Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ulcerative colitis (UC) is an important pediatric gastrointestinal disease, given its potential for significant morbidity and even mortality during childhood, its chronicity, and its premalignant nature. Although significant advances in our understanding of its immunologic basis have led to novel approaches to its therapy, UC remains medically incurable. Nevertheless, current medical and surgical therapeutic options have improved the overall outlook for children with this condition. Over the last 2 decades, several pediatric inflammatory bowel disease (IBD) multicentered studies, often prospective, have provided high quality and clinically relevant pediatric data that were strongly lacking. The most important study of this kind to date is the PROTECT study ( P redicting R esp o nse to Standardized P e diatric C olitis T herapy), which initiated in 2012 and completed enrollment in 2016. This study was designed to systematically examine the response of children and adolescents newly diagnosed with UC to standardized treatment regimens. During this prospective study, a large inception cohort was rigorously phenotyped by sampling clinical, genetic, microbiome and metabolome data, endoscopic appearance, and histologic assessment. This multicenter study from 29 pediatric centers across the United States and Canada enrolled 431 patients ages 4 to 17 years with a clinical history suggestive of UC. The data and analysis from this study, among others, will be presented during the course of this chapter.
As opposed to the documented rise in incidence of pediatric Crohn disease (CD) over the last few decades, incidence rates of UC in children appear to have remained stable in some parts of the world while rising in other areas. Population-based studies from Wisconsin and northern Stockholm describe incidence rates of 2.1 and 2.2 per 100,000 population per year, respectively. A follow-up report from the Wisconsin cohort documents the relative stability of UC incidence, with a reported rate of 2.4 per 100,000 per year when the assessment was extended to 7 years. These rates fall in the middle of the range of estimates of 0.5 to 4.3 per 100,000 population per year reported in earlier studies. Although the incidence rates for UC appear to be about half of that seen for Crohn disease in the same population, , anomalies exist, such as studies from Poland and Italy that report incidence rates of UC in children to be greater than that of Crohn disease. , Estimated prevalence rates in children are 18 to 30 per 100,000.
Males and females are equally affected. Although the majority of pediatric patients with UC present as adolescents (87% of subjects in a review from the Cleveland Clinic), very young children with UC are not unusual , and about 40% of children with UC present by the age of 10 years. Care must be exercised in diagnosing the youngest children, however, given the predilection for children younger than age 10 years with Crohn disease to present with isolated colitis. When followed over time, a significant number of these children with apparent UC will demonstrate features consistent with Crohn disease.
Overall, 10% to 15% of children with UC have first-degree relatives with IBD. , However, 22.6% of Jewish children with UC have affected relatives, compared with only 13.7% of non-Jewish children. Children with UC are more likely than their unaffected siblings to have had diarrhea during infancy. However, children who received formula feedings as infants appear to be at no greater risk of developing UC than those who were breast-fed.
Appendectomy before 20 years of age protects against the development of UC in adults. Of interest, it appears that protection against UC is not conferred by the appendectomy itself, but rather by the acute appendicitis or mesenteric lymphadenitis prompting appendectomy. This has been demonstrated convincingly using population-based data from 709,353 people from Denmark and Sweden, with a total duration of follow-up of 11.1 million years. Whereas appendectomy before age 20 years in patients with documented appendicitis or mesenteric lymphadenitis reduced the risk of subsequent UC by about half, appendectomy performed in patients without these underlying inflammatory disorders had no effect on the subsequent rate of developing UC. It is not clear, however, to what degree this factor protects against the development of UC during childhood.
The effect of either active or passive cigarette smoking on the risk of developing UC during childhood is also not clear. Epidemiologic studies have documented that passive smoking during childhood protects against developing UC as an adult. However, although passive smoking may increase the risk of developing Crohn disease as a child, a protective effect against the development of UC during childhood has not been clearly demonstrated. ,
Genetic factors are important in UC, although to a lesser degree than in Crohn disease. Studies consistently demonstrate a lower rate of IBD in relatives of probands with UC than in those with Crohn disease. , , Similarly, the rate of concordant disease is much less for monozygotic twin pairs with UC than for those with Crohn disease.
