Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver transplantation has made possible the functional cure of several metabolic diseases characterized by inherited genetic defects. In many pediatric transplantation centers, metabolic diseases, most notably α 1 -antitrypsin deficiency, are the second or third most common indication for liver transplantation after biliary atresia and fulminant hepatic failure. The Studies of Pediatric Liver Transplantation (SPLIT) database shows that of the more than 4000 U.S. and Canadian children transplanted between 1995 and 2011, more than 10% received a transplant for a metabolic disease. The number of children who underwent liver transplantation for each metabolic disease diagnosis is shown in Table 27-1 . The long-term survival and quality of life of children who undergo liver transplantation for metabolic disease is similar to that of children with other liver diseases. The manifestations of metabolic disease affecting the liver are diverse and range from acute liver failure to cirrhosis complicated by hepatoma. However, the indication for liver transplantation may go beyond the recognized complications of acute or chronic liver failure. Life-threatening extrahepatic disease as a result of a deficient enzyme localized to hepatocytes, as occurs, for example, in central nervous system (CNS) manifestations of the urea cycle defects, can be cured by liver transplantation. Liver transplantation has also been advocated to improve the severely impaired quality of life in children who must endure rigidly enforced protein-restricted diets to control the potentially devastating neurological consequences of the organic acidurias.
| Total number of children transplanted | 1187 | |
| Transplantation for metabolic disease | 141 | 11.9% ∗ |
| α 1 -Antitrypsin deficiency | 39 | 27.7% † |
| Urea cycle defects | 22 | 15.6% |
| Tyrosinemia | 16 | 11.3% |
| Cystic fibrosis | 12 | 8.5% |
| Wilson’s disease | 10 | 7.1% |
| Neonatal iron storage disease | 9 | 6.4% |
| Primary hyperoxaluria | 8 | 5.7% |
| Glycogen storage disease | 7 | 5.0% |
| Crigler-Najjar syndrome | 6 | 4.2% |
| Other | 12 | 8.5% |
∗ Percentage of total children transplanted who have metabolic liver disease.
† Percentage of children with a given diagnosis and transplanted for metabolic liver disease.
To determine when liver transplantation is appropriate treatment of metabolic disorders, it is useful to consider two general categories of disease : (1) metabolic disease with structural liver damage leading to end-stage liver disease (e.g., α 1 -antitrypsin deficiency, familial tyrosinemia, and Wilson’s disease) and (2) metabolic disease without structural liver damage (e.g., familial hypercholesterolemia, primary oxalosis, and urea cycle defects).
In the first group the genetic defect may be localized to the liver itself, such as occurs in the familial cholestatic syndromes (see Chapter 25 ), but more commonly the liver is one of the end-organs damaged as a result of a more widespread defect (e.g., tyrosinemia and α 1 -antitrypsin deficiency). When the liver is exclusively involved and also the only site of the metabolic defect, the decision to replace the liver is easily made, and liver transplantation can be expected to provide complete reversal of the metabolic defect. However, in diseases in which the liver is damaged as a consequence of a widespread enzymatic defect residing in a variety of cells other than hepatocytes, determination of whether liver transplantation is indicated is more complex. Essential to this decision is precise knowledge of the genetic defect itself, the somatic cells in which the cellular defect is expressed, the extent of organ involvement outside the liver, and whether liver replacement alone will be sufficient to either prevent further deterioration or improve dysfunction in extrahepatic organs. Tyrosinemia is illustrative of these principles. The deficient enzyme, fumarylacetoacetate hydrolase (FAH), is not localized to hepatocytes, and the kidneys and CNS are two other major organs affected. However, tyrosinemia is associated with a spectrum of severe liver disease for which liver transplantation is indicated, ranging from fulminant hepatitis to cirrhosis with hepatoma formation. The Fanconi syndrome–like kidney disease associated with tyrosinemia often persists after liver transplantation, although some functional improvement usually occurs. The neurological crises do not appear to recur after transplantation. Thus in tyrosinemia, liver replacement not only is lifesaving but also ameliorates the extrahepatic manifestations of the disease.
In contrast, in the mucopolysaccharidoses, successful liver transplantation circumvents the consequences of unremitting liver fibrosis but is unable to overcome the widespread extrahepatic expression of the enzymatic defect and therefore allows continued accumulation, particularly in the CNS, of abnormal sphingomyelin. Ongoing neurological deterioration can be anticipated. In this instance, liver transplantation alone would not be indicated, but when combined with a bone marrow transplant, it may be a more successful approach if attempted early in life.
Medical therapies that might preclude or delay transplantation should be optimized. In general, the success of these measures depends on early diagnosis. This is particularly relevant with the use of chelating agents in Wilson’s disease; treatment with 2-nitro-4-trifluoromethylbenzoyl-1,3-cyclohexanedione (NTBC), a compound that blocks the formation of toxic metabolites in tyrosinemia; and phototherapy in Crigler-Najjar syndrome.
