Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Traditionally, chapters similar to this one are entitled either “Tumors of the Peripheral Nervous System” or “Peripheral Nerve Sheath Tumors.” Such designations have been dropped in the present text so as to avoid any implications of histogenesis, which have progressively become less tenable, and to allow more logical inclusion of those tumors showing neuroectodermal differentiation, which arise at soft tissue locations without evidence or likelihood of origin from a peripheral nerve; tumors in the latter category include, for example, ectopic meningothelial lesions and clear cell sarcoma (malignant melanoma of soft parts).
With regard to those lesions that truly recapitulate some form of nerve sheath differentiation, these remain a very heterogeneous and complex group with variably precise diagnostic criteria. This reflects the sophisticated and intricate structure of peripheral nerves, which comprise myelinated or unmyelinated axons embedded in an endoneurial matrix composed of Schwann cells, and fibroblasts, in turn surrounded by a layer of perineurial cells; such nerve fascicles are then bound together within the fibroblastic connective tissue known as epineurium to form a peripheral nerve. The range of structure and cell types from which so-called nerve sheath tumors may either arise, or differentiate toward, is therefore quite broad.
Traumatic neuromas, as their name suggests, may arise at any location in which a peripheral nerve is severed and does not heal in an orderly manner. The two principal causes are trauma, either lacerating or penetrating, and surgery, especially limb amputation. The former occurs most often in young adults, while the latter is most common in the elderly and parallels the incidence of limb ischemia due to peripheral vascular disease. Irrespective of the cause, these lesions present as a generally small, painful nodule in superficial soft tissue. A clinically distinctive subgroup in the surgical category presents with right upper quadrant abdominal pain, or even jaundice, following previous cholecystectomy. The frequency of postsurgical examples at any location is reduced if care is taken with any cut nerve end.
Histologically, traumatic neuroma consists of a poorly circumscribed and disorderly outgrowth of all the normal components of a nerve fascicle (i.e., fibroblasts, Schwann cells, perineurial cells, and numerous small nerve fibers) ( Fig. 27.1 ). This proliferation generally takes place within collagenous fibrous tissue; on occasion the latter may become inflamed or myxoid, depending on external factors (such as repeated trauma or wound infection) to which the lesion may be subjected.
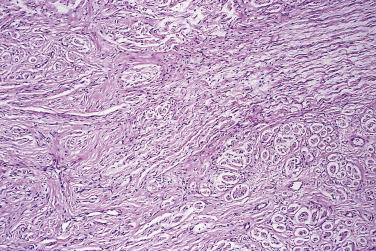
Morton neuroma is included in a book on tumors only because it may be identified clinically as a localized swelling. Morton neuroma presents with severe, lancinating pain in the sole of the foot, usually in the region of the metatarsal heads or metatarsophalangeal joints (metatarsalgia). The pain is exacerbated by walking and relieved by rest. This condition is confined to adults and shows a marked predilection for females. The use of footwear that necessitates extension of the foot at the metatarsophalangeal joints, such as high-heeled shoes, is probably contributory. At operation, the surgeon finds thickening or fusiform swelling of one or more of the digital plantar nerves, often with thickening of adjacent tenosynovial tissues. Resection is curative.
Histologically, the lesion is characterized by marked endoneurial, perineurial, and epineurial fibrosis and hyalinization associated with loss of axons ( Fig. 27.2 ). In addition, there is typically fibrosis and thickening of adjacent vessel walls and fibrosis within adipose tissue. Marked fibrointimal thickening may even lead to occlusion of smaller arteries.
