Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Once oxygen (O 2 ) has diffused from the alveoli into the pulmonary blood, it is transported to the tissue capillaries almost entirely in combination with hemoglobin. The presence of hemoglobin in the red blood cells allows the blood to transport 30 to 100 times as much O 2 as could be transported in the form of dissolved O 2 in the water of the blood.
In the body’s tissue cells, O 2 reacts with various foodstuffs to form large quantities of carbon dioxide (CO 2 ). This CO 2 enters the tissue capillaries and is transported back to the lungs. Carbon dioxide, like O 2 , also combines with chemical substances in the blood that increase CO 2 transport 15- to 20-fold.
This chapter presents the physical and chemical principles of O 2 and CO 2 transport in the blood and tissue fluids qualitatively and quantitatively.
In Chapter 40 , we pointed out that gases can move from one point to another by diffusion, and that the cause of this movement is always a partial pressure difference from the first point to the next. Thus, O 2 diffuses from the alveoli into the pulmonary capillary blood because the oxygen partial pressure (P o 2 ) in the alveoli is greater than the P o 2 in the pulmonary capillary blood. In the other tissues of the body, a higher P o 2 in the capillary blood than in the tissues causes O 2 to diffuse into the surrounding cells.
Conversely, when O 2 is metabolized in the cells to form CO 2 , the intracellular CO 2 partial pressure (P co 2 ) rises, causing CO 2 to diffuse into the tissue capillaries. After blood flows to the lungs, the CO 2 diffuses out of the blood into the alveoli because the P co 2 in the pulmonary capillary blood is greater than that in the alveoli. Thus, the transport of O 2 and CO 2 by the blood depends on both diffusion and the flow of blood. We now consider quantitatively the factors responsible for these effects.