In the future, total liver replacement may become obsolete for some categories of metabolic disease. In metabolic diseases in which the liver is structurally normal, hepatocyte transplantation is an attractive option. Normal allogeneic hepatocytes have been able to provide temporary metabolic support in animal models, as first shown in the Gunn rat model of Crigler-Najjar syndrome and later in case studies of children with Crigler-Najjar syndrome and those with ornithine transcarbamylase (OTC) deficiency. More recently a limited number of cases have emerged of hepatocyte transplantation in humans, with the leading indication being children with urea cycle defects. Worldwide there are reports of over 30 patients with metabolic liver disease safely treated with hepatocyte transplantation as a treatment for Crigler-Najjar syndrome type I, glycogen storage disease type IA, infantile Refsum’s disease, progressive familial intrahepatic cholestasis type 2, urea cycle defects, familial hypercholesterolemia, and congenital deficiency of clotting factors. Advantages of hepatocyte transplantation include that it is less invasive than liver transplantation and may be able to offer a bridge for survival in an acute setting. Major challenges include the fact that these patients still require immunosuppression and the function of the transplanted hepatocytes often declines by 9 months after infusion, meaning that the patients will go on to require liver transplantation.
A potentially more attractive option involves the use of gene therapy to modify the genetic program of the patient’s own hepatocytes. Harvested hepatocytes infected in vitro with a recombinant retrovirus or adenovirus carrying the normal human gene are then able to express the normal gene’s protein products. Autologous transplantation of these genetically reconstituted hepatocytes is then performed. This approach has been used successfully in animal models for such diseases as familial hypercholesterolemia, the urea cycle defects, Crigler-Najjar syndrome, and tyrosinemia. Alternatively, in vivo modification of hepatocytes might be achieved by a vector containing the normal gene. Such approaches would avoid the significant morbidity and mortality associated with orthotopic liver transplantation and a lifetime of immunosuppression.
To reverse the metabolic defect, animal models have shown that only a small percentage of the total liver cell mass needs to be replaced with cells containing viable enzyme. However, in clinical reports of hepatocyte transplantation for Crigler-Najjar disease, urea cycle defects, and hypercholesterolemia, despite transplantation of what should have been an adequate cell mass, only partial correction of the defect has been reported. Not only is the long-term viability of transplanted cells a problem to be overcome, but a limitation of hepatocyte transplantation is that only about 1% of the liver mass can be replaced by transplanted cells.
Recently attention has been focused on the concept of “liver repopulation,” whereby the transplanted cells are given a growth advantage over the recipient’s own cells. To be successful in animal models, this technique has two specific requirements to provide “the space” for the transplanted cells to proliferate. First, the transplanted cells must have an advantage in either proliferation or survival in comparison to the endogenous hepatocyte population. Second, removal of endogenous hepatocytes, usually by partial hepatectomy, is required to provide the stimulus for liver regeneration, which selectively allows the transplanted hepatocytes to proliferate. In animal models, approaches used to decrease the regenerative capacity of endogenous hepatocytes include drugs blocking DNA syntheses and irradiation. By applying these two principles in animal studies, up to 90% of the host liver cells can be replaced by transplanted cells. If such techniques prove applicable to humans, hepatocyte transplantation, including the transplantation of genetically altered autologous hepatocytes, would become a clinical reality.
The rapidly advancing field of stem cell transplantation may also have important implications for the correction of some liver-localized metabolic diseases. Stem cells, whether of bone marrow or liver origin, may prove to be the best candidate cells for transplantation into the liver.
The following sections systematically describe metabolic defects for which liver transplantation is indicated. For each disease entity, a description is provided of the metabolic defect and its genetics, inheritance and biochemical effects, pathology, clinical manifestations from infancy through the teenage years, indications for transplantation, and impact of transplantation on the course of the disease. A familiarity with metabolic liver disease and an understanding of these concepts, including indication and contraindications, is crucial for hepatologists and transplant surgeons alike to optimize patient selection and timing of liver transplantation. Liver transplantation for familial cholestasis syndromes and hemopoietic metabolic disease is discussed in Chapter 25 and later in this chapter.
α 1 -Antitrypsin deficiency is one of the most common lethal inherited diseases that affect the white population. It is characterized by liver disease in children and emphysema in adults. There is a rare association of α 1 -antitrypsin deficiency with glomerulonephritis in children and young adults. The frequency of the disease is between 1 in 2000 and 1 in 7000 in populations of European descent. Liver disease associated with α 1 -antitrypsin deficiency is the most common metabolic disease for which liver transplantation is performed in children.
α 1 -Antitrypsin is a major serine protease inhibitor that is produced primarily in the liver but also to some extent in neutrophils and macrophages. Its most important function is inhibition of neutrophil elastase, a powerful proteolytic enzyme capable of degrading extracellular structural proteins, particularly elastin. The effect of low circulating α 1 -antitrypsin levels is most dramatically seen in the lung, where the unopposed action of neutrophil elastase leads to progressive destruction of the lung parenchyma, which becomes clinically manifested as emphysema. In contrast, liver disease is the result of retention of the abnormal α 1 -antitrypsin molecule within hepatocytes.
