Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
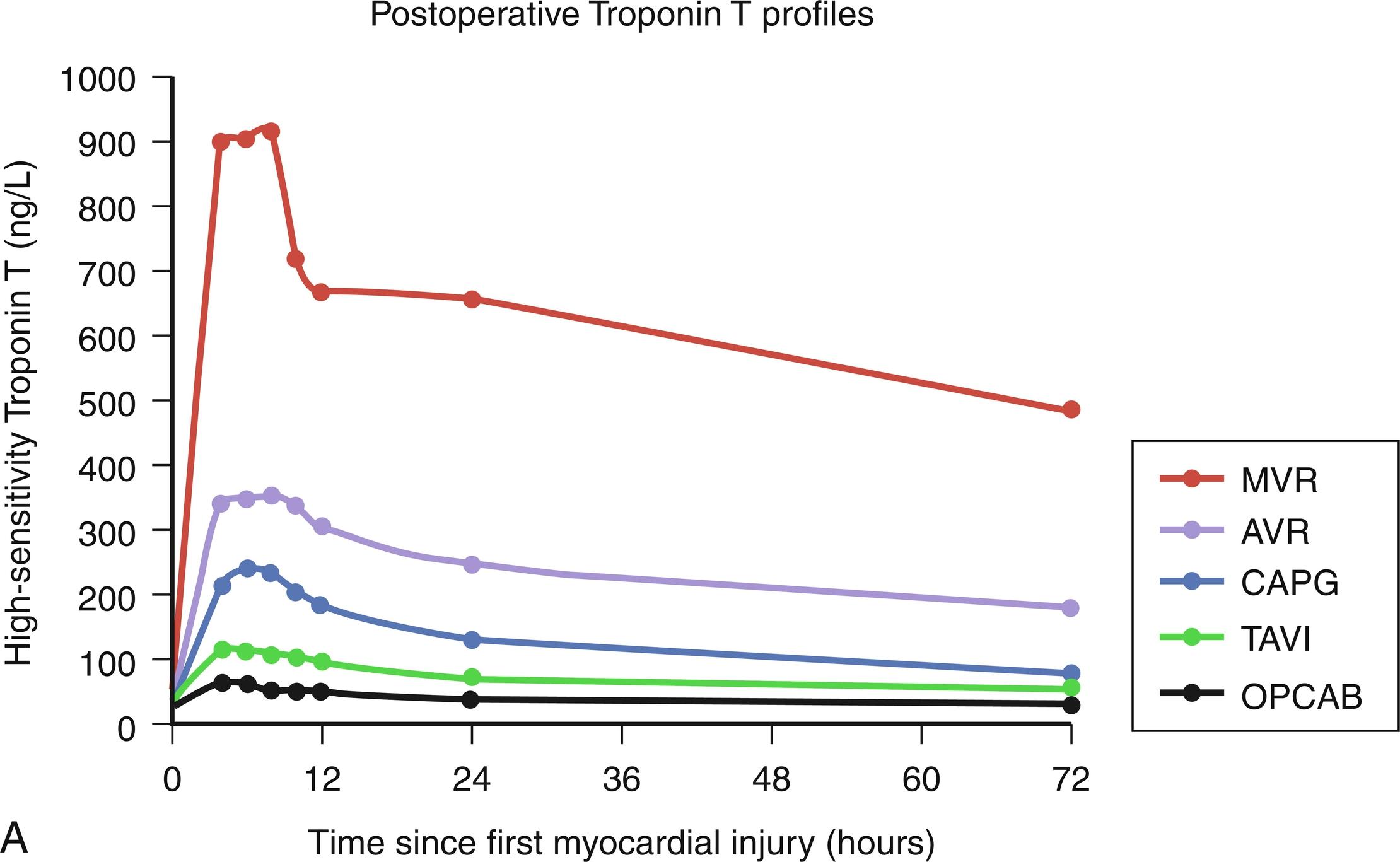
Perioperative myocardial ischemia is difficult to define, as it represents a continuum with clinically irrelevant events at one end of the spectrum and myocardial infarction (MI) leading to complete ventricular failure at the other end. The diagnosis of perioperative myocardial ischemia and/or infarction is challenging in the setting of cardiac surgery, specifically because of the elevation of cardiac enzymes that accompanies even uncomplicated procedures ( Fig. 13.1 A,B ). For instance, using high-sensitivity troponin release assays, Markman et al 1 demonstrated a rise in troponin even after transcutaneous aortic valve implantation. Similarly, the electrocardiographic (ECG) changes that typify ischemia and infarction in the nonoperative setting may instead reflect postoperative pericardial inflammation or subclinical myocardial injury incurred during routine surgical manipulation. Therefore, accurate determination of ongoing ischemia often requires a systematic, multifaceted approach, including serial evaluation of ECG changes, biochemical markers, echocardiography, and even angiography. Importantly, early detection of perioperative ischemia may prompt therapies to relieve the ischemia and minimize the incidence of subsequent infarction.
The interpretation of ECG changes remains valuable for defining ischemia in the perioperative period. Table 13.1 summarizes various clinical entities that may lead to ST-segment changes in this setting, including myocardial ischemia and infarction. After cardiac surgery, the initial ECG may be difficult to interpret because of lead placement changes related to surgical dressings, because of cardiac pacing, and because of the common presence of conduction abnormalities. For example, right bundle branch block and first-degree atrioventricular (AV) block occur very frequently but typically resolve within the first few postoperative hours.
| Condition | Electrocardiographic features |
|---|---|
| Left bundle branch block | Wide QRS complex |
| ST-segment deviation | |
| Acute pericarditis | Diffuse ST-segment elevation |
| Reciprocal ST-segment depression in lead aVR, not in lead aVL | |
| Elevation seldom > 5 mm | |
| PR-segment depression | |
| Hyperkalemia | Widened QRS and tall, peaked, tented T waves |
| Low-amplitude or absent P waves | |
| ST-segment usually downsloping | |
| Pulmonary embolism | Changes simulating myocardial infarction, seen often in both inferior and anteroseptal leads |
| Classically, right bundle branch block and S1Q3T3 pattern, right-axis deviation | |
| Cardioversion | Striking ST-segment elevation, often > 10 mm, lasting only minutes immediately after direct-current shock |
| Myocardial ischemia | Flattened, downsloping ST-segment |
| T-wave inversion | |
| Myocardial infarction | ST-segment elevation with a plateau, shoulder, or upsloping |
| Reciprocal behavior between aVL and III; progression to Q waves |
Other ECG abnormalities in cardiac surgery patients are not uncommon findings, as noted in the Bypass Angioplasty Revascularization Investigation (BARI) study ( Table 13.2 ), and often portend increased cardiac mortality, especially the development of postprocedural Minnesota code Q-wave abnormalities ( Table 13.3 ). Therefore, most centers perform preprocedural and serial postprocedural ECGs. ECG alone, however, may lack sufficient sensitivity and specificity for the detection of myocardial ischemia. Transesophageal echocardiography (TEE), which has become a standard component of perioperative monitoring at most centers, provides a very effective adjunct to the diagnosis of myocardial ischemia and infarction. During surgical revascularization, normal wall motion indicates effective revascularization, whereas newly detected regional wall motion abnormalities usually reflect underlying ischemia or infarction. These regional wall motion abnormalities can consist of akinesia or dyskinesia in a ventricular segment that was normokinetic or hypokinetic preoperatively. A study of 351 coronary artery bypass graft (CABG) patients monitored with intraoperative TEE and ECG demonstrated that wall motion abnormalities detected by TEE were more common than ST-segment changes, but there was only a 17% positive concordance between the two modalities. In addition, TEE was found to be twice as predictive as ECG in identifying patients who ultimately met criteria for MI. These results indicate that TEE may be a more sensitive method of detecting myocardial ischemia in the perioperative setting and should probably be routinely used in cardiac surgery monitoring. An important observation with TEE is that new interventricular septal wall abnormalities appear to be common postprocedurally but do not appear to be associated with irreversible myocardial damage. Importantly, technical development of perioperative TEE has led to recent advances in 3D echocardiography, tissue Doppler imaging (TDI), and contrast echocardiography, all of which are readily being investigated in their utility to better detect perioperative ischemia in a number of studies.
| New ECG abnormality | Incidence |
|---|---|
| n (%) | |
| Major Q wave | 65 (4.6) |
| ST elevation | 216 (15.1) |
| ST depression | 220 (15.4) |
| T-wave abnormality | 557 (39.0) |
| No ECG abnormality | 557 (39.0) |
| ECG | Frequency | Dead | Alive | Total | Disease | Disease | 95% CI | Incidence | Relative | |
|---|---|---|---|---|---|---|---|---|---|---|
| Abnormality | (%) | ( n ) | ( n ) | ( n ) | Odds | Odds | (per 100) | Risk | 95% CI | Ratio |
| None or one step | 54.4 | 74 | 1194 | 1268 | 0.062 | 1.00 a | — | 5.84 | 1.00 a | — |
| Two-step worsening | 23.6 | 18 | 532 | 550 | 0.034 | 0.55 b | 0.31-0.95 | 3.27 | 0.56 b | 0.34-0.93 |
| T wave | ||||||||||
| Two-step worsening | 14.2 | 11 | 320 | 331 | 0.034 | 0.55 | 0.27-1.09 | 3.32 | 0.57 | 0.31-1.06 |
| ST-segment elevation | ||||||||||
| Two-step worsening | 2.7 | 4 | 59 | 63 | 0.068 | 1.09 | 0.33-3.24 | 6.35 | 1.09 | 0.41-2.88 |
| ST-segment depression | ||||||||||
| Two-step worsening | 0.4 | 0 | 10 | 10 | 0.000 | 0.00 | — | 0.00 | 0.00 | — |
| ST-segment | ||||||||||
| elevation and | ||||||||||
| Two-step worsening | ||||||||||
| ST-segment | ||||||||||
| depression | ||||||||||
| Two-step worsening | 4.7 | 12 | 98 | 110 | 0.122 | 1.98 | 0.98-3.90 | 10.91 | 1.87 b | 1.05-3.33 |
| Q wave |
Precise diagnostic criteria for defining perioperative myocardial ischemia have not been uniformly adopted, and thus the published incidence varies. A recent study found that 6.4% of patients undergoing CABG ( n = 2052) experienced perioperative myocardial ischemia, using the following criteria: (1) an increase in the creatine kinase-to-creatine kinase-MB isoenzyme (CK/CK-MB) ratio of greater than 10%; (2) new onset of elevated ST-segment change of greater than 1 minute’s duration and involving a shift from baseline of at least 0.1 mV and a new associated postoperative Q wave; (3) recurrent or sustained ventricular tachyarrhythmia or fibrillation; and (4) hemodynamic deterioration on inotropic support. In a meta-analysis of randomized controlled trials in patients undergoing cardiac surgery, 20 trials ( n = 1522 patients) had a 17% overall incidence of ischemic events. Ischemia was defined as an ST-segment deviation on the ECG or new wall motion abnormalities on the TEE.