Despite this, a number of genetic breakthroughs in UC have been made. Among the first was the recognition of an association between human leukocyte antigen (HLA) class II alleles and UC. HLA-DR2 has been found in 40% of a U.S. population with UC, confirming previous studies in which 70% of a Japanese population with UC was found to have the same association. , The strength of this association was more recently demonstrated in a genome-wide analysis from PROTECT. In this analysis, an enhanced contribution of HLA was demonstrated in childhood compared with adult UC, with the odds ratio (OR) for HLA-DRB1∗0103 nearly twice as high (6.94 vs. 3.59) in children, and even higher in girls with extensive disease (OR = 9.46). Another gene of interest is the multidrug resistance 1 (MDR1) gene, located on chromosome 7 in a region identified by genome-wide scans as a potential site of an IBD susceptibility gene. Abnormal gene expression is characterized by decreased production of P-glycoprotein, an important barrier to microbial invasion of the intestine. Studies have identified genetic polymorphisms in the MDR1 gene more frequently in white patients with UC than in normal controls or those with Crohn disease.
A number of other genes or gene loci have been identified as potentially important in the susceptibility to or development of UC ( Box 43.1 ). A recent review identifies 200 independent loci associated with IBD, most of which are statistically significant risk factors for both Crohn disease and UC. Only 29 are thought to be UC specific. Variants associated with either increased or decreased ORs for association with UC have been identified. In most cases, the specific genes involved and the role that the gene products play in the development of UC have not been clarified. Many, however, appear to be involved in control of various aspects of innate or adaptive immunity, antigen sampling, mucosal barrier function, or mucosal integrity.
| Pathway | Gene Loci Associated With CD | Gene Loci Associated With UC | Gene Loci Shared Between CD and UC |
|---|---|---|---|
| Cellular innate immunity | NOD2, IRGM, ATG16, LRRK2 | ||
| Immune-mediated | IL2RA, IL18RAP, IL27, PTPN22, CCR6, ERAP2, CCL2/CCL7, TNFSF11, BACH2, ITLN1, TAGAP, VAMP3 | IL8RA/IL8RB, IL2/IL21, IFNY/IL26, IL7R, TNFRSF9, TNFRSF14, IRF5, LSP1, FCGR2A | IL10, CARD9, MST1, REL, ICOSLG, IL1R2, YDJC, PRDM1, TNFSF15, SMAD3, PTPN2, TNFRSF6B |
| Other | GCKR, THADA, SP140, PRDX5, ZPF36L1, ZMIZ1, CPEB4, FADS1, DENNDIB, DNMT3A, MUC1/SCAMP3 | OTUD3/PLA2G2E, PIM3, DAP, CAPN10, JAK2 | ORMDL3, RTEL1, PTGER4, KIF21B, NKX2-3, CREM, CDKAL1, STAT3, ZNF365, PSMG1 |
| Epithelial barrier | ECM1, HNF4A, CDH1, LAMB1, GNA12 | ||
| HLA/Th17 | HLA-DRB1 | HLA-DRB103, STAT3J, L23R, IL12B, AK2, FUT2, TYK2 |
One well-characterized genetic variation resulting in severe colitis in very young children involves mutations in the interleukin 10 (IL-10) signaling transduction pathway. IL-10, after complexing with its receptor (IL-10R), is involved in limiting hyperactive immune responses mediated by tumor necrosis factor α (TNFα) and IL-12. IL-10R mutations have been shown to be functionally significant and an important cause of early onset (younger than 18 years of age) and very early onset (VEO, younger than 6 years of age) IBD. , In these children, allogeneic stem cell transplantation has proven to be a successful treatment. Overall defects in about 20 different genes in addition to IL-10/IL-10R have been associated with monogenic VEO-IBD. Virtually all are expressed, at least initially, as colitis.
Despite significant advances in unraveling the pathophysiology of UC, its etiology remains unknown. Numerous theories have been proposed over the years. Both environmental and genetic factors have important interrelated roles in the development of pediatric IBD.