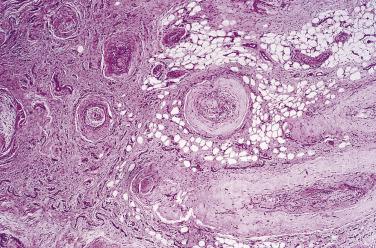
Pacinian neuroma is an uncommon but distinctive type of neuroma, which typically arises on the fingers, usually in adulthood, at the site of previous injury. The lesion presents as a small but very painful nodule. There is no tendency to recur following excision. Almost all reported examples have arisen following local trauma, and these neuromas are therefore regarded as reactive rather than neoplastic. Histologically comparable lesions may exceptionally occur in the retroperitoneum.
Histologically, digital pacinian neuroma consists simply of an unencapsulated hyperplastic collection of normal-sized pacinian corpuscles ( Fig. 27.3 ) associated with numerous small nerves in surrounding fibrous tissue.
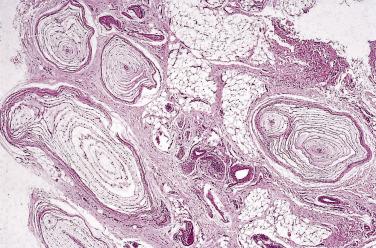
Under the heading of hamartomatous lesions it is intended to describe those lesions which, by their age of onset or morphologic pattern, appear to represent some kind of developmental anomaly. It is conceded, however, that the distinction from a benign neoplasm is often blurred or inconsistent.
Lipomatosis of nerve is a rare condition, which, although it has been the subject of many single case reports, has only once been documented properly in a large series. It is characterized by the development of a slow-growing fusiform swelling, most often of the median (or rarely the ulnar) nerve, accompanied by symptoms of a compression neuropathy similar to carpal tunnel syndrome. The onset is usually in early childhood, with presentation to a clinician in early adulthood. The sex incidence is equal, and up to 25% of patients have associated macrodactyly due to lipohypertrophy. Exceptional cases may occur outside the region of the wrist/forearm, usually in a more proximal location, and may even involve cranial nerves. Other than biopsy for diagnosis and attempted surgical decompression, no other treatment is effective. Inadvertent excision (or attempted excision) of the fusiform mass inevitably leads to a significant neurologic deficit.
Histologically, the epineurium of the affected nerve is expanded by copious, poorly circumscribed, mature fibrofatty tissue ( Fig. 27.4 ). The nerve fascicles themselves are preserved and show only perineurial fibrosis. Histologically significant axonal degeneration is rare.
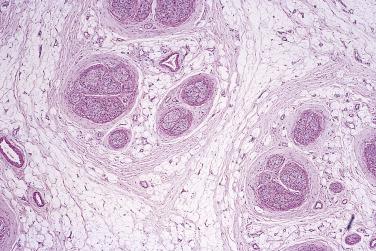
Mucosal neuromas are almost always multiple and characteristically form part of the multiple endocrine neoplasia syndrome type IIb (Gorlin), known to be the result of germline RET mutation. Such lesions are very rare and tend to be clustered around mucocutaneous junctions, especially the mouth, although other skin locations may be affected in exceptional cases. They are accompanied by comparable hypertrophy of the myenteric plexus, or sometimes by ganglioneuromatosis, in the intestines. Clinically, the most important component of this syndrome with autosomal dominant inheritance is medullary carcinoma of thyroid (see Chapter 18 ).
Histologically, each lesion consists of a poorly defined dermal or submucosal mass composed of hyperplastic nerve fibers arranged in a disorderly and irregularly ramifying manner ( Fig. 27.5 ). Some cases may show a superficial resemblance to traumatic neuroma, except that this proliferation is purely endoneurial and without fibrosis. The hyperplastic nerve bundles most often have a perineurial capsule, identified by its positivity for epithelial membrane antigen (EMA).
