Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The World Health Organization (WHO) classification of tumors of lung and pleura is commonly referenced in pathology practice. The fourth edition, published in 2015, was heavily influenced by joint recommendations from the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society. These recommendations represent a global perspective of pathologists, radiologists, oncologists, surgeons, epidemiologists, and molecular geneticists and aim to create a more specific and reproducible tumor classification system. This chapter will reflect the system put forth by the WHO, with an emphasis on important predictive biomarkers in lung cancers, known genotype-phenotype correlates across lung neoplasms, and immunophenotypic approaches to lung cancer subclassification.
In the United States, about 225,000 people are diagnosed with and 160,000 die annually from lung cancer. There are an estimated 1.8 million new lung cancer diagnoses per year worldwide, with the highest incidence in North America, Northern and Eastern Europe, and Turkey. Only 16% of patients have disease confined to the lung at the time of diagnosis. Estimated 5-year survival is approximately 18% and is strongly dependent upon stage of disease at diagnosis, ranging from 55% for patients with localized disease to 4% for patients with distant metastases. Survival rates are expected to improve with the routine use of immunotherapy in patients with advanced disease. The incidence of lung cancer is two- to threefold higher in men than in women, across all subtypes. In the United States, the median age of diagnosis of lung cancer is 70, and the median age of death due to lung cancer is 72. Lung cancer incidence and mortality have declined in men since the early 1990s, whereas in women both incidence and mortality increased significantly from the 1970s to the early 2000s; only in the last decade has this trend reversed. Smokers have a tenfold increased incidence of lung cancer; adenocarcinoma, however, is significantly associated with lower median pack years of smoking (41.5 versus 61.8 pack years for other histologies); in former smokers, adenocarcinoma tends to present after a greater lag time than other lung cancer types.
Lung cancers have been historically categorized as non–small cell and small cell carcinoma, based on some distinctive clinical characteristics and treatment regimens proven to have efficacy in the two groups. This simple dichotomy is inadequate, however, given current treatment guidelines requiring the parsing of non–small cell lung carcinomas (NSCLCs) into squamous and nonsquamous subtypes when possible. This classification is essential for identifying patients who should undergo molecular testing for selection of targeted therapies against EGFR, BRAF, ALK, and ROS1 alterations, as these occur almost exclusively in lung adenocarcinomas. In addition, the choice of chemotherapeutics is influenced by tumor histology: the antimetabolite pemetrexed is more effective in adenocarcinoma and large cell carcinoma (LCC) than squamous cell carcinoma, and the antiangiogenic agent bevacizumab is contraindicated in patients with squamous cell carcinoma in which it has been linked to fatal pulmonary hemorrhage.
The clinical presentation of lung carcinoma reflects site of origin in the central airways or periphery of the lung. Increasingly, however, tumors may be detected on radiographic screening studies or incidentally during workup for other indications. Low-dose computed tomography (CT) screening of high-risk populations has significantly increased rate of detection of early stage cancers, with a concomitant improvement in clinical outcomes. Detection of early lung cancers has significant implications for pathologists, and the diagnosis and classification of in situ and early stage adenocarcinomas have faced renewed focus in the latest WHO classification scheme.
The current trend of minimally invasive sampling, including transthoracic core needle biopsies, endobronchial ultrasound-guided biopsies, and fine needle aspirations, reduces the morbidity of surgical biopsies but risks generating inadequate tissue for diagnosis and molecular testing. Indeed, half or more of lung cancer diagnoses are rendered on small biopsy or cytology specimens, often with limited tumor content. Morphologic features that serve as the foundation of pathologic classification are often lacking. Revised approaches that depend heavily on immunophenotype have led to the creation of diagnostic language that reflects morphologic ambiguity, that is, non–small cell lung carcinomas that favor adenocarcinoma or favor squamous cell carcinoma in the case of carcinomas lacking glandular or squamous differentiation but showing nuclear TTF-1 or p40 expression, respectively. Indeed, specific terminology that acknowledges the morphologic ambiguity that results from limited sampling has been endorsed in the WHO 2015 classification ( Table 5.1 ). Current guidelines also recommend judicious use of immunohistochemistry at the time of diagnosis to conserve materials for predictive molecular (EGFR, BRAF, ALK, ROS1) and immunohistochemical (PD-L1) testing.
| Diagnosis on Small Biopsy/Cytology Specimens After Immunohistochemistry Evaluation, When Appropriate | WHO 2015 Classification for Resection Specimens |
|---|---|
| Adenocarcinoma with lepidic pattern | Adenocarcinoma in situ |
| Adenocarcinoma with lepidic or predominantly lepidic pattern (list all identified patterns) | Minimally invasive adenocarcinoma or lepidic-predominant adenocarcinoma |
| Non–small cell carcinoma (NSCC), not otherwise specified (NOS), favor adenocarcinoma | Solid adenocarcinoma |
| NSCC, NOS, favor squamous cell carcinoma | Nonkeratinizing squamous cell carcinoma |
| NSCC, NOS, with a note that both squamous cell and adenocarcinoma components are present, possibly representing adenosquamous carcinoma | Adenosquamous carcinoma (both components ≥10%) |
| NSCC, NOS | Large cell carcinoma |
| NSCC with neuroendocrine morphology and positive neuroendocrine markers, possible large cell neuroendocrine carcinoma | Large cell neuroendocrine carcinoma |
| NSCC with spindle cell and/or giant cell carcinoma with a note if squamous cell or adenocarcinoma is present |
Pleomorphic, spindle cell or giant cell carcinoma |
| NSCC with a sarcomatous component with a note to specify the heterologous elements and to mention the presence of squamous cell or adenocarcinoma |
Carcinosarcoma |
Adenocarcinoma is the most common type of lung carcinoma, comprising an estimated 45% of cases diagnosed in men and 55% of those diagnosed in women. When compared with other types of lung carcinoma, adenocarcinoma is associated with better clinical outcomes (22% to 29% 5-year relative survival versus 18% overall). In the mid-20th century, squamous cell carcinoma outnumbered adenocarcinoma diagnoses at a ratio of nearly 20 to 1; by the end of the century, this ratio had dropped to 1 to 1. Diagnostic trends mirror shifts in cigarette manufacturing, including use of more effective filters that reduce tar deposition in proximal airways but encourage larger “puff volumes,” delivering smoke carcinogens to the lung periphery. The morphologic heterogeneity of lung adenocarcinoma likely reflects the presence of several functionally distinct cell populations in the lung that may undergo malignant transformation including bronchioalveolar stem cells, type 2 alveolar epithelial cells, and Clara cells.
