Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
While benign bone tumors are relatively common, primary malignant tumors of bone are rare and occur far less frequently than osseous metastases. Approximately 3200 patients are diagnosed with primary bone sarcoma in the United States per year compared with approximately 222,000 new cases of lung cancer and 255,000 new cases of breast cancer. The overall annual incidence rate for primary bone sarcomas is 1 to 1.5 per 100,000 and has a sarcoma-specific bimodal age distribution. Due to the rarity of bone tumors, most surgical pathologists are unfamiliar with them, leading to an understandable uncertainty in diagnosing bone tumors. One of the most important things for pathologists to remember is that a multidisciplinary team approach is essential in the diagnosis and management of a patient with a bone tumor. A pathologist who attempts to diagnose a neoplasm of bone without knowledge of the clinical features and the radiologic findings is at a distinct disadvantage.
Predisposing factors to neoplasms of bone are tumor specific. Genetic predisposition to osteosarcoma occurs in patients with germline mutations in tumor suppressor genes, such as retinoblastoma syndrome, Li-Fraumeni syndrome, and Rothmund-Thomson syndrome, among others. In addition, patients with prior irradiation, chronic osteomyelitis, bone infarcts, and Paget disease of bone have an increased risk of osteosarcoma. Patients with syndromes associated with multiple enchondromas (the spectrum of enchondromatoses) and multiple osteochondromas also have an increased risk of malignant transformation to secondary chondrosarcoma.
Symptoms related to bone tumors are usually nonspecific; they include localized pain, a palpable mass or swelling, neurologic compromise, and pathologic fracture. A subset of bone tumors can have a specific clinical presentation. For example, a patient with an osteoid osteoma typically presents with exquisite pain, worse at night, that is promptly alleviated with nonsteroidal antiinflammatory drugs (NSAIDs).
While symptoms are of limited value, patient age and the exact location of the tumor provide valuable clues. Many of the aggressive sarcomas, such as Ewing sarcoma and osteosarcoma, more frequently occur in children and adolescents. In contrast, chondrosarcoma is much more common in adults. Both the anatomic skeletal site of the lesion and its specific location within the affected bone provide key information. For example, giant cell tumors of bone usually occur at the ends (epiphyses) of bones. In contrast, an osteoclastic giant cell–rich lesion involving the metaphysis or diaphysis is more likely to be an aneurysmal bone cyst, nonossifying fibroma, or variant of osteosarcoma. For certain types of tumors, anatomic site can also suggest biologic behavior: An intraosseous cartilaginous neoplasm involving the small bones of the hands and feet is most likely a benign enchondroma, whereas a cartilaginous neoplasm involving the sternum is a chondrosarcoma until proven otherwise.
The unifocality or multifocality of the tumor is also of great importance. Hematolymphoid neoplasms and metastases tend to be multifocal. While the majority of primary bone sarcomas form solitary tumors, primary vasoformative tumors of bone often form multiple lesions.
For all bone tumors, the importance of correlation with radiologic imaging findings cannot be overstated. Many advances have been made in bone imaging; while a radiograph (plain film) still plays a role in radiologic diagnosis, advanced cross-sectional imaging, such as computed tomography (CT) and magnetic resonance imaging (MRI), provide important information. While surgical pathologists should be familiar with the fundamental radiologic features of bone features, imaging review by an experienced musculoskeletal radiologist is essential.
Several radiologic features aid in distinguishing a benign from a malignant lesion. Generally, benign lesions tend to be well circumscribed and may have a sclerotic rim, whereas malignant tumors tend to be poorly circumscribed and destructive. Small round cell malignancies, such as Ewing sarcoma, tend to show a permeative destructive pattern, resulting in a moth-eaten appearance in the affected bone. In addition, the character of the periosteal reaction is important. Benign lesions, such as Langerhans cell histiocytosis, incite thick, regular periosteal new bone formation. Malignant tumors, such as Ewing sarcoma, tend to form multiple layers of poorly organized new bone in the periosteum.
Bone tumors have highly variable biologic behavior. Unlike soft tissue tumors, the French National Federation of Cancer Centers (FNCLCC) grading system has not been validated in bone tumors. By the current World Health Organization (WHO) classification system, bone tumors are grouped similarly to soft tissue tumors into benign, locally aggressive or rarely metastasizing lesions and malignant tumors (sarcomas). The histologic grading of bone tumors is based on assessment of cellularity, cytologic atypia, and degree of differentiation. Some tumors, such as Ewing sarcoma, dedifferentiated chondrosarcoma, and mesenchymal chondrosarcoma, are by definition high grade, whereas parosteal osteosarcoma is low grade, and the aforementioned criteria are not used. Nevertheless, the three-tier grading system of bone tumors has gained acceptance as the preferred grading model.
Histologic grade is an established prognostic factor: Low-grade bone sarcomas (grade 1) have a better prognosis than high-grade sarcomas (grades 2 and 3). In cases where neoadjuvant therapy significantly alters the tumoral morphology, the pretreatment histologic grade should be used.
Clinical and pathologic staging systems are used for patient prognostication. For nonhematolymphoid primary bone tumors, the American Joint Committee on Cancer (AJCC) TNM stage is used for pathologic staging, whereas the Enneking staging system is more widely used by surgeons and clinicians. For pathologic staging, the eighth edition of the AJCC staging system introduced specific staging systems for primary bone sarcomas arising in the pelvis, spine, or all other skeletal sites. In all three subcategories, the pathologic stage is assigned based on tumor size, local extent and multifocality, lymph node status, and presence and location of metastasis. Prognostic stage groups are assigned based on pathologic stage combined with histologic grade. In addition, histologic response to neoadjuvant chemotherapy is an established prognosticator in osteosarcoma and Ewing sarcoma. The binary system assigning either 90% or more tumoral necrosis or less than 90% necrosis is most commonly used and requires adequate tumor sampling.
In the Enneking system, an anatomic compartment system, histologic tumor grade, categories of surgical margins, and the presence of metastasis are used to assign patients to stage I, II, or III. A major limitation of this system is that it does not include tumor size, an important predictor of biologic behavior.
Bone lesions can be diagnosed on specimens obtained via fine-needle aspiration (FNA), image-guided needle core biopsy, or incisional (open) biopsy. However, bone tumor FNA cytology analysis, without accompanying tissue cores, is done in only a few large orthopedic centers by experienced musculoskeletal pathologists. The cited potential benefits include fast turnaround time and cost savings; however, specimen inadequacy can be a significant issue.
Image-guided needle biopsies have become the primary biopsy method of bone tumors at most centers. In comparison to FNA, needle core biopsy is more accurate in yielding a tissue-specific diagnosis, and if sufficient tissue is present, ancillary studies can be performed on the tissue cores.
Ideally, the radiologist targets the most aggressive-appearing portion of the lesion, avoids necrotic areas, and obtains samples from multiple areas in large lesions. As with all bone tumors, needle core biopsies should be interpreted in conjunction with clinical and radiographic features of the lesion, particularly given the possibility of sampling error as a result of limited tissue.
At times, an incisional open biopsy is necessary to obtain diagnostic tissue. While image-guided biopsies have less patient morbidity, reduced contamination of surrounding tissue, and are less expensive, at times they may not provide adequate tissue for diagnosis and are susceptible to crush artifact. Incisional biopsies provide larger amounts of tissue, and ancillary testing can readily be performed. In addition, intraoperative frozen section analysis can assess specimen adequacy at the time of the procedure.
Irrespective of the biopsy method, most biopsies of bone tumors will require some amount of decalcification. Due to its deleterious effects on nucleic acid integrity, the use of organic acids (e.g., formic acid) for decalcification is not recommended. Instead, calcium-chelating agents, such as ethylenediaminetetraacetic acid (EDTA), preserve cytologic and nuclear detail and are much less likely to hinder ancillary cytogenetic or molecular genetic testing. The downside is that EDTA-based decalcification is a much slower process than acid-based decalcification.
Plasma cell neoplasms can present as a solitary destructive bone lesion (plasmacytoma) or more commonly as an osseous manifestation of systemic disease. Diagnostic criteria for a solitary plasmacytoma of bone include (1) biopsy-proven solitary lesion of bone containing clonal plasma cells, (2) normal bone marrow with no evidence of clonal plasma cells, (3) normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion), and (4) clinical absence of end-organ damage such as hypercalcemia, renal insufficiency, and anemia that can be attributed to a lymphoplasmacytic proliferative disorder. Patients with solitary plasmacytoma tend to be somewhat younger than patients with multiple myeloma, and many of them have a protracted clinical course with approximately 10% progressing to multiple myeloma within 3 years. The syndrome of p olyneuropathy, o rganomegaly, e ndocrinopathy, M protein, and s kin changes (POEMS) is another plasma cell disorder characterized by osteosclerotic lesions. One-third of patients with POEMS syndrome have a solitary lesion, and at least a third have multiple sclerotic lesions. No histopathologic features distinguish between these various plasma cell dyscrasias involving bone. Therefore a complete radiologic, clinical, and laboratory workup is required for definitive classification.
Radiographs of plasmacytomas typically show punched-out, purely lytic lesions centered in bone marrow. MRI and CT images may identify lesions that are not evidenced on radiographs. A small percentage of patients with myeloma present with a picture of diffuse osteopenia. Macroscopically, multiple myeloma usually has a soft red-brown appearance. However, some myelomas show the fish-flesh appearance typical of lymphoma.
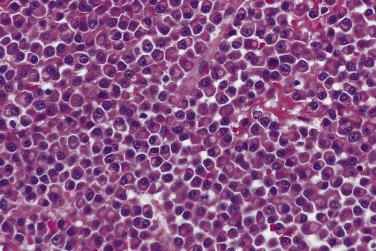
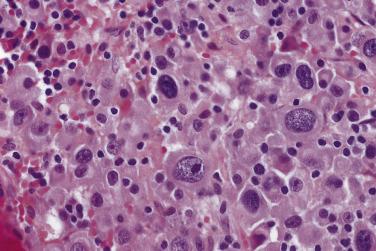
The spectrum of cytologic features in plasma cell myeloma ranges from those that closely resemble normal plasma cells to those that bear little resemblance. Well-differentiated plasma cells are easily recognized by their eccentric nuclei, “clock-face” chromatin, and amphophilic perinuclear Golgi apparatus (Hof) ( Fig. 25.1 ). Poorly differentiated myeloma is a more challenging diagnosis because the tumor cells often display significant cytologic atypia and pleomorphism ( Fig. 25.2 ). This can cause diagnostic problems because of overlapping features with lymphoma, carcinoma, and at times sarcoma. Some tumors exhibit a sinusoidal vascular pattern resembling a carcinoid tumor.