The top part of Figure 41-1 shows a pulmonary alveolus adjacent to a pulmonary capillary, demonstrating diffusion of O 2 between alveolar air and pulmonary blood. The P o 2 of the gaseous O 2 in the alveolus averages 104 mm Hg, whereas the P o 2 of the venous blood entering the pulmonary capillary at its arterial end averages only 40 mm Hg because a large amount of O 2 was removed from this blood as it passed through the peripheral tissues. Therefore, the initial pressure difference that causes O 2 to diffuse into the pulmonary capillary is 104 − 40 mm Hg, or 64 mm Hg. In the graph at the bottom of the figure, the curve shows the rapid rise in blood P o 2 as the blood passes through the capillary; the blood P o 2 rises almost to that of the alveolar air by the time the blood has moved a third of the distance through the capillary, becoming almost 104 mm Hg.
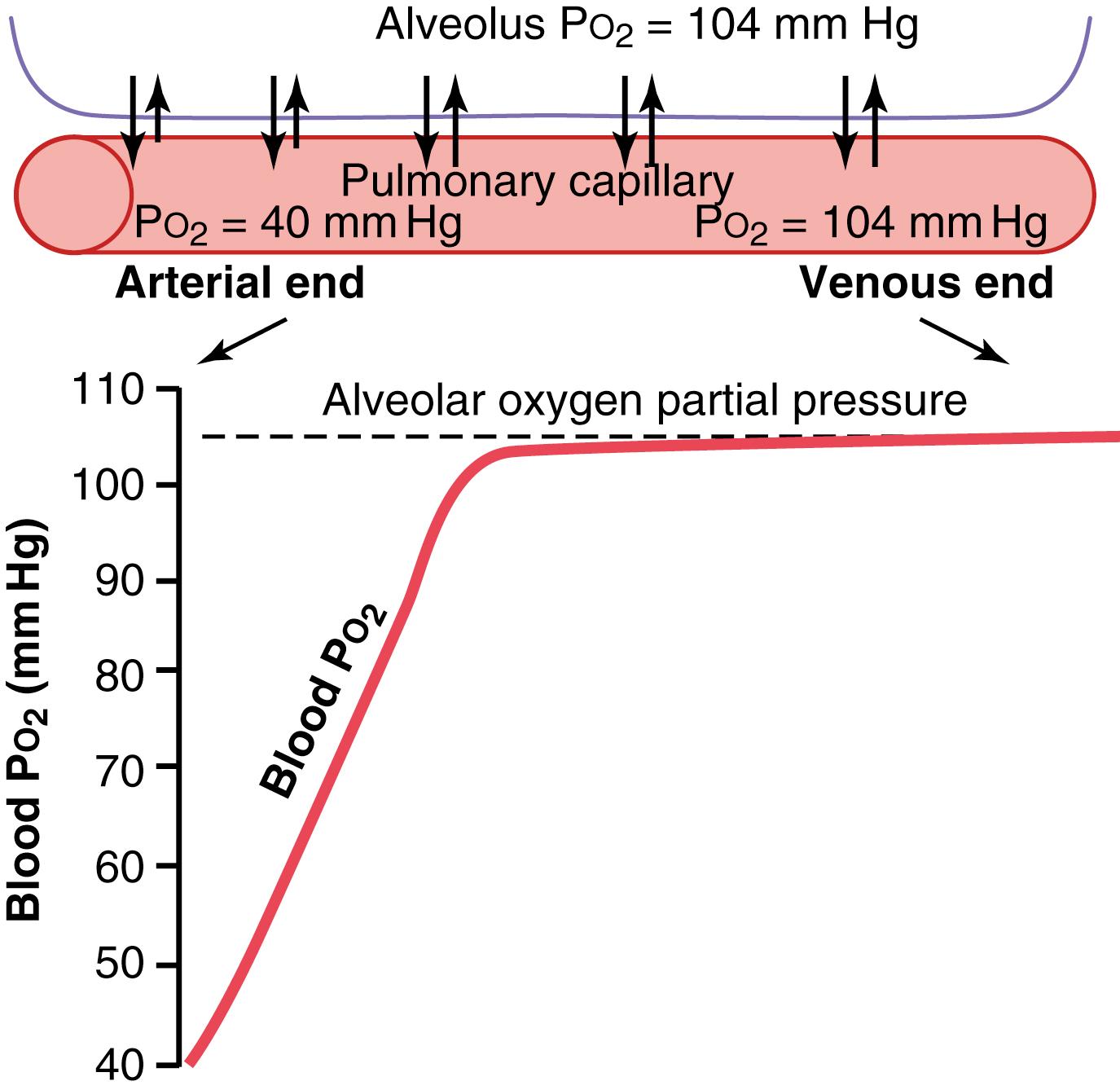
During strenuous exercise, a person’s body may require as much as 20 times the normal amount of oxygen. Also, because of increased cardiac output during exercise, the time that the blood remains in the pulmonary capillary may be reduced to less than one-half normal. Yet, because of the great safety factor for diffusion of O 2 through the pulmonary membrane, the blood still becomes almost saturated with O 2 by the time it leaves the pulmonary capillaries. This can be explained as follows.
First, in Chapter 40 , we pointed out that the diffusing capacity for O 2 increases almost threefold during exercise. This results mainly from increased surface area of capillaries participating in the diffusion and also from a more nearly ideal ventilation-perfusion ratio in the upper part of the lungs.
