Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter reviews advances in our understanding of the roles of neurotrophic proteins, retinoids, folate, lipids, and thyroid and steroid hormones in neural development. These molecules play central roles in the regulation of neuronal and glial lineages; when they are not maintained at optimal levels in the developing nervous system, neural structural abnormalities and permanent neurologic dysfunction may result. For example, maternal folate deficiency increases the frequency of neural tube defects (NTDs), particularly in genetically susceptible fetuses, and maternal folate prophylaxis sharply reduces the frequency of these defects. ,
The development of the nervous system entails the most complex array of cellular migrations and interactions of any organ system. The actions of neural morphogens, neurotrophic proteins, retinoids, folate, and thyroid and steroid hormones must be appreciated within this dynamic context. Actions of these molecules on the nervous system not only depend on their concentrations but are also specific to stage and location. , Altered actions of these molecules by mutations or environmental perturbations may delay brain development or cause irreversible brain injuries.
Induction of neuroectoderm, which occurs in the region of ectodermal contact with dorsal mesoderm, is the third major event of embryogenesis, after the establishment of embryonic dorsoventral and anteroposterior axes and the formation of mesoderm during gastrulation. The subsequent formation of the forebrain is induced by mesoderm anterior to the notochord, whereas Sonic hedgehog (Shh) secreted by notochord induces ventral structures of the hindbrain and spinal cord.
The neural plate, composed of multipotential neuroectodermal cells destined to form both the central nervous system (CNS) and peripheral nervous system, appears in the human embryo 18 days after fertilization, and genes begin to be transcribed in this structure that encode adhesion molecules, including neural cell adhesion molecule (NCAM), L1CAM, and N-cadherin. Mutations affecting one of these genes, L1, cause X-linked aqueductal stenosis and agenesis of the corpus callosum, which occurs approximately once in 20,000 births.