As with myocardial ischemia, there is considerable disagreement on the diagnostic criteria used to define perioperative myocardial infarction (PMI), which again explains discordant reports in the literature of its incidence and prognostic implications. In the same meta-analysis cited earlier, 22 trials of 1853 patients had a 5% overall incidence of PMI. Across the literature, the reported rate of PMI ranges from 5% to 15%. Despite advances in cardiac surgery, this incidence does not appear to be declining. The diagnosis of PMI has classically relied heavily on the development of new and persistent pathologic Q waves on an ECG, which may not always be truly representative of transmural necrosis. Indeed, in an autopsy study performed on cardiac surgery patients who died within 1 month after surgery, 23% of patients had significant transmural myocardial necrosis but no pathologic Q waves, and 20% of patients without transmural necrosis exhibited new postprocedural Q waves. Reliance on the presence of Q waves alone to diagnose PMI can dangerously overlook infarctions. In a study of cardiac surgery patients, transthoracic quantitative echocardiography analysis with ECG and radionuclide ventriculography demonstrated non–Q-wave PMI to be three times more common than Q-wave PMI, with equally deleterious effects on left ventricular (LV) function. A study evaluating the clinical significance of a new Q wave after cardiac surgery suggested that patients with this pathologic ECG change, without any concomitant elevation in myocardial enzyme markers or other indicators of PMI, had uneventful outcomes. These findings highlight the critical importance of combining various diagnostic modalities to define PMI.
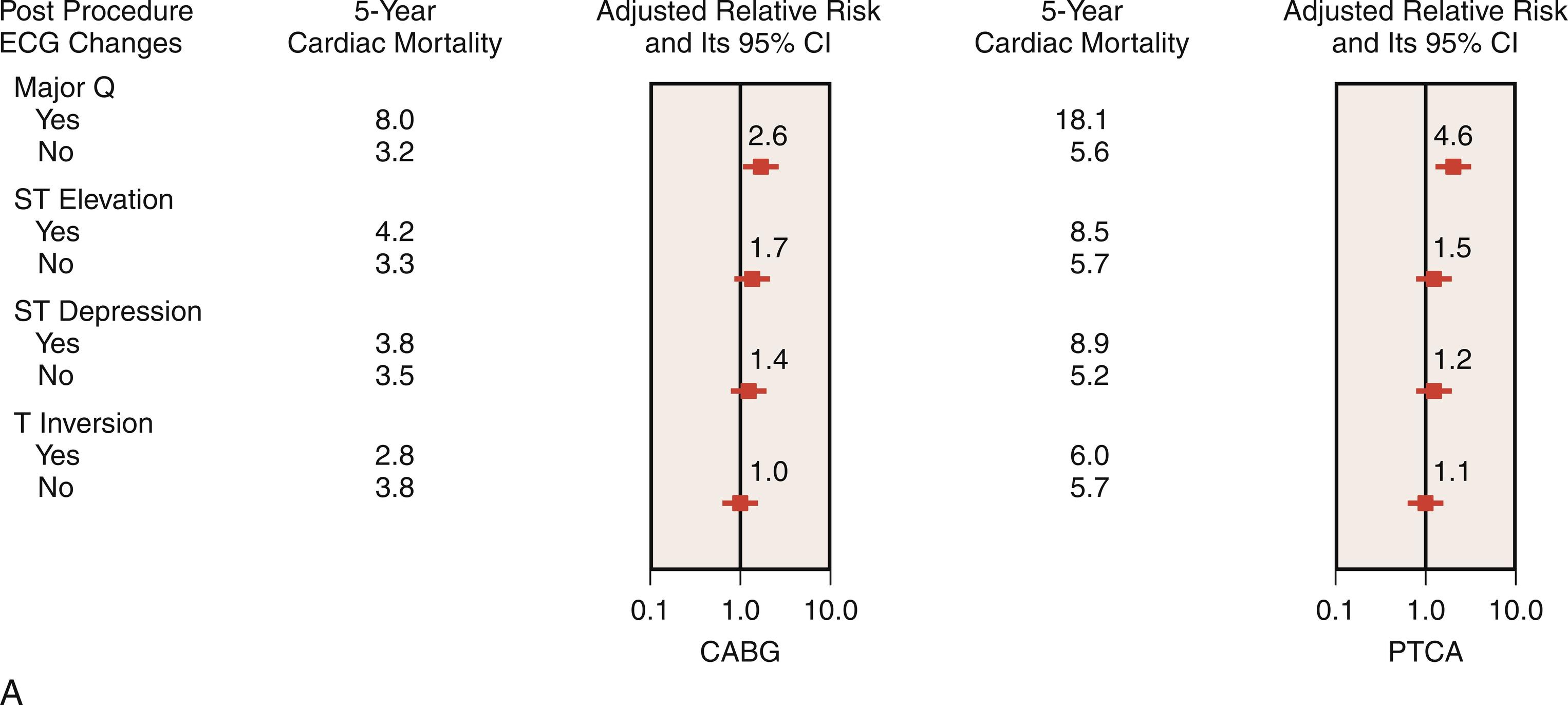
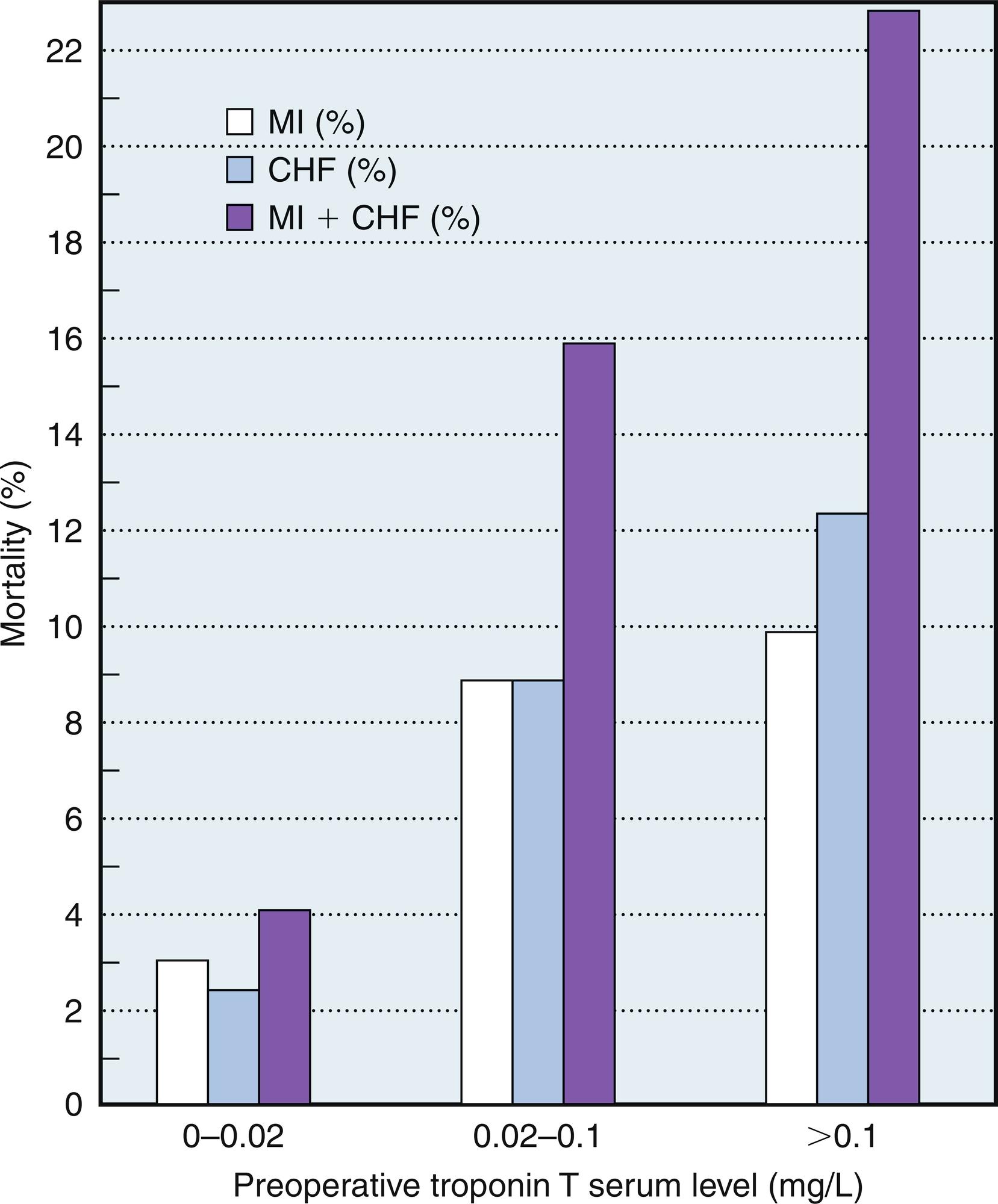
Although Q waves do not necessarily reflect transmural necrosis, appreciation of these and other ECG changes and their linkage to adverse outcomes cannot be disregarded. New perioperative ST-segment changes (> 0.1 mV) have been identified as an independent predictor of PMI, which accounts for up to 40% of preoperative CABG deaths. In the Coronary Artery Surgery Study (CASS; n = 1340 patients), 62 patients with a new Q wave postoperatively experienced 9.7% in-hospital mortality, compared with 1.0% in the remaining 1278 patients. In patients who survived to hospital discharge, the presence of new postoperative Q waves did not adversely affect 3-year survival. Among the 1427 CABG patients in the BARI trial, 5-year cardiac mortality was increased with new Q-wave development (8.2% versus 3.7% for no new ECG changes; adjusted relative risk, 2.6) ( Fig. 13.2 ). Results from the GUARD during Ischemia Against Necrosis (GUARDIAN) study of 2918 high-risk CABG patients further emphasize the negative impact of Q-wave development on survival ( Table 13.3 ). Namay et al 22 reported a 5-year survival rate of 76% in CABG patients ( n = 77) with new Q waves compared with 90% in the unaffected CABG patients ( n = 1790).
