Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The treatment of renal artery disease should be guided by the cause of the arterial obstruction and the health consequence(s) of the obstruction. A renal artery stenosis (RAS) of any cause that has no sequelae, such as hypertension or functional kidney impairment, generally requires no treatment. Although this circumstance might seem unusual, it is becoming more commonplace as imaging of the abdominal aorta becomes more frequent and incidental stenoses are identified. In children with congenital stenosis, surgical revascularization may be optimal. In patients with fibromuscular dysplasia, balloon angioplasty is the treatment of choice. In patients with atherosclerotic RAS, medical therapy that addresses the systemic nature of atherosclerosis is the ideal treatment; endovascular stenting is reserved for those who have failed medical therapy. In this chapter we review key concepts in the treatment of renal artery disease.
The kidneys have several critical functions, including filtration of blood and regulation of salt, water, and blood pressure. Disorders of the arterial circulation of the kidney may have an adverse impact on some or all of these functions (see Chapter 22 ). As early as the 1930s, Goldblatt determined that stenosis of a renal artery resulted in increases in systemic blood pressure. From this critical observation, it would seem intuitive that in the presence of RAS, the approach should be “find it, fix it.” Simply put, if the blockage can cause problems, then fix the blockage. Importantly though, the circulation of the kidney is somewhat more complex than many other vascular beds. Control of cortical renal blood flow is under the influence of both the afferent and efferent arterioles of the glomerulus. Generally, cortical blood flow extracts minimal amounts of oxygen and is mostly related to autoregulation of the filtering glomerulus. In addition, there is a separate circulation for the medulla of the kidney. As a consequence, the straightforward concept that “a blockage of a renal artery decreases function of the kidney” is often not correct. Due to the ability of the kidney to autoregulate, it is undoubtedly true that even a hemodynamically significant stenosis may not result in a decrement of excretory renal function. Finally, the renal circulation responds to vasoactive agents independently of other circulations. As an example, adenosine is a vasodilator in the coronary circulation and can cause vasoconstriction in the kidney.
Generally, medical therapy is directed at the pathophysiology of the ischemic kidney and biologically, to block the activity of the renin- angiotensin system. Thus, for all patients with RAS and hypertension, consideration should be given to the use of a long-acting angiotensin receptor blocker or angiotensin converting enzyme inhibitor that has a high trough-to-peak ratio to provide consistent blood pressure lowering and biologic effects. In some patients, additional treatment with a long-acting thiazide-type diuretic, such as chlorthalidone, can be quite useful. In patients with more advanced renal dysfunction, loop diuretics may play a role. Long-acting calcium channel antagonists can be quite useful, and on occasion the use of β-blockers or α-β blockers can provide an added benefit. Importantly, for many patients with RAS, hypertension control can often be achieved with current modern medical therapy and with few if any side effects.
When a patient fails to respond to medical therapy, there are several considerations that arise, first of which is noncompliance with treatment. This may be due to limited financial resources, drug-related side effects, and symptoms attributed to the medication(s) that are unrelated, among others. In some individuals, the lack of blood pressure control may be due to other concomitant issues such as sodium noncompliance, the presence of hyperaldosteronism, or excess alcohol consumption. Thus it is critical to carefully evaluate patients for other causes of drug-resistant hypertension when they appear to be failing medical therapy for blood pressure control. There are some patients that can be difficult to manage; these are principally patients with (1) advanced kidney disease, (2) severe global ischemia, or (3) both. However, these situations should not be used as a reason to avoid a trial of medical therapy, because many if not most patients will tolerate antihypertensive medications, including the use of angiotensin receptor blockers or angiotensin converting enzyme inhibitors. In some individuals there will be a modest rise in serum creatinine with blood pressure lowering and/or the use of renin-angiotensin inhibition, however, it most often stabilizes and remains stable for very long periods. Only patients who experience a rapid and significant worsening of kidney function should have treatment withdrawn. As will be discussed later in this chapter, for patients with atherosclerotic RAS, medical therapy should also address atherosclerosis risk per se , including lipid-lowering therapy, antiplatelet medication, smoking cessation, and diabetes control if indicated.
Fibromuscular dysplasia (FMD) is an important cause of hypertension in young adults and in middle-aged individuals (see Chapter 58 ). A common presentation would be a young female with unexpected hypertension that is moderate to severe, and may be accompanied by an abdominal bruit. Importantly, FMD may be unilateral or bilateral, and may occur in other vascular beds including the carotid and iliac arteries. FMD is infrequently associated with renal dysfunction, and commonly associated with hypertension. It is interesting that it has also now been associated with spontaneous arterial dissections in a variety of vascular beds, including the carotid and coronary arteries.