α 1 -Antitrypsin is a small, 52-kD glycosylated protein. It is encoded for by a single gene located on chromosome 14 with codominant expression of the two inherited alleles. At least 75 allelic variants have been described. Phenotyping, designated by the Pi (protease inhibitor) nomenclature, was originally described by the relative mobility of the α 1 -antitrypsin molecule along an acid starch gel gradient. Variants are described by letters of the alphabet. Approximately 70% to 80% of selected populations have the normal phenotype, PiMM. The α 1 -antitrypsin deficiency state is most often characterized by PiZZ. Other variations have been described (e.g., PiMZ, PiMS) and are variably associated with low α 1 -antitrypsin levels (between 15% and 60% of normal) and clinical disease.
The first association of α 1 -antitrypsin deficiency and liver disease was made by Freier et al in 1968 and expanded by Sharp et al in 1969. Soon thereafter, Sveger’s large prospective screening of 200,000 newborns in Sweden provided the study that still stands as the best description of the natural history of the disease. In this study 120 PiZZ infants were identified, 12% of whom presented with cholestasis within the first 3 months and an additional 6% had clinical evidence of liver disease (hepatosplenomegaly). In a follow-up study, 73% of PiZZ infants had transaminitis by 6 months of age that persisted until age 8 years in 59%. Overall, about 3% of infants with the PiZZ phenotype progressed to cirrhosis, which represented about 20% of the PiZZ infants with neonatal cholestasis. Since these first observations, additional information has allowed an easily remembered generalization to be made. Of PiZZ infants with cholestasis, cirrhosis will develop in 25% in the first decade, 25% will show persistent transaminitis progressing to cirrhosis in the second decade, 25% will have mild transaminitis without cirrhosis, and the biochemical abnormality will resolve completely and show only mild fibrosis on liver biopsy in 25%.
Typically in an infant with α 1 -antitrypsin deficiency and cholestasis, the jaundice resolves by about 6 months. However, the transaminitis usually persists. In those who progress to cirrhosis, the clinical development of portal hypertension, with or without recrudescence of jaundice, is a common manifestation later in childhood. In many children the progression to end-stage liver disease may be quite slow. Rarely, however, the course progresses rapidly to end-stage liver disease. The early development of ascites with cirrhosis on liver biopsy is an ominous sign and has been reported as early as 2 weeks of age, thus suggesting that in some infants the liver insult begins in utero.
The severity of the cholestatic liver disease in infancy correlates with the appearance of cirrhosis in later childhood. However, α 1 -antitrypsin deficiency must still be considered a cause of cirrhosis in childhood, even without an antecedent history of neonatal cholestasis.
Persistent abnormalities in urinary bile acids may also predict progression to cirrhosis. Other risk factors are female sex and siblings in whom cirrhosis has also developed. Whether early breast-feeding is protective remains debatable.
The PiZZ phenotype is most often correlated with liver disease. However, both PiMZ and PiSZ individuals have been reported with moderately depressed α 1 -antitrypsin serum levels, as well as clinical and histological evidence of liver disease. The association of hepatocellular carcinoma (HCC) in adults with “cryptogenic” cirrhosis has been linked to previously undiagnosed ZZ and MZ phenotypes.
The characteristic pathological changes of the liver in α 1 -antitrypsin–deficient patients with liver disease provide insight into the mechanism of liver injury. Abnormal globules of α 1 -antitrypsin, characteristically periodic acid–Schiff positive and diastase resistant, accumulate in periportal hepatocytes, which are the site of α 1 -antitrypsin production ( Fig. 27-1 ). On electron microscopy the rough endoplasmic reticulum of such cells is distended with similar granules. It has been postulated that abnormal folding of the mutant α 1 -antitrypsin molecule leads to accumulation in the rough endoplasmic reticulum. Retention of abnormally folded proteins in the endoplasmic reticulum is thought to be a protective mechanism that allows degradation of abnormal proteins to prevent further cellular damage. It is now proposed that patients with liver disease associated with α 1 -antitrypsin deficiency have a defect in the degradative pathway that causes greater accumulation of the putatively hepatotoxic mutant α 1 -antitrypsin molecule. Because liver disease develops in only a small minority of patients with the ZZ genotype, the defect in degradation is thought to be controlled by either other unlinked genetic traits or environmental factors.
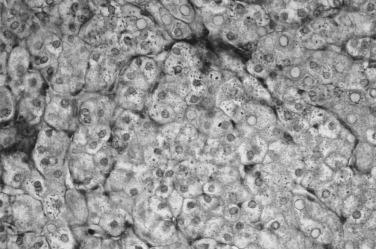
It is now clear that the mechanism of liver injury in α 1 -antitrypsin deficiency is not analogous to the mechanism of injury in the lung, which is caused by low tissue levels of α 1 -antitrypsin that allow destruction of the parenchyma by locally released proteases. This different mechanism is substantiated by studies of patients with the rare Pi-Null phenotype, in which no detectable α 1 -antitrypsin is present in serum or hepatocytes and no liver injury occurs. Augmentation of α 1 -antitrypsin serum levels by administering recombinant α 1 -antitrypsin can be expected to improve lung function but has not been shown to either turn off or promote secretion of α 1 -antitrypsin globules accumulated in the liver.