The dorsal aspect of the embryo develops lateral furls, and then invagination of the neural plate forms a fold ( Fig. 127.1 ). A tube appears along the length of the fetus by fusion of this fold, proceeding both forward and backward. The anterior neuropore closes at day 24, and the posterior neuropore closes at days 26 to 28. The neural tube initially consists of a single layer of neuroectodermal cells, which proliferate and generate neuroepithelial progenitors for neurons and neuroglial cells. Radial glial cells appear early during neural tube formation and stretch across the wall of the neural tube. Neurons originate at the inner luminal border of the neural tube from these radial glial cells and then migrate outward along the radial glial cells. In the cerebral cortex, which is highly laminated, early-born neurons occupy the deeper cortical layers and later-born neurons migrate past the deep layers to establish new superficial layers. Thus the deepest cortical region contains the ontogenically oldest neurons.
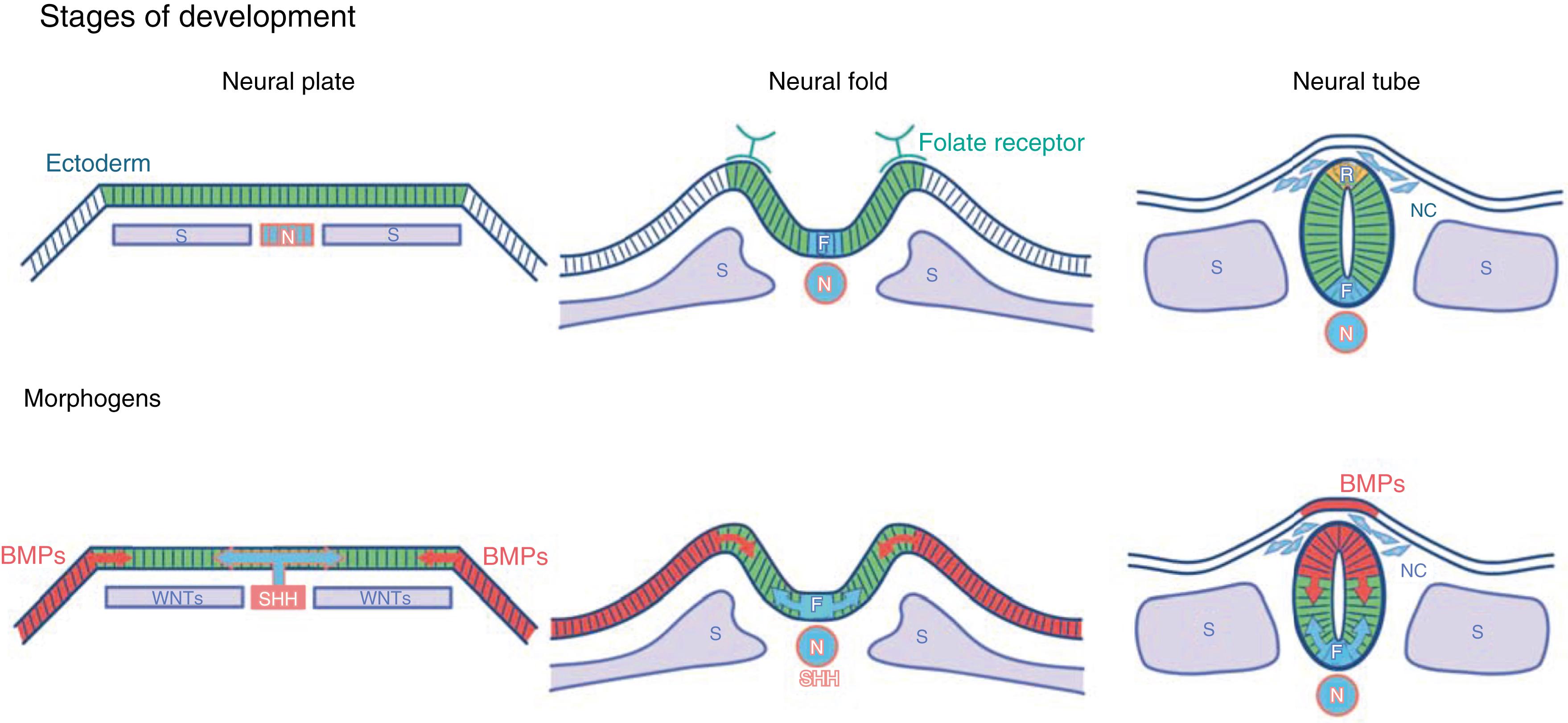
Neural crest cells are derived from neuroectoderm of the dorsolateral neural tube and form the peripheral and enteric nervous systems. The neural crest cells migrate from the neural tube into the surrounding mesenchyme at about the time of neural tube closure. The cephalic neural crest contributes to the formation of the facial skeleton, branchial arches, and, in conjunction with placode-derived cells, the lower cranial nerves. Neural crest cells migrating from the dorsolateral aspects of the spinal neural tube serve as progenitors for the peripheral and enteric nervous systems, the adrenal medulla, and melanocytes. ,
Our understanding of human brain development has recently been enhanced by methods for the single-neural-cell transcriptomic analyses of human postmortem fetal brain cells and for guiding human-induced pluripotent stem cells (hiPSCs) to form brain organoids. However, most experimental studies of neural development have used rodent and other experimental animal models. The mouse CNS at birth is at a developmental stage similar to that of the human CNS at 5 to 6 months’ gestation. By 10 days postpartum, the mouse CNS has matured to a stage equivalent to that of the human brain at term. Cerebral cortical neurogenesis is largely completed by birth in both human and mouse, although some new neurons continue to be generated throughout life from neural stem cells that persist in both the mouse and human CNS, particularly in the hippocampus and the subventricular zone bordering the cerebral ventricles. , CNS myelination in humans commences during the third trimester of gestation; in rats and mice, differentiated oligodendroglial cells first appear around the time of birth, and myelination is an entirely postnatal process. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here