FMD can present in a variety of forms, which have been described pathologically as intimal, medial, and periadventitial. More recently, an angiographic classification has been used to characterize the stenosis as uni- or multifocal. The most common type is the multifocal medial type, which has the appearance of a string of beads. In contrast to atherosclerotic stenosis, which is typically ostial in location, FMD most often occurs in mid-artery or beyond. This is important during diagnostic imaging because a complete assessment of the mid- and distal renal artery is necessary when FMD is suspected. Furthermore, because of the web-like nature of some FMD lesions, conventional angiographic or axial anatomic imaging may not be sufficient to gauge the severity of the stenosis. Oftentimes function assessment is needed, such as duplex ultrasonography or the use of a pressure guidewire when invasive imaging is performed. Additionally, intravascular ultrasound offers another adjunct to examine the luminal anatomy of the renal artery in FMD patients, which may offer considerable insight as to the location and severity of areas of flow restriction, which are often difficult to visualize. FMD may be familial or sporadic, with no known gene yet identified as causative. Likely, there is a genetic predilection, because the disorder is far more common in women than men.
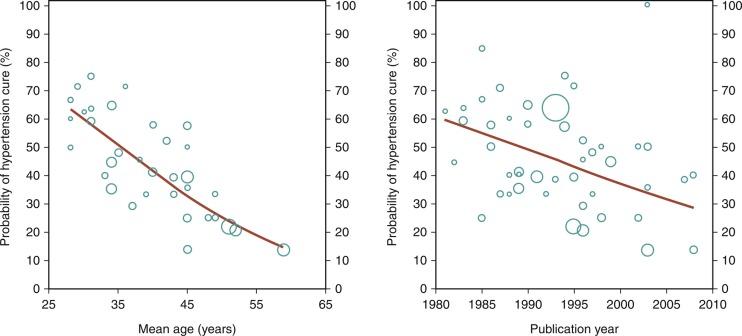
Most of the information regarding treatment of FMD is derived from single or small case series, but this is starting to change. Trinquart and colleagues performed a metaregression analysis that used a number of case series to demonstrate several important findings. First, they demonstrated that the likelihood of a hypertension cure with angioplasty declined with age ( Fig. 24.1 ). Second, they also showed that more contemporary angioplasty case series were associated with lower cure rates. This is almost certainly related to the fact that the thresholds for hypertension cure have become more stringent with lower and lower blood pressure values. Experienced operators now recognize that hypertension cures may be less common, but it is not uncommon for patients undergoing revacularization to experience an improvement in blood pressure along with a lessened need for antihypertensive therapy. Patients who are younger often have a better response to revascularization of FMD than do older patients. Whether this is due to vascular remodeling, the presence of concomitant essential hypertension, aging itself, or some other factor is unknown.
For normotensive patients with incidental FMD noted on imaging studies, observation alone is indicated. For hypertensive patients, antihypertensive medical therapy is important. As stated above, treatment should be with a long-acting angiotensin receptor blocker or angiotensin converting enzyme inhibitor that has a high trough-to-peak ratio. In younger women who are planning pregnancy or who are pregnant, other antihypertensive agents should be used due to the potential of harm to a fetus with the use of drugs that interrupt the renin-angiotensin system. Although there is little data to support this approach, some experts recommend the use of daily aspirin for patients with FMD.
If the blood pressure is elevated, or less commonly, if there is renal insufficiency, revascularization should be considered. In general, in these young patients, stenting should be avoided because it is unnecessary and the long-term presence of metallic stents in the mid- or distal artery may lead to stent fracture over years. The renal artery is often mobile during the respirophasic cycle, and may cause an implanted stent to bend during inspiration/expiration. The repetitive stress may cause tissue injury at the ends of the stent, or cause fracture(s) of the stent that result in adverse occurrences such as restenosis. Surgical revascularization may be reasonable for some complex cases of FMD or in those cases that cannot be treated with an endovascular approach. Patients with FMD and concomitant aneurysms may be quite challenging to treat adequately from an endovascular approach and as a consequence some may benefit from surgical repair.