Adenocarcinoma comprises 62% of lung cancer diagnoses in nonsmokers as compared to 19% in smokers. Gender and ethnicity influences risk of developing lung adenocarcinoma among never smokers: Over half of women with lung cancer are never smokers, as compared to 15% to 20% of men ; half or more of Asian/Pacific Islanders with lung cancer are never smokers, as compared to 10% to 40% of non-Hispanic whites. Risk factors for development of lung adenocarcinomas in nonsmokers include exposure to secondhand smoke, cooking fumes, and asbestos; however, in many cases no such environmental risk factor is identified.
The genomic profiles of adenocarcinomas vary significantly between smokers and nonsmokers. Lung adenocarcinoma in smokers ranks among the most highly mutated tumor types anywhere in the body, with a characteristic pattern of transversion substitutions. The most commonly mutated genes in smoking-related adenocarcinomas include KRAS , STK11/LKB1 , and KEAP1 . A high mutation rate is associated with increased tumor neoantigen presentation and tumor immunogenicity. Immunotherapy, or therapeutic antibody-mediated inhibition of checkpoint proteins such as PD-1, PD-L1, and CTLA4, promotes an immune attack against tumor cells. Immunotherapy appears to have greater efficacy in adenocarcinomas with high mutation rates and smoking-related mutation signatures.
Adenocarcinomas arising in nonsmokers have mutation rates five- to tenfold lower than in smokers and are far more likely to have activating EGFR mutations or oncogenic fusions in ALK , ROS1 , RET, and NTRK genes. Prior to the advent of immunotherapy, much of the improvement in outcomes among patients with advanced lung adenocarcinoma was attributable to identification of targetable alterations and use of appropriate tyrosine kinase inhibitor therapies. Retrospective analyses of immunotherapy trials suggest these drugs have limited efficacy in adenocarcinoma with EGFR mutations or ALK fusions.
Adenocarcinomas commonly arise in the lung periphery with clinical symptoms occurring only after the cancer has reached an advanced stage. As a result, many patients present with manifestations of distant spread such as neurologic symptoms from brain metastases or pain due to bone metastases. Local symptoms of lung adenocarcinoma include cough and hemoptysis when the tumor involves a central airway; chest pain resulting from invasion of pleura and surrounding structures; and dyspnea due to airway obstruction, airspace or lymphatic infiltration by tumor, or pleural effusion. About half of patients with early stage disease come to clinical attention due to symptoms; in the remainder, tumors are detected incidentally during imaging for other indications or as part of lung cancer screening programs. On CT imaging, peripheral invasive adenocarcinomas commonly present as solid or part solid lesions and less commonly as consolidations or lobar ground-glass opacification. Centrally located tumors that obstruct a major bronchus can lead to secondary signs on imaging including atelectasis, lobar collapse, and air trapping.
The gross appearance of lung adenocarcinoma depends upon the histologic subtype and pattern of growth and spread. Peripheral tumors may cause puckering or dimpling of the overlying pleura, and pleural invasion often manifests as local roughening of the pleural surface by fibrous adhesions. Invasive adenocarcinomas typically have a distinct border and appear fleshy and tan to white, sometimes with necrosis or scarring. The gross dimensions of centrally located, airway-obstructing tumors may be overestimated because of adjacent obstructive/organizing pneumonia. Intrapulmonary spread of tumor may occur via airspaces (aerogenous), lymphatics, or blood vessels. Aerogenous spread manifests as a dominant mass with smaller satellite nodules of similar appearance. Lymphangitic spread appears as irregular thickening of interlobular septa and bronchovascular bundles, with lower lung predilection and occasional peripheral infarcts due to vascular obstruction. Preferential involvement of the pleural lymphatics may lead to a gross pattern mimicking malignant mesothelioma. Hematogenous spread manifests as innumerable randomly distributed nodules (miliary disease).
Histologically, invasive adenocarcinoma is highly heterogeneous, with recognized patterns including in situ (lepidic) and invasive (acinar, papillary, micropapillary, and solid) growth. In the 2004 WHO classification schema, this intratumoral heterogeneity was captured in the designation “Adenocarcinoma, mixed subtype,” however this nonspecific approach failed to communicate prognostic information inherent in the histologic patterns. The 2015 WHO classification recommends recording each component pattern in 5% increments and rendering a diagnosis based on the predominant pattern. This semiquantitative description of histologic patterns permits reproducible prognostication and allows for detailed comparison to other pulmonary tumors when attempting to make a distinction between intrapulmonary metastatic disease and multiple synchronous primaries.
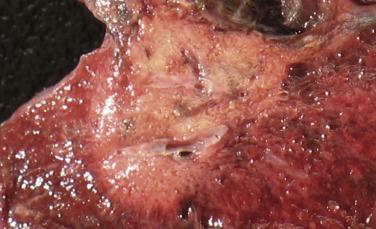
Lepidic-predominant adenocarcinoma represents 6% to 8% of adenocarcinoma diagnoses and is commonly an incidental finding by CT imaging, where it appears as a predominantly ground-glass lesion with focal solid component. Grossly, lepidic-predominant tumors appear as circumscribed areas of subtle alveolar wall thickening with focal or multifocal alveolar obliteration corresponding with areas of invasion. The overall size is rarely greater than 3 cm and pleural invasion is unusual. The invasive components can appear grossly scarlike or fleshy ( Fig. 5.1 ). Microscopically, neoplastic pneumocytes grow along preexisting alveolar structures, often with associated septal widening by inflammatory cell infiltrates ( Fig. 5.2 ). Lepidic-predominant adenocarcinomas are typically comprised of well-differentiated cells with mildly enlarged and hyperchromatic nuclei and may show focal mucinous differentiation. Areas of invasion, defined as tumor cells infiltrating within a myofibroblastic stroma, or any of the invasive patterns as described later in the chapter, must measure at least 5 mm. The presence of lymphovascular or pleural invasion, spread of tumor through airspaces in surrounding normal lung, or tumor necrosis triggers a diagnosis of invasive adenocarcinoma, irrespective of the diameter of defined invasion. The lepidic growth pattern can be identified reproducibly in interobserver studies; however, identification and quantification of invasion is associated with more variability. In practice, discriminating between lepidic-predominant adenocarcinoma, minimally invasive adenocarcinoma, and adenocarcinoma in situ may be challenging. Complete sampling of tumors measuring 3 cm or less should be performed and correlation with radiographic studies is recommended. Lepidic-predominant adenocarcinoma consistently shows TTF-1 expression by immunohistochemistry. p63 expression may be seen at the interface between lepidic tumor growth and normal lung. Lepidic-predominant adenocarcinomas have over a 90% disease-free survival following curative resection.
