Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Placement of a tracheostomy tube is a common procedure in critically ill patients. Common indications for this procedure include upper airway obstruction, head or neck trauma, and prolonged respiratory failure. Approximately one fourth of patients in the intensive care unit (ICU) will require a tracheostomy tube for prolonged respiratory support or weaning from mechanical ventilation. Advances in health care allow many patients with tracheostomies to live at home or in other relatively low-technology environments such as rehabilitation facilities and nursing homes. Tracheostomy care is often provided by a variety of caregivers, including family members, home health care nurses, and patient care technicians.
Patients with tracheostomies are seen in emergency departments (EDs) for a variety of problems related to the tracheostomy. Many emergency clinicians will have minimal experience with such patients and it should be emphasized that complications or problems with tracheostomies often require specialty consultation in the ED to be fully evaluated or definitively remedied.
Common complaints include difficulty breathing as a result of tube obstruction, tube displacement, or equipment failure; poor oxygenation from infection or altered patient anatomy; and bleeding. In some cases, complications can be life threatening. Emergency physicians must be knowledgeable regarding the evaluation and management of patients with tracheostomies. This chapter reviews the relevant tracheal anatomy and essential tracheostomy equipment, discusses pertinent tracheostomy care, provides a systematic approach to the evaluation and management of selected complications, and identifies high-risk patient populations.
Most tracheostomies are performed electively. For elective tracheostomies, the surgical site is between the first and second or the second and third tracheal rings. With an open, or surgical, tracheostomy, the anterior aspect of the trachea is generally left sutured to the skin until the tract matures, approximately 4 to 5 days after the procedure. In recent years, percutaneous dilational tracheostomy has become the preferred technique for many ICU patients. It can be performed at the bedside and eliminates the risks associated with transporting critically ill patients to an operating room. Postoperative complications vary and depend on the timing and insertion technique ( Box 7.1 ). Early postoperative complications tend to arise in the first few days to weeks. Sixteen to twenty percent of patients experience early complications, and 6% to 8% experience late complications. Although elective and emergency tracheostomies are generally performed with the same technique, the complication rate associated with emergency procedures may be higher than the rate for elective tracheostomies.
Hemorrhage—postoperative
Tube dislodgement or obstruction
Subcutaneous emphysema
Soft tissue infection
Pneumothorax, pneumomediastinum