The dominant strategy for treatment of FMD is balloon angioplasty, also referred to as percutaneous transluminal angioplasty (PTA). The goal of PTA is to sufficiently dilate the vessel and tear intimal webs to relieve the hemodynamic obstruction and postobstructive pressure decline that are associated with these lesions. As stated previously, the severity of the lesions, and as an extension, the anatomic completeness of PTA, are difficult to ascertain angiographically. Addition of intravascular ultrasound can provide significant information to assist in localizing lesions for treatment in cases where the culprit lesion is difficult to visualize. The use of 0.014-inch diameter pressure-sensing guidewires that can be left in during the procedure, and that can be used for delivery of the PTA balloons, have made the decision(s) of when to continue to use larger balloons and when to conclude the procedure more objective and easier. As a general approach, the lesion is sequentially dilated with balloons. In general, the balloon’s size should match the normal diameter of the vessel. If the stenosis is not adequately relieved, then a higher pressure can be used to more fully expand the initial balloon, or a slightly larger balloon can be used to achieve a larger diameter. Importantly, the index balloon should never be selected to match the diameter of poststenotic dilation, because such treatment may result in the dreaded complication of vessel rupture. In general, a successful procedure results in the reduction of the translesional gradient to less than 10 mm Hg. It is also important to note that the angiographic appearance after balloon dilation may not be markedly different than the pretreatment angiogram and is often still quite abnormal in appearance due to the continued presence of the disrupted or torn webs. However, if the lesion has been adequately dilated and the pressure gradient is significantly improved, then the angiographic appearance is of minimal significance.
Ostial lesions of the renal artery, especially in middle-aged and older individuals, are most often due to atherosclerosis. Many, if not most of these likely represent the encroachment of aortic plaque into the renal artery, thus the presence of RAS is often accompanied by moderate or even severe atherosclerotic plaquing of the abdominal aorta or at times, aneurysms of the abdominal aorta. As a consequence, the aorta may represent a minefield of risk in these individuals, especially related to atheroembolization.
The occurrence of atherosclerotic RAS has certain features that are similar and different from atherosclerosis in other vascular beds. First, atherosclerotic RAS tends to occur in older individuals, with a mean age of 70 years, which is about a decade older than the mean age of a first myocardial infarction. Tobacco use is a very important risk factor for atherosclerotic RAS, and is associated with an occurrence that is about 9-years prior to nonsmokers. Atherosclerotic RAS affects men and women equally. This is in sharp contrast to coronary artery disease, which tends to occur more often in men, and with FMD, which occurs more often in women. Why atherosclerotic RAS equally affects men and women is not known.
Atherosclerotic RAS often is diagnosed incidentally during imaging of the abdominal aorta for other indications. When it is identified, irrespective of other issues, it is certainly a marker of advanced atherosclerosis and the risk for atherosclerotic events. Several groups have developed prediction models to understand who is likely to have atherosclerotic RAS. Factors that are important include advanced age, atherosclerosis in other beds, presence of renal dysfunction, and hypertension.
There are several ways to establish a diagnosis when atherosclerotic RAS is suspected. The least invasive and least expensive is duplex ultrasonography (see Chapter 12 ). For many patients this is possible, however, certain measures should be taken to improve the likelihood of a definitive study. Foremost among these are measures to minimize bowel gas that can interfere with imaging. Steps that are helpful include withholding food the morning of the procedure, avoiding gassy foods for 24 hours prior to the study, and ingestion of simethicone the night before and morning of the procedure. When these steps are taken, and an experienced technician performs the study, the likelihood of a conclusive study is improved. Several duplex ultrasound criteria can be used to identify a significant stenosis, including peak systolic velocity, renal aortic ratio, and other indirect criteria that suggest the potential presence of a stenosis. The peak systolic velocity is quite helpful and the range for determining a positive result ranges from 150 to 300 cm/s. Generally, the higher the velocity threshold used, the better the specificity at the cost of a lower sensitivity. Importantly, however, with a positive study demonstrating high velocity, poststenotic turbulence, and vessel expansion, the diagnosis is made and the functional significance is established. Despite enhanced recognition of multiple arteries by color Doppler flow, only 40% of accessory renal vessels are currently identified by renal duplex ultrasound examination.
Other tools for diagnosis include computerized tomographic angiography (CTA) (see Chapter 14 ) and magnetic resonance angiography (MRA) (see Chapter 13 ). CTA has the advantage of rapid scan time, and good spatial and temporal resolution. However, the need for iodinated contrast makes it impractical for some patients with moderate or advanced chronic kidney disease who have a risk for contrast- induced nephropathy. MRA can be an excellent alternative, especially when a noncontrast imaging strategy is used. In some patients, gadolinium-based MRA contrast may pose the risk of progressive systemic sclerosis. Importantly, when tomographic imaging is performed, the assessment of stenosis severity should be based on the lumen of the stenosis compared to the lumen of the normal distal vessel. Comparisons made to the overall vessel diameter at the site of the stenosis will exaggerate the stenosis severity due to the Glagov phenomenon of positive vascular remodeling. Similarly, comparisons of lumen dimension to areas of poststenotic dilation will also potentially overestimate stenosis severity. Finally, with any imaging modality, the characteristics of the downstream kidney provide important clues to the lesion severity, including the size of the kidney. Generally, when the downstream kidney is less than 8 cm, it is often nonfunctional.