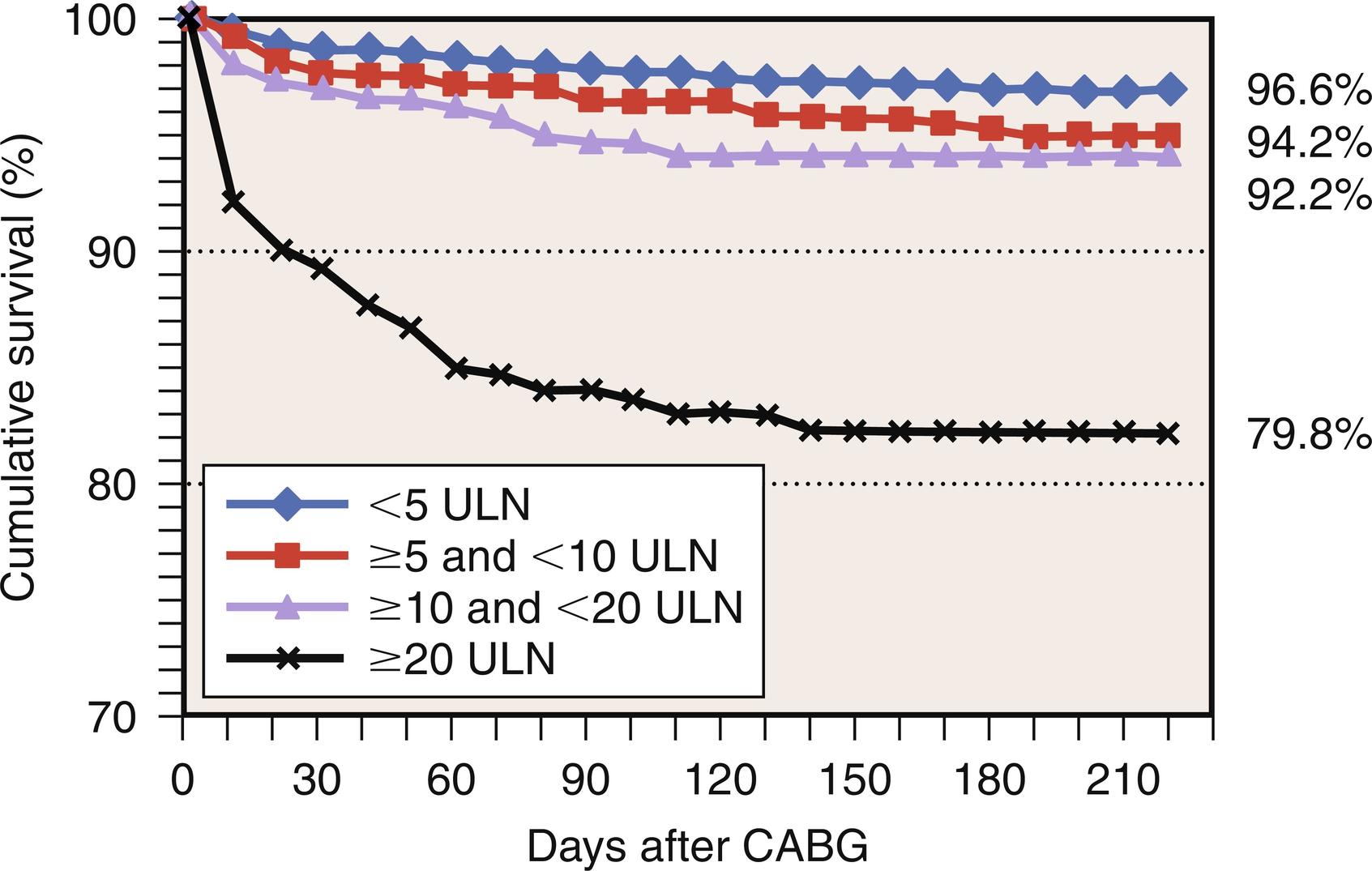
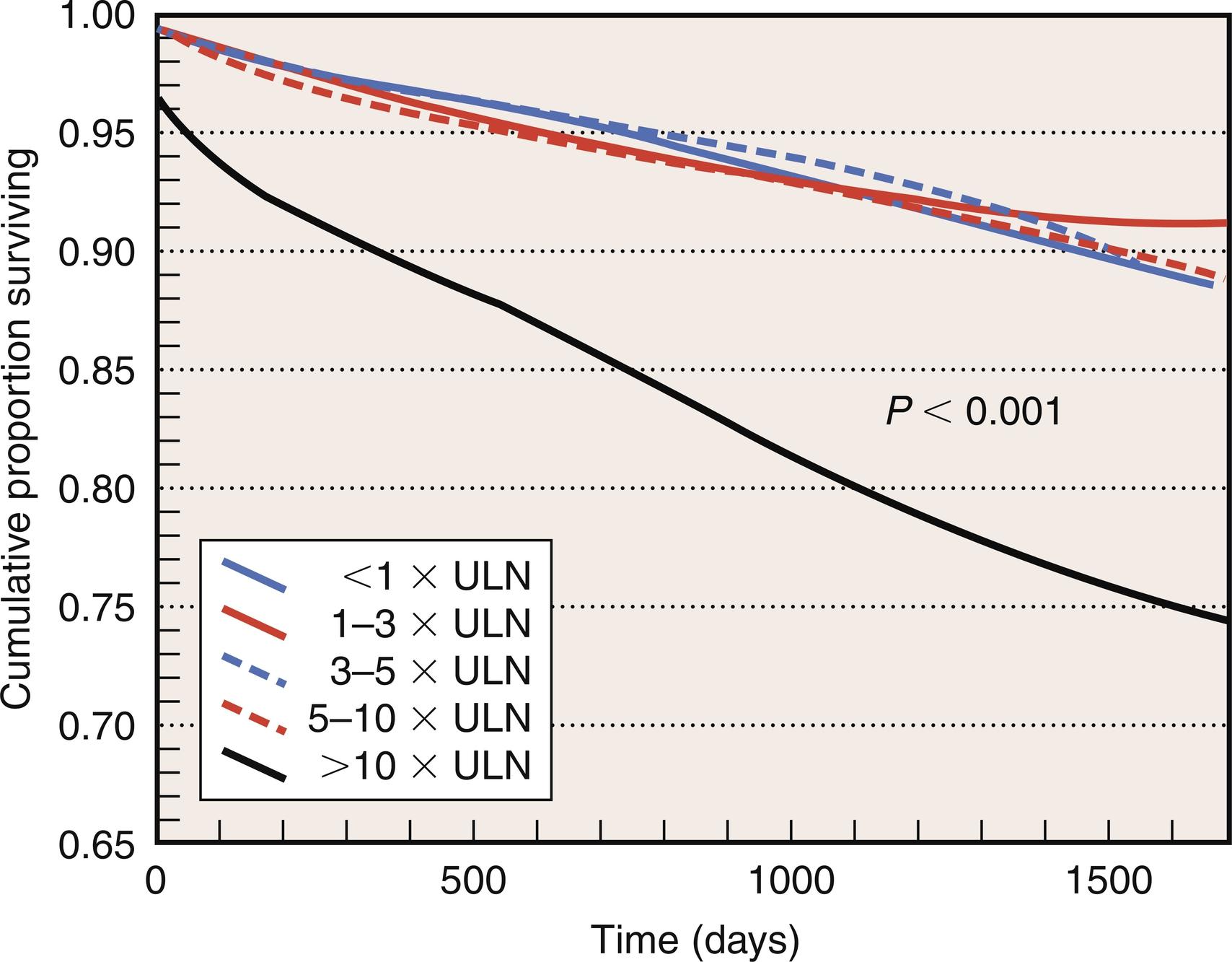
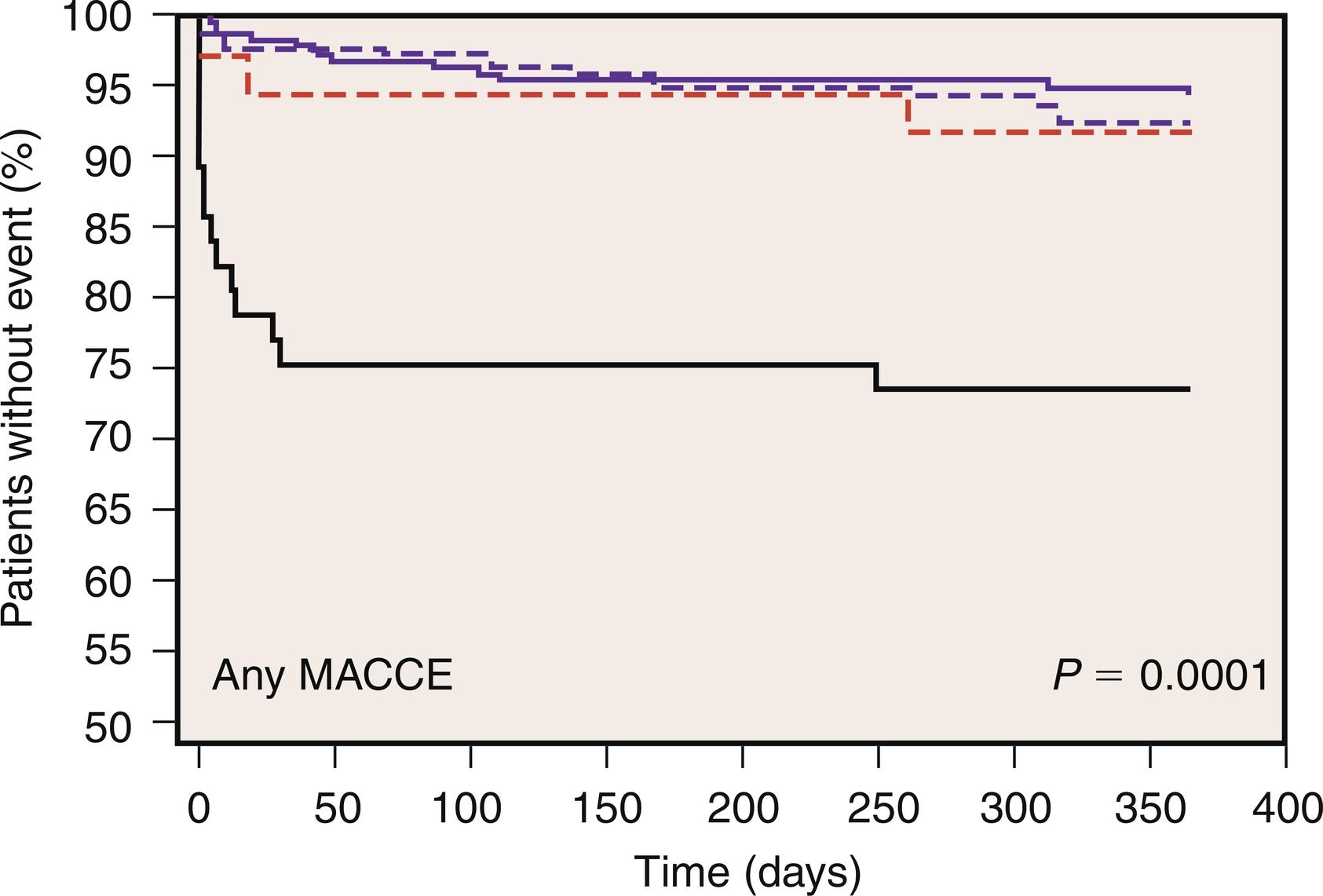
Along with new postoperative Q-wave development, enzymatic criteria are routinely used to define PMI and to enhance the overall sensitivity and specificity of PMI detection. In a study performed on 499 cardiac surgery patients at the Brigham and Women’s Hospital, PMI occurred in 5% of patients and was designated by the following criteria: total peak CK greater than 7000 μg/L, CK-MB greater than 30 ng/mL, and new Q waves on ECG. In the GUARDIAN study of 2918 CABG patients, the 6-month mortality associated with a postoperative peak CK-MB of less than 5, of between 5 and 10, of between 10 and 20, and of greater than 20 times the upper limit of normal (ULN) was 3.4%, 5.8%, 7.8%, and 20.2%, respectively ( Fig. 13.3 ). This highly significant association was conserved even after adjusting for other risk factors. The Cleveland Clinic experience with 3812 CABG patients revealed that a postoperative CK-MB level 10 times ULN was independently predictive of increased mortality at 3 years ( Table 13.4 and Fig. 13.4 ). The Arterial Revascularization Therapies Study (ARTS) prospectively evaluated 496 CABG patients and also demonstrated that increased levels of CK-MB (> 5 times ULN) was highly predictive of cardiac death and recurrent MI after the postoperative period ( Fig. 13.5 ).