Much of the knowledge regarding IBD pathogenesis has been accumulated from experimental animal models. Factors such as genetic aberrant immunologic responses to environmental triggers leading to specific inflammatory cascades, different components of the gut microbiome directly propagating inflammation, and disruption of the epithelial barrier have all been demonstrated experimentally. The resultant mediator of the inflammatory response is a combination of an aberrant innate immune system and T helper cell response. While the immunologic profile of patients with UC is characterized by a predominantly humoral response, an atypical type 2 inflammatory response defined by production of IL-5 and IL-13 but not IL-4 has also been demonstrated. This profile has been shown to be able to differentiate pediatric UC from pediatric Crohn colitis. In addition, studies in UC have shown that there is marked overproduction of immunoglobulin G 1 (IgG 1 ) by both intestinal lymphocytes and those in the peripheral circulation, especially when compared with Crohn disease. Autoantibodies have been identified that are directed against colonic epithelial proteins such as the cytoskeletal protein tropomyosin as a mucosal immune response to a self-antigen of cellular origin, bacterial origin, or both. In addition, these autoantibodies cross-react with antigens in tissues commonly affected by the extraintestinal manifestations of UC, including the biliary epithelium, skin, chondrocyte, and ciliary body of the eye. Which factor(s) initiate this autoimmune process remains to be elucidated. Specifically, human tropomyosin isoform 5 (hTM5) is the major isoform present in normal colonic cells and has increased expression in UC. Research into this pathway is producing knowledge regarding UC pathogenesis and identifying targets for novel treatment.
The genetic discoveries of the last few years, however, appear to support the hypothesis that UC is a consequence of a defect in immune regulation. The genes that have been shown to be associated with UC potentially play important roles in preventing enteric organisms from accessing the lamina propria (e.g., decreased production of P-glycoprotein due to defective MDR1 gene expression) or in immune response (e.g., HLA genes, IL-11, ARPC2, TNFα, IL-10R). , , , These functions suggest that the driving force for the unremitting inflammation characterizing UC appears to be the normal enteric flora rather than an enteric pathogen. How these genes promote the chronic inflammation of UC, and whether they predispose to the development of the aforementioned autoantibodies, remains to be determined.
Although there is great clinical similarity between UC and infectious colitides, no solid evidence supports the theory that an infectious agent is the primary cause of colonic inflammation in UC. Children can initially present with documented enteric infection, only to be found after clearance of the pathogen to have persistent, chronic inflammation. In fact, compared with controls that never had a documented infection with either Salmonella or Campylobacter , a population-based study from Denmark identified a nearly threefold increase in IBD (both UC and Crohn disease) in subjects in the 15-year period following one of these infections. Despite this, the majority of patients lack such a history. Viral infection has also been suggested as a potential initiator of UC, but no study has identified a definitive link. An evaluation of the fecal virome from baseline stool samples collected as part of the PROTECT study did not demonstrate an association between the presence or absence of virus in the stools and either the severity of UC at presentation or the risk for colectomy or rescue with a biologic therapy. Although cytomegalovirus is probably not an etiologic agent in the development of UC, it does appear to be associated with up to 25% of cases of steroid-resistant fulminant disease.
The interplay between the gut microbiome and the enteric immune system has been the focus of important research over the last decade. Intestinal dysbiosis characterized by an overall decrease in microbial biodiversity with a decrease of Firmicutes and increase in Enterobacteriaceae has been described. In children hospitalized with a severe UC exacerbation, the richness, evenness, and biodiversity of the gut microbiome was notably reduced compared with controls. Data from the treatment-naïve PROTECT cohort confirm the presence of a marked dysbiosis at the time of UC presentation, characterized by a decrease in many organisms from the Ruminococcaceae and Lachnospiraceae family, including two common commensals, Faecalibacterium prausnitzii and Dorea formicigenerans —two organisms known to play a crucial role in maintaining healthy gut homeostasis. The dysbiosis is more pronounced with increasing UC activity and worsened over time in subjects who progressed to colectomy.
Allergic reactions, especially to dietary antigens such as milk, have been investigated extensively, but data supporting an allergic etiology for UC are lacking. High titers of antibodies to dietary antigens such as milk are not specific to UC. Patients with UC at times respond to elemental or elimination diets, but when the particular food that appeared to induce symptoms is reintroduced, symptoms are only rarely reproduced consistently.
In the past, UC has been considered to be a psychosomatic disorder. However, although children with UC often demonstrate psychologic profiles that distinguish them from healthy children and other chronic disease controls, these traits do not appear to be the cause of the illness. An analysis of the literature on the psychosomatic etiology of UC demonstrates many methodologic deficiencies, including lack of controls, lack of diagnostic criteria, and nonblinded collection of data. None of the well-designed studies in the literature show an association between UC and psychiatric disturbance.
