Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Uncemented extensively porous-coated femoral components have a 98% stem survival rate at 20 years.
The implant can be used for all diagnoses and in all qualities of bone.
Defining characteristics of the stem include its nontapered cylindrical distal geometry with extensive porous coating.
The surgical technique is a reamed technique.
The goal of the surgical technique is to obtain 5 cm of diaphyseal scratch fit.
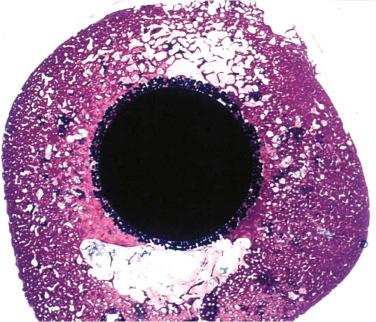
In response to early failures of some cemented femoral components, porous-coated femoral stem technology was developed in the 1970s. The concept behind the design was that live bone in contact with a 3-dimensional metal implant surface would grow into and interdigitate with the porous surface to provide a means of stable implant fixation ( Fig. 66.1 ).
In 1983, the US Food and Drug Administration approved the first porous-coated femoral implant for use without cement. This implant, the anatomic medullary locking stem (AML, DePuy, Warsaw, IN), was characterized by a circumferentially porous-coated, straight, nontapered distal cylindrical rod coupled with a circumferentially porous-coated proximal metaphyseal triangular shape. Long-term studies of the AML have documented a 98% femoral component survival rate at 20 years. Excellent results with this stem have been reported in scenarios historically not deemed appropriate for porous-coated fixation, as in patients with avascular necrosis or rheumatoid arthritis (RA) and in the elderly with osteoporosis.
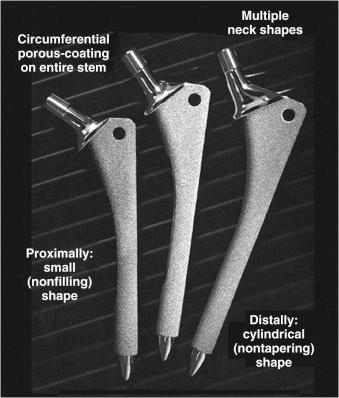
Today, extensively porous-coated femoral implants are available from many manufacturers. Hallmarks of these implants include the presence of a 3-dimensional beaded circumferential porous coating on two-thirds or more of the femoral stem. Because application of the porous surface requires reheating, which weakens the metal substrate, these stems are typically made of cobalt-chrome rather than titanium. The shape of the typical extensively porous-coated stem is a cylinder distally for fixation in the femoral diaphysis and a triangle proximally to fit the patient's femoral metaphysis. Diaphyseal diameters range from 10.5 mm to 22.5 mm, usually in 1- to 1.5-mm increments. Each diaphyseal diameter is accompanied by 2 to 3 metaphyseal sizes, which allow for different femoral offsets ( Fig. 66.2 ).
Surgical implantation of an extensively porous-coated stem is a reamed technique wherein the femur is machined to fit the implant. The cylindrical parallel sides of the implant contact the diaphyseal femoral cortex. Initial fixation is attained with a “scratch fit” of the porous coating, which contacts at least 5 cm of diaphyseal bone. The initial rigid fixation obtained with this technique allows subsequent ingrowth of bone or osseointegration of the stem. This osseointegration accounts for the extremely durable implant fixation .
Through analyses of well-functioning implants retrieved postmortem, extensively porous-coated femoral implants have been studied more thoroughly than many other designs of cementless stems. The extent and quality of bone ingrowth, the amount of implant micromotion, and the bone remodeling process have been well documented through such studies and serve to complement an ever growing clinical experience. Clinical experience has further addressed design concerns such as thigh pain and periprosthetic bone loss secondary to stress shielding. Both thigh pain and stress shielding are thought to result from the mismatch of modulus between the host bone and the much stiffer cobalt-chrome stem. To date, however, the proximal bone loss that occurs to some extent with all uncemented implant systems has not demonstrated negative long-term clinical consequences. Clinical experience has not shown practical complications such as failure of fixation, implant fracture, or femoral fracture from bone remodeling.
Indications, contraindications, and alternative treatment options for the use of extensively porous-coated femoral components are the same as they are for any other form of hip arthroplasty. The patient should be skeletally mature with clinical and radiographic evidence of hip joint deterioration. The patient should have had an adequate trial of nonoperative care and should not have active infection at the surgical site. Patients with a femoral diaphysis smaller than 10.5 mm are not candidates for this type of implant. For those with a femoral diaphysis larger than 22.5 mm, fixation with cement, proximal porous coating, or taper-fluted designs can be considered. Femoral angular and rotational deformities can be addressed with the use of a femoral osteotomy. Further, as this design is applicable to both the primary and revision setting, experience with its use in primary arthroplasty is useful and translatable to more difficult cases.
Finally, although this style of stem has an excellent 20-year published clinical track record and was among the first cementless fixation devices, we acknowledge that minimally invasive and direct anterior approaches are more difficult with this type of implant. The senior author of the chapter has transitioned to proximal fixation in most cases. Therefore, the most common indication in the senior author's practice at this point is wide femoral canals not amenable to proximal fixation. Further, the 6-inch primary stem is a very good option for revisions without diaphyseal bone loss.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here