| CABG patients ( N = 3812) | ||
|---|---|---|
| CK-MB level | At risk, no. (%) | Death (%) |
| ≤ 1 × ULN | 386 (10) | 7.2 |
| 1–3 × ULN | 1922 (50) | 7.7 |
| 3–5 × ULN | 853 (22) | 6.3 |
| 5–10 × ULN | 427 (11) | 7.5 |
| > 10 × ULN | 224 (6) | 20.8 |
| P (trend) | — | < .001 |
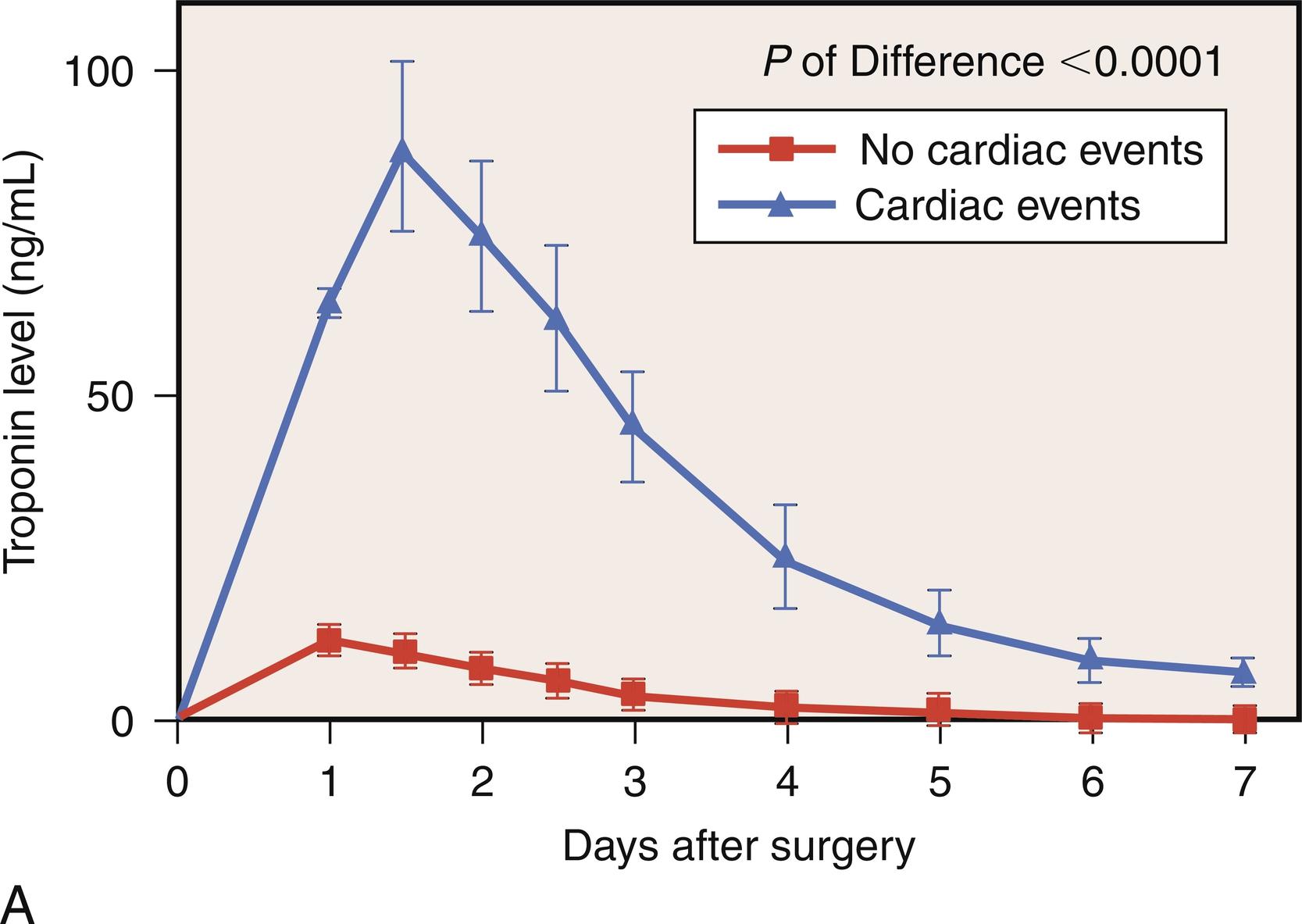
The specificity of CK and CK-MB as markers of myocardial injury is limited by the fact that these enzymes are also present in and released from skeletal muscle. Significant skeletal muscle injury and release of large quantities of CK-MB occurs during cardiac surgery. An abundance of experimental evidence has emerged in the literature on the superior sensitivity and specificity of serum troponins as biochemical markers of myocardial damage and predictors of adverse outcomes. Cardiac troponin isoforms I and T (cTnI, cTnT) are cardiac-specific proteins involved in regulation of muscle contraction via the tropomyosin complex. No cross-reactivity with skeletal muscle isoforms has been described, and the levels of cardiac troponins do not increase in healthy subjects, even when they are exposed to strenuous muscular activity or after noncardiac operations. The levels of cTnI also appear to be unaltered by renal dysfunction, and its plasma concentration decreases very slowly, allowing for retrospective detection of myocardial damage if necessary. Interestingly, troponin levels drawn preoperatively may also enable stratification of patients into subgroups with increased risk for postoperative cardiac complications, notably PMI ( Fig. 13.6 ). Outcomes for these high-risk patients, who presumably have undetected ongoing myocardial injury, might be improved with a tailored perioperative approach that would include pharmacologic support or an intra-aortic balloon pump (IABP).
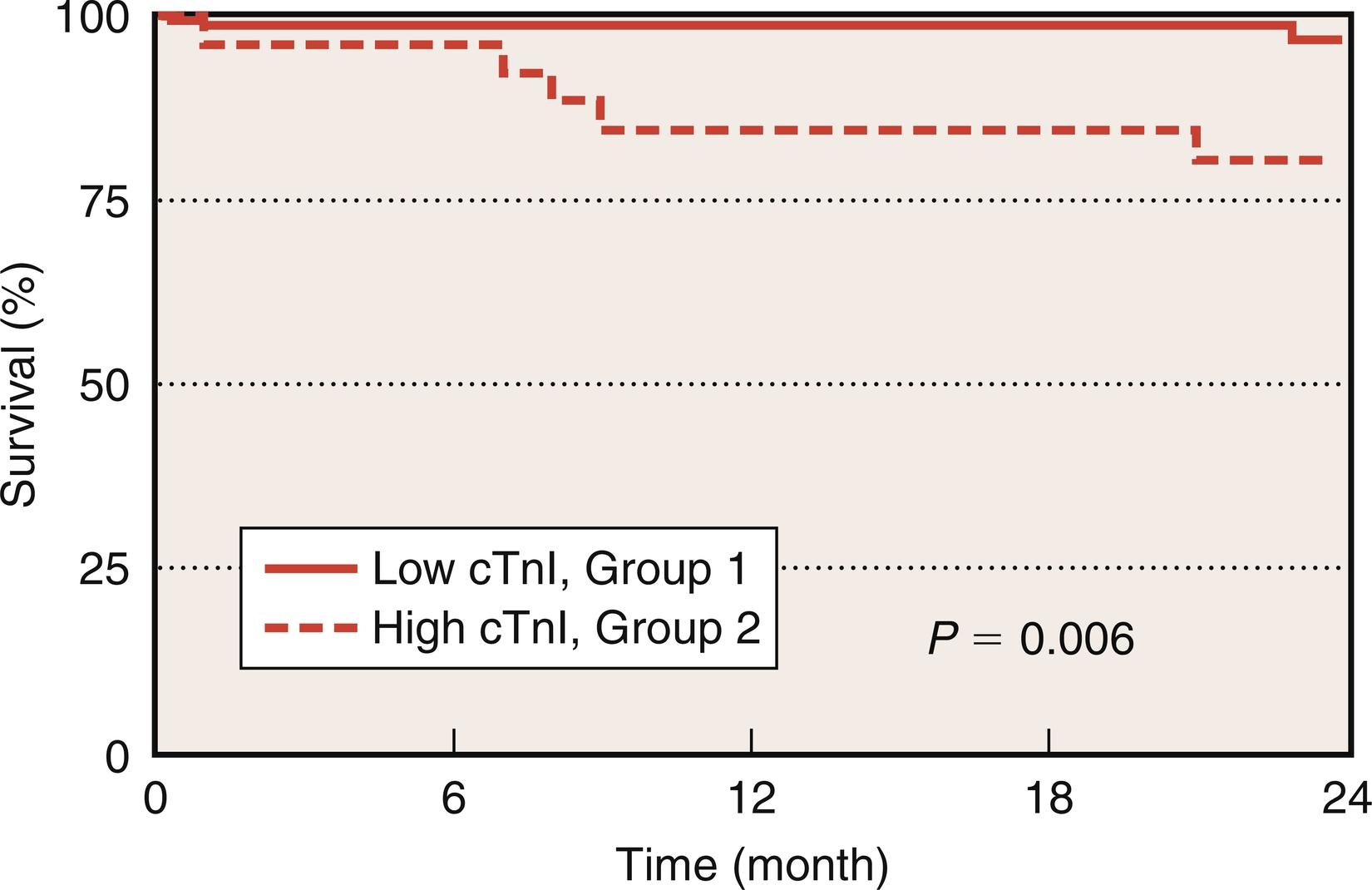
A study comparing the efficacies of cTnT and CK-MB in a series of 224 cardiac surgery patients indicated that serum cTnT concentrations greater than 1.58 ng/mL (which represented the upper quintile) were the most powerful independent predictor of postoperative death or shock within the first 24 hours after surgery. Greenson and colleagues demonstrated that stratification of cardiac surgery patients by peak cTnI levels of less than 40 and greater than 60 ng/mL was exquisitely predictive of intensive care unit (ICU) length of stay, prolonged mechanical ventilation, new arrhythmia development, and especially cardiac events ( Fig. 13.7 ). Similar results were obtained in a larger series of 502 patients undergoing conventional heart surgery, where the cTnI level proved to be an independent predictor of in-hospital mortality and associated with other major postoperative complications such as PMI, ventricular arrhythmias, low cardiac output, prolonged mechanical ventilation, and acute renal failure requiring dialysis ( Table 13.5 ). The long-term prognostic value of cTnI levels has also been validated, and it is clearly associated with increased mortality, death from cardiac causes, and nonfatal cardiac events within 2 years after CABG ( Fig. 13.8 ). The variable cutoffs of predictive troponin values reported by different studies is concerning and may be attributed to lack of standardization of troponin detection kits by different suppliers.