Short-chain fatty acids (SCFAs) extracted from the luminal contents are a major source of energy for the colonocyte. In children, total fecal SCFAs are markedly decreased in moderate-severe compared with mild UC, although fecal butyrate appears to be increased compared with healthy controls in at least some patients. These observations have suggested the possibility that UC might represent a form of colonic mucosal “malnutrition.” , It is not clear, however, that the observed changes are due to the colonocyte’s inability to utilize SCFA, as the dysbiosis of UC is characterized by loss of commensals responsible for SCFA production.
Little is known about the effect of diet on the development of UC in children. A prospective study in adults has demonstrated that subjects with the highest intake of n-6 polyunsaturated fatty acids (PUFAs, such as linoleic acid) were more than twice as likely (OR 2.49, 95% CI 1.23 to 5.07; P = .01) as those with the lowest intake to develop UC. By contrast, those with the highest intake of the n-3 PUFA docosahexaenoic acid had a significantly reduced OR (0.23, 95% CI 0.06 to 0.97) for the development of UC. Because n-6 PUFAs are converted into proinflammatory molecules including arachidonic acid, prostaglandin E2, leukotriene B4, and thromboxane A2, there is biologic plausibility for the hypothesis that high consumption of linoleic acid (found in red meats and cooking oils) might contribute to the development of UC. Similarly, n-3 PUFAs promote the production of antiinflammatory molecules, such that a diet high in these fatty acids might plausibly protect against the development of UC.
Fiber and vitamin D intake may have less effect on the development of UC. In a prospective study of almost 240,000 registered nurses, a high cumulative average intake of dietary fiber was associated with a lower incidence of Crohn disease, but no similar protective effect was observed for those who developed UC. Similarly, higher plasma 25(OH) vitamin D levels were significantly associated with a lower risk of developing CD but not UC. Data from the treatment-naïve pediatric UC cohort from PROTECT indicates that vitamin D deficiency was detected in almost half of the study sample. Strong associations were shown between clinical severity and levels of free and bioavailable 25-hydroxyvitamin D, indicating that vitamin D levels may be involved in UC pathophysiology.
The extent of UC at the time of diagnosis appears to have varied over the years, possibly as a function of the area of the world being described. Before the mid-1970s, the extent of disease in UC determined by barium enema and sigmoidoscopy estimated that 60% of children had pancolitis, 22% left-sided colitis, and 17% proctitis or proctosigmoiditis. , A study from the northeastern United States in the 1980s and early 1990s, however, found that at diagnosis only 41% of children have pancolitis, whereas 34% had left-sided disease and 26% proctitis or proctosigmoiditis. There has been a suggestion that pancolitis is more common in younger children, with one report from the United States identifying pancolitis in 71% of children younger than 10 years of age presenting with UC. However, a Danish study identified pancolitis in only 13% of children younger than 10 years, and a U.S. study of children younger than the age of 5 years at diagnosis identified pancolitis in only 40%. It is estimated that there is proximal extension of disease within 3 years of initial UC diagnosis in up to 25% of children initially presenting with proctosigmoiditis and 29% to 70% over the course of follow-up.
Although UC has classically been described as a diffuse inflammation confined to the rectum and colon, careful endoscopic and pathologic studies demonstrate that this is not entirely true. Upper gastrointestinal involvement has been found, with esophageal disease in 15% to 50% of cases and gastroduodenal inflammation in 25% to 69%. , Gastric biopsies examined by immunostaining reveal lymphocytes expressing markers characteristic of a T helper cell type 2 (Th2) immune response, similar to that found in the rectum of children with UC. Descriptions of distal colonic UC associated with periappendiceal or appendiceal inflammation have also been published. , Therefore, in a patient with colitis, inflammation in the proximal gastrointestinal tract or partial colonic involvement with associated “skip” lesions is not necessarily evidence of Crohn disease.
The inflammatory changes characteristic of UC are confined to the mucosal surface. Therefore, to the surgeon or pathologist, the external surface of the colon appears normal. On the other hand, macroscopic changes are immediately apparent to the endoscopist. Classically, mucosal abnormalities begin at the anal verge and extend proximally to a variable extent. In the untreated patient, rectal sparing should suggest Crohn disease, although a few children with well-documented UC have been described to have macroscopic rectal sparing at initial presentation. Because treatment (both systemic and rectal) can significantly change the appearance of the mucosa, particular care must be taken in interpreting the finding of rectal sparing in the child undergoing endoscopy once therapy has been initiated.