Over the past two decades there has been considerable debate about the appropriate treatments for patients with atherosclerotic RAS. What has become clear is that medical therapy is the cornerstone for all patients ( Table 24.1 ). Patients with atherosclerotic RAS have systemic atherosclerosis, and thus are in need of treatment for this systemic disorder. The indicated therapies include effective cholesterol lowering therapy, antiplatelet medication, smoking cessation, and diabetes management to goal for those with diabetes. In addition, reducing blood pressure with effective, long-acting agents is critically important, because nearly all patients with significant atherosclerotic RAS also have systolic hypertension. A key component of reducing blood pressure in these individuals is the use of potent, long-acting agents to interrupt the renin-angiotensin system, such as angiotensin receptor blockers or angiotensin converting enzyme inhibitors. The purpose of medical therapy is to prevent progression of atherosclerosis generally, and of the renal artery specifically, prevent thromboembolic complications, and minimize the deleterious effects of systemic hypertension on the heart and vascular system.
| Class of Recommendation | Level of Evidence | Recommendations |
|---|---|---|
| I | A | 1. Medical therapy is recommended for adults with atherosclerotic renal artery stenosis. |
| IIb | C-EO | 2. In adults with renal artery stenosis for whom medical management has failed (refractory hypertension, worsening renal function, and/or intractable HF) and those with nonatherosclerotic disease, including fibromuscular dysplasia, it may be reasonable to refer the patient for consideration of revascularization (percutaneous renal artery angioplasty and/or stent placement). |
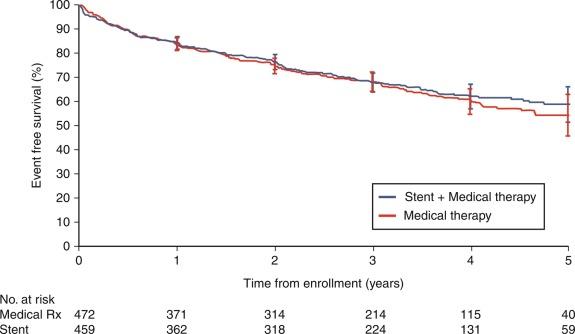
Several important trials were completed in the last decade that addressed whether revascularization with endovascular stenting conferred significant benefits when added to a background of medical therapy. These trials, including CORAL and ASTRAL, demonstrated that medical therapy was as effective as medical therapy with stenting ( Fig. 24.2 ). CORAL addressed this in patients with hypertension on medical treatment or with chronic kidney disease and ASTRAL did so in patients in whom the decision to revascularize was unclear. From these two studies it can be concluded that for most patients with atherosclerotic RAS, medical therapy is the preferred treatment strategy. This point was made clear in the recent American College of Cardiology/American Heart Association (ACC/AHA) Hypertension Guidelines of 2017 (see Table 24.1 ).
A controversial issue is whether there are some patients who should still be considered for revascularization, and if so, how are these individuals identified for treatment. Fundamentally, the kidneys require arterial blood flow to function, so presumably there must be a threshold at which a stenosis becomes significant enough to warrant treatment. Experienced operators may encounter occasional patients with nearly occluded renal arteries (or, rarely, occluded renal arteries) that, upon opening of the vessel, observe a marked and immediate beneficial effect that can be indicated by diuresis, a substantial drop in blood pressure, or a marked improvement in kidney function. However, these cases tend to be exceptional, and unfortunately, it is often difficult to predict those individuals who will experience such a benefit. Some authors have suggested a more detailed use of renal scintigraphy, combined with arterial imaging, to assess the function of the stenotic kidney and to predict its likelihood of improvement. An alternative approach is to discount treatment in patients with severe stenosis that are unlikely to benefit, because of factors such as a high resistive index, proteinuria, or a small distal kidney. Unfortunately, some patients are not well represented in the comparative clinical trials; thus, a decision to treat may be based on operator judgment and careful consent from the affected person. In the 2017 AHA/ACC Hypertension Guidelines, the use of revascularization for RAS was categorized as a Class of Recommendation IIb (weak), and Level of Evidence C-EO, (Consensus of Expert Opinion based on clinical experience) (see Table 24.1 ).