Occasionally patients with a plasma cell dyscrasia develop large masses of amyloid within bone, demonstrating radiologic features of a malignant bone tumor. Histologically, the amyloid deposits create paucicellular or acellular eosinophilic nodular masses often associated with a few plasma cells and, at times, foreign-body multinucleate giant cells. A positive Congo red stain confirms the diagnosis of amyloid, and mass spectrometry–based proteomic analysis is the best test methodology for typing the protein. Amyloidomas of bone usually occur in patients with AL type amyloidosis, and the majority of these patients have multiple myeloma, monoclonal gammopathies, or light chain disease. β2-microglobulin protein amyloid deposits can occur in patients on long-standing hemodialysis who do not have an underlying plasma cell dyscrasia.
The histologic differential diagnosis of plasma cell neoplasms primarily includes lymphoma, metastatic carcinoma, and occasionally chronic osteomyelitis. Monoclonal cytoplasmic staining (or in situ hybridization) for κ or λ light chains is essential to confirm a diagnosis of malignancy. Myeloma cells typically express pan-plasmacytic markers, including CD138, CD38, and MUM1. However, caution should be used when considering a diagnosis of metastatic carcinoma because the latter can be positive for CD138, and myeloma cells occasionally express epithelial membrane antigen (EMA). A panel of immunohistochemical studies should be used when separating lymphoma from myeloma because the pan-B-cell antigen CD20 is expressed in 20% to 30% of myelomas.
When lymphoma involves the skeleton, it is typically as a manifestation of systemic disease, presenting as multifocal destructive lesions or diffuse marrow infiltrates. Primary lymphoma of bone, defined as lymphoma originating in bone with no extraskeletal disease or disseminated bone marrow involvement, is rare. It accounts for approximately 5% of primary malignant bone tumors, 5% of extranodal lymphomas, and less than 1% of all malignant lymphomas. Half of the patients are over 40 years old, and there is a slight male predominance. However, it has also been well documented in the pediatric and young adult population, in whom it can be mistaken for more common pediatric bone tumors, particularly Ewing sarcoma. The bones most commonly affected include the metadiaphyses of long bones (femur, humerus, and tibia) and the axial skeleton (pelvis and vertebrae). Multiple bones are involved in up to 25% of cases. The most common presenting symptoms are localized pain and swelling; focal neurologic deficits may result from nerve involvement or cord compression in axial tumors, and 25% of patients present with pathologic fractures in weight-bearing bones.
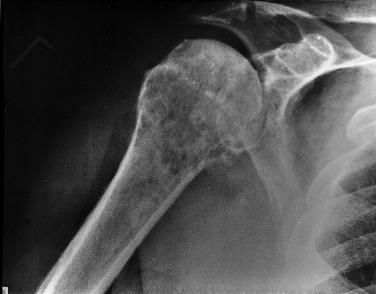
Typical radiographic findings include radiolucency with permeative margins, minimal onion skin periosteal reaction, and an associated soft tissue mass in 50% of patients ( Fig. 25.3 ). Occasionally the radiograph demonstrates only subtle changes or, at times, appears completely normal. CT and MRI are helpful in determining the extent of osseous and extraosseous involvement. Functional imaging (FDG-PET and FDG-PET-CT) has become increasingly important in staging and monitoring disease during and after treatment.
The gross appearance of lymphoma of bone is similar to that of lymphoma elsewhere (i.e., a fish-flesh appearance). Microscopically, the diagnosis can be difficult due to crush artifact, particularly when dealing with smaller needle biopsies. Open biopsy is usually preferred because the technique obtains more tissue and yields better cytologic preservation. Primary lymphomas of bone demonstrate the same histologic features as their extraosseous counterparts and are classified by the same criteria ( Fig. 25.4 ). The majority are B-cell lymphomas, most commonly diffuse large B-cell lymphoma, and these tumors have a better prognosis than their extranodal counterparts. As with extraosseous diffuse B-cell lymphomas, the primary types express CD45 and pan-B-cell markers (CD19, CD20, CD79a, PAX5) and lack immunoreactivity with T-cell markers (CD3 and CD5). T-cell primary lymphomas of bone are rare in Western societies, but more common in Japan and China. Anaplastic large cell lymphoma occasionally presents as a primary lymphoma of bone with histologic features resembling Ewing sarcoma. Tumor cells in anaplastic large cell lymphoma are positive for CD30 and generally positive for CD3, CD4, and ALK. Lymphoblastic lymphoma accounts for approximately 2% of primary lymphoma of bone. The majority of lymphoblastic lymphomas will be of T-cell origin (90%) and stain positively for CD10, CD43, CD99, FLI1, and terminal deoxynucleotidyl transferase. CD45 may be weakly positive or negative. It is important to remember that lymphoblastic lymphoma may be positive for CD99 and negative for CD45, an immunoprofile that overlaps with Ewing sarcoma. Additional non-Hodgkin B-cell lymphomas, including follicular lymphoma, marginal zone lymphoma, mantle cell lymphoma, and small lymphocytic lymphoma, only rarely (2%–5%) present as primary lymphoma of bone. Skeletal involvement by late-stage Hodgkin lymphoma is not uncommon and typically involves the spine. If a bone biopsy shows lymphoid cells admixed with eosinophils and scattered cells with pleomorphic nuclei, Hodgkin lymphoma and Langerhans cell histiocytosis should be considered. Immunohistochemical testing can readily distinguish the two entities.
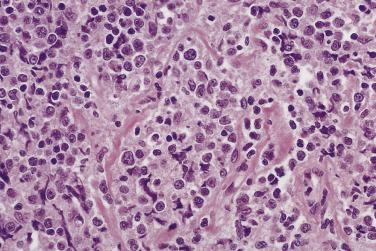
Occasionally, myeloid sarcoma (granulocytic sarcoma) presents as a mass lesion in bone. The tumor cells (myeloblasts) are positive for myeloperoxidase, lysozyme, and CD43; they stain variably for CD34 and are negative for CD3 and CD20. Thus myeloid sarcoma should be a diagnostic consideration for a CD45-positive tumor that looks like lymphoma but is negative with CD20 and CD3. Myeloid sarcoma has also been shown to express CD99, thus limiting the usefulness of this marker in distinguishing myeloid sarcoma from Ewing sarcoma.
Fibrosis elicited by the lymphoma also may lead to several diagnostic difficulties. Fibrous bands may envelop tumor cells, thus simulating an alveolar or organoid pattern, mimicking a metastatic carcinoma. Cytoplasmic clearing also may exist, another feature that can lead to confusion with metastatic adenocarcinoma. The problem is even further compounded by the fact that lymphoma and carcinoma often involve more than one bone. Fibrosis also may be associated with reactive new bone and distorted architecture, causing spindling and storiform patterns, leading to the morphologic consideration of a sarcoma ( Fig. 25.5 ). Lymphomas morphologically mimicking sarcoma usually contain an increased number of small lymphocytes, a feature that may serve as a reminder to rule out lymphoma with ancillary testing.
Ewing sarcoma is a rare primary neoplasm of bone representing approximately 6% to 8% of all malignant bone tumors. The tumor is slightly more prevalent in males than females. More than half of patients are in the second decade of life. It is unusual to see Ewing sarcoma in patients younger than 5 years. Any portion of the skeleton may be involved, but more than half of the tumors involve the long bones, usually the diaphysis or metadiaphysis. The flat bones also may be involved, especially the ilium and the ribs. Extraskeletal lesions are discussed in Chapter 24 . The patients usually present with localized pain or swelling. Pathologic fractures may occur. Some patients with Ewing sarcoma present with systemic symptoms such as fever and weight loss. Laboratory investigations may show anemia and an increased erythrocyte sedimentation rate. Taken together, these findings may lead to a mistaken diagnosis of osteomyelitis, thus delaying treatment.
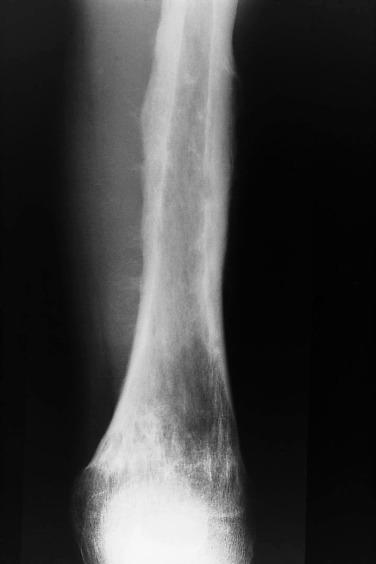
Imaging studies usually show an extensive lesion that may involve the entire bone ( Fig. 25.6 ). The tumor typically involves the shaft of the bone. The appearance is that of a permeative destructive process, similar to that seen in malignant lymphoma. However, some lesions appear as large areas of geographic destruction at presentation. Most tumors are associated with a soft tissue mass, a feature best appreciated by MRI and CT. Typically, Ewing sarcoma gives rise to prominent periosteal new bone formation. The new bone formation is in the form of multiple layers, producing an onion skin appearance.
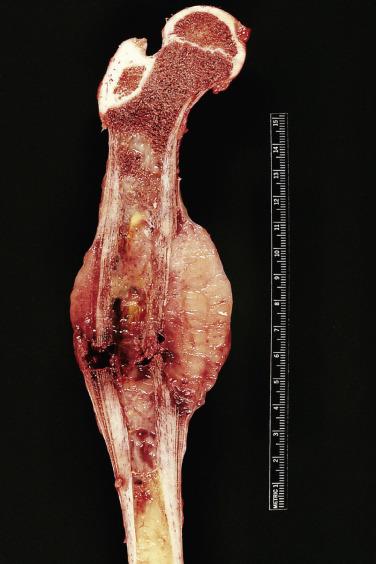
Macroscopically, Ewing sarcoma tends to be white, fleshy, and necrotic ( Fig. 25.7 ). The lesion may be so soft that it resembles pus. At biopsy, a surgeon may mistake the tumor material for pus and send all the material for microbiologic cultures.