Second, note in the curve of Figure 41-1 that under nonexercising conditions, the blood becomes almost saturated with O 2 by the time it has passed throughone-third of the pulmonary capillary, and little additional O 2 normally enters the blood during the latter two-thirds of its transit. That is, the blood normally stays in the lung capillaries about three times as long as needed to cause full oxygenation. Therefore, during exercise, even with a shortened time of exposure in the capillaries, the blood can still become almost fully oxygenated.
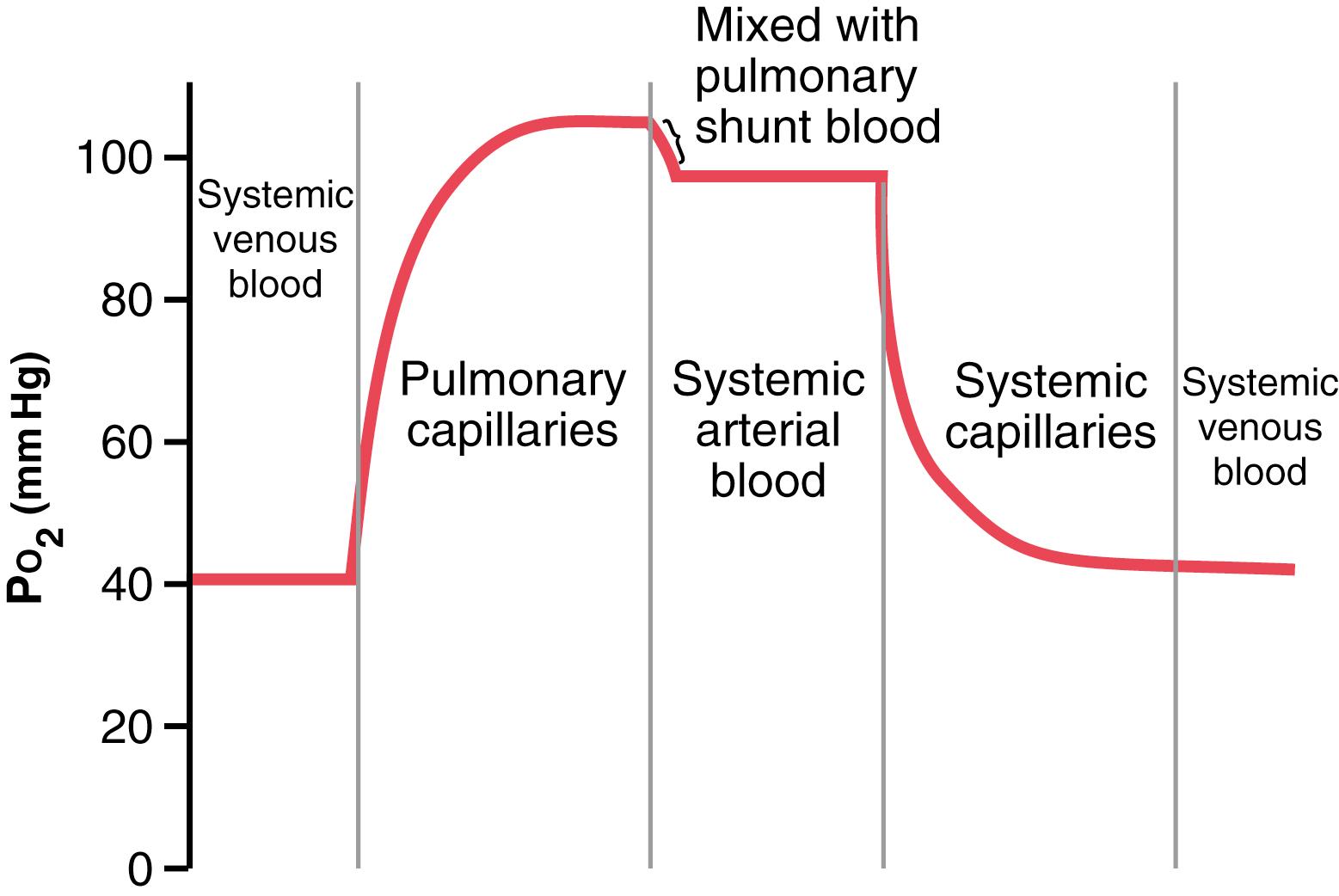
About 98% of the blood that enters the left atrium from the lungs has just passed through the alveolar capillaries and has become oxygenated up to a P o 2 of about 104 mm Hg. Another 2% of the blood has passed from the aorta through the bronchial circulation, which supplies mainly the deep tissues of the lungs and is not exposed to lung air. This blood flow is called shunt flow , meaning that blood is shunted past the gas exchange areas. On leaving the lungs, the P o 2 of the shunt blood is approximately that of normal systemic venous blood—about 40 mm Hg. When this blood combines in the pulmonary veins with the oxygenated blood from the alveolar capillaries, this so-called venous admixture of blood causes the P o 2 of the blood entering the left heart and pumped into the aorta to fall to about 95 mm Hg. These changes in blood P o 2 at different points in the circulatory system are shown in Figure 41-2 .
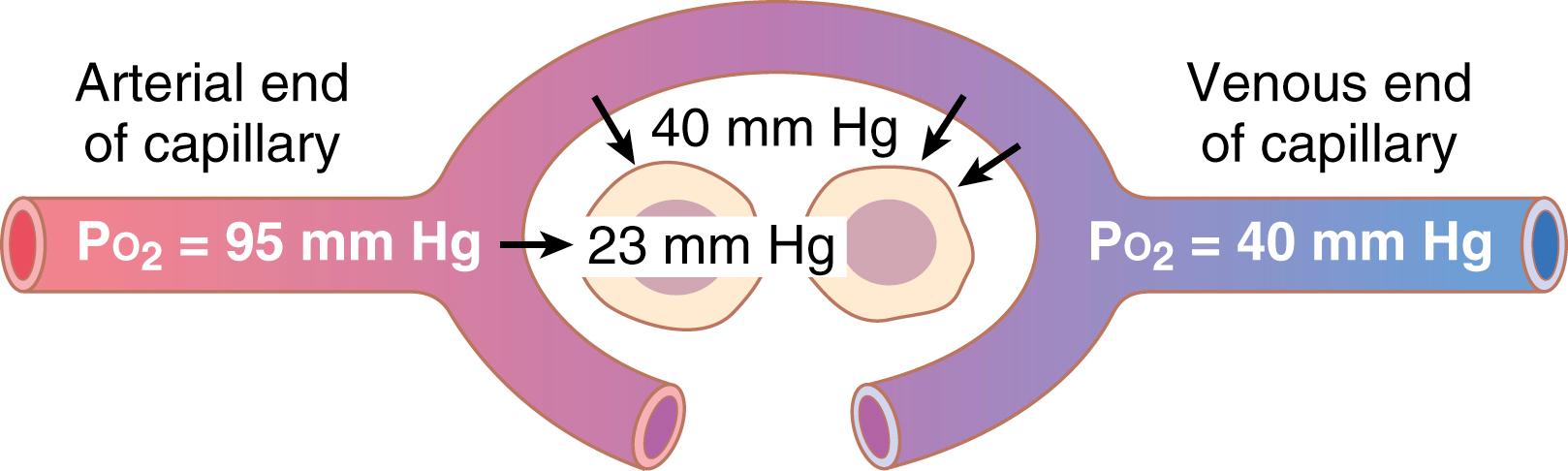
When the arterial blood reaches the peripheral tissues, its P o 2 in the capillaries is still 95 mm Hg. Yet, as shown in Figure 41-3 , the P o 2 in the interstitial fluid that surrounds the tissue cells averages only 40 mm Hg. Thus, there is a large initial pressure difference that causes O 2 to diffuse rapidly from the capillary blood into the tissues—so rapidly that the capillary P o 2 falls almost to equal the 40-mm Hg pressure in the interstitium. Therefore, the P o 2 of the blood leaving the tissue capillaries and entering the systemic veins is also about 40 mm Hg.