There is no specific medical therapy for α 1 -antitrypsin deficiency, and infants with this disease are initially managed with supportive care.
Liver transplantation as an effective cure for α 1 -antitrypsin deficiency was first performed in 1973. The transplanted liver produced normal α 1 -antitrypsin molecules, and the α 1 -antitrypsin serum level normalized. The recipient’s phenotype converted to that of the donor. As could be predicted by the restoration of circulating α 1 -antitrypsin levels to normal, no patients to date have contracted emphysema. However, it should be remembered that the transplanted patient’s original genotype is unchanged in the germ cell line, so when children transplanted for α 1 -antitrypsin deficiency reach reproductive age, genetic counseling should be offered.
Analysis of the United Network of Organ Sharing database reveals 1- and 5-year patient survival rates of 92% and 90%, respectively, for children with α 1 -antitrypsin deficiency who received a liver transplant. The excellent outcomes now reported from many centers for children undergoing liver transplantation for α 1 -antitrypsin deficiency associated with end-stage liver disease has changed the overall prognosis substantially for children with this disease. In a large single-center experience in children with clinical liver disease secondary to α 1 -antitrypsin deficiency, 27% underwent transplantation. The duration of jaundice and the severity of the histological features and biochemical abnormalities predicted outcome at an early stage of the disease. As a group, children undergoing transplantation for α 1 -antitrypsin deficiency have lower mortality and morbidity than other pediatric recipients do. This better outcome can be attributed to their generally older age at initial evaluation, which often shows portal hypertension and bleeding varices. Jaundice is usually mild and nutritional status better preserved than in younger children with biliary atresia. In addition, most have had no previous abdominal surgeries. However, as with other conditions associated with cirrhosis and portal hypertension, children with α 1 -antitrypsin deficiency have a propensity for the development of large arteriovenous pulmonary shunts and cyanosis before transplantation. The degree of shunt and arterial oxygenation should be evaluated before transplantation. Although these problems may resolve over time, large shunts complicate the early postoperative period and compromise weaning from the ventilator.
Rupture of a splenic artery aneurysm has also been described as a lethal complication after liver transplantation in a child with α 1 -antitrypsin deficiency. Such rupture is most likely a reflection of the commonly seen severe pretransplantation portal hypertension and not a function of the disease itself.
Other possibilities for the future treatment of liver disease associated with α 1 -antitrypsin deficiency might include gene therapy to suppress the abnormal Z gene so that the mutant molecule is not produced. It will also be difficult to prospectively determine which patients to treat because clearly, liver disease does not develop in all these children. For such preventive strategies to be successful, a better understanding of the other genetic and environmental triggers that predispose patients with abnormal phenotypes to the development of liver disease will be needed.
Copper accumulation in the liver, CNS, eyes, and kidneys is the cardinal clinical feature of Wilson’s disease, an autosomal recessive disease of copper metabolism with a prevalence of about 1 in 30,000 in most populations. The liver plays an essential role in copper homeostasis inasmuch as about 95% of copper in the portal vein is taken up by the liver and biliary excretion of copper is the only physiologically important route of copper elimination. About 90% of serum copper is bound to ceruloplasmin. Newborn infants show concentrations of copper within the liver similar to those of patients with Wilson’s disease. Normally the liver copper level falls toward adult levels by 6 months of age. In Wilson’s disease, copper first accumulates in the liver and subsequently in the CNS and other extrahepatic tissues.
In recent years, elucidation of the copper metabolic pathway in the liver and discovery of the genes that encode the proteins essential to normal copper metabolism have greatly enhanced our understanding of the molecular and genetic basis of Wilson’s disease. Mutations in the ATP7B gene give rise to Wilson’s disease. The gene product for ATP7B is an adenosine triphosphate (ATP)-dependent copper transporter that is required for the intrahepatocyte delivery of copper to the secretory pathway that incorporates copper into apoceruloplasmin, with subsequent transport across the lipid bilayer of the hepatocyte into bile. The frequency of the abnormal gene, irrespective of race, is about 1 in 200 to 400. Heterozygote carriers occur at a frequency of about 1 per 100 in the general population.
Because more than 200 mutations have been described, it is currently difficult to screen populations for Wilson’s disease. However, genetic analysis is useful in screening family members of affected individuals. A single dominant mutation (H1069Q) is found primarily in Slavic populations but in only about a third of North American populations. In addition, there is often a poor correlation between patients homozygous for specific alleles and the clinical manifestations of disease, thus implying that additional genetic or environmental factors play a role.
Evolution of the liver injury in Wilson’s disease appears to be related to redistribution of copper within the liver, which may induce oxidant injury in hepatocyte mitochondria. Clinical evidence of disease seldom occurs before 5 years of age, although one case of jaundice in a 2-year-old has been described. In children, the liver manifestations of the disease are most frequently manifested in their teenage years, whereas 40% of adults initially have neurological abnormalities. The symptoms of neurological disease are usually subtle in children. Personality and behavior changes or poor school performance may be present. The predominantly motor abnormalities of tremor, dystonia, and dysarthria become more pronounced with age and are related to the effects of copper accumulation in the extrapyramidal system. In adult patients with primarily neurological manifestations of Wilson’s disease, the condition may be misdiagnosed as mental retardation or psychiatric impairment, and the true origin of their neurological disease is never appreciated.