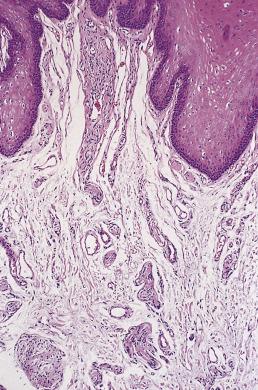
Neuromuscular hamartoma is an exceptionally rare lesion of infancy characterized by the congenital presence of a lump arising from a major nerve, such as the brachial plexus or sciatic nerve. The lesion consists of a complex admixture of normal skeletal muscle and numerous small nerve fibers, the latter often being present within perimysial sheaths. As the child grows, the lesion usually seems to disappear, although actual regression seems unlikely. In a very rare subset of these lesions, smooth muscle substitutes for skeletal muscle. A subset of these lesions is associated with the development of desmoid fibromatosis.
There are scattered case reports of a variety of curious, mainly cutaneous lesions, some of them congenital, which have usually been characterized by hyperplasia of either entire nerve fibers or Schwann cells. There are also rare congenital lesions composed of hyperplastic pacinian corpuscles, sometimes associated with neural tube defects.
The word tumor rather than neoplasm is used here since the precise biologic status of lesions, such as solitary circumscribed neuroma or even the common neurofibroma (and its variants), remains uncertain and open to argument. The tendency, which was widespread in the late 1960s and early 1970s, to lump together schwannoma, neurofibroma, and solitary circumscribed neuroma without discrimination as benign nerve sheath tumor is to be discouraged since it conceals significant clinicopathologic differences, particularly with regard to the risks of associated neurofibromatosis or of malignant change.
Solitary circumscribed neuroma, first described in 1972 by Reed et al, is quite common and has often been underrecognized by both clinicians and pathologists. It usually presents as a solitary painless skin nodule, less than 1 cm in diameter, in the muzzle or butterfly area of the face, although other mucocutaneous sites, including oral cavity and glans penis, may be affected. The peak incidence is between the ages of 30 and 60 years; there is no sex predilection. There is no association with any of the neurocristopathies, and the clinical course is entirely benign. Most lesions are initially mistaken for an intradermal melanocytic nevus. Rare patients with multiple lesions have been described.
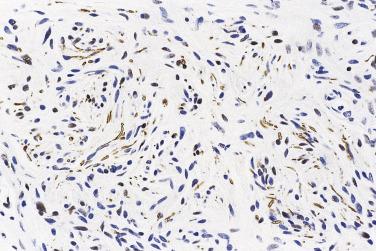
Most cases consist of a small, well-circumscribed nodule in the dermis, although some lesions are club shaped. The nodule occasionally pops out of the surrounding tissue at the time of surgery or pathology cutup. The nodule, in most examples, is subtotally encapsulated around its sides and base, but the superficial surface tends to merge with the dermis ( Fig. 27.6 ); often a nerve of origin can be identified adjacent to the base. The lesion itself is composed of bland eosinophilic spindle cells with poorly defined cell borders and small, wavy, hyperchromatic nuclei. The cells are arranged in fascicles set in a collagenous stroma and often separated by artifactual clefts ( Fig. 27.7 ); vessels are few in number. True nuclear palisading is not a feature, and there is no Antoni A and B zonation. In many cases there is overlying mild acanthosis, and rare cases are associated with pseudoepitheliomatous hyperplasia. Occasional examples show degenerative (ancient) nuclear atypia in tumor cells, and focal epithelioid morphology has been described. Immunohistochemistry demonstrates that the S100-positive lesional Schwann cells are admixed with numerous tiny axons (neurofilament positive) ( Fig. 27.8 ) not evident in hematoxylin and eosin (H&E) sections, hence the designation neuroma . Cells in the subtotal capsule are positive for EMA, confirming their perineurial nature. The relative circumscription of the lesion, combined with the presence of a prominent axonal component, allows ready distinction from schwannoma or neurofibroma.