If the blood flow through a particular tissue is increased, greater quantities of O 2 are transported into the tissue, and the tissue P o 2 becomes correspondingly higher. This effect is shown in Figure 41-4 . Note that an increase in flow to 400% of normal increases the P o 2 from 40 mm Hg (at point A in the figure) to 66 mm Hg (at point B). However, the upper limit to which the P o 2 can rise, even with maximal blood flow, is 95 mm Hg because this is the O 2 pressure in the arterial blood. Conversely, if blood flow through the tissue decreases, the tissue P o 2 also decreases, as shown at point C.
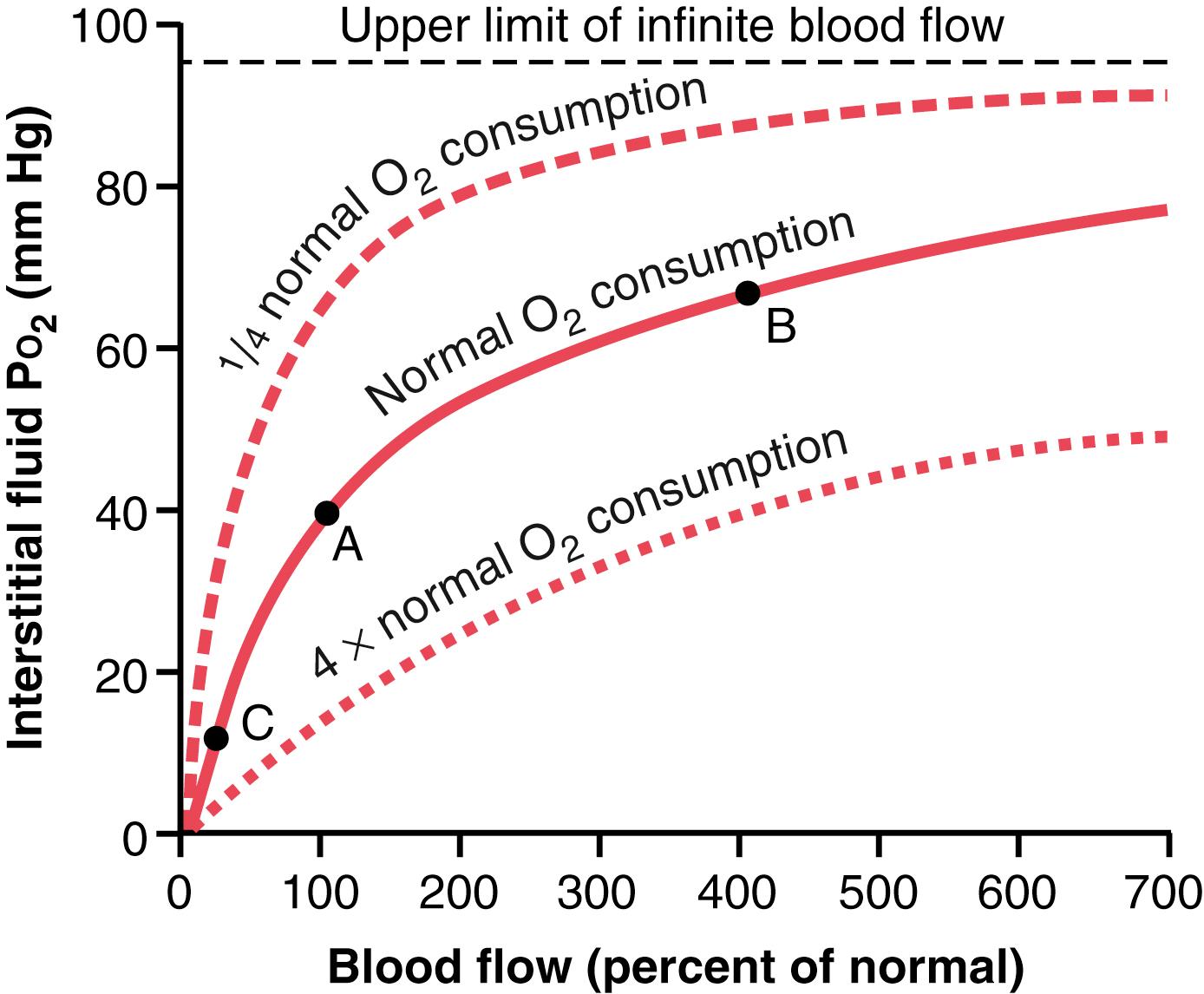
If the cells use more O 2 for metabolism than normal, the interstitial fluid P o 2 is reduced. Figure 41-4 also demonstrates this effect, showing reduced interstitial fluid P o 2 when the cellular oxygen consumption is increased and increased P o 2 when consumption is decreased.