The gross appearance of the mucosa in UC depends on the severity of inflammation ( Fig. 43.1 ). Mild disease is characterized by diffuse erythema and loss of the normal mucosal vascular pattern. A fine granularity can also be present. Moderate inflammation results in numerous small surface ulcerations, scattered flecks of exudate, and spontaneous or contact bleeding from the mucosal surface. With more active disease, larger, deep ulcerations covered with shaggy exudate become widespread. Because these ulcers surround less involved areas of mucosa, single or multiple pseudopolyps form ( Fig. 43.2 ). All of these changes are present diffusely in involved areas of the large bowel, but the severity of the inflammatory process can vary from location to location.
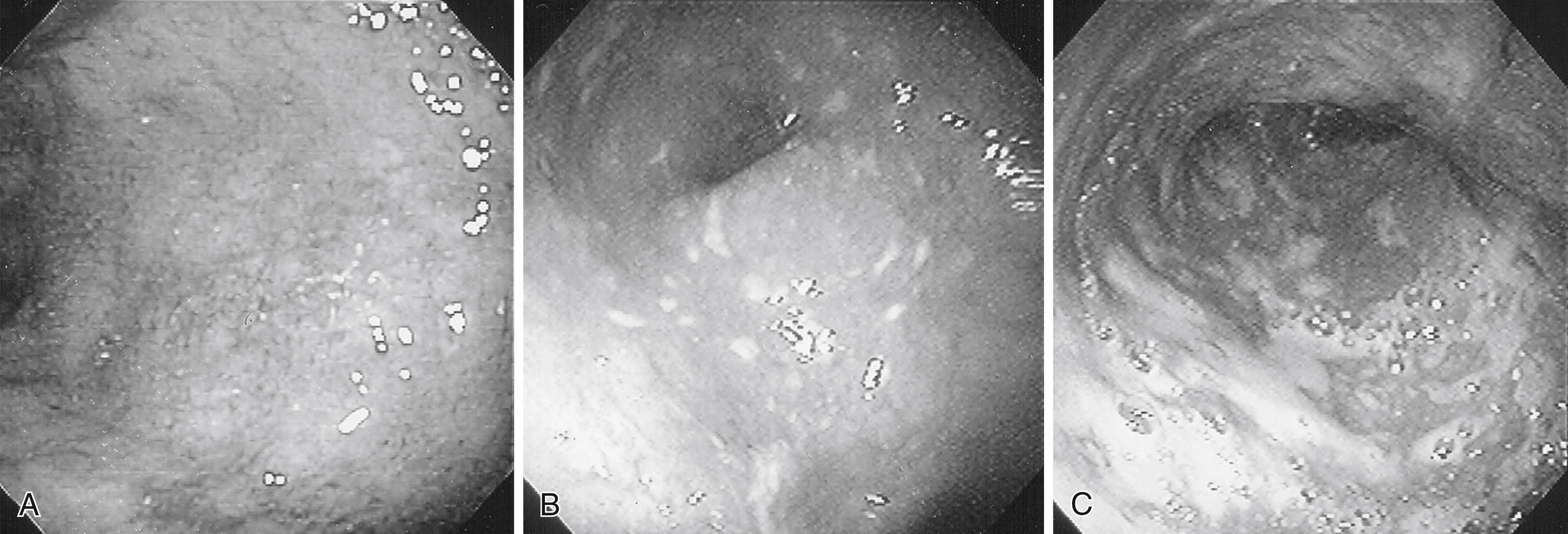
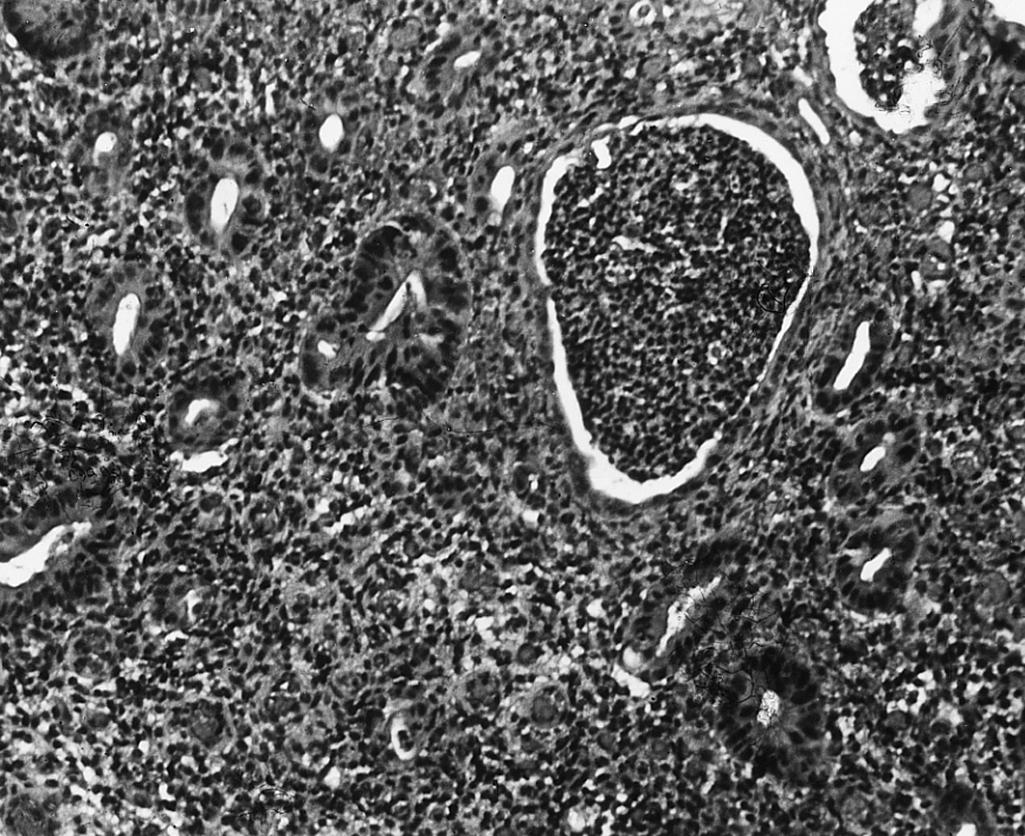
Both acute and chronic inflammatory changes characterize UC at the times of presentation. Neutrophilic infiltration of crypts (cryptitis) often accompanied by crypt abscesses, depletion of goblet cell mucin, and chronic inflammatory cells in the lamina propria constitute the primary histologic findings ( Fig. 43.3 ). In addition, signs of chronicity include evidence of crypt damage such as crypt distortion, a papillary configuration to the surface epithelium, and Paneth cell metaplasia. Data from the PROTECT cohort also highlight the significance of low (<32 eosinophils per high power field) or absent mucosal eosinophilia and surface villiform changes as findings associated with more severe disease. Granulomas are not seen in UC and, if found, imply that Crohn disease should be strongly considered. None of these findings is pathognomonic for UC, as similar changes can be seen in severe Crohn colitis. Infectious colitis may also have a similar appearance, although histologic differentiation of UC from acute self-limiting colitis is generally possible.