The clinical manifestations of liver impairment in Wilson’s disease are diverse and range from asymptomatic hepatosplenomegaly with an associated low-grade transaminitis to fulminant liver failure. The heterogenicity in findings frequently delays the diagnosis. Chronic active hepatitis progressing to cirrhosis may remain clinically silent for years before becoming manifested as acute onset of jaundice, which is often misdiagnosed as acute hepatitis. In some adolescents the acute hepatitis–like picture may progress over a period of weeks to severe liver failure, whereas in others, the first manifestation of the disease is fulminant liver failure. Portal hypertension and bleeding varices are also frequent initial signs.
Physical examination may not be especially helpful in making the diagnosis of Wilson’s disease. Hepatosplenomegaly is frequently present, but the liver may be shrunken in advanced cases. Kayser-Fleischer rings, seen as a rusty brown ring at the junction of the iris and cornea caused by deposition of copper in Descemet’s membrane, are often said to be pathognomonic of Wilson’s disease. The ring first appears as a crescent in the superior aspect of the eye but may be difficult to visualize in brown eyes. Slit-lamp examination is often required. However, Kayser-Fleischer rings do not usually appear until midadolescence and are not unique to Wilson’s disease.
The diagnosis of Wilson’s disease may also be confounded by an often-confusing constellation of test results. Classically, serum ceruloplasmin level is low (<20 mg/dL), serum copper level is low (<80 mg/L), and 24-hour urine copper level is high (>100 mg/24 hr). However, 15% of homozygotes with liver disease have normal ceruloplasmin levels, and about 20% of carriers of the Wilson’s disease gene have low ceruloplasmin levels. Low ceruloplasmin levels are likewise seen in severe copper deficiency and fulminant liver failure. Because ceruloplasmin is also an acute phase reactant, it may be elevated with ongoing inflammatory liver injury and in pregnancy or with estrogen administration. Total serum copper level is unreliable as well because free serum copper is often increased in untreated or fulminant Wilson’s disease. The 24-hour urine copper level is also elevated in chronic liver disease with cholestasis, acute liver failure, and severe proteinuria. Careful collection in copper-free containers is required.
The copper content of the liver is the most reliable diagnostic test. In Wilson’s disease a copper content greater than 250 μg/g dry tissue (normal, <50 μg/g) is frequently seen. Values up to 3000 μg/g are not uncommon. Although an increased copper concentration may also be seen with chronic active hepatitis and primary biliary cirrhosis, the ceruloplasmin level is either normal or increased in these diseases, and other distinctive features should allow differentiation.
If the ceruloplasmin level is normal, a useful adjuvant test is to measure the incorporation of radioactive copper into ceruloplasmin. Wilson’s disease is characterized by decreased accumulation of radioactive copper. This test is of no value if the serum ceruloplasmin concentration is low.
Abnormalities in liver function vary depending on the clinical manifestation. However, rapid diagnosis of Wilson’s disease in patients with fulminant liver failure is of critical importance. Acute hemolysis frequently accompanies fulminant Wilson’s disease because massive amounts of copper are released into the circulation and lyse red blood cell membranes. The association of acute hemolysis with liver failure in an adolescent should be diagnosed as Wilson’s disease until proved otherwise. In addition, the characteristic pattern of mildly elevated transaminase levels with a high serum bilirubin level and an unexpectedly normal alkaline phosphatase concentration should prompt consideration of Wilson’s disease over most other causes of acute fulminant liver failure. In one study of emergency liver transplantation, 11.4% of children with fulminant liver failure had Wilson’s disease. Of the 703 children enrolled in the latest report of the Pediatric Acute Liver Failure Study Group, 3.3% had Wilson’s disease.
Deiss et al describe four stages of Wilson’s disease before treatment. In stage 1, copper accumulates in the cytosol of the hepatocyte, and the patient remains asymptomatic. In stage 2, copper is redistributed into lysosomes, with some copper being released into the circulation. Liver fibrosis, cirrhosis, or liver failure may occur. In stage 3, copper accumulates asymptomatically in the CNS, which leads to neurological symptoms in stage 4.
In the liver the histological appearance of early Wilson’s disease includes fatty infiltration and glycogen-filled hepatocyte nuclei. Distinctive, but not unique, mitochondrial abnormalities are present on electron microscopy. As the disease progresses, there is continuing fibrosis, parenchymal collapse, inflammatory cell infiltrates, and nodular regeneration culminating in frank cirrhosis. In patients presenting with acute liver failure, liver necrosis is the predominant histological factor.