Tracheal stenosis or tracheal malacia (granulation tissue)
Tube dislodgement or obstruction
Equipment failure
Tracheoinnominate artery fistula
Tracheoesophageal fistula
Infection—pneumonia, aspiration
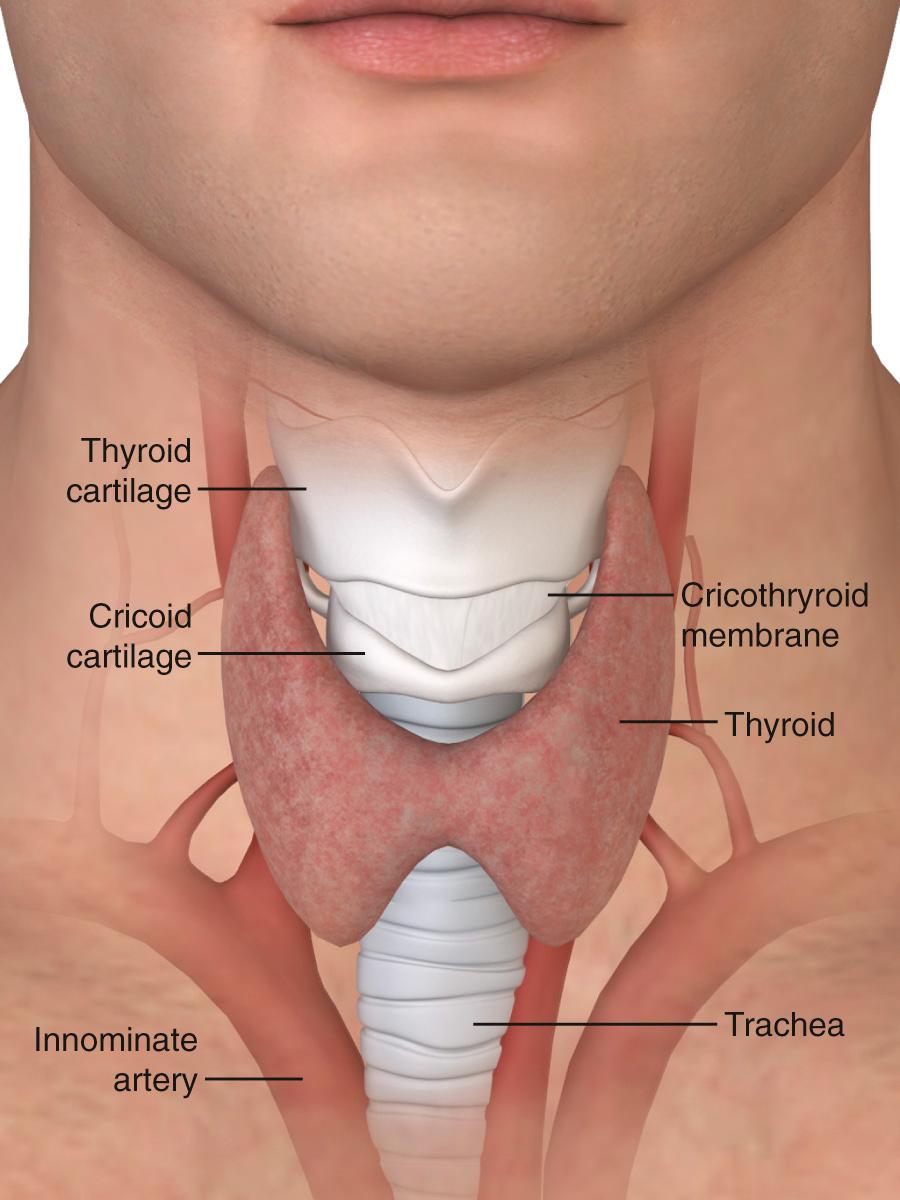
The lower respiratory tract begins at the vocal cords. Inferior to the vocal cords lies the cricoid cartilage, which encases the 1.5- to 2-cm subglottic space. Inferior to the cricoid cartilage is the trachea ( Fig. 7.1 ). The typical adult trachea is 10 to 12 cm in length. The anterior and lateral walls of the trachea are supported by 18 to 22 incomplete cartilaginous rings. A fibromuscular sheet lying anterior to the esophagus completes the posterior wall. The interior diameter of the adult trachea is 12 to 25 mm, and it is lined with mucosa covered by respiratory epithelium. Blood is supplied to the trachea by branches of the inferior thyroid, innominate (brachiocephalic), bronchial, and subclavian arteries. Critically, the innominate artery (IA) lies in close proximity to the tracheostomy stoma. From its origin at the aortic arch, the IA courses between the sternum and the anterior aspect of the trachea and veers right at the sternomanubrial joint. The location of the IA is important because erosion of the anterior tracheal wall can lead to life-threatening bleeding. The recurrent laryngeal nerve innervates the intrinsic laryngeal muscles and mucosa below the vocal cords. Efferent vagal fibers stimulate bronchoconstriction, mucosal secretions, and vasodilation. Efferent sympathetic fibers of the pulmonary plexus stimulate tracheal bronchodilation and vasoconstriction.
The upper airway, including the oropharynx and nasal passages, filters particulate matter, humidifies inspired air, and aids in the expectoration of secretions. These functions are reduced in patients with a tracheostomy. Placement of a tracheostomy bypasses humidification and results in the formation of thick, dry secretions. In the absence of humidification, squamous metaplasia and chronic inflammatory changes develop in the trachea. Bronchoconstriction resulting in reduced airflow can occur if the inspired air temperature is below room temperature. Normal mucociliary clearance is also impaired because of the increased viscosity of respiratory secretions, which underlies chronic illness and respiratory infections, particularly with Mycoplasma or viral pathogens.
The tracheostomy procedure weakens the anterior tracheal wall and blunts the normal cough mechanism, an important component for clearance of secretions by the trachea. Normally, the epiglottis and vocal cords close to trap air in the lungs and raise intrathoracic pressure before a cough. Patients with a tracheostomy tube are generally unable to generate sufficient pressure to initiate a strong cough and facilitate airway clearance. In addition to an impaired cough mechanism, immune responses are often blunted in patients with tracheostomies as the result of underlying illnesses, chronic lung disease, chemotherapy, or acquired immunosuppression.