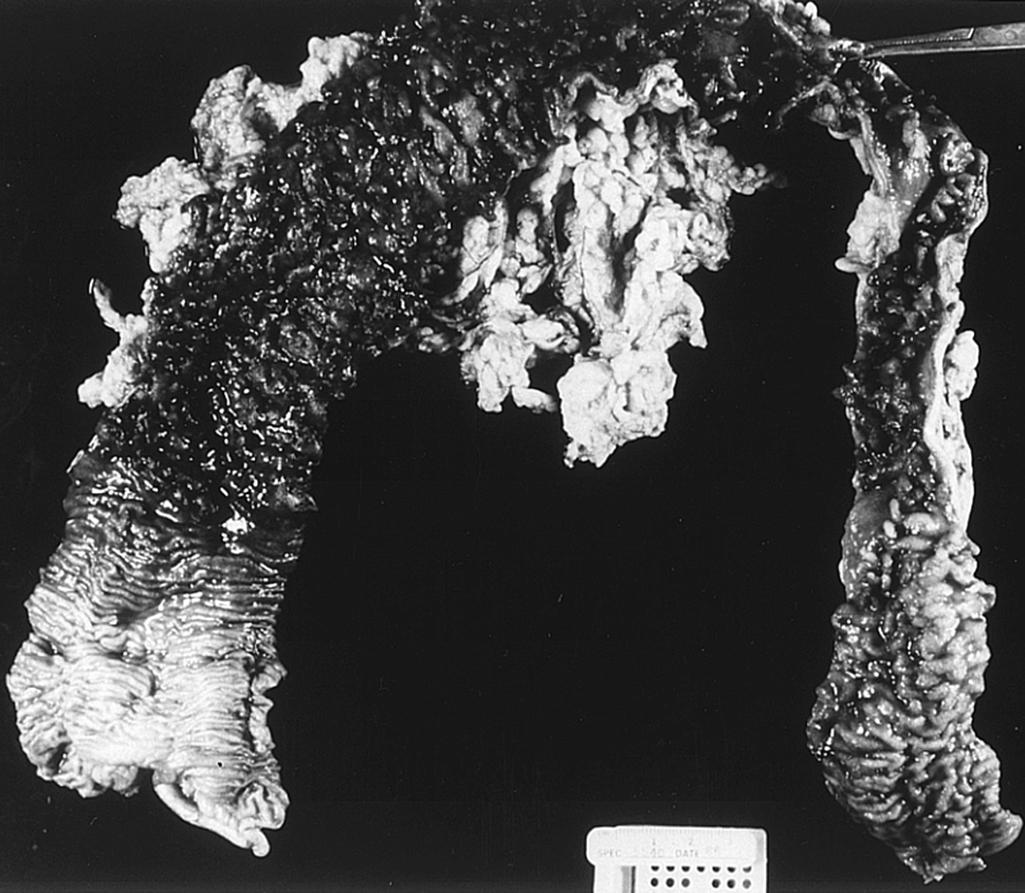
Although diffuse histologic involvement of the affected bowel is typical in the untreated patient, a few children have manifested patchy inflammation and rectal sparing at the time of initial diagnosis. In surgical specimens obtained from patients with severe or fulminant disease, ulceration can, at times, extend into the submucosa or rarely the deeper layers of the bowel wall.
Children with UC most commonly present with diarrhea, rectal bleeding, and abdominal pain ( Table 43.1 ). Frequent watery stools can contain either streaks of blood or clots and are most common on arising in the morning, after eating, and during the night. Children often describe both tenesmus and urgency, although the former symptom is at times misinterpreted as constipation by the child or parent. Acute weight loss is common, but abnormalities of linear growth are unusual (see later discussion).
| Toronto ∗ (Diagnosed 1970–1978) | Cleveland † (Diagnosed Before 1967) | |||
|---|---|---|---|---|
| No. of Patients ( n = 87) | % of Population | No. of Patients ( n = 125) | % of Population | |
| Hematochezia | 84 | 96 | 107 | 86 |
| Diarrhea | 82 | 94 | 116 | 93 |
| Abdominal pain | 77 | 88 | 107 | 86 |
| Anorexia | 44 | 50 | — | — |
| Nocturnal diarrhea | 43 | 49 | — | — |
| Weight loss | 37 | 42 | 64 | 51 |
| Fever | 12 | 13 | 46 | 37 |
| Vomiting | 10 | 11 | 53 | 42 |
∗ Data from Hamilton JR, Bruce GA, Abdourhaman M, Gall DG. Advances in pediatrics. In: Barness LA, editor. Inflammatory Bowel Disease in Children and Adolescents , vol. 26. Chicago: Year Book Medical; 1979:311–341.
† Data from Michener WM. Ulcerative colitis in children. Problems in management. Pediatr Clin North Am . 1967;14:159–173.
The severity of symptoms at presentation is variable. Some 40% to 50% of children and adolescents present with mild symptoms, characterized by fewer than four stools per day, only intermittent hematochezia, and minimal (if any) systemic symptoms or weight loss. These children generally have normal findings on physical examination, or only minimal tenderness on palpation of the lower abdomen. Stools may have streaks of blood or may be positive only for occult blood. Laboratory studies can reveal mild anemia and raised acute-phase reactants such as the erythrocyte sedimentation rate. However, some children have entirely normal laboratory findings.