Orthotopic liver transplantation provides a cure for Wilson’s disease in patients in whom medical therapy has failed or in those found to have advanced decompensated liver disease at initial evaluation. Medical management in patients in whom Wilson’s disease is diagnosed in the early stages of the disease—and continued lifelong—may abrogate the need for liver transplantation entirely. d -Penicillamine, trientine dihydrochloride, and tetrathiomolybdate are chelating agents with proven success. More recently the use of oral zinc has been advocated in asymptomatic patients after an initial cupruresis has been induced with chelating agents or in combination with a chelator in patients with symptoms related to either liver or neurological disease. In one report, combination therapy averted liver transplantation in several patients. Oral zinc induces the formation of metallothionein, a copper-binding substrate, within the enterocyte. Ingested copper is then bound within the enterocyte to metallothionein and sloughed into the gastrointestinal tract, never reaching the systemic circulation.
Various attempts have been made to ascertain which patients with Wilson’s disease should be considered for transplantation. Indications include failure of medical treatment to improve either liver or neurological function, fulminant liver failure, and decompensated cirrhosis at initial evaluation. On the basis of a scoring system, Nazer et al accurately predicted which patients had a poor prognosis when treated medically. Elevations in bilirubin level, serum glutamic-oxaloacetic transaminase level, and prothrombin time predicted increased mortality. Jaundice and ascites also correlated with a poor outcome. More recently the group at King’s College Hospital has updated its Wilson’s Disease prognostic index with over 35 years' (n = 74 patients) worth of pediatric data. The updated scoring system incorporates serum bilirubin level, international normalized ratio, aspartate aminotransferase level, and white cell count at presentation and is 93% sensitive and 98% specific, with a positive predictive value of 88% in predicting the need for liver transplant.
Successful liver transplantation for Wilson’s disease with complete reversal of the metabolic manifestations was first reported by Dubois et al in 1971. By the early 1980s, orthotopic liver transplantation had become the standard treatment of Wilson’s disease manifested as fulminant liver failure or decompensated chronic disease. The results of transplantation, particularly in patients with fulminant Wilson’s disease, have been impressive. In reported series, fulminant liver failure, which occurs more frequently in females by a 2:1 ratio, is the indication for liver transplantation in the majority of patients. In one of the largest single-center reports, in which 45 patients underwent transplantation for Wilson’s disease, 42.2% were younger than 18 years at transplantation, and two thirds of the patients received transplants for either fulminant or subfulminant liver failure; 73.3% of these patients survived more than 5 years after transplantation. Based on Organ Procurement and Transplantation Network data from 2007, 1-and 5-year patient survival rates for Wilson’s disease patients who require liver transplantation are 89% and 84%, respectively. Aggressive temporizing strategies may be needed to maintain patients with acute fulminant liver failure secondary to Wilson’s disease until a liver can be found. Treatments have included d -penicillamine in conjunction with hemofiltration in patients with kidney failure and heterotopic transplantation.
In successful liver recipients, an initial period of cupruresis is followed by normalization of serum copper, ceruloplasmin, and liver copper content. The characteristic Kayser-Fleischer rings resolve slowly, in some cases more than 3 years. Adult patients with neurological or psychological impairments have shown complete or partial recovery, although such recovery may take several months. In general, patients transplanted for Wilson’s disease enjoy an excellent quality of life. A still-contentious issue is whether liver transplantation is justified for neurological disease without severe liver disease. This debate is exemplified by a report of a 15-year-old without significant liver disease who was bedridden with severe incapacitating dysarthria despite maximal medical therapy. This patient reportedly returned almost to normal after liver transplantation.
The use of a living related donor for Wilson’s disease, usually a parent who is a heterozygote carrier, carries the risk that copper metabolism may remain abnormal after transplantation. In a recent report of two children who each received a graft from a parent, it was shown that the liver copper content was slightly elevated, not exceeding 250 μg/g dry weight. However, serum copper and ceruloplasmin levels were lower and urinary copper excretion higher than normal. The long-term outcome is as yet unknown. It would seem prudent to avoid living related donors whenever possible for Wilson’s disease or to perform gene analysis preoperatively if the procedure is to be attempted.
The future holds the promise that gene therapy or isolated hepatocyte transplantation may offer definitive therapy for patients identified very early in the disease process. Transplantation of normal hepatocytes into Long-Evans cinnamon rats, an animal model of Wilson’s disease, showed that a viable transplanted hepatocyte mass of 4% to 20% prevented the development of Wilson’s disease.
Identification of any child with Wilson’s disease, regardless of whether liver transplantation is a therapeutic option, should always prompt a systematic investigation of all family members. Early detection and treatment of asymptomatic homozygotes may prevent the necessity for subsequent liver transplantation.
Hereditary tyrosinemia type I is an autosomal recessive disease in which FAH, the terminal enzyme in tyrosine metabolism, is deficient. The more than 30 mutations of the gene located on chromosome 15 account for the wide clinical variability of the disease. The distribution of the genetic abnormality varies considerably with population groups. In Quebec, where the disease was first described in 1967 by Larochelle et al, the incidence of the disease is 1 in 10,000 but rises to 1 in 800 births in one geographically isolated area. In comparison, the incidence in Scandinavia is 1 in 50,000.