In summary, tissue P o 2 is determined by a balance between (1) the rate of O 2 transport to the tissues in the blood, and (2) the rate at which the O 2 is used by the tissues.
Oxygen is always being used by the cells. Therefore, the intracellular P o 2 in peripheral tissues remains lower than the P o 2 in peripheral capillaries. Also, in many cases, there is considerable physical distance between the capillaries and cells. Therefore, the normal intracellular P o 2 ranges from as low as 5 mm Hg to as high as 40 mm Hg, averaging (by direct measurement in experimental animals) 23 mm Hg. Because only 1 to 3 mm Hg of O 2 pressure is normally required for full support of the chemical processes that use oxygen in the cell, even this low intracellular P o 2 of 23 mm Hg is more than adequate and provides a large safety factor.
When O 2 is used by the cells, virtually all of it becomes CO 2 , and this transformation increases the intracellular P co 2 ; because of this elevated tissue cell P co 2 , CO 2 diffuses from the cells into the capillaries and is then carried by the blood to the lungs. In the lungs, it diffuses from the pulmonary capillaries into the alveoli and is expired.
Thus, at each point in the gas transport chain, CO 2 diffuses in the direction exactly opposite to the diffusion of O 2 . Yet, there is one major difference between diffusion of CO 2 and of O 2 — CO 2 can diffuse about 20 times as rapidly as O 2 . Therefore, the pressure differences required to cause CO 2 diffusion are, in each case, far less than the pressure differences required to cause O 2 diffusion. The CO 2 pressures are approximately the following:
Intracellular P co 2 , 46 mm Hg; interstitial P co 2 , 45 mm Hg. Thus, there is only a 1 mm Hg pressure differential, as shown in Figure 41-5 .
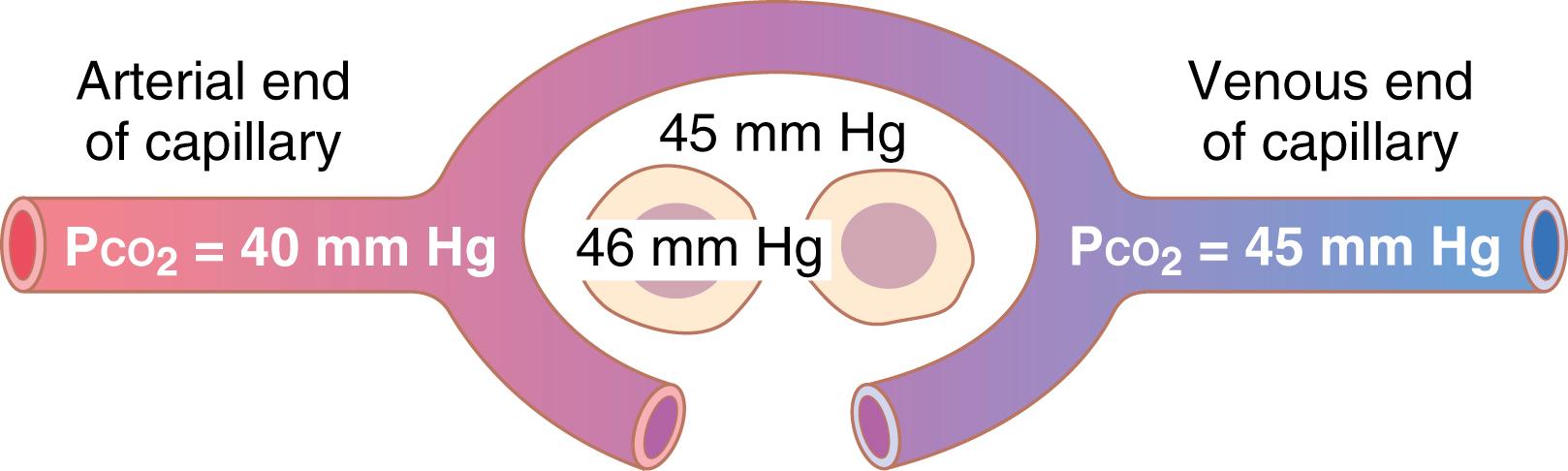
P co 2 of the arterial blood entering the tissues, 40 mm Hg; P co 2 of the venous blood leaving the tissues, 45 mm Hg. Thus, as shown in Figure 41-5 , the tissue capillary blood comes almost exactly to equilibrium with the interstitial P co 2 of 45 mm Hg.