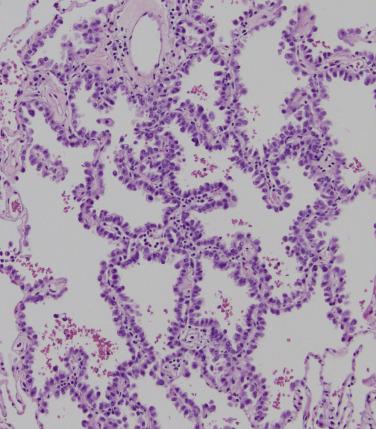
Acinar-predominant adenocarcinoma represents 33% to 45% of lung adenocarcinomas. Acinar growth correlates with solid density by CT imaging and grossly appears as a firm, scarlike or fleshy mass. Acinar growth is characterized by simple, ovoid glands or as complex, irregular branching structures with central lumens; the quantity and character of stromal and inflammatory cells vary widely. The neoplastic glandular epithelium can be flat to cuboidal, hobnail or columnar with variable cytoplasmic volume and coloration ( Fig. 5.3 ). Back-to-back growth of glands to form a sievelike or cribriform pattern ( Fig. 5.4 ) resembles solid growth and is associated with more aggressive tumor behavior. More than 90% of acinar-predominant adenocarcinomas show TTF-1 expression. Surgically resected acinar-predominant adenocarcinoma has an intermediate survival (70% to 80% 5-year disease-free survival) among invasive adenocarcinoma subtypes.
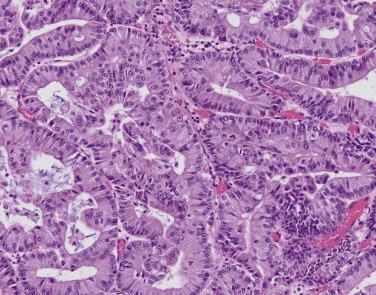
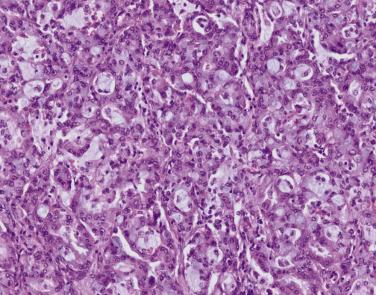
Rates of papillary-predominant adenocarcinoma diagnosis vary widely between studies (5% to 27%). Papillary growth appears solid on CT imaging and firm and fleshy on gross examination. Microscopically, neoplastic epithelium surfaces fibrovascular cores that protrude into airspaces ( Fig. 5.5 ). Papillary pattern may be difficult to distinguish from lepidic, acinar, and micropapillary growth and may show subtle continuity with these other patterns. Tumor cytology and differentiation is variable. Close to 100% of papillary-predominant adenocarcinomas express TTF-1. Papillary-predominant adenocarcinoma is considered an intermediate risk subtype, similar to acinar, and has been associated with EGFR mutation and ROS1 rearrangements.
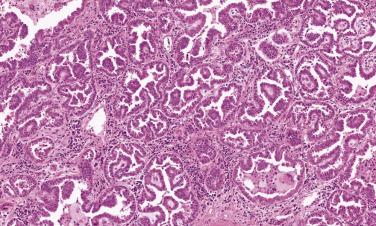
Micropapillary-predominant adenocarcinoma is rare in most series (2% to 7%). However, interobserver variability in the diagnosis of this subtype is significant and some large series report micropapillary-predominant histology in up to 20% of lung adenocarcinomas. Relative to other subtypes, micropapillary-predominant adenocarcinoma is more likely to present at a larger size with visceral pleural invasion and lymph node metastases. Micropapillary growth occurs as small clusters of tumor cells lacking fibrovascular cores floating freely in airspaces within the tumor and at the interface with normal lung ( Fig. 5.6 ). TTF-1 is expressed in over 90% of micropapillary-predominant lung adenocarcinomas. Micropapillary and papillary patterns may be difficult to distinguish. Micropapillary growth portends aggressive behavior. Even when present as 5% of an adenocarcinoma with another predominant histologic subtype, micropapillary pattern is associated with poor prognosis in early stage adenocarcinoma and tends to be overrepresented in paired metastatic samples.
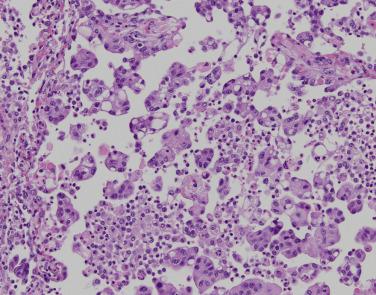
Solid-predominant tumors represent over a third of lung adenocarcinomas in some series, but are less common in studies examining predominantly early stage disease. Solid adenocarcinomas appear grossly fleshy and well circumscribed; necrosis may be apparent. Microscopically, solid pattern shows nests and sheets of large, round to polygonal cells with abundant cytoplasm growing in an interlocking pavingstone-like manner. Cytoplasmic clearing (clear cell change), intracytoplasmic mucin droplets, and central necrosis are common. Solid pattern tumors may also show ovoid to irregular cells with less-defined cell borders, growing in infiltrative nests ( Fig. 5.7 ). Solid-predominant adenocarcinoma, particularly when mucin droplets are sparse, resembles large cell carcinoma. TTF-1 is expressed in 86% of solid-predominant adenocarcinomas. Absence of TTF-1 expression predicts more aggressive behavior. Rarely, TTF-1 may be expressed only in the cytoplasm; although not specific for lung differentiation, this pattern of TTF-1 expression is seen in hepatoid adenocarcinoma of the lung, a mimic of metastatic hepatocellular carcinoma. Hepatoid lung adenocarcinoma occurs in heavy smokers, often presents with distant metastases, and is associated with elevated serum AFP. Identification of a dominant lung mass and staining with monoclonal CEA and EpCAM markers such as MOC31 support a lung primary origin. Solid-predominant growth is associated with aggressive behavior, with ~40% to 70% 5-year disease-free survival in surgically resected tumors. Among smokers, solid growth correlates with KRAS mutations. Solid growth also predominates in ALK , ROS1, and RET -fusion positive tumors, particularly in those tumors with signet ring cell differentiation ( Table 5.2 ).