Another third of children are moderately ill, often displaying weight loss, more frequent diarrhea, and systemic symptoms. Physical examination demonstrates abdominal tenderness, whereas laboratory studies often are characterized by moderate leukocytosis, mild anemia, and raised acute-phase reactants.
The final 10% to 15% of the pediatric UC population has an acute fulminant disease presentation. These patients appear moderately to severely toxic and have severe crampy abdominal pain, fever, more than six diarrheal stools per day, and, at times, copious rectal bleeding. They frequently manifest tachycardia, orthostatic hypotension, diffuse abdominal tenderness without peritoneal signs, and distension. Laboratory studies reveal leukocytosis, often with numerous band forms, anemia, thrombocytosis, and hypoproteinemia. Toxic megacolon represents the most dangerous extreme of acute fulminant colitis and is quite rare in the pediatric age group.
Extraintestinal manifestations are common in children with UC and can affect almost every organ system. The more common sites of involvement are the skin, eye, biliary tree, and joints. Although the etiology of these extraintestinal manifestations remains unknown, it has been shown that an anti-colonocyte antibody detectable in the serum of patients with UC cross-reacts with antigens present in the skin, ciliary body of the eye, bile duct, and joints. , , Many of the extraintestinal manifestations tend to occur at times of increased colitis activity. It is therefore tempting to speculate that extraintestinal symptoms develop when autoantibodies capable of recognizing these nonintestinal tissues are produced as part of the humoral response characteristic of UC.
The most serious hepatobiliary diseases associated with UC are primary sclerosing cholangitis (PSC) and autoimmune hepatitis (AIH). The presentation and severity of these manifestations are generally independent of the activity of colitis and often do not appear to be affected by medical management of UC or by colectomy. PSC has been reported in up to 9.8% and AIH in less than 1% of children and adolescents with UC. In a large pediatric IBD registry, pediatric IBD-related liver disease was more likely to be found in UC than in CD (4% vs. 0.8%, P < .001), with a distribution of 72% PSC, 21% overlap syndrome, and 7% AIH. PSC and AIH may be present before or at the time of the initial diagnosis of UC or can develop at any time during the course of the illness. ,
Both illnesses cause variable degrees of chronic liver disease, ranging from mild to end-stage liver disease requiring transplantation or death. PSC also is a risk factor for the development of cholangiocarcinoma.
Compared with patients with UC alone, adults with PSC and UC tend to present at a younger age and have a greater likelihood of having more extensive but less severe colitis. It has also been shown that the presence of PSC enhances the risk of colorectal aneuploidy, dysplasia, and cancer in patients with UC. The absolute cumulative risk for colorectal cancer in patients with UC and PSC is 9%, 31%, and 50% after 10, 20, and 25 years of disease, respectively, compared with 2%, 5%, and 10% in patients with UC without PSC. In patients with UC and PSC who have a colectomy and ileal pouch, the risk of severe mucosal atrophy, aneuploidy, and dysplasia in the pouch also appears to be increased. In addition, those with PSC and UC complicated by colorectal cancer are at increased risk of cholangiocarcinoma, compared with patients with PSC and UC but no colorectal malignancy. Although treatment with ursodeoxycholic acid has shown some potential benefit in decreasing the risk of colonic cancer in adult patients with UC and PSC, earlier initiation and increased frequency of colonic surveillance is indicated. Data are accumulating in favor of using vancomycin to treat PSC, with effectiveness measured as improved liver enzymes, imaging, and biopsy. However, these data remain insufficient to broadly recommend vancomycin as an established therapy. [CR]
In addition, liver function abnormalities can be seen in a variety of other clinical circumstances, for instance during periods of increased colitis activity, as well as in association with specific therapies used for colitis including CSs, sulfasalazine, parenteral hyperalimentation, azathioprine, and 6-mercaptopurine (6-MP); with biologic medications; or with fatty changes associated from concomitant nonalcoholic fatty liver disease (NAFLD) or following massive acute weight gain.
Arthralgia has been described in up to 32% of children with UC at some time during their course. Arthritis, either a peripheral migratory type affecting the large joints or a monoarticular nondeforming arthritis primarily affecting the knees or ankles, has been reported in 10% to 20% of children. , The presence and activity of arthritis and arthralgia generally, but not invariably, correlate with the activity of the bowel disease. Ankylosing spondylitis occurs in up to 6% of adults with UC but is rare during childhood.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here