Every ED patient with a tracheostomy who has a respiratory complaint or a complaint related to the tracheostomy should be thoroughly evaluated. Begin the primary assessment with a review of the patient's vital signs and evaluation of the airway. Examine the tracheostomy and consider dislodgement, obstruction, and fracture of the tube. Next, assess the patient's respiratory status. If the patient is receiving mechanical ventilation on arrival and is in acute distress, remove the patient from the ventilator and replace it with manual ventilation with a bag-valve-mask device. Initiate continuous cardiac monitoring, pulse oximetry, and capnography, if available, in all tracheostomy patients in respiratory distress.
In the history of the present illness, include the indications for placement of a tracheostomy, the length of time from placement to arrival at the ED, and any previous complications. Discuss any planned or existing voice prosthesis, previous bleeding complications or strictures, and whether a permanent tracheostomy or decannulation of the tracheostomy is anticipated. Ask whether there have been any recent changes in ventilator settings or tracheostomy care, including increased oxygen use, increased suctioning, equipment failure, or changes in equipment.
After the primary assessment and focused history, perform a thorough physical examination to differentiate between tracheostomy complications and other causes of the patient's respiratory complaint. The differential diagnosis may include pneumonia, exacerbation of chronic obstructive pulmonary disease, congestive heart failure, pulmonary embolism, pneumothorax, and acute coronary syndrome. During the secondary evaluation, evaluate the patient's stoma for signs of bleeding, infection, or skin breakdown. Make a note of the specific type and model of equipment used in the event that a replacement tube is needed.
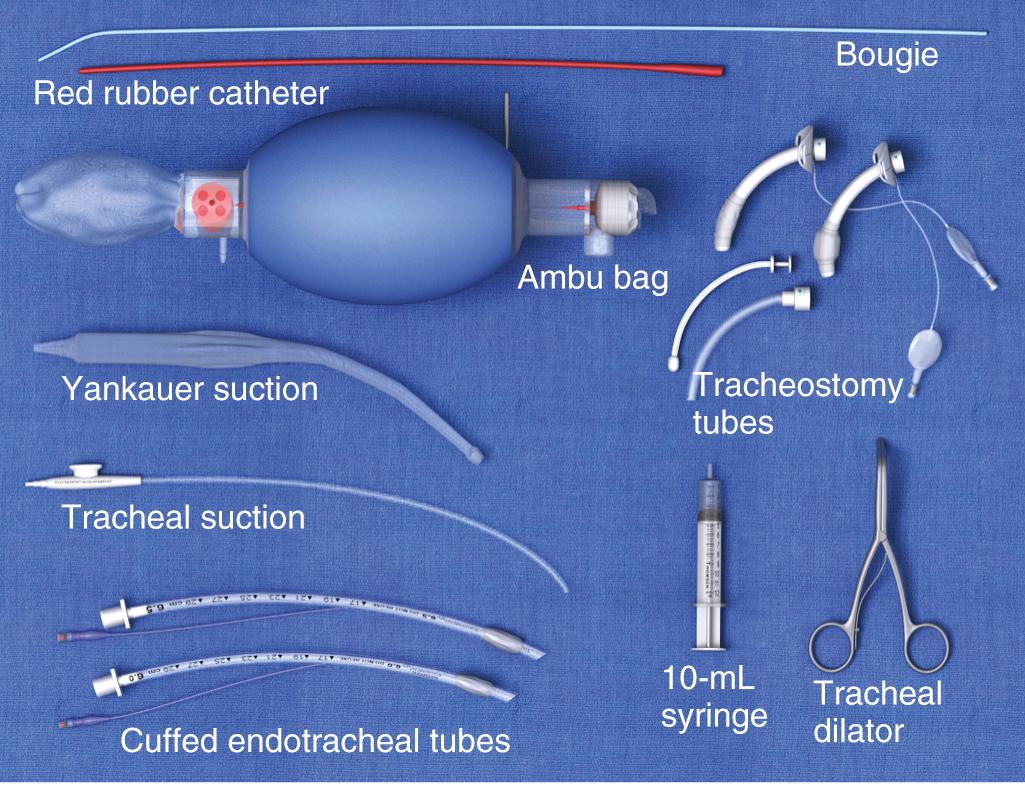
Before performing any procedure on the tracheostomy tube, it is important that the emergency physician ensure that essential equipment is readily available at the patient's bedside. These items are shown in Fig. 7.2 . Adequate preparation is crucial in preventing a poor outcome should complications arise. Most of this equipment can be placed in a designated airway box that can easily be accessed within the room or ED.