| Histologic Subtype | Significant Genomic Associations |
|---|---|
| Adenocarcinoma in situ | EGFR mutations |
| Minimally invasive adenocarcinoma Lepidic-predominant adenocarcinoma |
|
| Acinar-predominant adenocarcinoma | EGFR mutations |
| KRAS mutations | |
| ROS1 fusions | |
| ALK fusions | |
| RET fusions | |
| Papillary-predominant adenocarcinoma | EGFR mutations |
| Micropapillary-predominant adenocarcinoma | EGFR mutations |
| BRAF mutations | |
| ALK fusions | |
| Solid-predominant adenocarcinoma | ROS1 fusions |
| ALK fusions | |
| RET fusions | |
| KRAS mutations | |
| Invasive mucinous adenocarcinoma | KRAS mutations |
| KRAS transition mutations | |
| KRAS + NKX2-1 mutations | |
| ERBB2 mutations | |
| NRG1 fusions |
Invasive mucinous adenocarcinoma, formerly known as mucinous bronchioloalveolar carcinoma, characteristically presents with cough productive of abundant, thin mucus (bronchorrhea) and is equally common in smokers and nonsmokers. On computed tomography, invasive mucinous adenocarcinoma appears as a partly solid to solid nodule(s), or as lobar consolidation with air bronchograms. On gross examination, the cut surface appears spongy and glistening; aerogenous spread of tumor leads to a consolidated appearance mimicking lobar pneumonia. Microscopically, invasive mucinous adenocarcinoma grows in a lepidic fashion; however, most cases will show areas of frank invasion with collapse of the normal architecture. Tumor cells typically lack any significant pleomorphism. Cells show columnar morphology with low nuclear to cytoplasmic ratio, basally located nuclei, and clear to pale cytoplasm containing abundant mucin. The neoplastic epithelium grows as clusters and strips that appear abruptly on otherwise unremarkable-appearing alveolar septa ( Fig. 5.8 ). Airspaces are filled with mucin and muciphages. Most cases are TTF-1 negative; instead, these tumors recapitulate a gastrointestinal phenotype, including mixed CK7 and CK20, CDX2, MUC5AC, MUC6, and HNF4α expression. Two-thirds of invasive mucinous adenocarcinomas contain activating KRAS mutations, the majority of which are transition mutations rather than the transversion substitutions characteristic of smoking-related carcinoma. TP53 mutations are rare, in contrast to the findings in other forms of lung adenocarcinoma. Loss of function mutations in NKX2-1, the gene that encodes for TTF-1, occur uniquely in mucinous tumors of the lung. Invasive mucinous adenocarcinomas lacking KRAS mutations show a high frequency of fusion events, including those involving NRG1 and ALK . A subset of cases harbor GNAS mutations indistinguishable from those seen in intestinal and pancreatic tumors.
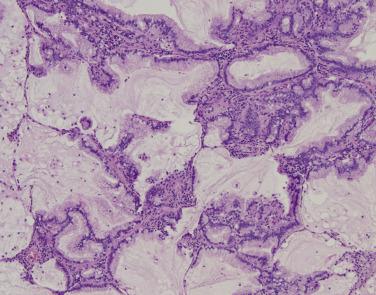
Preinvasive lesions include atypical adenomatous hyperplasia and adenocarcinoma in situ; tumors with less than 5 mm of invasion are termed minimally invasive adenocarcinoma . All are asymptomatic, detected either on radiographic or pathologic review. Atypical adenomatous hyperplasia is considered the earliest precursor to pulmonary adenocarcinoma. With current clinical imaging techniques, these lesions often go undetected; rather, atypical adenomatous hyperplasia more commonly presents as an incidental microscopic finding in pulmonary resections for other indications, especially lung adenocarcinoma. Adenocarcinoma in situ and minimally invasive adenocarcinoma are typically detected incidentally by computed tomography during lung cancer screening or for other indications, where they appear as well-defined ground-glass opacities, possibly with minor solid density.
Microscopically, atypical adenomatous hyperplasia appears as a discreet area in the lung periphery comprised of atypical epithelial cells lining mildly thickened alveolar septa, in general measuring less than 5 mm in diameter ( Fig. 5.9 ). Ultrastructurally, the atypical cells contain electron-dense granules and lamellar bodies, a feature shared with adenocarcinoma suggesting combined Clara cell and type II pneumocyte differentiation. The epithelial cells contain mildly enlarged, hyperchromatic nuclei with eosinophilic nuclear inclusions and small to moderate amounts of bubbly cytoplasm. Significant nuclear pleomorphism and mitotic figures are absent. These lesions express TTF-1 and harbor oncogenic alterations also commonly seen in lung adenocarcinoma, including EGFR and KRAS mutations.
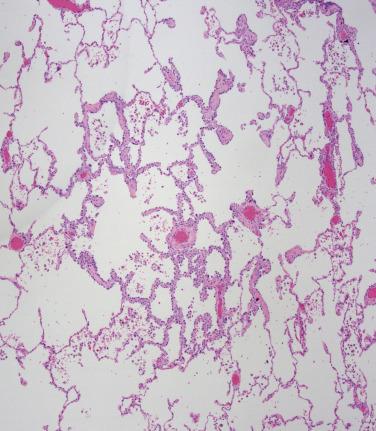
Adenocarcinoma in situ is defined as a proliferation of neoplastic epithelium confined to the surface of the alveolar structures, measuring 3 cm or less in greatest diameter. Grossly, adenocarcinoma in situ appears as a defined area of subtle pallor containing thickened alveolar septa and preserved airspaces. Microscopically, atypical adenomatous hyperplasia and adenocarcinoma in situ share similar architecture, cytology, and immunohistochemistry ( Fig. 5.10 ); practically these may be distinguished by size, with lesions measuring greater than 5 mm falling into the adenocarcinoma in situ category. This is not a hard cutpoint however, as severely atypical proliferations may be diagnosed as adenocarcinoma in situ even when measuring less than 5 mm.
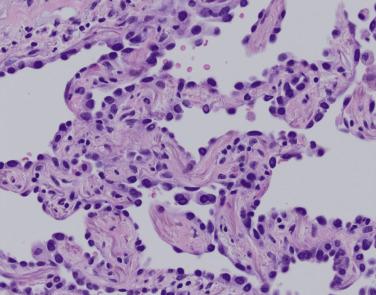
Minimally invasive adenocarcinoma manifests grossly as a circumscribed area of subtle alveolar wall thickening measuring 3 cm or less, with areas of alveolar obliteration. Histologically, minimally invasive adenocarcinoma shows a predominantly lepidic proliferation of atypical epithelial cells indistinguishable from adenocarcinoma in situ, with the addition of focal or multifocal invasion, defined by acinar, papillary, micropapillary, or solid pattern of growth, and measuring less than 5 mm.
Both adenocarcinoma in situ and minimally invasive adenocarcinoma may very rarely be mucinous. Oncogenic driver mutations in EGFR and KRAS are common in adenocarcinoma in situ and minimally invasive adenocarcinoma, as are mutations in the tumor suppressor gene TP53 . Based on mutational frequency and genomic complexity, these lesions likely represent intermediaries on a neoplastic continuum between atypical adenomatous hyperplasia and invasive adenocarcinoma. Following curative resection, both adenocarcinoma in situ and minimally invasive adenocarcinoma have 100% 5-year survival.