| cTnI Concentration a ( ng/mL) | ||
|---|---|---|
| Complication | With | Without |
| complication | complication | |
| (No. of patients) | (No. of patients) | |
| Q-wave perioperative | 56.5 [43; 175.5] b | 4.2 [2.4; 7.6] |
| myocardial | ( n = 7) | ( n = 495) |
| infarction | ||
| Ventricular | 8.4 [4.2; 28.2] b | 4.3 [2.4; 8.0] |
| arrhythmias | ( n = 17) | ( n = 485) |
| Low cardiac output | 9.5 [4.4; 26.78] b | 4.3 [2.4; 7.7] |
| ( n = 81) | ( n = 421) | |
| Prolonged (48-h) | 56.5 [5.2; 22.3] b | 4.5 [2.5; 8.1] |
| mechanical | ( n = 46) | ( n = 456) |
| ventilation | ||
| Acute renal failure | 18.2 [9.1; 33.5] b | 4.6 [2.5; 8.2] |
| requiring dialysis | ( n = 18) | ( n = 484) |
a cTnI values are expressed as median [25th; 75th percentiles].
During the preprocedural phase of cardiac surgery, myocardial ischemia usually represents extension of an acute coronary presentation triggered by hypertension, hypotension, hypoxia, tachycardia, bleeding, or surgical manipulation. Causes for postprocedural myocardial ischemic injury include inadequate myocardial protection during surgery, coronary graft failure, native coronary or graft spasm, extension of a preoperative infarction, inadequate revascularization, coronary injury secondary to a valve procedure, and distal coronary embolization. These etiologic considerations are notorious predictors of myocardial ischemia, PMI, and other adverse outcomes in cardiac surgery. Predictors of PMI have been defined and include advanced age, emergent procedure, previous revascularization, longer duration of cardiopulmonary bypass (CPB) or aortic cross-clamp time, need for inotropic support, need for coronary relocation (or reimplantation), prior MI, and preoperative ventricular dysfunction. Awareness of high-risk patients may increase monitoring and enable earlier intervention.
Prior to the widespread implementation of cold potassium cardioplegia and other advancements in myocardial preservation, PMI often resulted from suboptimal myocardial preservation. Consistent with this etiology was the pathologic finding of contraction band necrosis, which indicated intraoperative ischemia and subsequent reperfusion injury. A high percentage of infarctions analyzed from patients in these early studies were in areas supplied by patent grafts, again suggestive of injury mediated by an ischemia-reperfusion phenomenon. Improvements in techniques of myocardial preservation have reduced the incidence of perioperative myocardial ischemia and PMI.
Myocardial preservation techniques have undergone significant evolution during the past five decades. The implementation of cold crystalloid cardioplegia was an important early advancement that improved overall outcomes. The introduction of blood cardioplegia led to further improvements in maintaining myocardial energy stores and reducing anaerobic lactate production. Clinically, the advantage of blood cardioplegia is supported in studies that show a reduction in PMI and other morbidity when directly compared with crystalloid cardioplegia. The advent of retrograde delivery of cardioplegia via the coronary sinus was another important advance in myocardial protection, allowing fewer cardioplegic interruptions, perfusion to regions supplied by stenosed arteries, and enhanced cardioplegic delivery to the subendocardial region.
An important, relatively recent advance in cardioplegia has been the introduction and wide adoption of del Nido cardioplegia in the adult cardiac surgery population. The crystalloid components of this solution include a 1-L Plasma-Lyte base solution with mannitol, magnesium sulfate, sodium bicarbonate, potassium chloride, and lidocaine added. Unlike other cardioplegia solutions, del Nido is given as a single dose, with less need for frequent redosing. Even in low-risk, first-time CABG patients, the use of del Nido cardioplegia was associated with less defibrillation and lower costs. Its use has significantly increased in minimally invasive cardiac surgery, mainly due to decreased need for additional dosing. In a recent study by Vistarini et al, del Nido cardioplegia was found to be safe and easy in a group of consecutive patients undergoing minimally invasive aortic valve replacement. Most of the data related to the use of del Nido in adult cardiac surgery patients has been retrospective in nature, with the lack of large-scale randomized controlled trials.
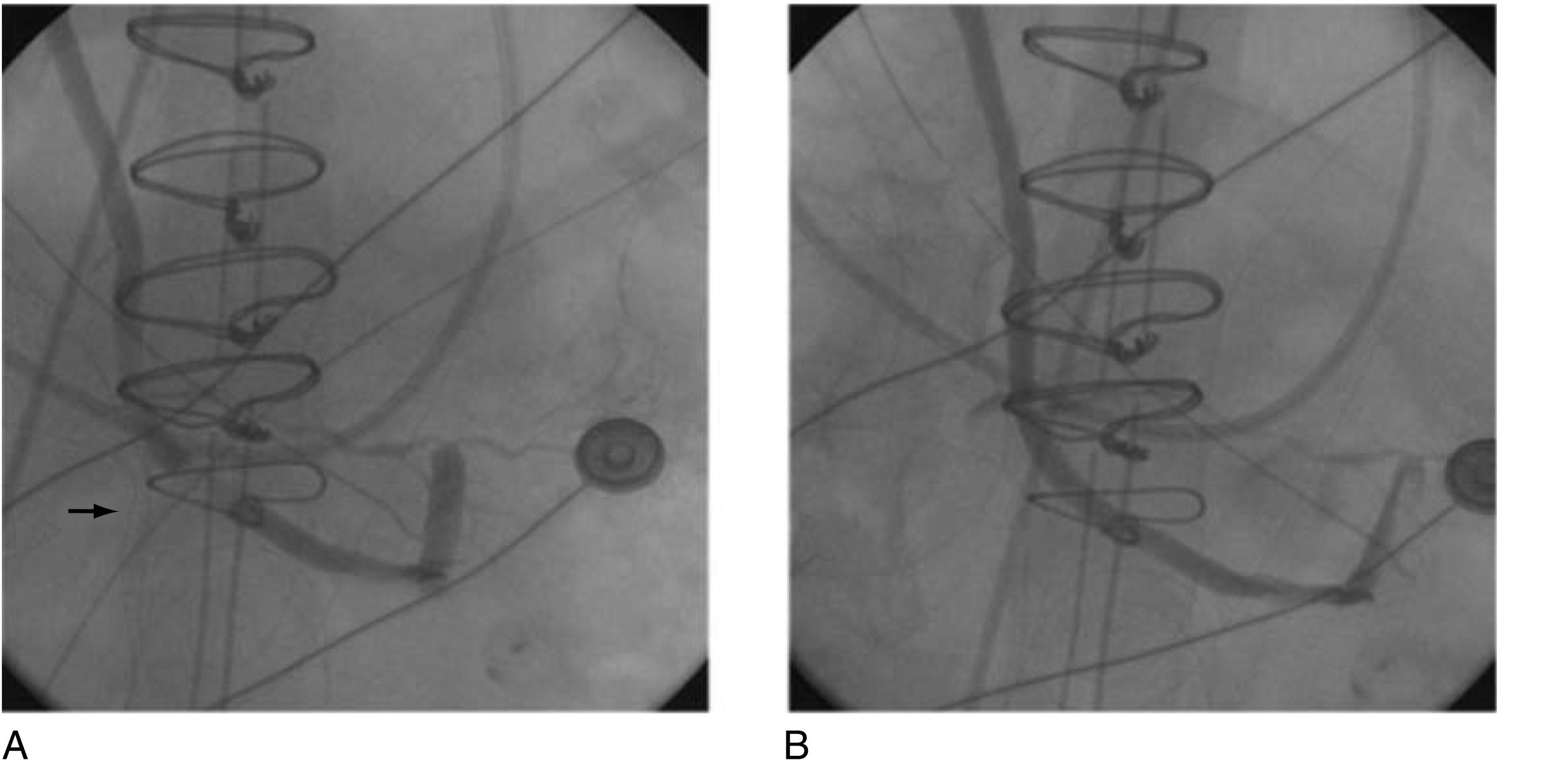
Ischemic injury in the current era may be more frequently the result of technical issues, early graft occlusion, anastomotic stenosis, graft, or native coronary spasm ( Figs. 13.9 to 13.11 ), poor distal runoff, incomplete revascularization, steal phenomena, or embolization. Graft compression by mediastinal chest drains has even been described as a cause of ischemic injury ( Fig. 13.12 ). Atheromatous embolization in the coronary microcirculation can also occur intraoperatively and lead to PMI. Potential embolic sources include atherosclerotic plaque present in the ascending aorta or major epicardial coronary arteries. In the reoperative setting, mechanical disruption of atheroma in old vein grafts has also been described. Retrograde cardioplegia may be protective in cases of coronary or graft embolization.