Recent work suggests a limited role for surgical revascularization, due to the associated high morbidity and mortality in this population. PTA alone is also of limited utility due to the high rates of restenosis, presumably due to recoil of aortic plaque into the ostium of the vessel. As a consequence, stenting is the dominant strategy when revascularization is planned for atherosclerotic RAS. When stenting is contemplated, careful attention must be given to the approach, to maximize the likelihood of success and minimize the risk for aorta-induced complications such as atheroembolization.
When endovascular revascularization of atherosclerotic RAS is planned, the approach requires careful consideration. Generally, there are two approaches, from above using upper extremity access, and from below using femoral access. Femoral access provides a shorter route and often better torque control on the guiding catheter. In contrast, upper extremity access can be easier in the setting of a downward directed renal artery. From the upper extremity, left arm access may be easier because the leftward placement of that subclavian artery may pose fewer challenges in manipulating into and down the thoracic aorta. From the femoral approach, depending upon the geometry of the aorta and iliac vessels, the femoral artery ipsilateral or contralateral to the side of the renal artery may impact the procedural success. This is especially critical in the presence of significant aortic tortuosity or an abdominal aortic aneurysm that can markedly change the ideal approach.
The use of effective antiplatelet therapy(s) should be strongly considered prior to treatment with an endovascular stent. In addition, there are a number of technical details that can impact the ease and success of renal artery stenting. First, the origin of the renal artery must be clearly identified with the fewest catheter manipulations and using the least amount of contrast. Consideration should be given for a nonselective aortogram, given in low volume and at a high rate, to outline all the renal arteries and their relationship(s) to the aorta and other visible structures such as the underlying vertebrae. From this, the origin can be carefully selected with a diagnostic catheter, or using a “no touch technique,” to minimize aortic wall contact. This is critical to avoid atheroembolization and other complications. Once the renal artery is engaged, a guiding catheter or sheath can be telescoped into the treatment site. An experienced operator should be able to accomplish these steps in most cases using minimal contrast media.
Once a guidewire is positioned in the renal artery, treatment may proceed with direct stenting or balloon predilation. Although predilation introduces another step in the procedure, it does have several potential benefits. First, it is an opportunity to demonstrate that the lesion can actually be dilated. Although infrequent, there are rare cases where the ostial stenosis does not respond to balloon dilation, and this becomes far more complex if the first step is to deploy a stent that cannot be adequately inflated. Second, the balloon dilation can be angiographically recorded with the balloon partially inflated. This gives an excellent outline of the lesion and can be quite useful for detailing the landing site of the stent. The stent, once advanced into the lesion, should be deployed with 1 to 2 mm of strut outside the ostium to ensure that the aortic plaque is adequately covered. Unfortunately, restenosis is sometimes related to overlooked lesions when the stent is deployed too far into the lesion. During dilation of the stent, the goal is to safely expand the stent to match the diameter of the distal normal vessel. Achieving a larger lumen area is clearly tied to improved rates of long-term in-stent restenosis. In contrast, the stent should not be deployed to match the diameter of poststenotic dilation, because this overexpansion can lead to vessel rupture. Finally, when the stent is being deployed, the patient should be asked about back pain, because this can be a sign of impending vessel rupture. Should vessel rupture occur, immediate balloon tamponade is helpful, often with the stent delivery balloon or an oversized balloon at low pressures. If the rupture does not seal with balloon inflation, then use of a covered stent or surgical repair may be necessary. At no time should vessel rupture be left without definitive repair.
Similarly, careful attention to the details of technique are necessary to avoid other complications such as kidney perforation, side branch occlusion with renal infarction, and contrast nephropathy. Perforation of the kidney typically occurs with the use of either hydrophilic guide wires or when careful attention is not placed on the distal end of the wire and it perforates the kidney parenchyma. Unfortunately, when the kidney is perforated, and a subcapsular hematoma develops, the subsequent pressure on the kidney can lead to the loss of the kidney. Side branch occlusion typically occurs with early branching renal arteries when side-branch vessels are jailed by a stent. Again, lesion selection and careful attention to treatment planning are critical because these side branch occlusions may result in loss of kidney function or persistent hypertension. Finally, contrast nephropathy is a real concern. Many patients who require treatment have advanced kidney disease and are intolerant of large or even moderate doses of contrast media. Careful planning, along with meticulous attention to detail, can markedly reduce contrast use; in some instances, alternative contrast media, such as CO 2 , can be of use.
After a successful revascularization procedure, the late complications of stent treatment can include restenosis within the stent, progression to end-stage kidney disease, and stent fracture. Fortunately, these complications are infrequent when experienced operators use careful technique. Alternatively, the cavalier use of stents, poorly placed and deployed, can result in significant early and late complications.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here