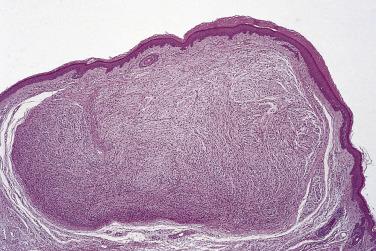
Schwannoma is classically regarded as a benign, nonrecurring tumor of adulthood with no sex predilection, although exceptional cases (usually the cellular variant) may recur locally. The anatomic distribution is very wide, including such diverse locations as the cranial nerves, bone, or the gastrointestinal tract, particularly the stomach, but the overwhelming majority of cases develop in subcutaneous tissue, or less often muscle, with a slight predilection for the distal extremities or head and neck region. With ever-increasing use of computed tomography (CT) and magnetic resonance imaging (MRI) scans, small incidental schwannomas in the retroperitoneum are being identified more frequently. Bilateral schwannomas of the acoustic nerve are the cardinal (or pathognomonic) feature of neurofibromatosis type 2 (NF2). Limb lesions are most often found on the flexor aspect. At operation those tumors arising from an identifiable nerve can usually be separated easily therefrom, leaving no neurologic deficit. Rare patients develop multiple peripherally located schwannomas but, other than those associated with coexistent vestibular schwannoma(s), these are not associated with neurofibromatosis. Instead, this is a separate entity known as schwannomatosis, which is asssociated with SMARCB1 ( INI1 ) mutation as well as inactivation of NF2 and which is sometimes familial. Most cases of solitary schwannoma are asymptomatic, and the majority are less than 5 cm in diameter. Convincingly recorded examples of benign schwannoma undergoing malignant change are very rare.
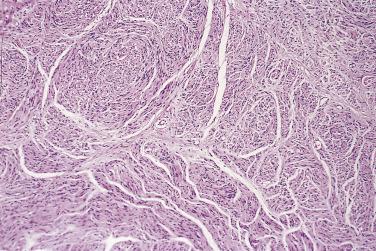
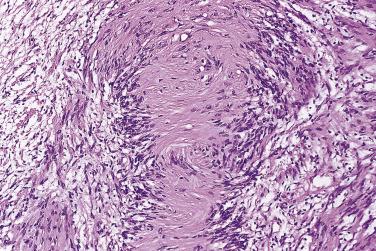
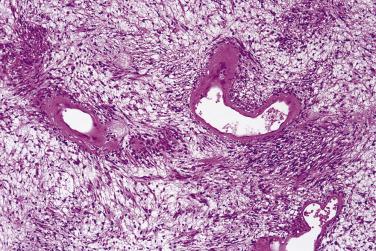
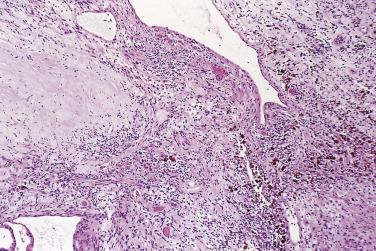
Classical schwannoma is an encapsulated neoplasm having two components, known as Antoni A and B tissue, in variable proportions ( Fig. 27.9 ). Antoni A tissue is cellular and consists of monomorphic spindle-shaped Schwann cells, with poorly defined eosinophilic cytoplasm and pointed basophilic nuclei, set in a variably collagenous stroma ( Fig. 27.10 ). These cells commonly show nuclear palisading, and parallel arrays of such palisades with intervening eosinophilic cytoplasm (cell processes) are known as Verocay bodies ( Fig. 27.11 ). Antoni B areas are also composed of Schwann cells, but their cytoplasm is inconspicuous, and the nuclei appear suspended in a copious myxoid, often microcystic, matrix. These areas are probably degenerative in nature. A common feature, usually most prominent in Antoni B areas, is the presence of blood vessels with thick hyaline walls ( Fig. 27.12 ). Notably, schwannomas arising in the gastrointestinal or upper respiratory tracts tend distinctively to be unencapsulated, and those in the gastrointestinal (GI) tract have a prominent peripheral lymphoid cuff. Normal mitotic figures are a common finding in benign schwannoma, especially in Antoni A areas, but it is exceptional for these to exceed 5 per 10 high-power fields (hpf) in number. Although the nerve of origin is occasionally evident stretched over the capsule, it has traditionally been believed that schwannomas do not contain axons. However, a more recent study demonstrated neurofilament-positive axons in almost 50% of schwannomas, most often the conventional and cellular subytpes. Progressive degenerative changes, most often reflecting lesional duration or a frequently traumatized location, are common in schwannoma. These changes, which are initially focal, include hyalinization, stromal hemorrhage, cystic change, and calcification ( Fig. 27.13 ). The point at which such tumors become classified as ancient schwannoma (see later discussion) is blurred.