The pure form of fetal adenocarcinoma of the lung is a low-grade tumor presenting on average in the fourth decade with a slight female predilection. It is radiographically and grossly indistinguishable from other forms of early stage invasive adenocarcinoma. Microscopically, low-grade fetal adenocarcinoma is comprised of monomorphic columnar cells organized into glands and trabeculae resembling the pseudoglandular phase of fetal lung, surrounded by loose fibroblast-rich stroma. Squamoid morules may be apparent ( Fig. 5.11 ). Tumor cells contain aggregates of intracellular glycogen in the subnuclear cytoplasm. TTF-1 is positive and a subset of cases shows synaptophysin and chromogranin expression. Low-grade fetal adenocarcinoma characteristically expresses strong nuclear and cytoplasmic beta-catenin protein, consistent with activation of the WNT signaling pathway and a high frequency of CTNNB1 mutations. This tumor has also been reported as a manifestation of germline DICER1 mutation syndrome. Low-grade fetal adenocarcinoma is associated with an indolent clinical course.
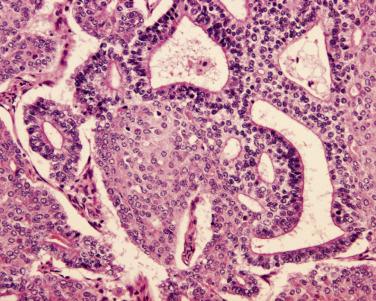
In contrast, high-grade fetal adenocarcinoma arises in the seventh decade on average, is more common in men, and has a predilection for heavy smokers. This tumor type represents less than 1% of lung carcinomas, with case series derived primarily from Japanese populations. Clinically, serum AFP may be elevated. Grossly, extensive necrosis may be apparent. Microscopically, high-grade fetal adenocarcinoma has focal to pure fetal lung–like morphology with high-grade nuclear features, disorganized architecture, and necrosis ( Fig. 5.12 ). Conventional patterns of adenocarcinoma are interspersed in most cases and occasional cases show admixed adenosquamous carcinoma. Oncofetal protein expression is common, with both conventional and fetal adenocarcinoma components frequently expressing AFP, GPC-3, and SALL4. About half of these cases express TTF-1 and 25% to 40% express CDX-2; however, TTF-1 is reportedly absent in pure high-grade fetal adenocarcinoma. The majority also express at least one neuroendocrine marker (chromogranin, synaptophysin, or CD56). Five-year disease-free survival for surgically resected tumors is approximately 50% to 65%. KRAS mutations are rare and EGFR mutations have been reported only in cases with admixed conventional patterns. The genetic basis of this tumor type is thus uncertain.

This very rare tumor type occurs predominantly in smokers on average in the seventh decade and usually presents as a cystic mass on imaging studies. Grossly, colloid adenocarcinoma is well circumscribed with a gelatinous cut surface and cystic change. Microscopically, circumscribed, partially encapsulated mucin pools distend and destroy alveolar parenchyma. Well-differentiated cuboidal to columnar cells line the cyst walls and float freely as detached micropapillary clusters within mucin ( Fig. 5.13 ). Signet ring cells are frequent. A subset of a cases shows admixed conventional adenocarcinoma. Colloid adenocarcinoma expresses CK7, CK20, and CDX-2 and is negative to weakly positive for TTF-1 and napsin. Colloid carcinomas of the lung are morphologically and immunophenotypically similar to those arising in the gastrointestinal tract, therefore an extrathoracic primary site should be excluded clinically. Molecular correlations are limited, but KRAS mutations are reported. Pulmonary mucinous cystadenoma, an exceptionally rare proliferation of benign mucinous epithelial cells lining a mucin-filled cyst, should lack any significant cytologic atypia or micropapillary tufting.
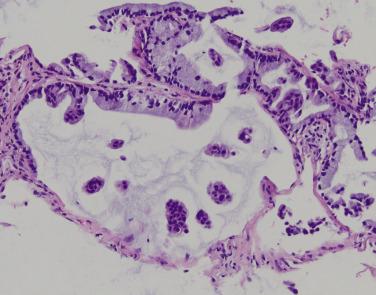
Enteric adenocarcinoma is rare, less than 1% of lung adenocarcinomas, and occurs disproportionately in men and heavy smokers. The presentation is similar to conventional invasive adenocarcinoma. Necrosis may be grossly evident. Microscopically, enteric adenocarcinoma of the lung is comprised of 50% or more intestinal-type epithelium characterized by tall columnar cells with brush borders, eosinophilic cytoplasm, and stratified vesicular nuclei ( Fig. 5.14 ) Dual CK7 and CDX2 expression supports this diagnosis; however, these markers are not consistently positive. TTF-1 is expressed in fewer than half; patchy CK20, MUC2, and villin staining may be seen. KRAS activating mutations are reported in enteric adenocarcinomas ; ALK rearrangements were identified in 13% of cases in one series. Metastatic colorectal adenocarcinoma is in the morphologic differential diagnosis; however, TTF-1 and CK7 expression favors lung origin.

Although some correlations between histologic subtype and tumor genetic alterations are reported (see Table 5.2 ), there are few strict genotype-phenotype associations in lung adenocarcinoma. As a result, testing for actionable genomic alterations is recommended in all patients with advanced (clinical stage IIIB or IV) disease. KRAS is mutated in 20% to 30% of lung adenocarcinomas in Western populations, where it is highly correlated with smoking history in most tumor subtypes. To date, attempts to target RAS or downstream MEK signaling pathways in patients with KRAS -mutated lung tumors have shown limited efficacy. However, KRAS mutations may correlate with increased efficacy of immunotherapies. The presence of a KRAS mutation essentially excludes the presence of another targetable oncogenic driver within the tumor.
Patients whose tumors harbor EGFR mutations or ALK or ROS1 fusions have improved survival when treated with targeted tyrosine kinase inhibitor therapy. EGFR mutations are reported in 15% to 20% of lung adenocarcinomas from patients in the United States and Europe, in half of those from Asian-Pacific patients, and more commonly in female never smokers. Mutations that sensitize tumors to EGFR tyrosine kinase inhibitors occur in the receptor kinase domain. About 90% of EGFR mutations occur as the L858R point mutation in exon 21 or as small in frame insertion-deletion mutations in exon 19 involving the ELREA amino acid sequence. Most of the remaining mutations with demonstrated oncogenic activity in lung adenocarcinoma occur at scattered hotspots throughout exons 18 to 21, although these less common alterations generally demonstrate less sensitivity to EGFR tyrosine kinase inhibitors. BRAF V600E mutation, found in 2% of lung adenocarcinomas, predicts response to combined RAF- and MEK-targeted therapies and is now considered an important biomarker in this tumor type. Molecular profiling is recommended for detection of this point mutation.