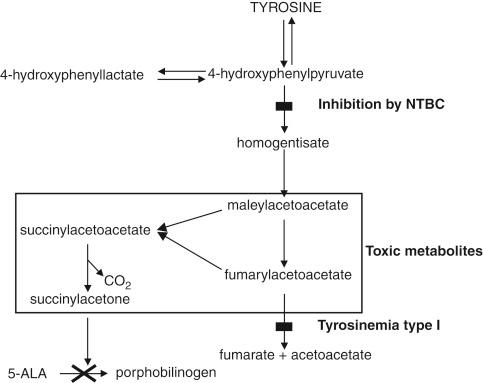
The diagnosis of tyrosinemia is made by demonstrating reduced activity of FAH, the enzyme responsible for cleaving fumarylacetoacetate into fumaric and acetoacetic acids. The accumulation of these acids leads to an increase in succinylacetone, a byproduct whose presence is suggestive of tyrosinemia. In Quebec, neonatal screening programs on dried blood spots are important for early detection in this high-risk population.
Lethal liver disease, neurological crises, and a Fanconi syndrome with hypophosphatemic rickets characterize the disease. Although serum tyrosine, methionine, and phenylalanine levels are elevated in serum, these elevations are not thought to mediate the toxic injury. Accumulation of fumarylacetoacetate and maleylacetoacetate most likely mediates the cellular toxicity. These alkylating agents are thought to inflict damage at the DNA level in hepatocytes and renal tubular epithelial cells.
Liver disease in tyrosinemia can be manifested as either acute or chronic disease. Tanguay et al demonstrated no FAH activity in the acute form, whereas in the chronic form, FAH activity was about 20% of normal.
Acute disease becomes apparent in infancy with the onset of fulminant liver failure. Frequently infants present with a bleeding diathesis, and liver failure is diagnosed secondarily. Tyrosinemia should be considered in an infant with coagulopathy even without other clinical evidence of liver failure. A few of these infants respond to a diet low in tyrosine, phenylalanine, and methionine, but most succumb if no other intervention is made. In these infants the liver may be pale and enlarged with already-apparent micronodular cirrhosis, bile duct proliferation, steatosis, and pseudoacinar arrangements of hepatocytes.
The chronic form of tyrosinemia-induced liver disease has a more insidious onset. Although it often develops after the first year of life, it should be considered even in infants or toddlers—particularly those with unexplained rickets or Fanconi syndrome. The liver is enlarged and coarsely nodular with progression from micronodular to macronodular cirrhosis. There may be clinical evidence of portal hypertension and decompensated cirrhosis with jaundice, ascites, and loss of synthetic function. Before the use of NTBC (see later), even with strict dietary management children with tyrosinemia showed a frightening propensity for the development of HCC after the age of 2 years. The malignancy is multifocal within the liver and may have metastasized at the time of diagnosis.
Neurological crises, which usually occur after 1 year of age, may also be a fatal consequence of tyrosinemia. The defining features of the syndrome are the acute onset of profound weakness or paralysis, painful dysesthesias, often with hypertonic posturing, and self-mutilation. Respiratory muscle paralysis may lead to sudden death. Seizures and sustained arterial hypertension are other common features. Mitchell et al reported a 42% incidence of neurological crises with an associated mortality of 70% in 48 tyrosinemic children hospitalized in Quebec.
The clinical features of the neurological crises closely resemble those of acute porphyria, and indeed, as in porphyria, elevated serum δ-aminolevulinic acid is common. These elevated levels are due to inhibition of aminolevulinic acid dehydrase by succinylacetone, a metabolite of tyrosine degradation. Treatment of neurological crises is largely supportive, although hematin, which decreases aminolevulinic acid production, may shorten the course. Emergency liver transplantation may be required for severely affected children, particularly those in respiratory failure, and averts any further crises.
The kidneys are the third major organ affected by hereditary tyrosinemia. Kidney biopsies have shown glomerulosclerosis and interstitial fibrosis. Autotoxicity of the kidneys by the local production of succinylacetone has been proposed as the mechanism of tubular dysfunction.
A major advance in the medical management of tyrosinemia has been made since 1991 when the first patient was treated with NTBC, a compound that inhibits tyrosine degradation and blocks the enzymes responsible for accumulation of the toxic metabolites (particularly succinylacetoacetate) that induce the liver injury ( Fig. 27-2 ).
By 2000, more than 300 patients had been enrolled in an international study, and more than 100 of these patients had been treated for longer than 5 years. The starting dose is 1 mg/kg/day, but up to 2 mg/kg/day may be required in infants. The greatest benefit was seen in children in whom the disease was diagnosed and treated before 6 months of age. In this cohort 90% responded—including some with an acute manifestation. Of the 10% with no clinical response, five children died and three others underwent liver transplantation. The least amount of benefit was seen in those beginning therapy after 2 years of age. This population was heterogeneous, with tyrosinemia newly diagnosed in some children and others managed for often long periods by dietary restriction alone. In this group the main reason for withdrawal from NTBC therapy was suspicion of HCC. Maintenance of a tyrosine-restricted diet is still essential with NTBC treatment, as shown in both animal and human studies. Rigorous monitoring of tyrosine levels is required. It is important to avoid tyrosine levels greater than 500 μmol/L, which are associated with corneal lesions, hyperkeratotic lesions of the palms and soles, and potentially nervous system abnormalities.