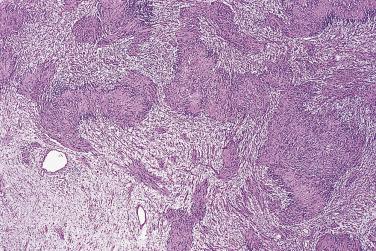

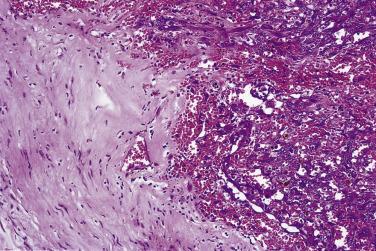
The very rare examples of schwannoma that undergo malignant change most often show features of high-grade epithelioid malignant peripheral nerve sheath tumor (MPNST) or, distinctively, epithelioid angiosarcoma ( Fig. 27.14 ). Exceptional cases may have small, round cell morphology. Very occasionally schwannomas may show what appears to be in situ epithelioid malignant change characterized by singly scattered large atypical epithelioid Schwann cells with vesicular nuclei and prominent nucleoli. To date such lesions have pursued a benign clinical course, and they might best be termed atypical schwannoma with epithelioid cells .
Immunohistochemically, schwannomas show diffuse and strong S100 protein and SOX10 positivity. Those located in the gastrointestinal tract are also often glial fibrillary acidic protein (GFAP) positive, while those in the retroperitoneum and mediastinum are very commonly keratin positive. The perineurial capsule of schwannomas is EMA positive. Ultrastructurally, benign schwannoma (including its variants) is composed of cells with small cell bodies and elongated, interdigitating cytoplasmic processes invested by a complete external lamina, which is often reduplicated. These processes are commonly connected by desmosome-like junctions. There are often bundles of long-spaced collagen (known as Luse bodies) in the stroma. By cytogenetic analysis, most schwannomas show either monosomy 22 or loss of 22q material, where the NF2 gene is located. Interestingly, this aberration is shared by other types of benign nerve sheath tumor and also by meningioma (see Chapter 26 ). Mutation (or inactivation) of NF2 leads to loss of merlin expression, thought to be a key event in schwannoma pathogenesis, although this is seemingly rare in GI schwannomas.
This essentially represents benign schwannoma in which there has been severe degenerative change. As mentioned, there are no strict criteria for this diagnosis, but this author tends to reserve its use for those cases in which either there is significant nuclear atypia or pleomorphism ( Fig. 27.15 ) or in which the cellular (Antoni A) component has become so inconspicuous or focal as to render the diagnosis difficult other than by S100 staining ( Fig. 27.16 ). The degenerative nuclear atypia is characterized by intense nuclear hyperchromasia and coarse clumping of chromatin; nucleoli are inconspicuous or absent, and mitoses generally cannot be found. This type of schwannoma may be found at any location but is often deep seated. There is no tendency to recur.