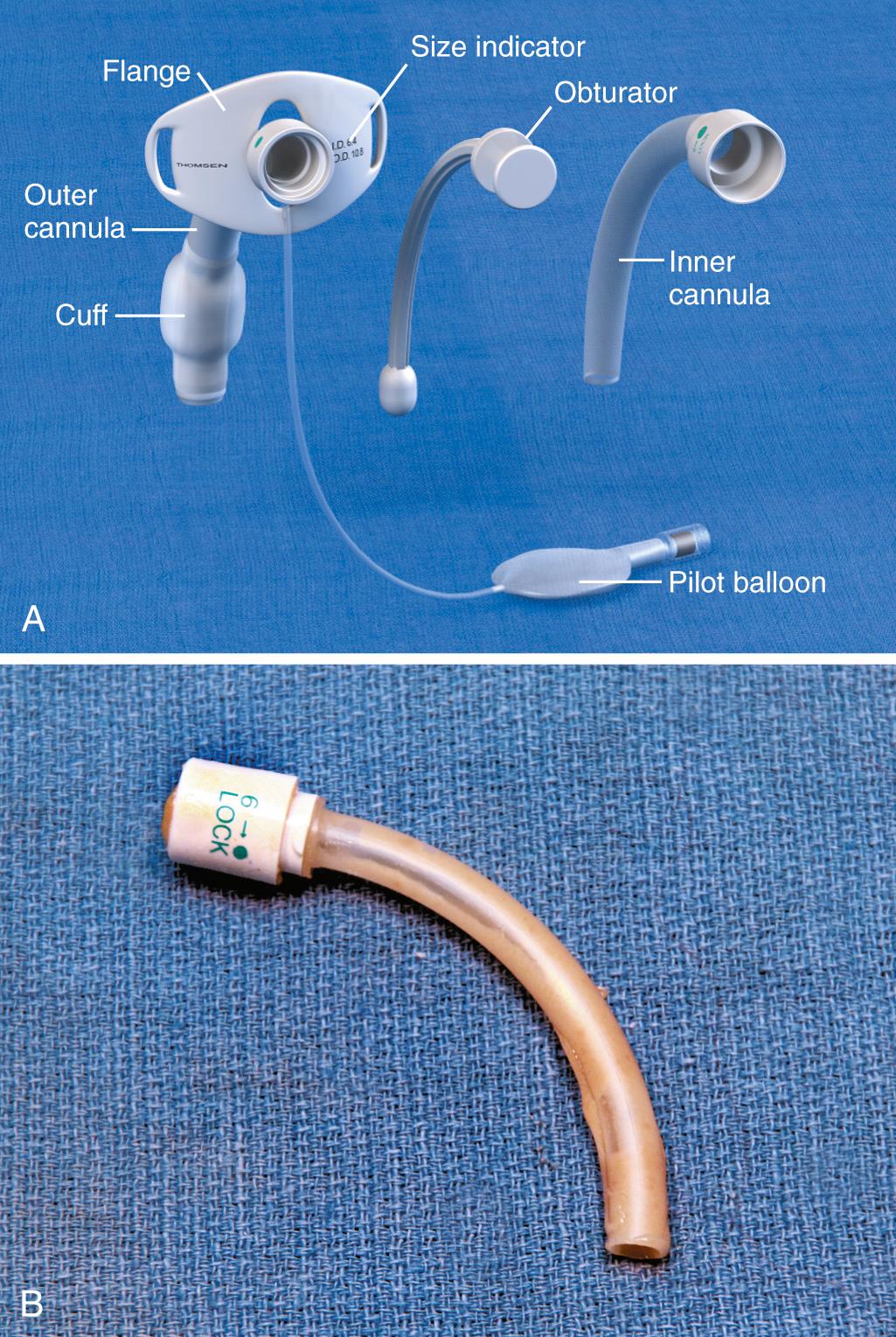
Routine tracheostomy maintenance involves (1) regular cleaning of the tube, (2) frequent stomal care, and (3) periodic monitoring of cuff pressure. There are many different types of tracheostomy tubes, and the focus of this section is on the most common, those with a removable inner cannula ( Fig. 7.3 A ). Regular cleaning of the tracheostomy tube and inner cannula can prevent the accumulation of dried secretions. Lack of cleaning and maintenance of the inner cannula is the primary cause of tube obstruction (see Fig. 7.3 B ). Under normal circumstances the inner cannula should be in place. It sits snugly within the tracheostomy tube and can easily be removed without disturbing the tube itself. The inner cannula should be cleaned daily. Soak it in a half-strength hydrogen peroxide solution for 10 to 15 minutes and remove encrustations with a soft-bristle tracheostomy brush. Clean dried debris and blood from the tracheostomy tube flanges as well. To prevent damage to the tracheal mucosa, rinse all airway equipment with sterile saline before reinsertion. Important stomal wound care includes changing contaminated tube ties, cleaning the tube flanges regularly, and using pre-cut tracheostomy gauze. Loose fibers from hand-cut gauze may induce inflammatory changes at the stomal site.