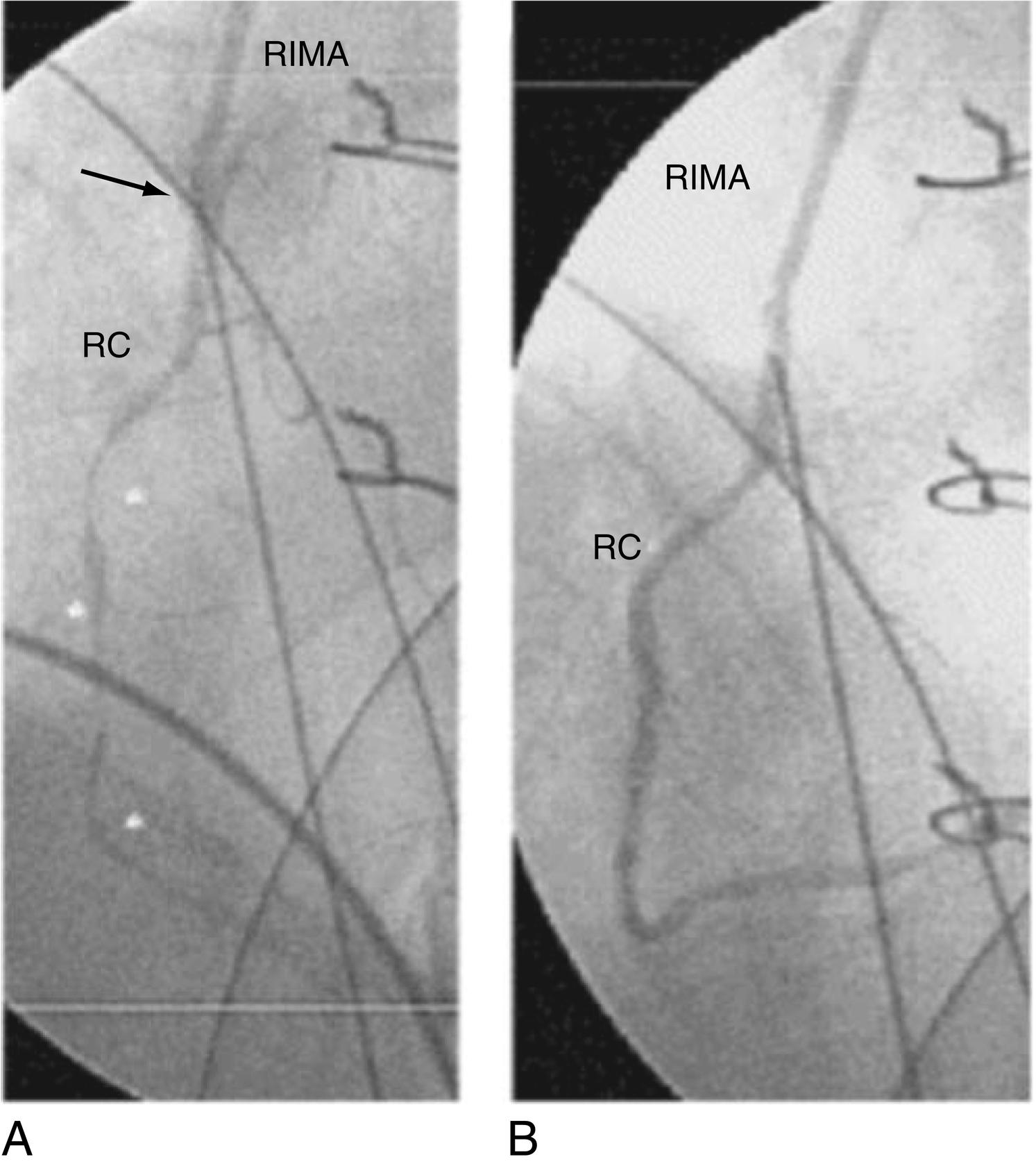
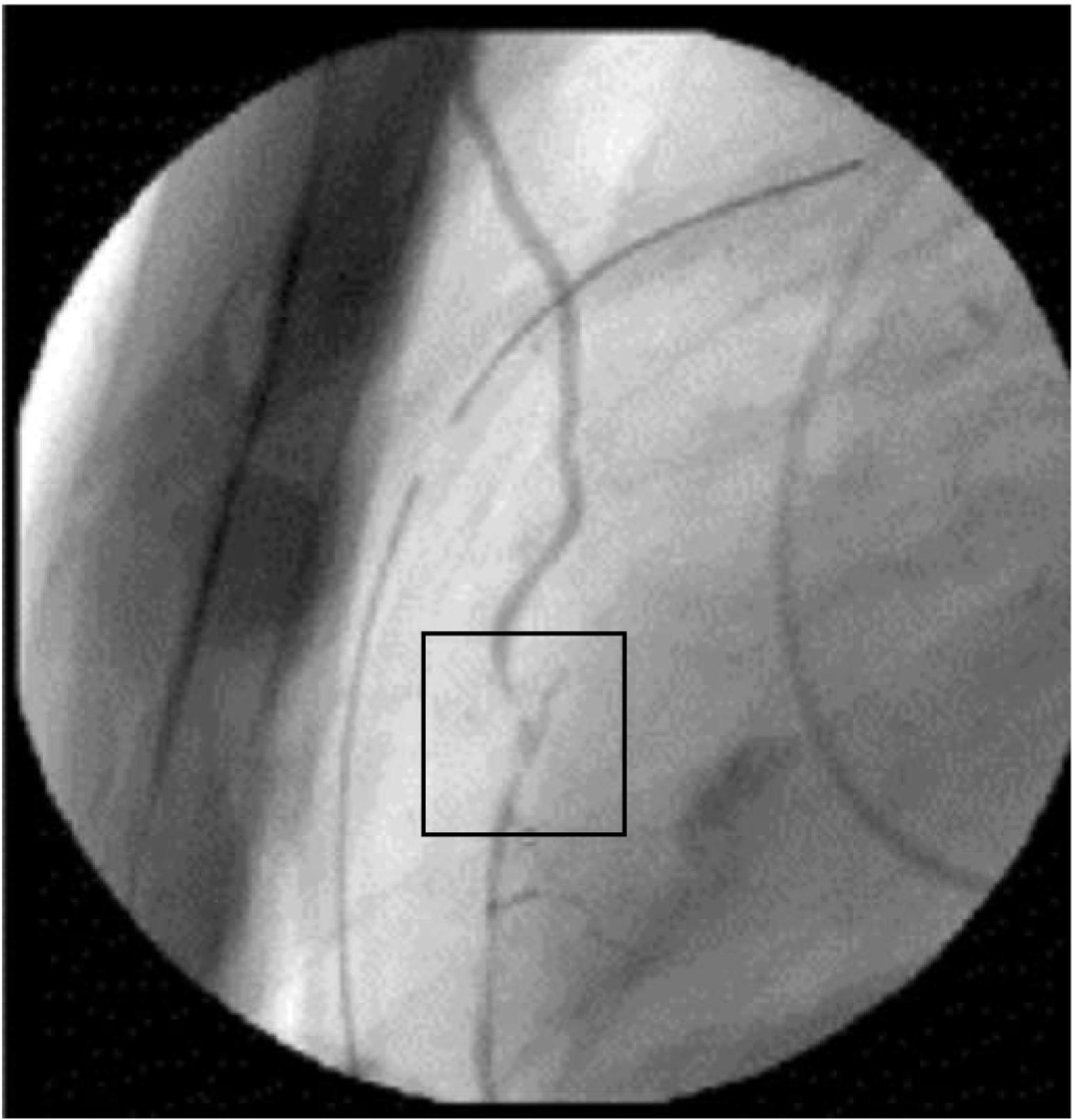
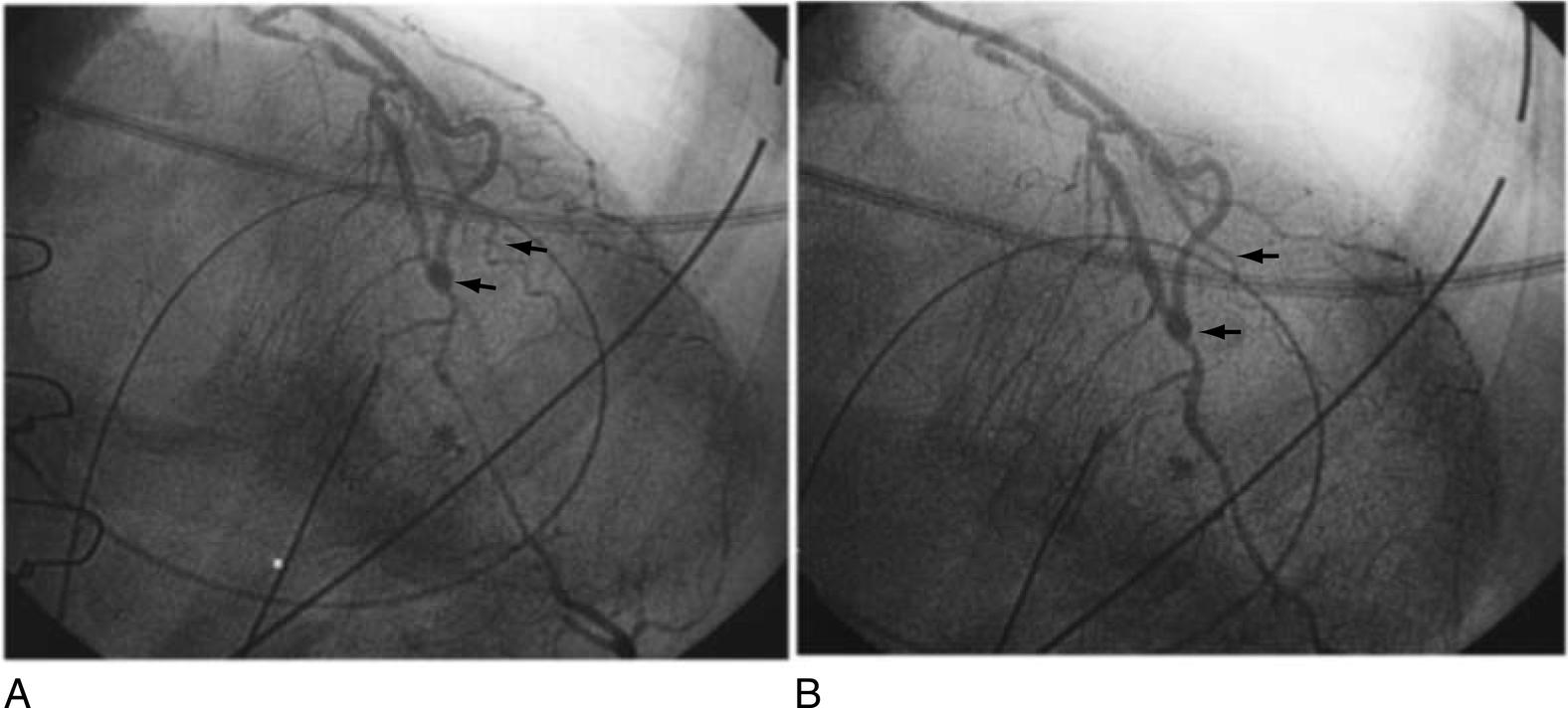
PMI caused by coronary spasm occurs with an incidence as high as 1% and involves both grafted and ungrafted vessels. Coronary spasm can lead to sudden hemodynamic collapse and is often heralded by acute hypotension. Conventional treatment methods for perioperative hypotension, such as pressor agents, may only exacerbate the vasospastic reaction in this scenario. Similarly, in patients experiencing PMI associated with a hyperdynamic state, elevated circulating catecholamine levels create an environment conducive to persistent coronary spasm. Mechanistically, abrupt withdrawal of preoperative calcium channel blockers can lead to rebound vasoconstriction of the native coronary arteries and is an important factor to consider in preoperative planning. Additional factors that may render cardiac surgical patients particularly susceptible to coronary vasospasm include thromboxane release caused by heparin–protamine interactions, CPB, platelet activation, anaphylactic reactions, calcium administration, and increased alpha-adrenergic tone from vasoconstrictor administration.