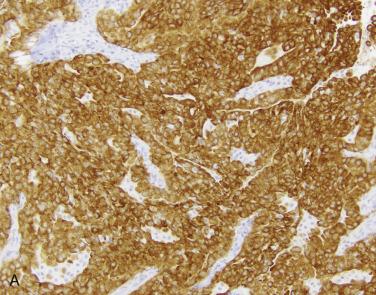
Rearrangements involving the receptor tyrosine kinases ALK and ROS1 represent ~5% and less than 2% of lung adenocarcinomas, respectively, and occur in a younger age group than other molecular subtypes of lung cancer. Never smokers are far more likely to have ALK or ROS1 rearranged tumors; however, patients with a smoking history may also have these alterations and derive benefit from targeted therapy. As a result, molecular and/or immunohistochemical testing for targetable alterations should be performed irrespective of smoking status. The majority of patients with ALK or ROS1 rearranged adenocarcinomas experience partial or complete response to targeted therapies, and the latter group appears to enjoy sustained response. Molecular methods (sequencing, targeted PCR) are recommended for detection of EGFR mutations, whereas fluorescence in situ hybridization and immunohistochemistry are preferred in many settings for ALK and ROS1 rearrangement detection. ALK protein overexpression is highly sensitive and specific for the presence of an activating rearrangement event in lung cancer ( Fig. 5.15A ) ; therefore immunohistochemistry for ALK protein using a well-validated assay can be used to select patients for ALK-targeted therapy. In contrast, ROS1 protein overexpression is highly sensitive but less specific for an activating rearrangement, due to low levels of full-length ROS1 protein expression in a subset of lung tumors. Tumors with ROS1 overexpression by immunohistochemistry (see Fig. 5.15B ) should undergo confirmatory testing with cytogenetic or molecular methods.
Patients receiving targeted therapies inevitably develop drug resistance and tumor relapse. Acquired mutations within the tyrosine kinase domain of the targeted receptor represent a common form of resistance to EGFR and ALK/ROS1 inhibitors. Acquisition of EGFR T790M mutation is the most common resistance mechanism in patients receiving EGFR tyrosine kinase inhibitors; however, morphologic transformation to small cell carcinoma occurs in approximately 3% to 14% of relapsed EGFR -mutated tumors, predicting some response to small cell treatment regimens but portending an overall grim prognosis.
Other emerging biomarkers for targeted therapies in lung adenocarcinomas include RET rearrangements, MET exon 14 splice mutations (~3%), and ERBB2 exon 20 insertion mutations (1% to 2%). Molecular methods such as sequencing are recommended for detection of these alterations.
In accordance with the WHO 2015 publication, squamous cell carcinomas of the lung are classified as conventional or basaloid. The former is further subclassified as keratinizing and nonkeratinizing. Basaloid squamous cell carcinomas should contain less than 50% conventional components.
Squamous cell carcinomas account for 25% to 30% of all lung cancers and are the most strongly associated with cigarette smoking among the major non–small cell lung cancer subtypes, with over 90% of these tumors occurring in smokers. The incidence of squamous cell carcinoma has decreased since the 1960s due to changes in smoking habits and increased detection of small peripheral adenocarcinomas with advanced imaging modalities. Clinically, patients with centrally located tumors present with cough, shortness of breath, and fever secondary to obstructive pneumonia. Tumor necrosis may cause hemoptysis. In general, squamous cell carcinomas tend to be locally aggressive with involvement of adjacent structures through direct invasion; distant metastases are less frequent relative to adenocarcinoma. Data on basaloid squamous cell carcinomas are limited, but their prognosis appears to be worse than that of other NSCLCs.
On gross examination, squamous cell carcinomas are typically located in the proximal airways, but an increasing number of peripheral tumors have been reported. The cut surface is usually white to grey, soft and friable, but may be markedly fibrotic and firm. Cavitary necrosis may occur in large tumors. Central tumors may form intraluminal polypoid lesions or infiltrate the peribronchial tissue. Secondary atelectasis, bronchiectasis, obstructive pneumonia, and bronchopneumonia may be present. Hilar lymph nodes may be enlarged due to direct invasion, metastasis, or reactive lymphadenopathy and often appear radiographically and grossly confluent with the primary tumor.
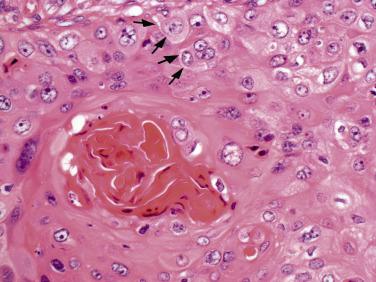
Histologically, keratinizing squamous cell carcinoma is characterized by the presence of layered keratin, cytoplasmic keratin in individual tumor cells, intercellular bridges, and keratin pearl formation ( Fig. 5.16 ). Keratinization varies based on degree of differentiation; poorly differentiated tumors only focally exhibit keratinization. In nonkeratinizing squamous cell carcinoma, epithelial nests typically lack maturation. The assessment of keratinization is subject to tissue sampling. The degree of keratinization has not been associated with patient outcomes, whereas single cell invasion, high-grade budding, large nuclear size, and spreading through air spaces have been reported as predictors of unfavorable prognosis. An alveolar space-filling growth pattern is characterized by cohesive aggregates of malignant squamous cells filling airspaces with intact alveolar septa. This pattern is occasionally seen in peripheral squamous cell carcinomas and is associated with a favorable prognosis. Squamous cell carcinomas may also spread superficially along the airways, thus assessment of the bronchial resection margin is extremely important.
Morphologic assessment alone is typically sufficient to render a diagnosis of keratinizing squamous cell carcinoma. In nonkeratinizing examples, immunohistochemistry should be performed to confirm squamous differentiation and exclude solid adenocarcinoma (including cases with a pseudosquamous appearance ) given the differences in molecular profiles and therapeutic strategies between the two entities. Nonkeratinizing squamous cell carcinoma should show diffuse staining for p40—this being more specific for squamous cell carcinoma than p63 or CK5/6—and absence of TTF-1 and napsin A; however, weak and focal TTF-1 expression may be seen in rare cases depending on choice of antibody clone.
Metastatic urothelial carcinoma, metastases from or direct extension of thymic squamous cell carcinoma, and metastases from squamous cell carcinomas of other organs enter into the differential diagnoses. The differentiation of primary lung squamous cell carcinoma from metastases from head and neck, esophagus, cervix, or cutaneous sites can be challenging and may require molecular correlation with mutation profiles, loss of heterozygosity analysis, or HPV genotyping. Well-differentiated endobronchial squamous carcinoma with papillary configuration can be distinguished from squamous papilloma based on the presence of invasion. Squamous metaplasia secondary to diffuse alveolar damage (DAD) or other infectious or inflammatory lung injury may acquire cytologic atypia resembling squamous cell carcinoma.