The tracheostomy cuff provides a tight seal to allow positive pressure ventilation and prevent aspiration. Cuff pressure should ideally be maintained below 25 mm Hg. Overinflation is common and can cause disastrous injury to the tracheal wall and mucosa and lead to tracheomalacia, tracheal stenosis, or the development of a fistula between surrounding anatomic structures. It is a good practice to regularly check and document cuff pressure with a handheld pressure manometer to document inflation volumes. If an air leak occurs at the maximum recommended cuff pressure, the tube may have become dislodged, which requires further evaluation.
It is essential that adequate air humidification be provided to patients with a tracheostomy. Inadequate humidification can result in obstruction of the tube from thick secretions, sputum retention, keratinization or ulceration of the tracheal mucosa, and impaired gas exchange as a result of lung atelectasis. Ambulatory patients and those who require low-flow oxygen can be fitted with a heat-moisture exchanger that attaches to the external opening of the tracheostomy tube. Patients receiving long-term ventilation or high-flow oxygen require regular saline nebulizer treatments delivered by an in-line humidification system.
To properly ventilate ED patients with a tracheostomy, it is important to determine the make, model, and type of tube ( Fig. 7.4 ). For proper ventilation, a 15-mm tube/Ambu bag or ventilator tube adapter must be present, either on the tube itself or on the end of an inner cannula that has an inflatable cuff. The inner cannula adapter will accept an Ambu bag or ventilator tubing, and the cuff will allow positive pressure ventilation. If the patient requires manual or mechanical ventilation and the tracheostomy tube is not suitable for ventilatory support, immediately replace it with a 6-0 cuffed endotracheal (ET) tube for ventilation.
Tracheal suctioning is required to remove secretions or aspirated material from the upper airway in patients whose cough is impaired or in whom an artificial airway is in place. Tracheal suctioning can be performed through an ET tube, a tracheostomy tube, a minitracheostomy placed in the cricothyroid membrane, or the nasopharynx. The indications, equipment, procedure, and complications are similar for each technique.
The primary indications for tracheal suctioning are to remove secretions, enhance oxygenation, or obtain samples of lower respiratory tract secretions for diagnostic tests. In the ED, tracheal suctioning should be performed to enhance oxygenation in any tracheostomy patient in respiratory distress. In addition, tracheal suctioning should be performed when the patient has coarse rales, rhonchi, or tubular breath sounds; acute or worsening dyspnea; or arterial oxygen desaturation. It is important to emphasize that tracheal suctioning should be performed only when it is clinically indicated; frequent, routine suctioning is not recommended.
