulation is a functional therapy, exerting its effects by reversing inadequate muscle activation. This makes hypoglossal nerve stimulation (HNS) uniquely different than CPAP, site-specific surgery, or oral appliances that address the anatomical or passive mechanical factors contributing to airway collapse ( ). Neurostimulation applied to the hypoglossal nerve (HN) represents a treatment paradigm shift and provides an opportunity to mitigate many of the issues of patient discomfort and stigma associated with previous treatment options.
Four pairs of extrinsic muscles and four intrinsic muscles comprise the human tongue; the extrinsic muscles of the tongue originate from bone and insert into muscle, while intrinsic muscles have both origin and insertion points in muscle ( ). The palatoglossus muscle, an extrinsic muscle, is innervated by the cranial nerve (CN) X/XI complex; the remaining muscles of the tongue are innervated by the HN or CN XII as illustrated in Fig. 108.1 . The extrinsic muscles are primary controllers of tongue position for the various functions of swallowing, speech, and breathing. Two extrinsic muscles, the styloglossus (connecting the tongue to the base of the skull) and hyoglossus (connecting the tongue to the hyoid bone), are innervated by branches from the lateral division of CN XII and are mainly retrusors. The genioglossus muscle (connecting the tongue to the mandible), is an extrinsic muscle and the primary tongue protrusor, composed of oblique and horizontal compartments which receive about five to six nerve branches from the medial division of CN XII ( ).
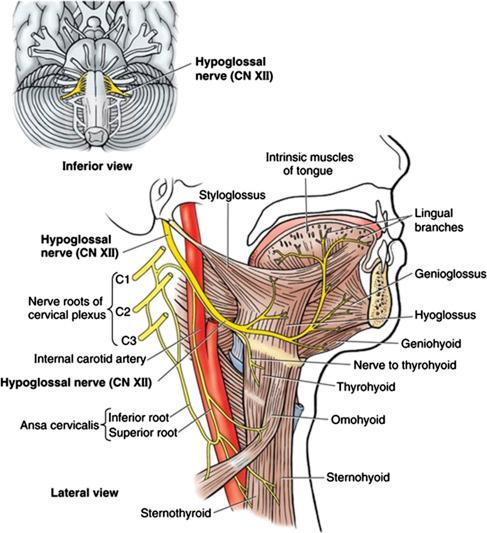
Activation of the genioglossus muscle pulls the tongue forward, thus widening the pharyngeal airway ( ). Combined stimulation of both protrusor and retrusor muscles has been observed to stiffen the pharynx, contributing to maintenance of airway patency during sleep. Consequently, the point at which electrical stimulation is placed on the branches of CN XII produces distinct differences in tongue movement ( ).
Control of protrusive tongue movement occurs in neurons in the ventral motor nucleus of CN XII ( ). Electrophysiologic studies indicate that activity patterns in the motor nucleus are modulated by premotor respiratory neurons controlling inspiratory drive and tonic activity, thus closing the loop between tongue function and respiration ( ). Anatomic evidence exists for connection to the Kölliker-Fuse nucleus, an area in the brainstem respiratory central pattern generator responsible for coordination rather than initiation of a breath ( ).
The direct relationship between loss of genioglossus muscle activation during sleep and subsequent airway obstruction was first described in 1978 ( ). These studies led to the first effort to directly stimulate pharyngeal muscles in humans as a treatment for OSA. In 1988, skin surface electrodes were placed on the submental region and connected to a demand-type electrical stimulator triggered by tracheal breath sounds ( ). Initial reports suggested this approach decreased both frequency and duration of apnea episodes, decreased frequency of oxygen desaturation episodes, and promoted deeper sleep ( ).
Continued investigations into transcutaneous electrical stimulation (TES) of the submental area, the subhyoid area, and intraoral region provided additional insights, although efficacy outcomes were inconsistent. Transcutaneous stimulation applied during sleep did not appear to change upper airway size, prevent apneas or improve sleep architecture in one report ( ), although others reported a decrease in inspiratory resistance dependent on voltage and stimulation frequency, suggesting a widening effect on the supraglottic airway ( ). Other investigators observed that while an intraoral electrode placed at the floor of the mouth induced tongue protrusion and an increase in the size of the posterior oropharyngeal airway, electroencephalographic (EEG) arousals occurred each time submental stimulation broke an apnea episode ( ). Subsequent studies were unable to consistently replicate findings of improved sleep architecture, decreased snoring, reduced daytime sleepiness, improved oxygenation, and reduced AHI in all studies ( ).
Two reports from the same center illustrate current attempts to develop TES approaches. A feasibility report was generally supportive with outcomes of significantly reduced snoring, improved oxygenation, and decreased respiratory disturbance index and AHI when bilateral surface patch electrodes were placed halfway between the chin and the angle of the mandible over the submental areas ( ). Stimulation (mean current, 10.1 mA; mean frequency, 30 Hz) was manually applied for 10 min periods during stage N2 sleep when upper airway occlusion was observed during attended polysomnography (PSG). However, in the subsequent randomized clinical trial of a full night of transcutaneous stimulation applied during occlusive events, the total AHI, snoring, average, and nadir oxygen saturations did not change, although significant improvements were seen in the 4% oxygen desaturation index (4% ODI) ( ). When considering the a priori primary outcome of 4% ODI, 17 of 36 participants (47.2%) responded to the therapy, primarily those in the mild–moderate OSA category. Baseline ODI directly predicted response with approximately 10% of variance explained. Stimulation failed to improve AHI during rapid eye movement (REM) sleep ( ). TES may provide an additional way to screen for responsiveness to implantable neurostimulation therapy ( ).
The next major step in the development of upper airway electrical stimulation occurred in , when Decker et al. performed the first trial of nerve stimulation in humans. HNs were directly stimulated during both wakefulness and sleep by current applied through fine-wire electrodes ( ). The study also assessed the amount of electrical current, applied to surface electrodes and then to fine-wire electrodes, required to induce an arousal from sleep. Key findings from that study revealed that (1) direct neurostimulation of the HN via percutaneous fine-wire electrodes resulted in genioglossus muscle recruitment and tongue protrusion; (2) when using fine-wire electrodes, the electrical current necessary to increase upper airway size was lower than that which induced arousal from sleep; and (3) neither surface stimulation nor fine-wire stimulation could consistently terminate obstructive apnea during sleep. CAT scan observation of pharyngeal changes during fine-wire stimulation in two OSA patients showed both increases and decreases in upper airway size with tongue protrusion, suggesting that electrode position on the various branches of the nerve determined whether upper airway size increased or decreased with neurostimulation ( ).
In 1997, a cuff electrode was surgically placed around the HN for testing during one sleep period ( ). Successful tongue protrusion occurred during stimulation of the distal branch of the HN that supplies the genioglossus muscle, and tongue retrusion occurred during stimulation of the main trunk of the HN. Another key finding was that neurostimulation did indeed open the airway despite occurrence of tongue retrusion ( ).
Simultaneously, biomedical engineers began exploring the feasibility and applicability of single or multielectrode cuffs, biocompatible hardware for implanting, development of both open-loop and closed-loop systems to trigger the neurostimulator, and determination of optimal stimulating parameters. Contributions from these investigations included observations that nonselective stimulation delivered by a multielectrode cuff on the HN yielded the greater benefit to airflow during expiration; during inspiration, improved airflow resulted when the whole nerve was stimulated along with selective coactivation of protrusors (genioglossus) plus retrusor muscles (hyoglossus/styloglossus) ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here