Historically, Ewing sarcoma was classified into classic or conventional (typical) Ewing sarcoma, primitive neuroectodermal tumor (PNET), and atypical Ewing sarcoma. With the identification of recurrent chromosomal translocations, including EWSR1, among other loci, these tumors are now classified as Ewing family of tumors. However, based on molecular features some of those in the atypical Ewing sarcoma category likely fall into the group of Ewing-like sarcomas. Each subtype is considered a high-grade tumor.
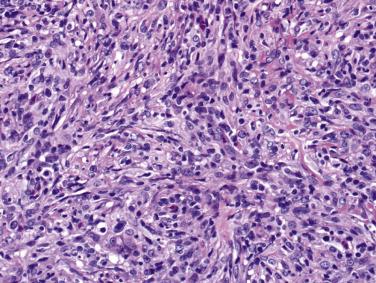
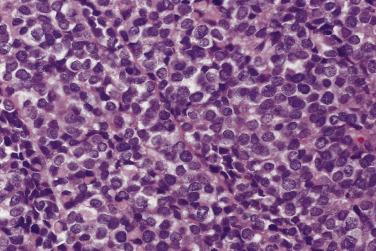
Classic Ewing sarcoma is composed of small, round, uniform cells ( Fig. 25.8 ). The nuclei are round with smudged chromatin, and the nucleoli are inconspicuous. The cytoplasmic boundaries are indistinct, with a syncytial appearance; they are arranged in a diffuse sheetlike pattern, with variable amounts of necrosis and mitotic activity. A variety of histologic variants have been described in the Ewing sarcoma family of tumors. Homer Wright rosettes with a central core of neuropil are present in approximately 10% to 15% of tumors (formerly known as primitive neuroectodermal tumors) ( Fig. 25.9 ). Another 15% to 20% of tumors display a greater degree of cytologic variability and/or unusual growth patterns; a small subset may contain areas with varying morphologic patterns, such as adamantinoma-like, vascular-like, spindle cell, and sclerosing often occurring in combination with areas of classic Ewing sarcoma.
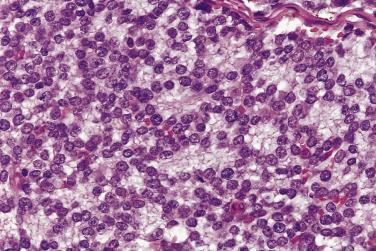
Immunohistochemical stains are essential in the diagnosis of Ewing sarcoma. As with all immunostains, they must be interpreted in combination with the histologic features. The most useful and sensitive marker is CD99. Greater than 90% of Ewing tumors express CD99 in a strong diffuse membranous pattern. Therefore, if a tumor is negative for CD99, it is highly unlikely to be Ewing sarcoma. Although CD99 is a sensitive marker for this tumor, it is not specific. Various tumors containing small round cells may be immunoreactive with this marker, including lymphoblastic lymphoma, small cell osteosarcoma, mesenchymal chondrosarcoma, myeloid sarcoma, and metastatic neuroendocrine tumors. Therefore a panel of immunostains and molecular genetic studies should be used when sorting through the differential diagnosis. FLI1 has been reported to be positive in 75% of Ewing sarcomas. However, it is also often expressed in lymphoblastic lymphoma, a tumor commonly included in the histologic differential of Ewing sarcoma. Care should also be taken when interpreting epithelial markers. Cytokeratin immunoreactivity has been reported in 20% to 40% of genetically confirmed Ewing sarcomas.
Ewing sarcoma was the first sarcoma to be associated with a recurrent chromosomal translocation. The most common is t(11;22)(q24;q12), which results in the formation of the EWSR1 - FLI1 fusion gene. This fusion gene occurs in more than 90% of cases and contains multiple splice variants. The two most common are type I ( EWSR1 exon 7 fused to FLI1 exon 6) and type II ( EWSR1 exon 7 fused to FLI1 exon 5), which together account for about 90% of EWSR1 - FLI1 fusions. Several other partner genes fused to EWSR1 have been identified in Ewing sarcoma, including ERG, ETV1, 1E1AF, FEV, among others. EWSR1FLI1 (>90%) and EWSR1 - ERG (3%–5%) are by far the most common fusion genes. Rare cases of Ewing sarcoma with FUS-ERG fusion from t(16;21) also have been reported ( EWSR1 -negative Ewing sarcomas).
A subset of Ewing-like tumors, having predominantly round cell morphology but lacking EWSR1 fusions , have been identified. The clinical and biologic significance of these newly recognized tumors, characterized by CIC or BCOR genetic alterations, including fusions with various partner loci and internal tandem duplications, are not yet fully understood. However, there is emerging evidence that the prognosis may differ among the different subtypes. In a large series evaluated by Antonescu and colleagues, CIC -rearranged sarcomas, which primarily arise in the soft tissues, had a more aggressive clinical course and poor clinical outcomes compared to EWSR1 -rearranged Ewing sarcomas.
Among the EWSR1 -negative round cell sarcomas, tumors showing BCOR-CCNB3 X-chromosomal paracentric inversion appear to preferentially involve bone and affect mainly young males. Beyond round cell morphology, BCOR -rearranged tumors can also have a distinctive fascicle-forming spindle cell component, mimicking poorly differentiated synovial sarcoma ( Fig. 25.10 ). In such cases, molecular testing for BCOR genetic rearrangements can be useful. Alternatively, an immunohistochemical BCOR stain is a useful surrogate for BCOR -rearranged tumors.
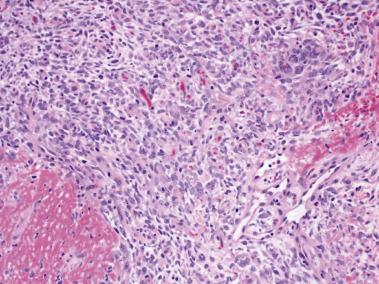
Lymphoma is the main consideration in the differential diagnosis of Ewing sarcoma involving bone. Ancillary testing, albeit molecular genetic testing or immunohistochemistry, is essential to make the distinction. Hematopoietic neoplasms do not have EWSR1 rearrangements. With the exception of lymphoblastic lymphoma, the majority of osseous lymphomas are negative for CD99 and positive for CD45 and either B-cell or T-cell markers.
Other less common considerations in the differential diagnosis include metastatic disease (e.g., carcinoma in adults and neuroblastoma in children) and other rare small cell sarcomas that occur in bone. Metastatic neuroblastoma typically occurs in children younger than 2 years of age, a very uncommon age group for Ewing sarcoma, and clinicians are usually aware of the underlying disease. CD99 can be helpful in separating carcinoma from Ewing sarcoma because both tumors can express epithelial markers. However, some neuroendocrine carcinomas may show some immunoreactivity with CD99. Mesenchymal chondrosarcoma and small cell osteosarcoma may be confused with atypical Ewing sarcoma when biopsy tissue lacks matrix as a result of sampling error.
Overall, cytogenetic in situ hybridization studies, reverse-transcriptase polymerase chain reaction (RT-PCR), or next generation sequencing methods are the most accurate means of diagnosing Ewing sarcoma. Fluorescence in situ hybridization (FISH) for EWSR1 genomic rearrangements is highly sensitive (>95%) but nonspecific because other tumors may show rearrangement of this locus. RT-PCR for EWSR1 and FUS fusion genes is highly sensitive (>95%) and specific (100%).
Patients who have localized nonpelvic disease are treated with multimodal therapeutic regimens, including induction chemotherapy and local control with surgery, radiotherapy, or a combination of both modalities. Current 5-year overall survival for patients with localized disease is 65% to 75%. Patients with metastases have a poorer 5-year survival of less than 30%. Tumor necrosis induced by primary chemotherapy is one of the most important treatment-related factors influencing prognosis. To date, no prognostic or predictive information has been associated with any specific Ewing family or Ewing-like fusion genes.
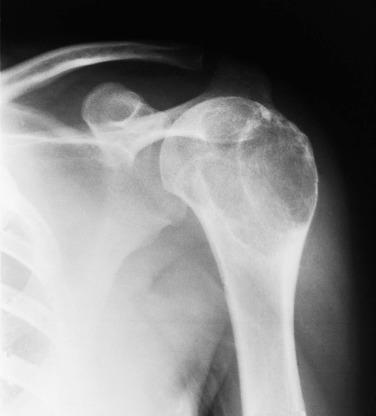
Osteochondroma accounts for approximately 35% of benign bone tumors. However, when they are small, osteochondromas are asymptomatic; hence their true incidence is likely underestimated. They most commonly occur as a solitary lesion, but approximately 15% of patients present with multiple lesions, characteristic of hereditary multiple osteochondromas syndrome, an autosomal dominant condition, resulting from germline inactivating mutations or deletions of the EXT1 and EXT2 genes and, less commonly, EXT3. Mutations in these genes lead to abnormalities in the IHh/PTHrP pathway, which controls chondrocyte differentiation and proliferation. In contrast, sporadic osteochondromas lack such mutations, but instead show homozygous deletions of EXT1 .
Osteochondromas may produce symptoms by their sheer size (by infringing on nearby structures such as nerves) or may become painful because of a fracture through the stalk. Most osteochondromas seen in surgical practice are in young patients, and there is a slight male predominance. While osteochondromas can arise anywhere in the skeleton, the metaphysis of long bones is the preferred site, and they are exceptionally rare in the hands and feet.
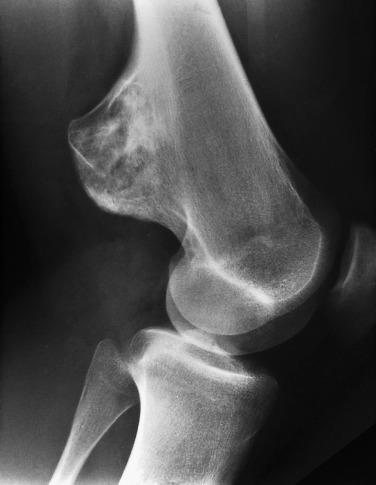
Imaging studies characteristically show a broad-based (sessile) or pedunculated osseous projection from the surface of the bone ( Fig. 25.11 ). In either case, there is a corticomedullary continuity between the underlying bone and the osteochondroma. When the lesion involves the metaphysis of a long bone, it typically grows away from the nearest joint.