The critical question remains whether early NTBC treatment can eliminate the risk for HCC. In children treated early in life, HCC developed in 2 (1%) during the first year of treatment. In a single-center report, 2 of 10 children failed NTBC treatment, with hepatic dysplasia developing in 1—the other was a nonresponder. Until further information is available, it would seem prudent to be vigilant in monitoring for the development of HCC in children treated with NTBC—particularly after 2 years of age. Serial imaging studies of the liver, immediate biopsy of any suspicious lesions, and frequent measurement of serum α-fetoprotein levels (in children with low levels during NTBC therapy) remains necessary.
In a recent report of 45 French children with tyrosinemia who were treated with NTBC for a mean of 4 years 9 months, only 3 patients required liver transplantation for cirrhosis or HCC. However, 17 of the 45 showed persistent abnormalities on liver imaging, and 15 of these children had persistently elevated α-fetoprotein levels, highlighting the question of the risk for developing HCC.
Before the advent of NTBC therapy, liver transplantation was lifesaving in children with hereditary tyrosinemia and remains so for children who are nonresponders to NTBC, those who already have cirrhosis at initial evaluation, and children with evidence of hepatic dysplasia or malignant change. In 1976 the first patient with tyrosinemia underwent transplantation. The metabolic abnormalities were promptly reversed, although the patient did have HCC with a pulmonary metastasis at the time of transplantation. In 1985 Starzl et al reported the successful outcome of four children with chronic tyrosinemia and made the important point that transplantation should be considered early, before hepatoma develops. The concern for malignant transformation is further justified by reports of liver cell dysplasia and HCC in explanted livers from children undergoing transplantation. Esquivel et al reported tumor in 5 of 10 children who underwent transplantation for tyrosinemia. All 5 children were younger than 2 years at transplantation, and in 3, both lobes were involved. Recurrent tumor has occurred in 1 child. A further observation that 37% of children older than 2 years will have hepatoma prompted most authors, before the use of NTBC therapy, to recommend elective transplantation at about 2 years of age, a concept supported by the excellent results reported from several centers.
One hundred twenty-five children with tyrosinemia in the United Network for Organ Sharing (UNOS) database have received liver transplants at a mean age of 2.5 ± 3.6 years. Overall, 5-year patient survival was 90%. Importantly, the rate of liver transplantation for children with tyrosinemia has decreased and age at transplant increased over the last decade because of early diagnosis and treatment with NTBC.
The dilemma still persists regarding when to offer transplantation to children with chronic tyrosinemia in whom decompensated end-stage liver disease has not yet occurred. Evidence from NTBC studies suggests that children older than 2 years do not have a clear benefit from NTBC therapy, and because the risk for HCC is of increasing concern after 2 years of age, liver transplantation rather than NTBC therapy is the preferred choice. The diagnosis of hepatoma itself is fraught with difficulties because serum α-fetoprotein levels cannot generally be used as a marker. α-Fetoprotein levels, often in the thousands, are characteristic of children with tyrosinemia, even without tumor. Similarly, computed tomographic scans and ultrasonograms may show liver nodules, even very early in the course of the disease, that may not be malignant.
Unfortunately, maintenance of normal tyrosine levels by dietary means is no protection against the development of hepatoma or the progression of liver disease. In a review of 10 patients, 9 of whom had been on a strict diet, 3 had HCC before transplantation, 2 had incidental carcinoma diagnosed at transplantation, and 9 had hepatocyte dysplasia.
The decision regarding transplantation is more easily made when the infant presents with fulminant liver failure. In such infants the severity of their disease usually precludes a trial of NTBC. As experience has accrued with liver transplantation in small infants, the fear of a poor outcome in such young recipients has been allayed. Esquivel et al reported an 80% survival rate in infants younger than 1 year who underwent transplantation for tyrosinemia. In very young infants a trial of dietary management and NTBC is reasonable only if some stabilization in liver function can be achieved.
Although many of the clinical features of tyrosinemia resolve, liver transplantation does not guarantee complete reversal of the kidney impairment seen in hereditary tyrosinemia. Not all patients reported have shown complete normalization of tubular dysfunction or the glomerular filtration rate (GFR), and many continue to have some, albeit reduced, amounts of succinylacetone in their urine. The ongoing endogenous production of succinylacetone is the most likely explanation, but heterogeneity in local expression of tyrosinemia in the kidney is evident by the variation in kidney function reported after liver transplantation. However, because the GFR is frequently impaired before transplantation and ongoing posttransplant kidney impairment secondary to calcineurin inhibitor use is likely, careful posttransplant management of renal function is necessary. Paradis et al monitored the GFR in tyrosinemic children after transplantation and fractionated their cyclosporine doses, which avoided further nephrotoxicity.
A mouse model of FAH deficiency is now allowing experimental approaches to gene therapy for tyrosinemia. This approach will be of particular benefit in high-risk populations in which routine screening of newborns, siblings of index cases, and infants with early manifestation of disease is performed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here