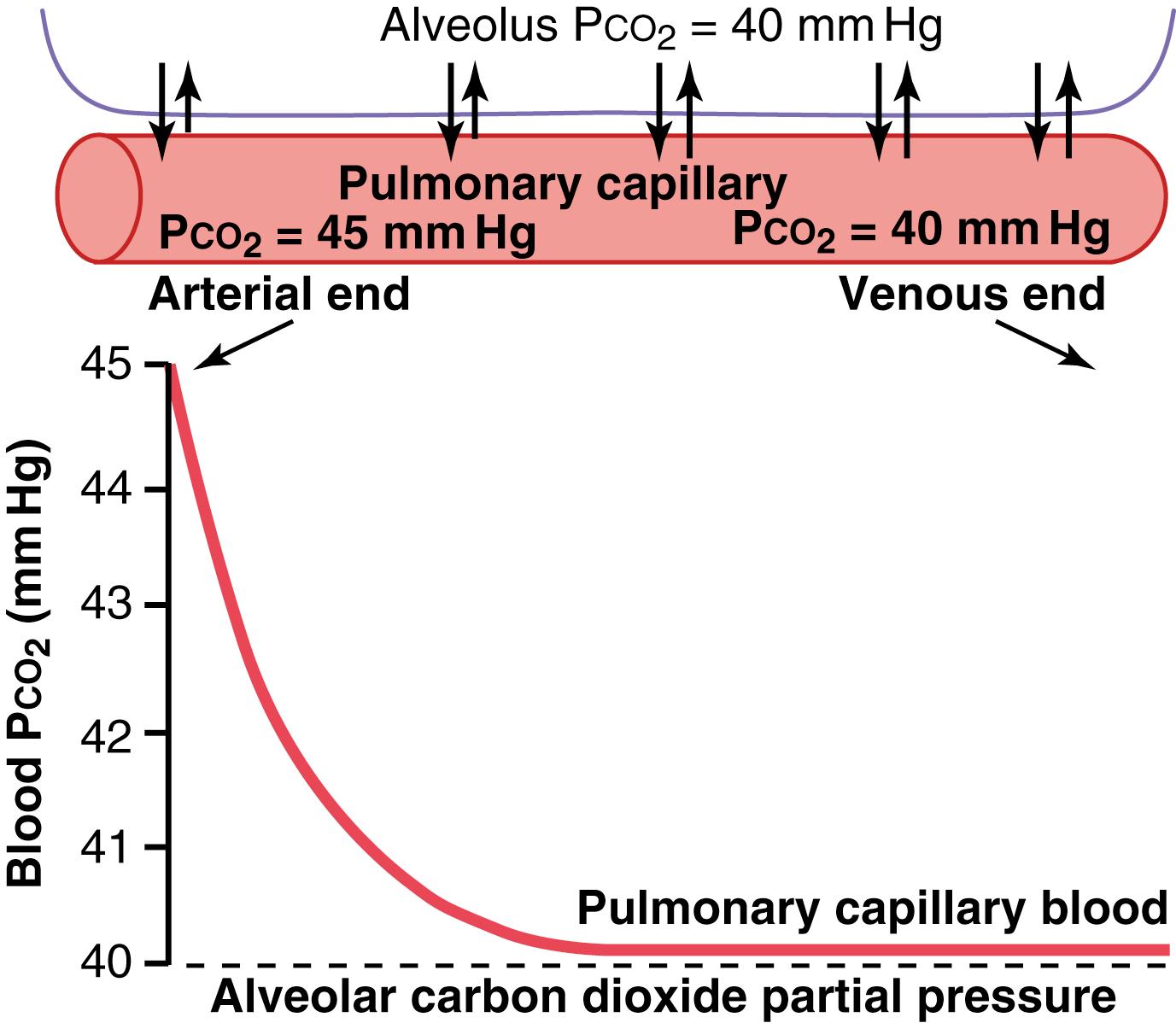
P co 2 of the blood entering the pulmonary capillaries at the arterial end, 45 mm Hg; P co 2 of the alveolar air, 40 mm Hg. Thus, only a 5 mm Hg pressure difference causes all the required CO 2 diffusion out of the pulmonary capillaries into the alveoli. Furthermore, as shown in Figure 41-6 , the P co 2 of the pulmonary capillary blood falls to almost exactly equal the alveolar P co 2 of 40 mm Hg before it has passed more than about one-third of the distance through the capillaries. This is the same effect that was observed earlier for O 2 diffusion, except that it is in the opposite direction.