Another well-described etiology related to perioperative ischemic injury is the internal mammary artery (IMA) malperfusion syndrome, caused by inadequate myocardial perfusion through the IMA graft. In a series of 2326 CABG patients in which the IMA was used, 45 patients (1.9%) with this syndrome were identified. Although the IMA is the proven conduit of choice for myocardial revascularization, immediate flow through this arterial graft is generally less than through a saphenous vein graft and may not adequately meet myocardial demand. Other factors that may precipitate this syndrome include vasospasm, kinking, or overstretching of the IMA; steal phenomena via flow diversion into major intercostal side branches; and residual competitive flow from the native coronary artery. As with radial artery grafts, direct mechanical manipulation of the IMA during harvesting may produce transient endothelial dysfunction and provoke a vasospastic response.
A number of large angiographic studies have correlated clinical suspicion of myocardial ischemia or PMI with angiographic evidence of graft failure. In a series of 2003 CABG patients, 71 (3.5%) had suspected myocardial ischemia and underwent acute reangiography ( n = 59) or an immediate reoperation ( n = 12) if they were hemodynamically unstable. Angiographic findings in the first group included the following: occluded vein graft(s) (32%), poor distal runoff to the grafted coronary vessel (17%), IMA stenosis (7%), IMA occlusion (5%), vein graft stenoses (5%), and IMA subclavian artery steal (3%). In the immediately reoperated group, 92% of patients had graft occlusions. A more recent study including 2052 CABG patients had similar findings. These studies emphasize that the majority of patients who experience myocardial ischemia or PMI following revascularization have underlying graft failure warranting angiography and either percutaneous or surgical reintervention.
Valvular procedures may lead to technical complications with a specific myocardial ischemia. The proximity of the posterior mitral annulus and the left circumflex coronary artery predisposes to circumflex injury and LV lateral wall ischemia with mitral reconstruction. An analogous situation exists for tricuspid valve surgery and the right coronary artery. Aortic valve procedures may compromise either the left main coronary or the right coronary ostia, and cases of coronary ostial thrombosis with severe ischemia have been reported. Finally, debrided valvular material may also be involved in deleterious coronary embolization.
The preprocedural phase is a critical period during which appropriate clinical management can prevent myocardial ischemia and PMI. Stabilization of blood pressure and maintenance of normal sinus rhythm should be the goal. A rate–pressure product below 12,000 may minimize perioperative myocardial ischemia. The importance of precise and continuous hemodynamic measurements cannot be overemphasized. It should at least include continuous monitoring of the ECG, cuff blood pressure, direct arterial pressure, central venous pressure (CVP), continuous pulse oximetry, and end-tidal CO 2 . Placement of a pulmonary artery (PA) catheter is warranted for high-risk patients, for example, in cases of unstable angina, ventricular hypertrophy, poor ventricular function, and combined valvular and coronary artery disease pathology. The PA catheter enables continuous assessment and maintenance of cardiac filling pressures, cardiac output, and mixed venous oxygen saturation (MVO 2 ) to prevent myocardial ischemia.
Pharmacotherapy with vasodilators such as intravenous nitroglycerin (NTG) may be required preoperatively for patients with unstable angina. The reduction in total ischemic burden may improve prognosis with regard to infarct progression and may prevent deterioration of LV function. Intravenous NTG has also been shown to improve postinduction regional wall motion abnormalities in a prospective study of CABG patients. On the basis of these results, TEE-guided NTG use after induction may yield a more optimal baseline echocardiogram. Other important pharmacotherapies to reduce ischemia include intravenous beta-blockers, with esmolol as the most titratable agent. Beta-blockers reduce heart rate, contractility, and blood pressure and thereby greatly reduce overall myocardial work. Beta-blockers also reduce the incidence of ischemic ventricular and atrial arrhythmias. Use of intravenous NTG and beta-blockers requires careful monitoring and titration, as both agents can induce hypotension; in patients with fixed coronary stenosis, reduced perfusion pressure can result in dangerous ischemia and subsequent myocardial dysfunction.
In patients with cardiogenic shock or medically refractory ischemia, preoperative stabilization with an IABP has been shown to reduce overall surgical mortality and improve outcomes in high-risk coronary patients. IABP counterpulsation enhances myocardial perfusion via augmentation of diastolic blood pressure and redistribution of coronary blood flow toward regions of myocardial ischemia. IABP provides partial LV assistance. Proper timing consists of balloon inflation just after aortic valve closure and deflation immediately prior to systole ( Fig. 13.13 ). Balloon inflation during diastole augments coronary perfusion, whereas deflation during systole reduces afterload, decreases cardiac work (myocardial oxygen consumption), and increases stroke volume and cardiac output. Efficacy of IABP support is determined by several factors, including balloon volume; location in the aorta; rate of inflation and deflation; and, most important, timing relative to the cardiac cycle. IABP counterpulsation is less effective in the presence of moderate or severe aortic regurgitation and should be avoided. Furthermore, timing may be difficult because of tachycardia or arrhythmias. Aortoiliac or femoral occlusive disease may make placement more difficult and increases the risk for limb ischemia.
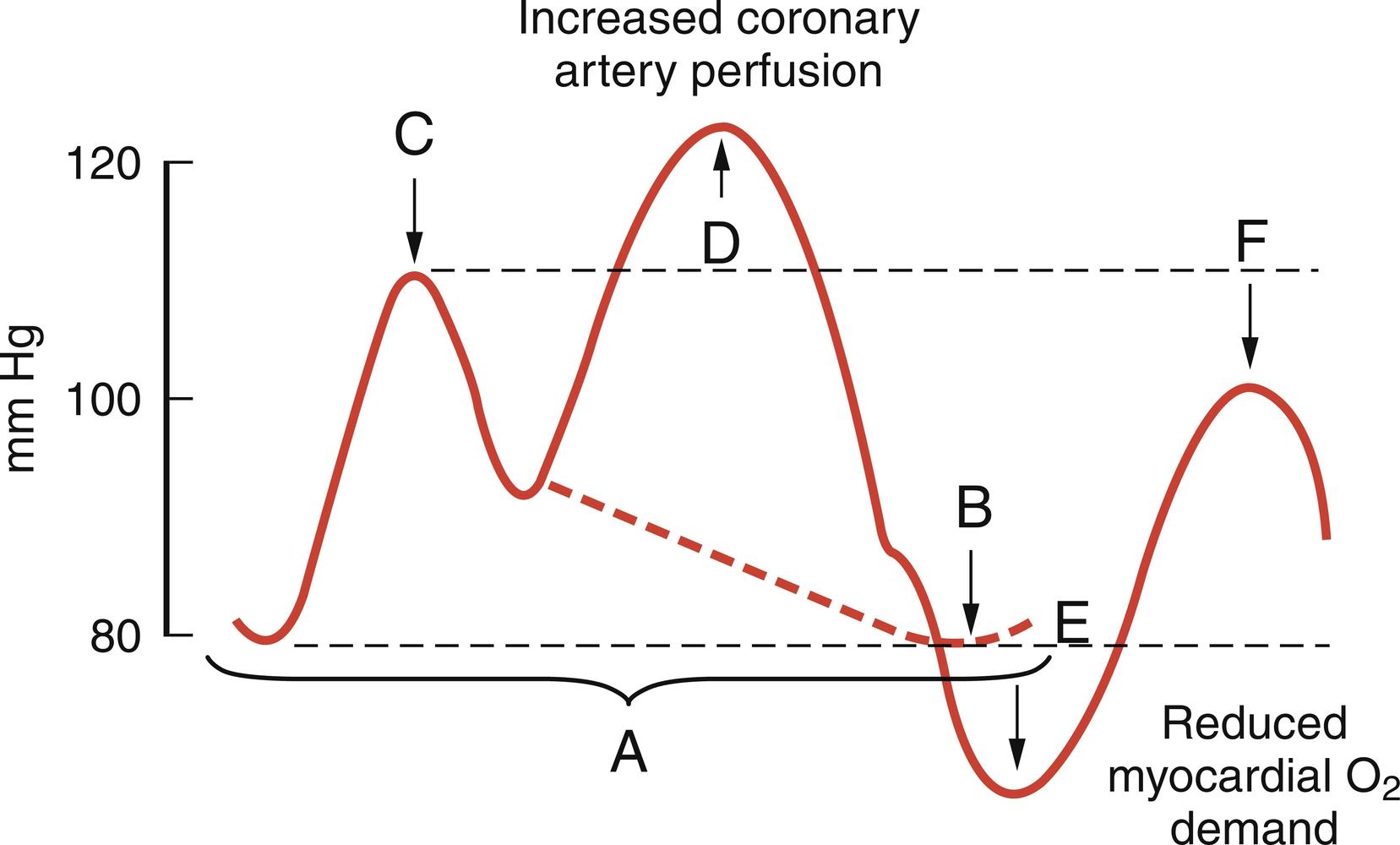
Retrospective review of 4756 cases of IABP support at the Massachusetts General Hospital suggests that a 24- to 48-hour period of preoperative IABP support is preferable in unstable or postinfarction angina and enables preoperative recovery of ischemic myocardium. In this study, preoperative IABP placement was associated with a lower mortality (13.6%) than intraoperative (35.7%) or postoperative (35.9%) use. A prospective, randomized study was performed in 60 high-risk CABG patients to evaluate optimal timing for preoperative IABP placement. In this and other studies, preoperative IABP support led to reduced CPB time, higher postoperative cardiac index, decreased incidence of postoperative low cardiac output, and a diminished period of mechanical ventilation and hospital stay. These studies suggest the beneficial impact of preoperative IABP support in high-risk coronary patients. Preoperative risk factors that designated high-risk status in these studies included preoperative left ventricular ejection fraction (LVEF) less than 30%, unstable angina, left main coronary artery stenosis greater than 70%, diffuse coronary artery disease, and reoperative CABG.