Basaloid squamous cell carcinoma is characterized by a proliferation of small cells with a lobular architecture or anastomotic trabecular growth ( Fig. 5.17A ). The tumor may have a minor component of unequivocal keratinizing and/or nonkeratinizing squamous cell carcinoma. Basaloid cells show scant cytoplasm, high nuclear/cytoplasmic ratio, and hyperchromatic nuclei lacking prominent nucleoli (see Fig. 5.17B ). Mitotic activity is brisk (15–50 per 2 mm 2 ), and Ki-67 proliferative index ranges from 50% to 80%. Comedo-type necrosis is common. Peripheral palisading and rosette formation may be seen in some cases, but nuclear molding is essentially absent. Most basaloid squamous cell carcinomas have hyalinized or mucoid stroma (see Fig. 5.17B ). Extensive squamous cell carcinoma in situ is often seen in the adjacent bronchial epithelium.

The differential diagnosis of basaloid squamous cell carcinoma includes large cell neuroendocrine carcinoma, small cell carcinoma, NUT carcinoma, and adenoid cystic carcinoma of the solid or basaloid type. Careful evaluation of cytomorphology and immunohistochemical evidence of squamous differentiation permits distinction between basaloid squamous cell carcinoma and high-grade neuroendocrine carcinomas. NUT carcinoma may be distinguished using NUTM1 fluorescence in situ hybridization (FISH) and/or a highly specific monoclonal NUT antibody. Ancillary tests that help to distinguish basaloid squamous cell carcinoma from adenoid cystic carcinoma include immunohistochemistry for CD117 and myoepithelial markers such as smooth muscle actin and FISH for MYB gene rearrangements.
Preinvasive lesions of squamous cell carcinoma consist of squamous dysplasia and squamous cell carcinoma in situ (CIS). Dysplastic foci typically develop as multiple lesions and may persist in the airway for many years, but progression of individual lesions to malignancy is rare. The likelihood of progression to invasive squamous cell carcinoma increases with the degree of dysplasia; as many as 50% of patients with severe dysplasia and the majority of those with CIS develop invasive lesions. Foci of CIS usually arise near bifurcations of segmental bronchi, subsequently extending both proximally and distally; the trachea is less frequently involved. Preinvasive lesions may not be identified macroscopically, but those that are usually consist of focal or multifocal plaque-like lesions resembling leukoplakia or nonspecific erythema; nodular or polypoid lesions may be seen in about one quarter.
Grading of preinvasive squamous lesions of the lung is similar to that in other sites such as the cervix and esophagus. Some prefer a four-tiered grading system (mild, moderate, and severe dysplasia, and CIS), while others tend to lump the grades together into low-grade and high-grade lesions, the latter category including CIS. With increasing grades of dysplasia, neoplastic cells involve and replace more of the epithelium, but the lesion remains confined to the epithelium. Grading is based on the constellation of cytoarchitectural alterations including cell size and maturation, nuclear atypia, and cell polarity. Epithelial thickness tends to increase with progression of dysplasia; however, CIS may or may not appear thickened. Some CIS lesions exhibit an exophytic, polypoid, or papillary growth without mucosal invasion. Ki-67 expression increases with advancing grades of dysplasia, and aberrant p53 expression is typically seen in high-grade lesions.
Lung squamous cell carcinomas typically exhibit a very high mutational rate, reflecting the mutagenic effects of cigarette smoking, and lack recurrent oncogenic driver alterations such as KRAS and EGFR mutations or ALK and ROS1 rearrangements. Recurrent gene copy number alterations include amplification of SOX2 , FGFR1 , EGFR , CCND1 , HER2, and PDGFRA and deletion of CDKN2A and PTEN . Recurrent somatic mutations occur in TP53 (>50%), CDKN2A , PIK3CA , PTEN , KEAP1 , MLL2 , HLA-A , NFE2L2 , NOTCH1, and RB1 . Basaloid squamous cell carcinoma shares genetic alterations with conventional squamous cell carcinoma, but appears to have a distinct gene expression profile.
NUT carcinoma (NUT-midline carcinoma) is a rare and highly aggressive tumor reported at multiple sites in both pediatric and adult populations, with a predilection for the head and neck and mediastinum. In adult series, NUT carcinoma is rare, accounting for less than 1% of lung carcinomas, and presents on average in the fourth decade. Most patients have no significant smoking history. Presenting symptoms include cough with or without hemoptysis and in some cases chest or shoulder pain. NUT carcinoma arising in the lung presents as a large, irregular, centrally located mass with ipsilateral pleural effusion, commonly with pleural thickening or tumor studding. Confluent hilar and mediastinal adenopathy is prominent. The contralateral lung is largely uninvolved. Distant metastases are common. Microscopically, intermediate-sized epithelioid tumor cells grow in nests and sheets with frequent necrosis. Cytologically, cells appear monomorphic with high nuclear to cytoplasmic ratio, scant pale amphophilic cytoplasm, open chromatin, and small to prominent nucleoli. Crush artifact and nuclear molding may be prominent, particularly on small biopsies ( Fig. 5.18 ). Focal, abrupt squamous differentiation may be apparent. Florid reactive epithelial hyperplasia in the adjacent lung may be mistaken for a neoplastic glandular component.
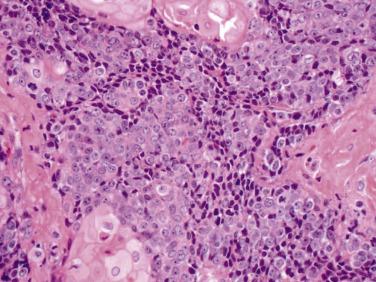
NUT carcinomas typically express at least focal p63 or p40, particularly in areas with morphologically apparent squamous differentiation. Focal TTF-1 can be seen, including within areas of p63 or p40 expression. Speckled nuclear NUT protein expression is highly sensitive and specific for the diagnosis of NUT carcinoma based on the corresponding identification of a fusion event involving NUTM1 , the gene that encodes for NUT, and bromodomain family members, most commonly BRD4 . Activation of bromodomain proteins triggers global epigenetic reprogramming and loss of cell differentiation. The differential diagnosis includes poorly differentiated non–small cell carcinoma, basaloid squamous cell carcinoma, small cell lung carcinoma (SCLC), adenosquamous carcinoma, thymic carcinoma, germ cell tumor, round cell sarcomas, and high-grade lymphomas. Median survival time in one series of pulmonary NUT carcinoma was 2.2 months from time of diagnosis. NUT carcinoma is generally refractory to conventional cancer therapies.