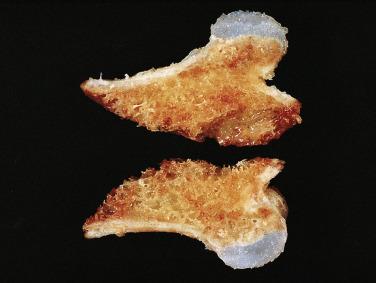
Grossly, an osteochondroma has a thin, smooth cartilage cap (typically <2 cm) and an underlying bony stalk ( Fig. 25.12 ). Microscopically, the cartilage cap contains chondrocytes in lacunae. Toward the base of the cap, the cartilage undergoes endochondral ossification to form trabecular bone. It is not unusual to see islands of residual cartilage in the middle of the bony elements within the stalk. The spaces between the bony trabeculae contain either fatty or hematopoietic marrow ( Fig. 25.13 ). Occasionally, degenerative change is present within the cartilage cap, a feature that is not indicative of malignancy.
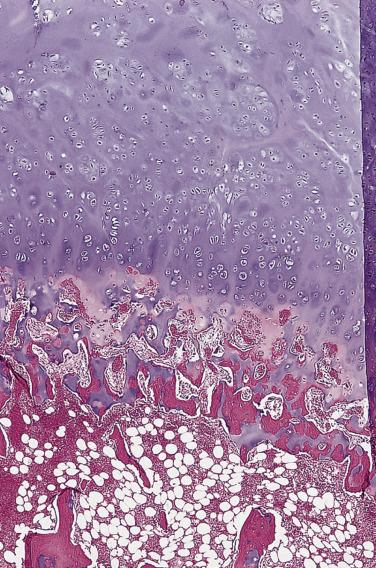
Benign lesions in the differential diagnosis include bizarre parosteal osteochondromatous proliferation (BPOP) and subungual exostosis (discussed later). Unlike osteochondromas, BPOP usually involves the small bones of the hands, and subungual exostosis typically occurs under the nail bed, especially of the great toe. The histologic resemblance of these lesions to an osteochondroma results from a combination of proliferating cartilage and maturation into trabecular bone. However, the cartilage in BPOP is frequently abundant, hypercellular with bizarre-appearing chondrocytes, and has a characteristic basophilic (blue) tinctorial quality. In contrast to osteochondroma, a cytologically bland intertrabecular spindle cell proliferation is commonly seen in both lesions.
Parosteal osteosarcoma can simulate osteochondroma, particularly because approximately 25% of parosteal osteosarcomas have a cartilage cap. Careful attention to the histologic features below the cap as well as the imaging features will lead to a correct diagnosis. The intertrabecular space within parosteal osteosarcoma is occupied by spindled cells with minimal nuclear atypia within a collagenous stroma. This differs from the stalk of an osteochondroma, in which the intertrabecular space is filled with hematopoietic marrow and fat.
The majority of osteochondromas are benign, and complete excision is typically curative. However, the cartilage cap of an estimated 1% of sporadic osteochondromas and 5% of syndrome-associated osteochondromas undergo morphologic malignant transformation into secondary peripheral chondrosarcoma (see later discussion). Most frequently, this occurs in osteochondromas arising in the pelvis or shoulder girdle.
Bizarre parosteal osteochondromatous proliferation was first described by Nora and colleagues in 1983 as an unusual reactive process involving the small bones of the hands and, less commonly, the feet. However, subsequent cytogenetic studies have shown a recurrent chromosomal translocation t(1;17)(q32;q21), supporting the clonal neoplastic nature of the lesion.
Although the majority of lesions involve the small tubular bones, approximately 25% involve the long bones. A slight female predominance is seen, and the lesion can involve patients in any age group. Radiography shows a heavily calcified mass attached to the underlying cortex by a broad base ( Fig. 25.14 ). Histologically, under low power, the lesion simulates the appearance of an osteochondroma. A large amount of cartilage may be arranged in the form of a cap or lobules. Typically, the cartilage undergoes endochondral ossification, producing bone with a characteristic dark blue tinctorial quality, especially at the interface with the cartilage ( Fig. 25.15 ). The intertrabecular spaces contain proliferating spindle cells that lack cytologic atypia. This lesion differs histologically from florid reactive periostitis and fibro-osseous pseudotumor of the digits.
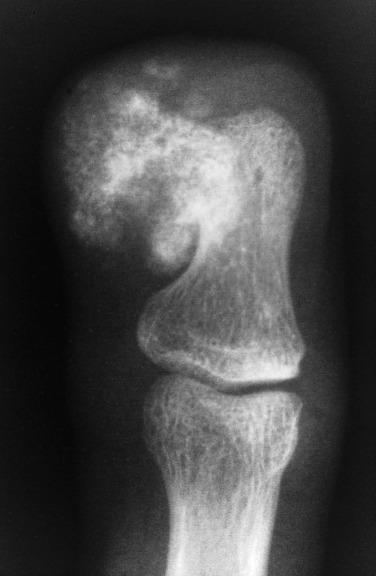
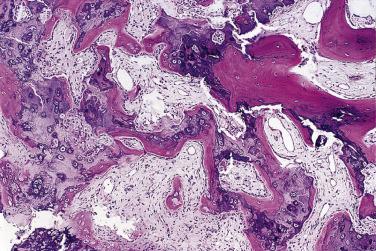
The proliferative nature of the hyaline cartilage and the presence of spindle cells between trabeculae may lead to a mistaken diagnosis of either chondrosarcoma or osteosarcoma. However, the typical radiographic appearance and the maturation of cartilage into bone with the unusual blue color should lead to the right diagnosis. Recurrences are common, but malignant transformation has never been described.
Subungual exostosis is an uncommon lesion that occurs exclusively under the nail bed, with a marked predilection for the great toe. Patients are usually in the second decade of life and present with a painful lesion that may lift off the nail and ulcerate the skin. The lesion may be black and confused clinically with a subungual melanoma. Radiographs show a mineralized lesion that is attached to the distal phalanx of the toe but lacks the corticomedullary continuity of an osteochondroma. The gross appearance may appear osteochondromatous with a thick, irregular cartilage cap and a bony stalk. Histologically, the hyaline cartilage is proliferative, with increased cellularity and frequent binucleate chondrocytes; however, orderly maturation with endochondral ossification into woven bone trabeculae occurs ( Fig. 25.16 ). The intertrabecular spaces contain proliferating spindle cells. This combination of bone, cartilage, and spindle cell proliferation may lead to a mistaken diagnosis of osteosarcoma. However, the typical location and the benign radiographic appearance suggest the correct diagnosis. Notably, a rare form of subungual melanoma that produces metaplastic bone and cartilage has been described that may be mistaken for subungual exostosis.
Cytogenetic analysis has shown that subungual exostosis is characterized by the chromosomal translocation t(X;6)(q24-q26;q15-21). This rearrangement seems to cause elevated expression of insulin receptor substrate 4 (IRS4) , likely caused by disruption of the COL12A1 locus.
The term chondroma refers to a benign cartilaginous neoplasm. The lesions may occur within the bone, in which case the term enchondroma is used; on the surface of the bone, as periosteal chondromas ; or in the soft tissues, as soft tissue chondromas .
Enchondromas are usually asymptomatic lesions, thus their true incidence is unknown. There is no sex or age predilection. Most enchondromas involve the small bones of the hands and feet. Asymptomatic enchondromas of larger bones may be incidental radiologic findings and are frequently identified on radionuclide skeletal scans in routine workup for metastatic disease. Radiographically, enchondromas are well-circumscribed lesions, confined to the medullary cavity with no significant erosion of the cortex. They typically show uniform calcification throughout the lesion ( Fig. 25.17 ).
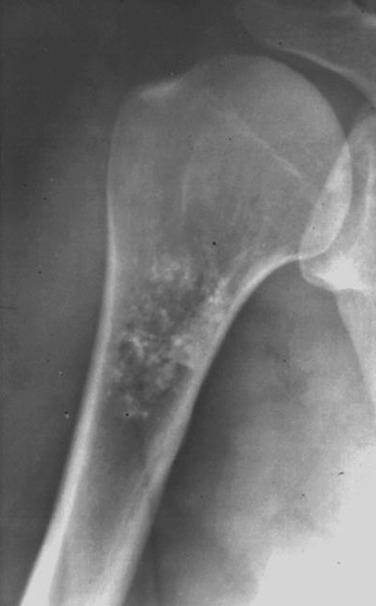
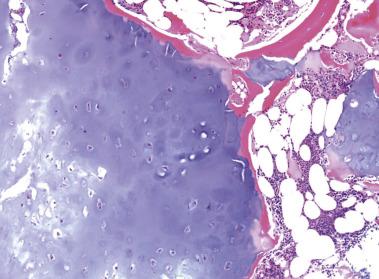
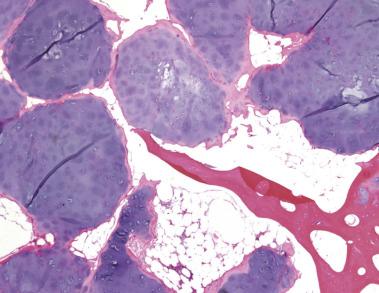
Grossly, enchondromas have the characteristic pale blue, glassy appearance of hyaline cartilage. Flecks of chalky-white calcification may be seen. Microscopically, enchondromas are lobulated, hypocellular lesions with chondrocytes arranged in clusters with abundant intercellular matrix. The matrix does not show extensive myxoid change or cyst formation. A rim of ossification at the periphery of the cartilage lobules is common (host bone encasement) ( Fig. 25.18 ). Enchondromas grow by slow expansion and compressive destruction of medullary bone and thus do not permeate between or entrap bony trabeculae ( Fig. 25.19 ). The chondrocyte nuclei are inconspicuous, small, and round with rare binucleation. The matrix lacks liquefactive myxoid change, but degenerative necrosis can be seen in older tumors.
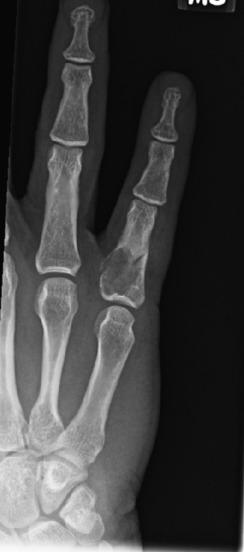
In contrast to enchondromas of the long bones, patients with enchondromas of the small tubular bones of the hands and feet often present with pain secondary to a pathologic fracture. Radiologic images show a medullary cartilaginous tumor, often with marked thinning of the overlying bony cortex ( Fig. 25.20 ). While this feature in a cartilage tumor of a larger bone might be worrisome, in a small bone, the diagnosis of chondrosarcoma should only be entertained if there is cortical breach with lesional infiltration into the soft tissues.