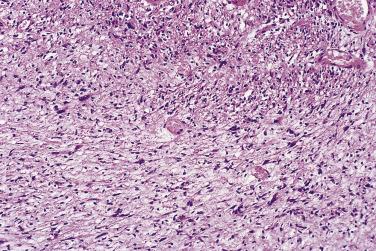
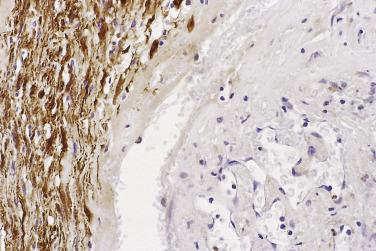
First characterized in 1981, this variant of schwannoma has been mistaken for sarcoma (at least in the past) in up to 30% of cases. Classically, it is a large, deep-seated tumor, which shows a propensity to occur in the retroperitoneum or mediastinum and often arises from a major nerve. There is a slight predominance in females, and occasional cases have been associated with neurofibromatosis. Although the tumor is usually encapsulated, some cases may be focally infiltrative and erode or invade bone. If excision is incomplete, then there may sometimes be local recurrence.
Histologically, these lesions differ from ordinary schwannoma by being hypercellular almost throughout (with an inconspicuous or only focal Antoni B component) and by showing a markedly fascicular ( Fig. 27.17 ) or whorled growth pattern with only very rare nuclear palisading. Mitoses may number up to 10 per 10 hpf, and there may be nuclear pleomorphism; the latter is degenerative (pyknotic) in nature and is usually dissociated from the mitotic areas. Other common features are the presence of a thick fibrous capsule, in which there may be a dense lymphocytic infiltrate, and the finding of quite numerous foamy (xanthomatous) cells within the tumor ( Fig. 27.18 ). Focal necrosis is an occasional, albeit infrequent, feature.
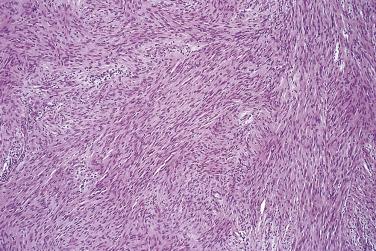
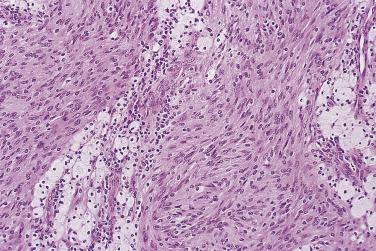
The circumscription of the lesion, presence of hyaline vessel walls, small Antoni B foci, and the relative absence of neurofibroma-like areas, combined with the reasonably distinctive clinicopathologic context and both strong and diffuse S100 protein immunopositivity, allow distinction from a low-grade MPNST in almost all cases. Positive immunostaining for S100 protein and desmin negativity prevent a misdiagnosis of leiomyosarcoma, which otherwise is easily made in the context of a fascicular eosinophilic spindle cell neoplasm showing quite frequent mitoses.
It is important to note that rare examples of cellular schwannoma may have a strikingly plexiform appearance. Such lesions, which have been mistakenly labeled as malignant in the past, most often occur in the extremities of infants and quite often recur locally.
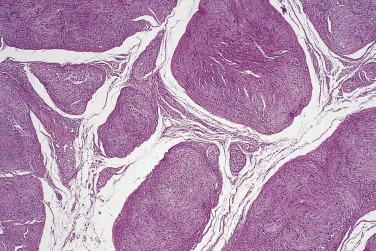
This uncommon type of schwannoma clinically tends to affect slightly younger patients than ordinary schwannoma. It has a slight predilection for the trunk and is notable for its origin in the dermis in at least two-thirds of cases. Deep-seated examples are quite infrequent. With very rare exceptions, it is not associated with neurofibromatosis type 1, hence the importance of its distinction from plexiform neurofibroma. However, as many as 5% are associated with schwannomatosis or neurofibromatosis type 2. The tumor usually measures less than 3 cm in diameter, is sometimes painful, and consists histologically of multiple discohesive nodules composed mainly of Antoni A-type tissue ( Fig. 27.19 ). Each nodule is encapsulated, and mitoses may be present as in ordinary schwannoma. Local recurrence is very infrequent, and malignant change is exceptional.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here