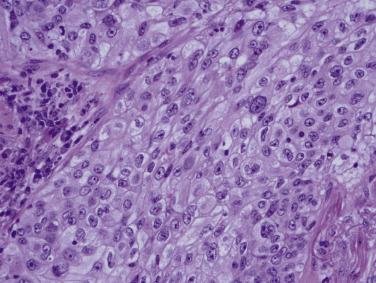
Large cell carcinoma incidence declined from around 10% to under 3% of lung cancers in the United States in the 30 years prior to the 2015 WHO classification, likely reflecting changes in use of immunohistochemistry in diagnostic practice. It is diagnosed on average in the seventh decade and is strongly associated with a history of cigarette smoking without a clear gender predilection. The clinical and radiographic presentation is similar to invasive lung adenocarcinoma. Grossly, large cell carcinoma appears well circumscribed, firm, fleshy, and tan-white. Necrosis may be present. Microscopic evaluation shows large, undifferentiated, polygonal tumor cells arranged in nests or sheets. Cells have vesicular nuclei, distinct nucleoli, and moderate to large amounts of eosinophilic cytoplasm and frequent clear cell change ( Fig. 5.19 ). Other morphologic features of glandular or squamous differentiation must be lacking. If intracellular mucin droplets are not visible by hematoxylin and eosin (H&E) staining, a mucicarmine stain should be performed. Tumors with fewer than five intracellular mucin droplets in two high-power fields are classified as large cell carcinoma.
The WHO 2015 classification recommends performing immunohistochemistry in any morphologically undifferentiated tumor, using TTF-1 to exclude adenocarcinoma and p40 or p63 to exclude squamous cell carcinoma. Only tumors that are negative for these markers should be classified as large cell carcinoma. The diagnosis also requires evaluation of a complete surgical resection specimen to exclude a minor component of glandular or squamous differentiation; small biopsies or cytology specimens with morphologic features of LCC and absent or unclear immunohistochemical differentiation should be diagnosed as non–small cell lung carcinoma, not otherwise specified (NOS). Prior studies have suggested that large cell carcinomas demonstrate more aggressive behavior relative to other forms of non–small cell carcinoma, stage for stage; however, immunohistochemical and genomics studies demonstrate that the morphologic large cell category is contaminated by solid adenocarcinoma, large cell neuroendocrine carcinoma, and basaloid carcinoma. As a result, past data on large cell carcinoma may not reliably reflect the behavior of tumors diagnosed by contemporary criteria. KRAS transversion mutations are detected in approximately 40% of large cell carcinomas. Alterations in EGFR and ALK are rare in this tumor type.
Although the presence of rhabdoid cytology was once used to define a separate subtype of large cell carcinoma, the subsequent recognition that rhabdoid change can occur across most types of lung carcinoma suggests it is not specific to this class of tumors. However, a newly described class of undifferentiated thoracic malignancies driven by SMARCA4 loss shows pronounced rhabdoid morphology. These arise predominantly in the mediastinum, although examples may occur within the lung where they resemble aggressive NSCLC clinically and grossly. Microscopically, SMARCA4-deficient thoracic malignancies are comprised of sheets of undifferentiated cells with rounded, vesicular nuclei; distinct nucleoli; and eosinophilic cytoplasmic inclusions. SMARCA4 protein expression is uniformly lost, and SOX2 expression may be strong and diffuse. Loss of function mutations in SMARCA4 are identified in all cases; transcriptionally, these tumors resemble the family of BAF-deficient sarcomas. Importantly, these rhabdoid-predominant tumors are almost uniformly fatal and are transcriptionally distinct from the 5% to 10% of morphologically differentiated lung carcinomas containing loss of function SMARCA4 mutations.
Adenosquamous carcinoma accounts for up to 4% of lung cancers. Its clinical features are not significantly different from those of other non–small cell carcinomas, but it appears to have an adverse prognosis. It is more commonly located in the peripheral lung. The histologic diagnosis of adenosquamous carcinoma requires identification of at least 10% each of squamous cell and adenocarcinoma components. Notably, adenocarcinoma-type driver mutations have been reported in a substantial minority of squamous cell carcinomas with a sub-10% glandular component, therefore histologic subtypes comprising less than 10% of the tumor should be reported. Adenosquamous carcinoma typically exhibits unequivocal foci of squamous cell carcinoma and adenocarcinoma that may be separate or intermingled. The degree of differentiation of each component can vary however, and the presence of solid adenocarcinoma or nonkeratinizing squamous cell carcinoma makes the diagnosis challenging. Immunohistochemistry for TTF-1 and p40 and mucin staining are recommended to distinguish adenocarcinoma and squamous cell carcinoma components. p40 may be expressed focally or multifocally in a subset of adenocarcinomas; a diagnosis of adenosquamous carcinoma is appropriate only if two distinct morphologic and immunophenotypic populations are apparent.
The differential diagnosis of adenosquamous carcinoma includes pure squamous cell carcinoma with entrapped reactive, atypical pneumocytes and pure adenocarcinoma with entrapped bronchioles exhibiting squamous metaplasia. Mucoepidermoid carcinoma may be in the differential diagnosis of centrally located biphenotypic tumors, but these tumors lack TTF-1 expression; testing for mammalian mastermind-like 2 ( MAML2) rearrangement—a specific feature of mucoepidermoid carcinoma—can confirm this diagnosis.
EGFR and KRAS mutations, both characteristic of adenocarcinoma, have been reported in adenosquamous carcinoma. EGFR amplification appears to be enriched in adenosquamous carcinoma. Detailed molecular analyses using microdissection have shown identical mutations in both morphologic components of adenosquamous carcinoma, indicating a phenotypically heterogeneous but genetically clonal tumor.
Sarcomatoid carcinomas of the lung are malignant epithelial tumors with variable amounts of sarcomatoid elements and include pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma. It is thought that the sarcomatoid component emerges from epithelial-to-mesenchymal transition of a carcinoma clone. Pulmonary sarcomatoid carcinomas are rare and account for 0.4% of all lung cancers in epidemiology databases. The clinical and radiographic presentation is similar to that of other non–small cell carcinomas, albeit with a more aggressive clinical course and worse prognosis. Sarcomatoid carcinomas occur in older adults (median age of 60–70 years), more commonly in men and smokers. Grossly, sarcomatoid carcinomas tend to be large, with a mean size of 5 to 8 cm. Pleomorphic carcinomas are the most common form of sarcomatoid carcinoma, accounting for 2% to 3% of resected lung cancers; other types are very rare. Pleomorphic carcinoma and pulmonary blastoma are more often located in the peripheral lung; pleomorphic carcinoma often invades the chest wall and mediastinum. Carcinosarcoma more often occurs centrally, sometimes with endobronchial involvement. On gross inspection, these tumors are typically circumscribed and unencapsulated with a variegated, grey-white cut surface; hemorrhage and necrosis are often present. The differential diagnosis of sarcomatoid carcinoma is broad ( Table 5.3 ) and is informed by clinical and radiographic features and appropriate immunohistochemistry. The distinction from synovial sarcoma may require assessment of SS18 - SSX gene fusion/t(X;18) translocation.
| Pleomorphic Carcinoma and Spindle Cell Carcinoma | Giant Cell Carcinoma | Carcinosarcoma | Pulmonary Blastoma |
|---|---|---|---|
|
|
|
|
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here