A recent meta-analysis reviewed studies between 1977 and 2015 on the use of IABP in patients undergoing cardia surgery. A total of 46,067 patients were included with 11 randomized controlled trials and 22 observational studies. Analyses from the included randomized controlled trials suggested that IABP prior to CABG was associated with shorter ICU (weighted mean difference [WMD], − 1.47 d; 95% confidence interval [CI], − 1.82 to − 1.12 d; P < .00001) and hospital length of stay (WMD, − 3.25 d; 95% CI, − 5.18 to − 1.33 d; P = .0009). Importantly, IABP use was also found to have a significant reduction in hospital mortality (odds ratio [OR], 0.20; 95% CI, 0.09–0.44; P < .0001) and 30-day mortality compared with no preoperative IABP (OR, 0.43; 95% CI, 0.25–0.76; P = .003).
Patients with profound preprocedural myocardial ischemia or evolving MI probably benefit from a strategy that rapidly establishes CPB. Currently, many surgical revascularizations are performed without CPB, and for others, the initial period of conduit harvest is conducted off CPB. In unstable patients, immediate institution of CPB may dramatically reduce myocardial workload and risk for ischemic injury. Conduit harvest and further surgical preparation can be performed with the heart protected on CPB. In the setting of reoperative cardiac surgery and prior sternotomy, groin dissection and cannulation of the femoral vessels may be the safest and most rapid method to establish CPB. Aggressive use of mechanical unloading may help avoid ischemic ventricular arrhythmias and ventricular dysfunction, which may be difficult to reverse once established. Notably, CPB does not directly unload the left heart and further LV unloading can be achieved with the addition of an LV vent.
During the intraoperative postprocedural phase, rigorous attention to ECG pattern, hemodynamic parameters, and TEE findings must be continued. As discussed previously, the collective information obtainable with these modalities can be sensitive and specific for intraoperative myocardial ischemia and PMI. For cases of revascularization, intraoperative ischemic ECG patterns, new regional wall motion abnormalities, or hemodynamic instability requires assessment of the grafts to rule out graft failure as the cause. Assessment of graft integrity and graft patency can be readily accomplished using Doppler flow probes. These probes provide a rapid and highly sensitive method of detecting graft failure, as shown in a recent study of patients undergoing off-pump CABG. In this study, flow probe results were correlated with intraoperative angiographic findings, and Doppler analysis was 100% accurate for confirming graft patency and detecting failed grafts. To qualify as a normal Doppler study, the observed pattern of flow should be predominantly diastolic, with a diastolic flow velocity of greater than 15 cm/s. Additional flow parameters to consider when evaluating graft integrity include total flow, systolic flow, diastolic-to-total flow ratio, systolic peak flow, diastolic peak flow, systolic-to-diastolic peak flow index, and pulsatility index (PI; defined as difference between systolic peak flow and diastolic flow divided by mean flow). The PI value should be between 1 and 5, where higher values are indicative of a graft problem. When an abnormal Doppler flow study is encountered, consideration should be given to revision of the offending graft. This decision is, however, complex and ultimately relates to the surgeon’s confidence in achieving a better technical result. Small or diffusely diseased target coronary arteries predictably result in grafts with limited flow. In a recent analysis for a large multicenter CABG trial, 1-year graft patency and intraoperative revision rates in patients undergoing CABG based on intraoperative flow probe assessment were evaluated. For the 1607 patient (2738 grafts) with available flow probe data, 1-year FitzGibbon grade A patency and intraoperative revision were compared based on TTF measurements (< 20 [low flow] vs ≥ 20 mL/min [normal flow]) and PI values (< 3, 3–5, and > 5). FitzGibbon grade A patency was much less common in grafts with a low flow (71.3%) vs (87.2%) for graft having a normal flow. An inverse relationship was seen between 1-year patency and intraoperative PI values, with 85.6% of grafts having FitzGibbon A patency when the PI < 3, 74.7% with a PI of 3–5, and 67.9% with a PI > 5 ( P ≤ .01). As expected, intraoperative graft revision was more frequent in grafts with low flow at 7.7% than in those with normal flow (0.4%; P < .01). What remains unknown is the percentage of grafts that had a meaningful improvement after revision versus those that did not.
Perhaps one of the most common causes of postprocedural myocardial ischemia is coronary air embolization. This event is important to recognize; the right coronary artery or right coronary graft is specifically affected. Air may actually be seen within the graft, and needle aspiration of the graft can help limit the problem. The left coronary system is more dependent and infrequently involved. Profound right ventricular (RV) dysfunction can follow. TEE may show inferior LV wall myocardial air. Venting of the heart, aorta, or graft is indicated. Often, transiently raising perfusion pressure with an alpha-adrenergic receptor agonist alleviates the problem, presumably by forcing the obstructing air out of the major coronary arterial branches. Performing procedures under CO 2 , which dissolves more readily in blood, may reduce this complication. Importantly, in the absence of continued air embolization, this ischemia should resolve rapidly, and persistent evidence for ischemia warrants consideration of other causes.
The early postoperative phase should include careful hemodynamic and clinical monitoring. Adequate oxygen delivery and systemic blood flow must be ensured: MVO 2 , cardiac output, blood pressure, heart rate, urine output, acid–base status, and pulse should all be carefully monitored. The presence of myocardial ischemia may be indicated by ST-segment changes (via ECG monitoring), ventricular arrhythmia, or hemodynamic collapse. In other cases, more subtle hemodynamic changes or increased inotropic requirement may be the only clues. Many postcardiac surgery units obtain routine postprocedural 12-lead ECGs and serial serum cardiac enzyme results. Concerns for myocardial ischemia warrant echocardiography to assess LV function and regional wall motion.
Administration of calcium antagonists in the postoperative period may reduce rates of myocardial ischemia, PMI, and long-term mortality. These agents enhance the balance between myocardial oxygen supply and demand by mediating negative chronotropy, negative inotropy, afterload reduction, and coronary vasodilation. Recent studies have advocated the use of this therapy to prevent post-CABG graft vasospasm. Native coronary or graft spasm should always be suspected in the setting of postoperative ischemia, and aggressive management with NTG and a sublingual calcium channel blocker should be instituted. These agents are routinely recommended when radial arterial grafts have been used. Unfortunately, postoperative myocardial ischemia may be secondary to hypotension or low cardiac output, limiting the use of calcium channel blockers and NTG.
Postoperative ischemia or PMI accompanied by hemodynamic instability necessitates IABP placement and urgent reoperation. In more stable patients with evidence of significant myocardial ischemia, coronary angiography should be strongly considered. Early angiography can identify technical issues and guide further therapy before permanent myocardial damage has occurred. Specifically, graft or native artery occlusions or stenoses may be identified, and angioplasty or stenting of the native vessels or new grafts can be safely achieved. Similarly, angiography can identify native coronary or graft vasospasm and enables targeted intraluminal infusion of NTG or calcium channel blocker (see Figs. 13.9 to 13.11 ). In cases of IMA steal phenomenon, transarterial catheter embolization to obliterate undivided vessels is another effective treatment that is feasible in the angiography suite. Furthermore, specific problems may be identified angiographically that require return to the operating room for surgical revision. Important examples include incomplete revascularization and the IMA malperfusion syndrome, in which placement of an additional saphenous vein graft is widely regarded as the optimal treatment option.
Clinical studies have evaluated other alternative pharmacotherapies to prevent ischemia-reperfusion injury and PMI in the setting of cardiac surgery. The Pexelizumab for Reduction in Infarction and Mortality in Coronary Artery Bypass Graft surgery (PRIMO-CABG) study was a randomized, double-blind, placebo-controlled prospective trial evaluating whether pexelizumab, a C5 complement inhibitor, would decrease PMI in CABG patients. Therapy with this agent resulted in a significant reduction in the 30-day incidence of death or MI in 3099 patients undergoing CABG surgery with or without valve surgery. A follow-up trial in higher-risk patients (PRIMO-CABG II) failed to meet the primary endpoint of death or MI at 30 days or the development of new or worsening congestive heart failure. However, in a combined analysis of both trials (PRIMO-CABG I and II; N = 7353), death at 30 days was significantly reduced for the greatest risk subset ( n = 2156; pexelizumab 5.7% vs placebo 8.1%; P = .024).
Sodium–hydrogen exchange inhibition with the agent cariporide has also demonstrated promising results. In a study evaluating the CABG cohort of the GUARDIAN trial, therapy with cariporide yielded a modest risk reduction in all-cause mortality and MI that was evident on postoperative day 1 and persisted at 6 months postoperatively. These therapies remain far from wider clinical adoption, however.
Postoperative ventricular failure with low cardiac output state after cardiac surgery is an important predictor of perioperative mortality and sudden death ( Figs. 13.14 and 13.15 ). Even patients with normal preoperative ventricular function exhibit transient dysfunction after uneventful cardiac operations. When ventricular dysfunction is encountered in the perioperative period, treatment should consist of restoring adequate oxygen delivery to prevent end-organ damage and rapid recognition and correction of the underlying cause. In addition, recognition of well-described preoperative risk factors for the development of low cardiac output syndrome may prevent its occurrence via earlier intervention and more aggressive preoperative optimization. A retrospective review of 4558 CABG patients identified eight independent predictors of postoperative ventricular dysfunction. These risk factors included preoperative LVEF less than 20%, redo operation, emergency operation, female sex, age greater than 70, left main coronary artery stenosis, recent MI, and extensive three-vessel coronary artery disease. The observed incidence of low cardiac output was 9.1% in this population, with an operative mortality of 16.9%, as opposed to 0.9% in the remaining patients with normal ventricular function. In another study of over 20,000 CABG patients, the incidence of low cardiac output syndrome varied from 6% with preoperative LVEF greater than 40% to 23% in patients with LVEF less than 20%. Importantly, these studies highlight that advances in therapeutic management strategies have led to a decline in mortality rates and perioperative low cardiac output states despite the increasing risk profile of cardiac surgery patients.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here