Grossly, enchondromas of the small tubular bones usually fill and expand the affected bone with marked thinning of the overlying cortex. Microscopically, enchondromas of the small bones are hypercellular relative to those of the long bones ( Fig. 25.21 ). Binucleate chondrocytes are frequent, and focal myxoid change may be seen. The expansile growth pattern may lead to a lobulated pushing border into surrounding soft tissue. However, only destructive permeation through the bony cortex into soft tissue and/or host bone entrapment should prompt a diagnosis of chondrosarcoma.
One of the more difficult problems in bone tumor pathology is the differentiation of an enchondroma from chondrosarcoma. When evaluating an intraosseous cartilage tumor, review of the imaging studies by an experienced radiologist is imperative. Imaging features demonstrating extensive destruction of the cortex or soft tissue infiltration by the cartilage tumor should be considered evidence of malignancy. On gross examination, a diffuse myxoid quality or cyst formation of the matrix must be considered worrisome for malignancy. Microscopically, permeation of surrounding structures, including entrapment of host lamellar bone, is probably the best single criterion to differentiate a chondrosarcoma from an enchondroma.
Enchondromas are cytogenetically characterized by simple karyotypes with frequent rearrangements of chromosomes 6 and 12. HMGA2 on chromosome 12q15 is often rearranged, but this finding has limited diagnostic value. Enchondromas frequently harbor heterozygous mutations in genes encoding isocitrate dehydrogenase (IDH1/2), an enzyme involved in the tricarboxylic acid cycle. Mutations in IDH result in aberrant production and accumulation of D2-hydroxyglutarate, a putative oncometabolite that is associated with histone and DNA hypermethylation. Somatic mosaic IDH mutations in cartilaginous tumors were initially identified in patients with Ollier or Maffucci syndrome. However, an estimated 50% to 70% of sporadic benign and malignant central cartilaginous tumors also contain IDH mutations. As they occur in both benign and malignant central and periosteal cartilaginous tumors (see upcoming discussion), IDH mutational testing has no value in the differentiation of enchondroma from chondrosarcoma.
Periosteal chondromas are rare, benign cartilaginous tumors arising on the surfaces of bone. They usually arise in the proximal humerus or the distal femur and occasionally involve the small bones of the hands and feet. Radiologic imaging shows a well-circumscribed lesion on the surface of bone, with saucerization of the underlying cortex. Periosteal chondromas are typically small lesions, measuring less than 5 cm. Macroscopically, the lesion has the typical appearance of a cartilage tumor and shows no tendency to permeate surrounding tissues. Histologically, periosteal chondromas tend to be more cellular then enchondromas of the long bones. The chondrocytic nuclei are usually hyperchromatic, and binucleated cells are common. Taken out of context, these cytologic features mimic those of a low-grade chondrosarcoma. However, in small (<5 cm), well-circumscribed periosteal chondromas, these cytologic features are not indicators of malignancy. Once again, radiologic correlation is essential
Most soft tissue chondromas occur in the hands or feet. It is extremely unusual to see benign hyaline cartilage tumors of the soft tissues at any other anatomic site. Grossly, soft tissue chondromas are lobulated and may have multiple nodules. Calcification is a common finding. Histologically, the chondrocytes are clustered but may show mild to moderate cytologic atypia. The histologic features strongly resemble those of synovial chondromatosis (see later discussion). Occasionally, soft tissue chondromas show features resembling those of chondroblastoma. Soft tissue chondromas are benign, but local recurrences are not unusual. Rearrangements of HMGA2 have been identified in soft tissue chondroma with one case harboring a HMGA2-LPP fusion. Unlike enchondromas, soft tissue chondromas do not appear to have IDH1/2 mutations.
Enchondromatosis, defined by the presence of multiple enchondromas, encompasses a number of rare syndromes categorized by the skeletal distribution of enchondromas (e.g., spinal involvement), associated clinical findings, and mode of inheritance. The tumors in enchondromatosis typically involve the ends of long bones and flat bones, may be confined to one limb or half the body, or may be bilateral. Among the enchondromatoses, Ollier disease and Maffucci syndrome are by far the most common subtypes. Both conditions are nonhereditary disorders characterized by multiple enchondromas, particularly involving the extremities; however, patients with Maffucci syndrome also present with multiple hemangiomas, including spindle cell hemangiomas, and lymphangiomas of the soft tissues, skin, or viscera. Both disorders are also associated with a spectrum of other tumor types. As mentioned earlier, somatic mosaic mutations of IDH1 and IDH2 frequently occur in these patients; approximately 80% to 90% of cartilaginous and noncartilaginous tumors from patients with Ollier disease or Maffucci syndrome have IDH1 or IDH2 mutations.
Radiographically, the metaphyseal-diaphyseal portions of the long bones appear expanded and show longitudinal striations of mineralization ( Fig. 25.22 ). Histologically, in comparison to sporadic examples, enchondromas arising in Ollier disease and Maffucci syndrome are usually hypercellular, with binucleated chondrocytes and enlarged hyperchromatic nuclei. However, in the setting of enchondromatosis, these histologic features do not denote malignancy, and the lesions may regress following skeletal maturation. Nevertheless, patients with Ollier disease and Maffucci syndrome have a higher risk of malignant transformation of one or more enchondroma(s) to secondary central chondrosarcoma, particularly in tumors affecting the long bones (see later discussion).
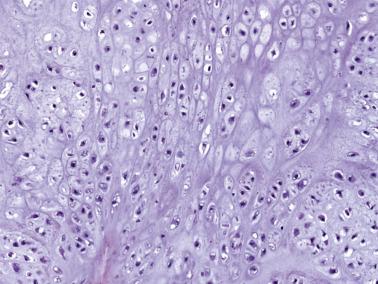
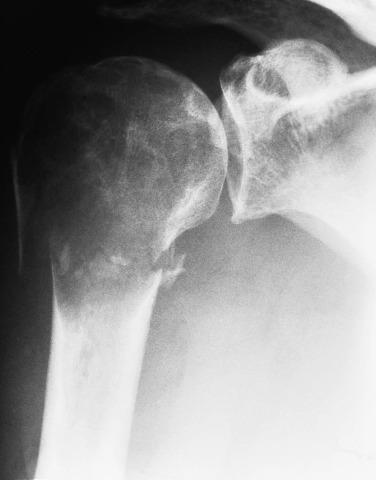
Chondroblastoma is a benign neoplasm that typically occurs in skeletally immature patients. There is a slight male predominance, and the clinical presentation is nonspecific. Chondroblastomas most commonly arise in the femur, proximal tibia, and proximal humerus; however, any skeletal site may be involved, and the calcaneus, talus, and patella are also classic sites of involvement. The incidence of chondroblastomas involving the temporal bone is lower than previously reported as several have been reclassified as tenosynovial giant cell tumors. The vast majority of chondroblastomas arise in the epiphysis or epiphyseal-equivalent apophysis (e.g., greater trochanter of the femur and tuberosity of the humerus) ( Fig. 25.23 ), although metaphyseal or diaphyseal examples have been described. The radiologic features are quite characteristic: a well-circumscribed lytic lesion, sometimes with a sclerotic border, and extensive perilesional edema. Typically, chondroblastomas have an epiphyseal epicenter, wherein over 50% extend to the metaphysis through an open epiphyseal plate, and one-third show mineralization.
Grossly, chondroblastomas tend to be white and firm with occasional macroscopic cyst formation. Microscopically, chondroblastomas are composed of a mixture of chondroblasts and variable numbers of osteoclast-type giant cells. Cytologically, the mononuclear cells have oval nuclei with longitudinal grooving, similar to the cells of Langerhans cell histiocytosis. The cytoplasmic membranes are usually distinct, and the cytoplasm is eosinophilic or clear. Scattered chondroblasts with atypical hyperchromatic nuclei can be seen but are not an indicator of malignancy. Mitoses are commonly seen, but atypical mitoses are absent.
Chondroid differentiation is a characteristic feature, forming amorphous islands of chondroid material juxtaposed to the mononuclear cell proliferation. Mature hyaline cartilage formation is rare. Notably, the oft-described pericellular chicken-wire calcification is conspicuously absent in more than half of chondroblastomas, and its absence should not preclude the diagnosis ( Fig. 25.24 ).
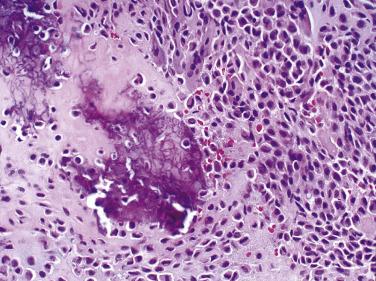
Approximately one-third of chondroblastomas undergo secondary aneurysmal bone cyst formation. This may be a focal phenomenon or a predominant feature and does not affect prognosis. Chondroblastomas are benign lesions that typically are treated with simple curettage followed by bone grafting. Local recurrence rates are low, with the exception of chondroblastomas involving the temporal bone, which can recur in up to 50% of patients. Notably, pulmonary metastasis from histologically benign chondroblastomas has been well documented in the literature, but simple surgical excision appears to be curative in the majority of cases.
The differential diagnosis of chondroblastoma chiefly includes chondromyxoid fibroma and chondrosarcoma, especially clear cell chondrosarcoma. Chondromyxoid fibromas are metaphyseal lesions, whereas chondroblastomas arise in the epiphysis. The two tumors have overlapping histologic features; however, chondroblastoma does not show the lobulation of chondromyxoid fibroma, and chondromyxoid fibroma lacks pericellular calcification. Radiologic correlation can be very helpful in difficult cases (see later discussion). In contrast, both chondroblastoma and clear cell chondrosarcoma are epiphyseal tumors. However, clear cell chondrosarcoma usually occurs in skeletally mature patients with closed growth plates and often has areas of conventional chondrosarcoma.