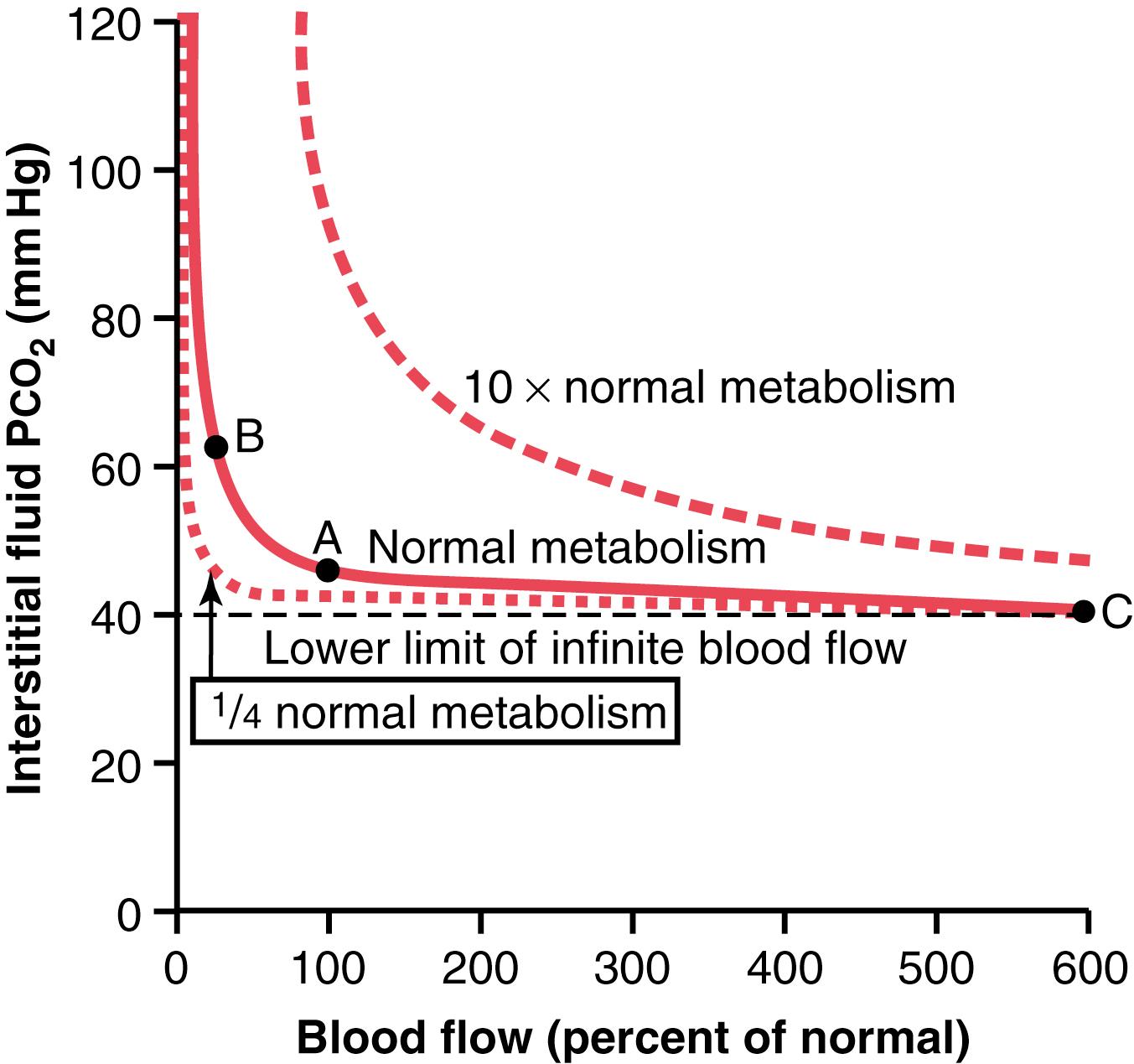
Tissue capillary blood flow and tissue metabolism affect the P co 2 in ways exactly opposite to their effect on tissue P o 2 . Figure 41-7 shows these effects, as follows:
A decrease in blood flow from normal (point A) to one-quarter normal (point B) increases peripheral tissue P co 2 from the normal value of 45 mm Hg to an elevated level of 60 mm Hg. Conversely, increasing the blood flow to six times normal (point C) decreases the interstitial P co 2 from the normal value of 45 to 41 mm Hg, almost equal to the P co 2 in the arterial blood (40 mm Hg) entering the tissue capillaries.
Note also that a 10-fold increase in tissue metabolic rate greatly elevates the interstitial fluid P co 2 at all rates of blood flow, whereas decreasing the metabolism to one-quarter normal causes the interstitial fluid P co 2 to fall to about 41 mm Hg, closely approaching that of the arterial blood, 40 mm Hg.
Normally, about 97% of the oxygen transported from the lungs to the tissues is carried in chemical combination with hemoglobin in the red blood cells. The remaining 3% is transported in the dissolved state in the water of the plasma and blood cells. Thus, under normal conditions , oxygen is carried to the tissues almost entirely by hemoglobin.