Chondroblastomas very often show cytogenetic abnormalities involving chromosomes 5 and 8. Recently, mutations in H3F3A/B , encoding the histone 3.3 protein, have been identified as frequent events in chondroblastoma, with the vast majority of cases harboring H3F3B mutations. Most commonly, these H3F3 mutations result in the p.K36M amino acid substitution, and immunohistochemical testing with a H3K36M mutant antibody has a high sensitivity and specificity for chondroblastoma.
Chondromyxoid fibromas are extremely unusual and occur only half as frequently as chondroblastomas. Patients are usually in the second or third decade of life. These lesions are most frequently located in the metaphysis of long bones, particularly the proximal tibia and distal femur. The small bones of the feet and ilium are also commonly involved. Occasional lesions are juxtacortical and extend into soft tissue. They can be especially difficult to recognize when located at uncommon sites such as the craniofacial bones. Radiographic images show an extremely well-circumscribed, purely lytic defect involving the metaphysis. The lesion usually has a sclerotic border, which may be undulating and give rise to a scalloped appearance ( Fig. 25.25 ).
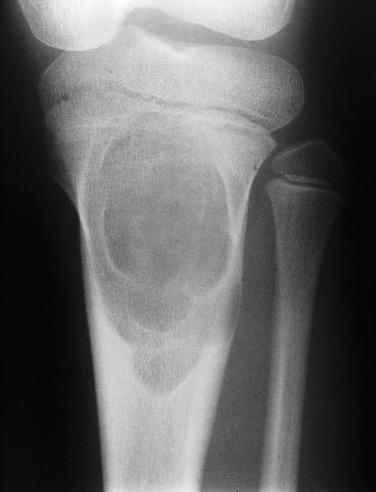
Macroscopically, chondromyxoid fibromas tend to be grayish white, well circumscribed, lobulated, and may appear to “pop out” of the surrounding bone. Microscopically, chondromyxoid fibromas form variably sized lobules ( Fig. 25.26 ), which are centrally hypocellular, with increasing cellularity toward the lobule periphery, imparting the appearance of hypercellularity between adjacent lobules. The chondromyxoid stroma is basophilic, and coarse calcification occurs in a subset of cases, but liquefactive myxoid degeneration is unusual. Mature hyaline cartilage differentiation is not usually seen in chondromyxoid fibroma. Secondary aneurysmal bone cyst changes may be seen but are not as common as in chondroblastoma. Cytologically, the cells are spindle or stellate shaped. While they may be enlarged or hyperchromatic, these features are not indicators of malignancy. In cellular examples, the cytologic features can resemble those of chondroblastoma.
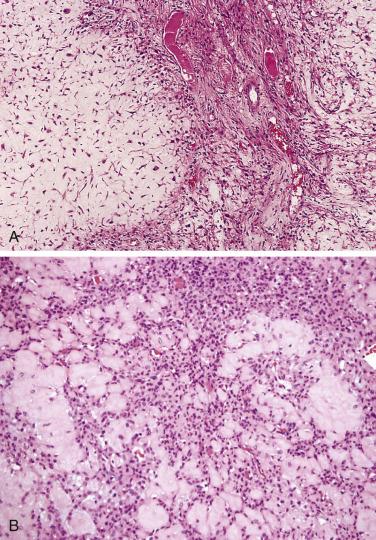
Cytogenetic analysis has shown that these lesions often have rearrangements of chromosome 6. More recent work has shown rearrangement of the glutamate receptor GRM1 with several gene partners in a promoter swapping mechanism, leading to the overexpression of the intact protein.
The differential diagnosis chiefly includes chondroblastoma, phosphaturic mesenchymal tumor, conventional chondrosarcoma with myxoid features, and chondromyxoid fibroma-like osteosarcoma. Chondrosarcomas almost always have an aggressive-looking radiographic appearance, whereas chondromyxoid fibromas always have a benign radiographic appearance. Histologically, in contrast to chondromyxoid fibroma, the lobules of chondrosarcoma are uniformly hypercellular, typically lack osteoclastic giant cells, and show an infiltrative growth pattern. Similarly, chondromyxoid fibroma-like osteosarcoma has an aggressive radiologic appearance and microscopic features of an infiltrative tumor with cytologic atypia beyond what is acceptable in a chondromyxoid fibroma.
Chondroblastomas usually do not show the distinct lobulation of chondromyxoid fibroma. However, in some cases, or with minimal tissue, the distinction may be very difficult. In these cases, radiologic correlation can be helpful. Moreover, as H3F3A/B mutations have not been identified in chondromyxoid fibromas, by immunohistochemistry the tumors are negative for H3K36M.
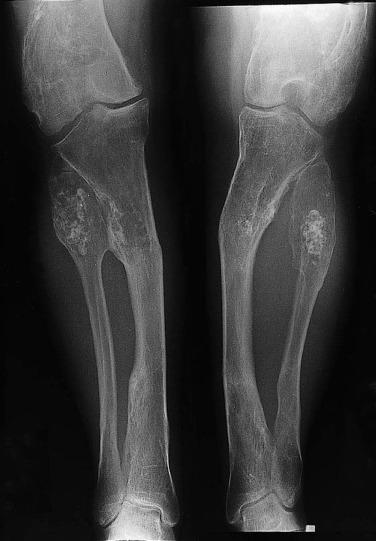
Chondrosarcoma is a common primary sarcoma of bone, second only to osteosarcoma. By the current WHO classification, conventional chondrosarcoma is subclassified by its location on or in bone (central/intraosseous, periosteal, or peripheral chondrosarcomas), whether it arises de novo (primary chondrosarcoma) or within a preexisting lesion (secondary chondrosarcoma) and the presence of dedifferentiation. Other rare subtypes of chondrosarcoma include clear cell chondrosarcoma, dedifferentiated chondrosarcoma, and mesenchymal chondrosarcoma, which will be considered separately.
Conventional central chondrosarcomas comprise the largest group and are defined as malignant hyaline cartilage-producing tumors without neoplastic osteoid matrix production, arising within the bone. Conventional chondrosarcomas typically occur in the shoulder and pelvic girdles; however, they can arise at any skeletal site. Patients with chondrosarcoma are usually in the fourth to sixth decades of life and present with long-standing or progressing pain at the affected site. Pediatric chondrosarcomas are rare. Pathologic fracture is unusual.
Radiographically, chondrosarcomas are more aggressive and destructive appearing than enchondromas. They typically demonstrate significant endosteal scalloping, cortical thickening, cortical destruction, and periosteal reaction ( Fig. 25.27 ). Although present in only a fifth of cases, the combination of expansion of bone and thickening of the cortex is highly suggestive of chondrosarcoma. The tumor usually shows heterogeneous calcific densities. Calcific densities in a benign cartilage tumor are usually evenly distributed within the lesion. When a cartilage tumor shows lucent areas and areas of mineralization, chondrosarcoma should be considered. Magnetic resonance imaging can delineate the extent of medullary involvement and soft tissue extension.
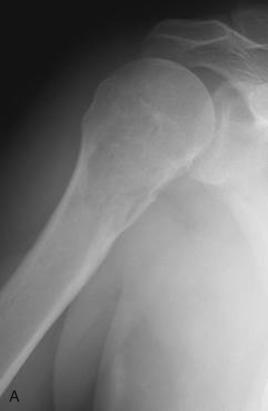
Chondrosarcomas usually have a characteristic macroscopic appearance; they are composed of blue-white hyaline cartilage with gritty white calcification. Liquefied, oozing, or mucoid-appearing lesions, often with cystic areas, are additional gross features ( Fig. 25.28 ).
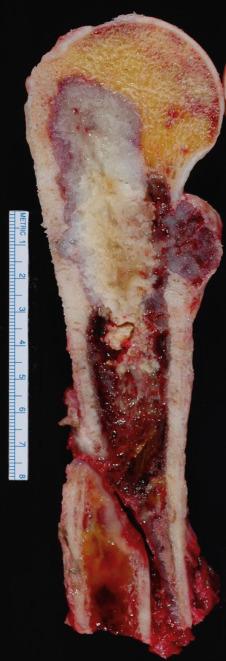
The microscopic appearance of chondrosarcoma varies, depending on the histologic grade of the neoplasm. As described earlier, low-grade chondrosarcomas can be difficult to differentiate from enchondromas. At low magnification, low-grade chondrosarcomas are more cellular than enchondromas. They tend to fill the marrow spaces and permeate preexisting bone, entrapping bony trabeculae ( Fig. 25.29 ). In curettage specimens, artifactual pseudoentrapment of bone can be caused due to tissue fragmentation, and is a common pitfall. True entrapment requires circumferential entrapment of the bone trabeculae by neoplastic cartilage. The nuclei of chondrocytes in enchondroma are small, round, and regular; in chondrosarcomas, the nuclei are larger and may in fact be less densely stained than the benign cells. Chondrocytes are located within lacunae and do not generally show spindled morphology. Binucleated cells may be found. Extensive myxoid change usually manifests as a grainy, stringy, or bubbly appearance to the matrix ( Fig. 25.30 ). Many authors have pointed out the difficulty in differentiating a low-grade chondrosarcoma from an enchondroma. The cytologic changes (cellularity and atypia), myxoid quality of the matrix, and permeative characteristics are important. The presence of myxoid matrix degeneration in more than 20% of the lesion or host bone entrapment favors a diagnosis of chondrosarcoma over enchondroma. The diagnosis also relies heavily on the radiologic interpretation, and the anatomic site provides an additional clue. Large cartilaginous tumors in the axial skeleton are likely to be chondrosarcomas, whereas tumors involving the small bones of the hands and feet are much more likely to be benign.
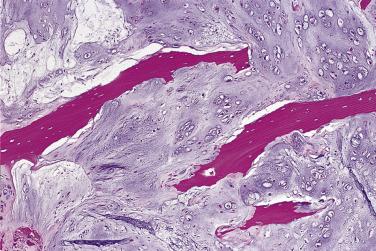
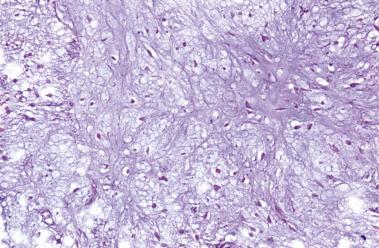
The most important predictor of biologic behavior in chondrosarcoma is histologic grade. Several different grading systems have been suggested for chondrosarcoma. Historically, they have been graded on a scale of 1 to 3. However, in the current WHO classification system, grade 1 chondrosarcoma is designated as atypical cartilaginous tumor/chondrosarcoma grade 1 due to its negligible potential for metastasis. Furthermore, this term was placed into the biologic potential group of an intermediate (locally aggressive) category rather than malignant.
Grading is assessed by tumor cellularity, nuclear size, and nuclear hyperchromasia. The role of mitotic activity in grading chondrosarcomas is debatable. The majority of chondrosarcomas are either grade 1 or 2 tumors. Atypical cartilaginous tumor/grade 1 chondrosarcomas show an overall increased cellularity compared with enchondromas. The chondrocytes are enlarged and more irregular. Grade 2 chondrosarcomas show even more cellularity than grade 1 chondrosarcomas, and the nuclear atypia is more pronounced ( Fig. 25.31 ). Grade 3 chondrosarcomas show marked cellularity, marked pleomorphism of the nuclei, and some spindling at the periphery of the lobules ( Fig. 25.32 ).
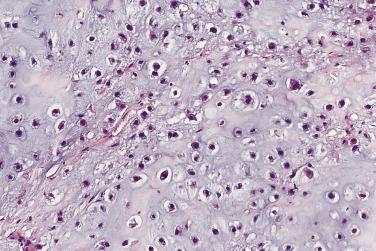
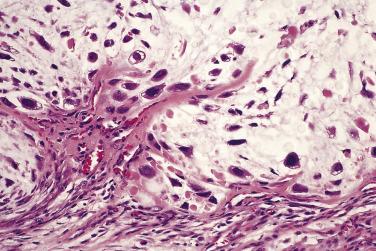
While the distinction between enchondroma and grade 1 chondrosarcoma is often difficult, it does not carry the prognostic significance attached to separating grade 1 from grade 2 tumors. In a large retrospective study of patients with chondrosarcoma from the Netherlands Cancer Registry, the 5- and 10-year survival rates were, respectively, 93% and 88% for ACT/CS I, 74% and 62% for grade 2 CS, and 31% and 26% for grade 3 CS. Similarly, Björnsson and colleagues found a 5-year survival rate of 89% for grade 1 chondrosarcoma and 57% for the combined group of patients with grade 2 and 3 tumors. Intralesional curettage with adjuvant local therapy (i.e., intralesional cryotherapy, phenol, liquid nitrogen, or argon-beam laser) has emerged as an effective treatment for tumors of the long bones classified as Grade 1 chondrosarcomas or atypical/borderline tumors, provided the tumors show no radiologic evidence of aggression (i.e., cortical permeation or soft tissue extension). In contrast, patients with higher grade chondrosarcomas of the extremities or chondrosarcoma of any grade involving the axial skeleton are usually treated with en bloc resection. Chondrosarcomas do not respond to chemotherapy. Local recurrence rates vary from 15% to 28%, depending on the type of surgical procedure. On occasion, recurrent tumor is of a higher histologic grade.
Although the majority of cartilage lesions of the small bones of the hands and feet are benign, chondrosarcomas do occur at this anatomic site. However, the criteria for malignancy of a cartilage tumor of the small bone differ from those for a larger bone. As previously mentioned, enchondromas of small bones frequently thin the cortex, and histologically benign tumors have increased cellularity and mild cytologic atypia. Thus extensive myxoid degeneration of the stroma and unequivocal radiologic or histologic evidence of tumoral cortical permeation and soft tissue infiltration are the main features for diagnosis of chondrosarcoma in a small bone. Although the prognosis for patients with these tumors is generally very good, metastasis does occur rarely.
Chondrosarcomas often show complex and nonspecific genetic abnormalities. With regard to IDH mutational testing, as both benign and malignant central and periosteal cartilage tumors frequently harbor IDH1/2 mutations, testing is not useful to support a diagnosis of malignancy. However, in most series to date, chondroblastic osteosarcomas lack IDH mutations. Thus IDH mutational testing may be of use in distinguishing a high-grade or dedifferentiated chondrosarcoma with an osteosarcomatous component from a chondroblastic osteosarcoma.
Most chondrosarcomas arise de novo in bone as primary chondrosarcomas. However, a small percentage of chondrosarcomas arise in the presence of a preexisting lesion and are designated secondary chondrosarcomas. In the Mayo Clinic files, approximately 14% of chondrosarcomas were considered secondary tumors. Secondary chondrosarcomas arising within a surface lesion (i.e., osteochondroma) are peripheral secondary chondrosarcomas, whereas those arising within enchondromas are central secondary chondrosarcomas. This latter event occurs most frequently in the context of Ollier disease or Maffucci syndrome.
Approximately 1% of solitary and up to 5% of multiple osteochondromas undergo malignant transformation to a chondrosarcoma, usually low grade. Rarely, osteochondroma undergoes malignant transformation into a dedifferentiated chondrosarcoma (dedifferentiated peripheral chondrosarcoma).
When malignant transformation does occur in osteochondroma, it develops earlier in patients with hereditary multiple osteochondromas syndrome (average 25–30 years) than those with sporadic osteochondroma (average 50–55 years). Generally, a previously quiescent osteochondroma that starts to grow in adulthood should raise suspicion of chondrosarcoma.
As most peripheral secondary chondrosarcomas are extremely well differentiated, the histologic diagnosis is difficult, and radiologic correlation is essential. Worrisome radiographic findings include an irregular or indistinct lesional surface, focal areas of radiolucency within the mass, erosion or destruction of adjacent bone, and a soft tissue mass with scattered or irregular calcifications. In skeletally mature patients, osteochondromas typically have a smooth, thin cartilage cap that measures less than 2 cm in maximal thickness. When chondrosarcomatous transformation occurs in the cap, the cartilage cap thickens and develops indistinct borders, often with significant myxoid and cystic change, and infiltration of cartilaginous nodules into the surrounding soft tissue. In early malignant transformation, measurement of the cartilage cap can be very helpful: A cap thickness exceeding 2 cm should prompt consideration of chondrosarcoma ( Fig. 25.33 ). Low-grade secondary peripheral chondrosarcomas are usually treated with en bloc surgical resection. Adjuvant therapy is typically not indicated. The prognosis in secondary peripheral chondrosarcoma is generally good but depends on the anatomic site and histologic grade. Rarely, secondary peripheral chondrosarcomas are dedifferentiated tumors; they have different clinicopathologic and treatment considerations and are included in the discussion of dedifferentiated chondrosarcoma.
Patients with multiple enchondromas in the setting of Ollier disease or Maffucci syndrome have a markedly elevated risk of malignant transformation, presumably in part due to their increased numbers of enchondromas. Patients with Ollier disease have an estimated 40% lifetime cumulative risk of chondrosarcomatous transformation, while the risk is 50% for patients with Maffucci syndrome. Due to the hypercellularity and mild cytologic atypia of enchondromas seen in Ollier disease and Maffucci syndrome, the histologic diagnosis of low-grade chondrosarcoma may be difficult. Radiologic images show destruction and invasion. Marked myxoid change of the matrix and invasion of surrounding tissue should be considered signs of malignancy.
Chondrosarcomas arising de novo on the surface of a bone are extremely unusual and are classified as periosteal chondrosarcoma. These tumors tend to be large (>5 cm), and size is an important criterion in distinguishing periosteal chondromas from periosteal chondrosarcomas. The most common sites of involvement include the metaphysis of the distal femur and proximal humerus. Radiologically, periosteal chondrosarcomas are large, poorly defined, surface-based cartilaginous tumors with heterogeneous calcification. Histologically, the tumors frequently show myxoid change and intramedullary extension (26%) with concomitant host bone entrapment (40%). With regard to molecular genetics, similar to other cartilaginous tumors, in a series of 38 periosteal chondrosarcomas, IDH1 mutations were found in 15% of cases. Rarely, these tumors metastasize to the lungs.
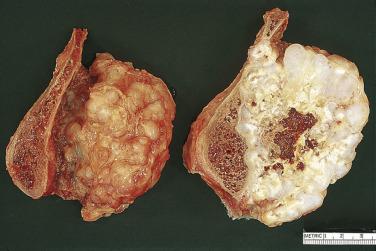
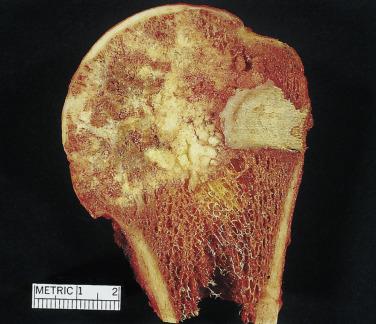
Clear cell chondrosarcoma is an unusual chondroid neoplasm, representing approximately 2% of all chondrosarcomas. The lesion tends to occur at the ends of long bones, similar to chondroblastomas and giant cell tumors ( Fig. 25.34 ). The radiographic appearance may mimic that of a chondroblastoma in that the lesion is usually well circumscribed and may even have a sclerotic border. Several years may pass before the radiographic appearance suggests malignancy. Grossly, clear cell chondrosarcomas are composed of hyaline cartilage with gray fleshy firm areas and cystic components ( Fig. 25.35 ).
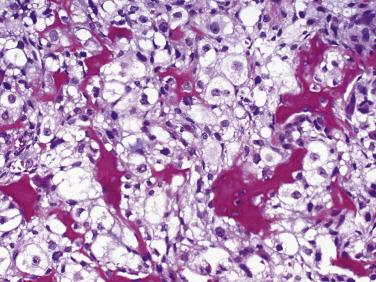
Microscopically, clear cell chondrosarcomas are lobulated tumors. Osteoclastic giant cells are usually found at the edge of the lobules, which would be an unusual finding in conventional chondrosarcoma. The center of the lobule usually shows osteoid or bone formation. The tumor cells have well-defined cytoplasmic borders and a centrally placed round nucleus ( Fig. 25.36 ), often containing prominent nucleoli. Approximately 50% of clear cell chondrosarcomas show areas of conventional chondrosarcoma. Aneurysmal bone cystlike changes are often found in clear cell chondrosarcoma and can be so prominent that residual tumor forms only a small mural nodule. The clinical behavior of clear cell chondrosarcoma is typically that of a low-grade chondrosarcoma. IDH1/2 mutations have not been found in these tumors. Only a few rare examples of dedifferentiated clear cell chondrosarcoma have been described.