The chemistry of hemoglobin is presented in Chapter 33 , where we pointed out that the O 2 molecule combines loosely and reversibly with the heme portion of hemoglobin. When P o 2 is high, as in the pulmonary capillaries, O 2 binds with hemoglobin, but when P o 2 is low, as in the tissue capillaries, O 2 is released from hemoglobin. This is the basis for almost all O 2 transport from the lungs to the tissues.
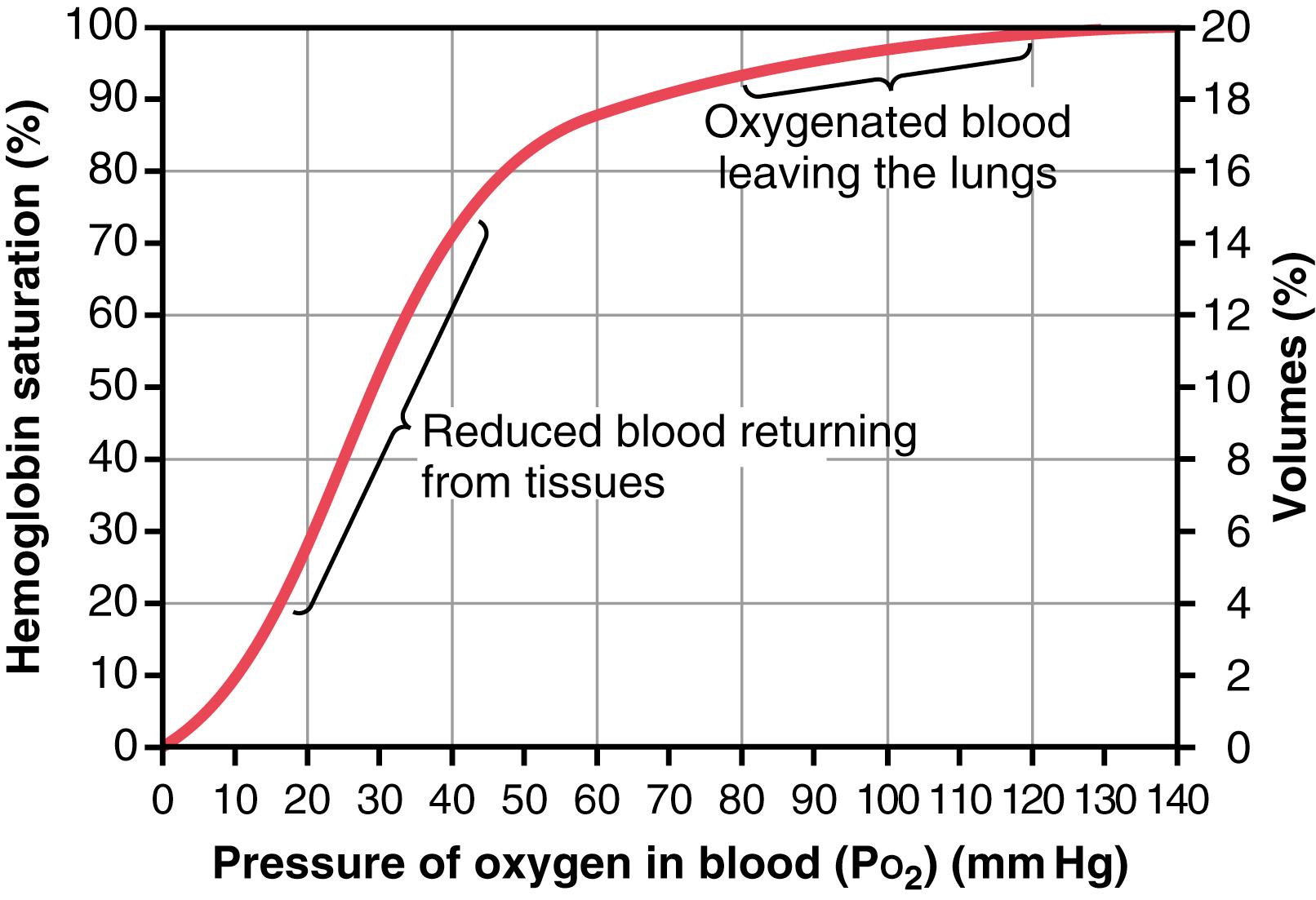
Figure 41-8 shows the O 2 -hemoglobin dissociation curve, which demonstrates a progressive increase in the percentage of hemoglobin bound with O 2 as blood P o 2 increases, called the percent saturation of hemoglobin . Because the blood leaving the lungs and entering the systemic arteries usually has a P o 2 of about 95 mm Hg, it can be seen from the dissociation curve that the usual O 2 saturation of systemic arterial blood averages 97% . Conversely, in normal venous blood returning from the peripheral tissues, the P o 2 is about 40 mm Hg, and the saturation of hemoglobin averages 75% .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here