Dedifferentiated chondrosarcoma was first delineated as a distinct clinicopathologic entity in 1971. Dedifferentiated chondrosarcoma occurs in older adults, often presenting a decade later than patients with conventional chondrosarcoma. Although most dedifferentiated chondrosarcomas arise from conventional central chondrosarcomas, they also may occasionally form as a secondary peripheral (dedifferentiated) chondrosarcoma arising in a preexisting osteochondroma.
Dedifferentiated chondrosarcoma arises at the same skeletal sites as conventional chondrosarcoma. Patients may report symptoms for several years and then have a sudden increase in the number or intensity of symptoms. The imaging studies usually show classic features of chondrosarcoma with a juxtaposed, destructive-appearing lytic area ( Fig. 25.37 ). The presence of a large unmineralized soft tissue mass, usually picked up on MRI or CT scans, associated with an intraosseous cartilage tumor is highly suspicious for dedifferentiated chondrosarcoma. However, in one-third of dedifferentiated chondrosarcomas, radiologic studies do not identify a dedifferentiated component. Nevertheless, for patients with radiologic features suggestive of dedifferentiation, targeted biopsy of these areas is strongly encouraged to facilitate appropriate patient management.
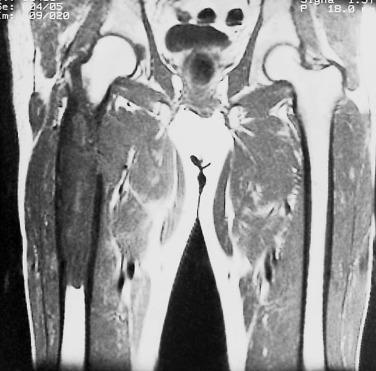
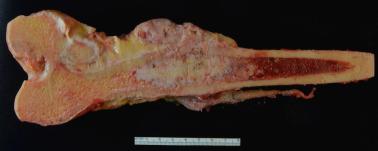
Grossly, dedifferentiated chondrosarcomas have a bimorphic appearance; they are composed of a low-grade chondrosarcoma component with nodules of glassy, pale blue cartilage juxtaposed to a soft, fleshy sarcoma-like tumor ( Fig. 25.38 ). Similarly, the histologic features are those of a distinctly bimorphic tumor. The well-differentiated chondrosarcoma component is usually low grade, although grade 2 chondrosarcomas with dedifferentiation are seen in a quarter of cases. These areas show an abrupt transition to a high-grade sarcoma ( Fig. 25.39 ). Most often, the dedifferentiated component resembles a high-grade spindle cell sarcoma, undifferentiated pleomorphic sarcoma, or osteosarcoma. Rarely, it may show features of rhabdomyosarcoma, leiomyosarcoma, angiosarcoma, adamantinoma-like or epithelial differentiation.
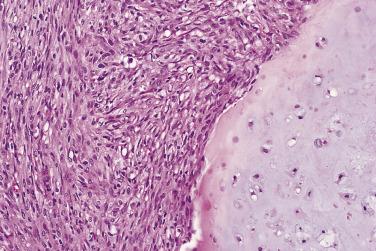
Dedifferentiated chondrosarcoma must be distinguished from chondroblastic osteosarcoma. Chondroblastic osteosarcoma usually affects adolescents, whereas dedifferentiated chondrosarcoma involves older adults. In chondroblastic osteosarcoma, the cartilaginous component has high-grade cytology and merges into a spindle cell sarcoma. In contrast, in dedifferentiated chondrosarcoma, the cartilaginous component is typically well differentiated, and the spindle cell malignancy is abruptly juxtaposed to it rather than merging into it. This distinction is important because the prognosis and management protocols differ between dedifferentiated chondrosarcoma and chondroblastic osteosarcoma. It is important to keep dedifferentiated chondrosarcoma in the differential diagnosis of a biopsy sample in an adult patient who shows a high-grade sarcoma. Imaging studies are often helpful in determining if there is an underlying cartilaginous component. Sarcomatoid carcinoma is also in the differential diagnosis when a biopsy specimen contains only the high-grade sarcoma component. Notably, immunohistochemical detection of cytokeratins is not always helpful, because a significant subset of the spindled cells in dedifferentiated chondrosarcomas show robust immunoreactivity with cytokeratin antibodies. Therefore, radiologic correlation should always be performed in the evaluation of malignant spindle cell lesions involving bone. IDH mutational testing may also be helpful because IDH1/2 mutations frequently occur in dedifferentiated chondrosarcoma and are absent in other types of sarcoma, including chondroblastic osteosarcoma.
Patients with dedifferentiated chondrosarcoma have a poor prognosis with 5-year survival rates ranging from 7% to 24%. Radiation and chemotherapy have not been shown to improve prognosis.
Originally described in 1959, mesenchymal chondrosarcoma primarily affects adolescents and young adults, with an equal sex distribution. About one-third of mesenchymal chondrosarcomas occur as primary soft tissue tumors or arise in the meninges, and they show similar clinicopathologic features to their osseous counterparts. In bone, mesenchymal chondrosarcoma tends to involve the axial skeleton, particularly the craniofacial bones, spine, and ribs. Rare examples of periosteal mesenchymal chondrosarcoma have also been described. Radiologically, the features are nonspecific and show a malignant-appearing tumor, with or without mineralization. Grossly, the lesion is usually pink and fleshy but may show foci of calcification.
The microscopic appearance of mesenchymal chondrosarcoma shows a characteristic bimorphism composed of islands of a well-differentiated cartilaginous component, resembling a low-grade chondrosarcoma, admixed with a primitive small cell component ( Fig. 25.40 ). The relative abundance of each component varies considerably. This variation can be a diagnostic pitfall in the evaluation of limited tissue.
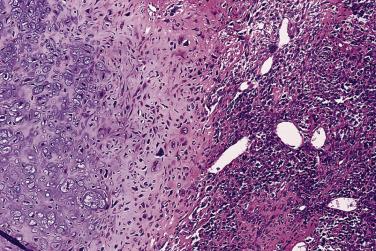
Foci of ossification and calcification within the cartilage may be seen. The primitive appearing small cells have hyperchromatic nuclei. While they are usually round-to-ovoid, areas of frank spindling may be found in mesenchymal chondrosarcoma. Characteristically, the small cells are arranged around variably gaping, staghorn-shaped vascular spaces in a hemangiopericytoma-like pattern ( Fig. 25.41 ).
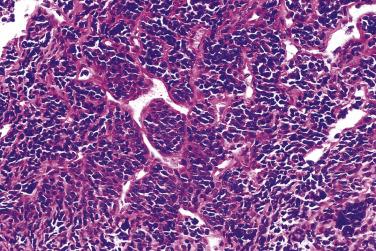
The differential diagnosis includes other small cell malignancies, such as Ewing sarcoma, and lymphoma, particularly when the cartilaginous component is not evident because of sampling error. Potential misdiagnosis of Ewing sarcoma is exacerbated by the fact that more than 90% of mesenchymal chondrosarcomas show strong, diffuse membranous immunoreactivity for CD99. Alternatively, FLI1 is positive in 75% of Ewing sarcomas, and mesenchymal chondrosarcoma is negative. Sox9 has been shown to be a helpful marker in the differential diagnosis with other small round cell tumors. Areas of ossification can be seen in mesenchymal chondrosarcoma, so it is important to pay close attention to the cytologic features of the tumor cells and consider ancillary testing when the differential diagnosis includes osteosarcoma.
The identification of a recurrent chromosomal translocation in mesenchymal chondrosarcoma has greatly facilitated its diagnosis. Until quite recently, only a few reports with cytogenetic data on mesenchymal chondrosarcoma had been published, most of them showing complex cytogenetic alterations, including identical robertsonian translocation t(13;21)(q10;q10) in a few cases. However, a genome-wide screen of exon-level expression data identified the novel HEY1 - NCOA2 gene fusion as a recurrent event in these tumors. NCOA2 is a member of the p160 nuclear hormone receptor transcriptional coactivator family that facilitates chromatin remodeling and transcription of nuclear receptor target genes. HEY1 seems to participate in the regulation of bone morphogenetic protein 9 (BMP9)–induced osteoblastic-lineage differentiation of mesenchymal stem cells. Future studies are needed to decipher the molecular events driven by this gene fusion. The HEY1 - NCOA2 fusion can be detected with molecular genetic techniques in most cases and is a valuable tool for the diagnosis of mesenchymal chondrosarcoma. More recently, a novel fusion IRF2BP2-CDX1 has been described in an example of mesenchymal chondrosarcoma with t(1;5).
The prognosis in mesenchymal chondrosarcoma is unpredictable, with published 10-year overall survival ranging from 21% to 54%. While a subset of patients presents with disseminated metastasis and dies within months, other patients have a protracted clinical course. Improved survival has been reported in patients who receive chemotherapy.
As the name implies, the vast majority of extraskeletal myxoid chondrosarcomas arise in the soft tissues. On rare occasions, extraskeletal myxoid chondrosarcomas occur as a primary osseous sarcoma. The histologic, immunohistochemical, and molecular genetic features of the tumoral subset arising in bone are identical to those arising in soft tissue (see Chapter 24 for discussion). The histologic differential diagnosis when it occurs as a primary in bone includes metastatic mucinous adenocarcinoma, myoepithelioma, epithelioid hemangioendothelioma, and chordoma.
Extraskeletal myxoid chondrosarcoma is characterized by the EWSR1 - NR4A3 fusion gene as a result of the t(9;22)(q12;q22). The fusion gene is present in approximately 75% of cases. As is often seen in chimeric transcripts involving EWSR1, the transactivation domain of EWSR1 is fused to the DNA binding domain of NR4A3 . NR4A3 is an orphan nuclear receptor that can activate the FOS promoter and plays a poorly understood role in the regulation of hematopoietic growth and differentiation. Alternate NR4A3 gene partners include TAF2N, t(9;17)(q22;q11), TCF12, t(9;15)(q22;q21), and TFG gene on chromosome 3q11-q12.
FISH with break-apart probes for the EWSR1 locus may be used as a diagnostic option in the appropriate histologic context, but as a subset of myoepitheliomas has EWSR1 rearrangements, a NR4A3 break-apart probe is a more specific approach for extraskeletal myxoid chondrosarcoma. RT-PCR is a less attractive option because of the large number of fusion partners.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here