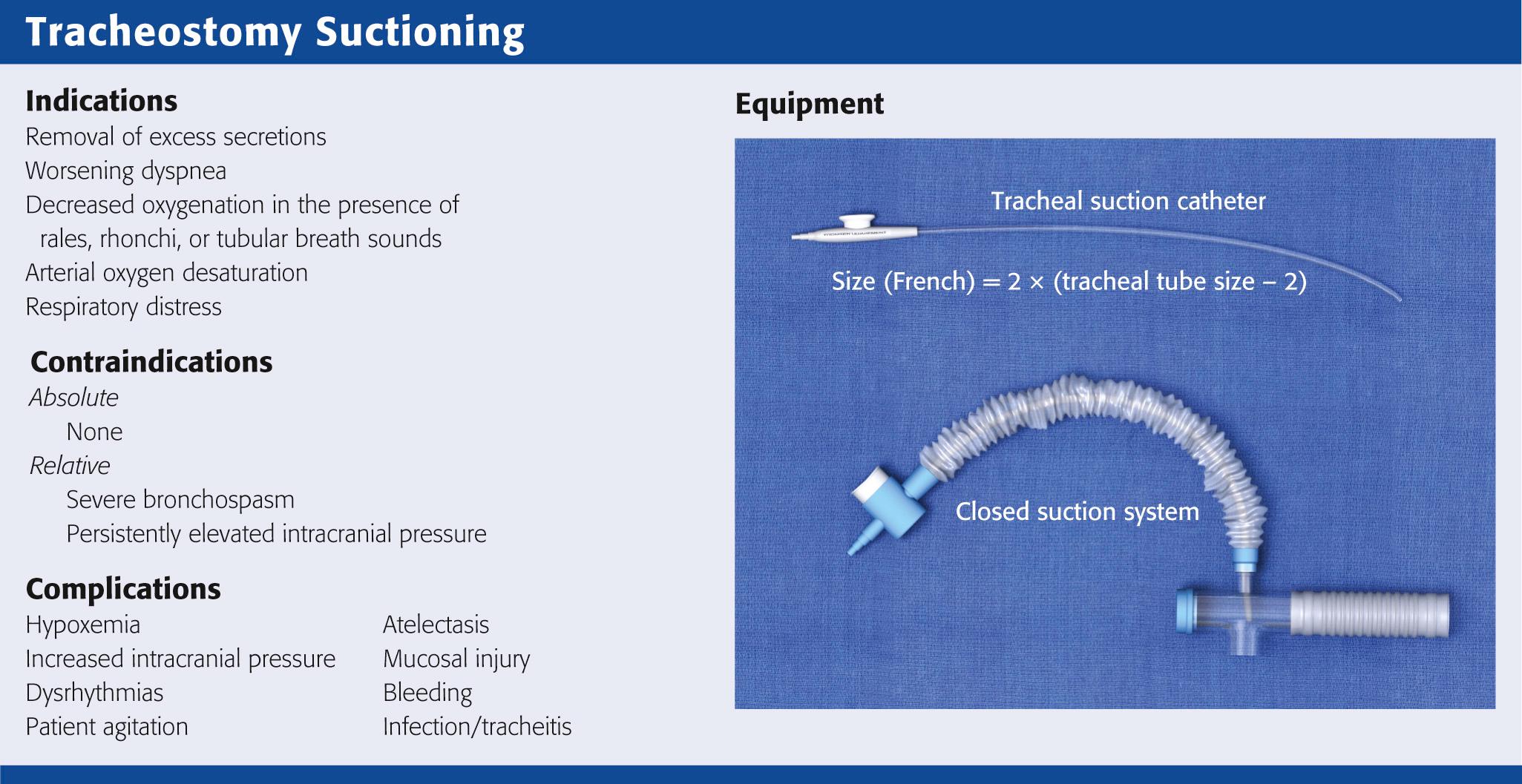
There are no absolute contraindications to tracheal suctioning. Relative contraindications include severe bronchospasm, which may worsen with suctioning, and persistently elevated intracranial pressure (ICP), which is exacerbated by suctioning. Bronchodilators, sedatives, and paralytics may alleviate these symptoms. Tracheal suctioning should be undertaken with caution in patients with cardiovascular instability because of an increased risk for associated dysrhythmias.
A suction catheter and vacuum system, open or closed, is required to perform tracheal suctioning ( Fig. 7.5 ). It is recommended that the diameter of the suction catheter be no larger than half the inner diameter of the tracheostomy tube. The size of the suction catheter in French gauge (Fr) can be calculated as follows:
For example, a 7-0 tracheostomy tube will require a 10-Fr suction catheter because 2 × (7 − 2) = 10 Fr. If the catheter is too small, it will not remove excess secretions adequately. If the catheter is too large, it can obstruct airflow during insertion and cause alveolar collapse with resultant hypoxemia.
In adults, the suction catheter should be inserted only 10 to 15 cm, depending on the length of the tracheostomy tube. The goal of suctioning is to remove secretions only from the proximal airways. Shallow suctioning occurs when the catheter is placed just beyond the hub of the tracheostomy tube to remove proximal secretions. Premeasured suctioning occurs when a catheter is inserted such that the distal side ports are beyond the caudal end of the tracheostomy tube. Deep suctioning occurs when the suction catheter is advanced until resistance is met. It is used for clearing excess secretions in the lower airways. Deep suctioning has not been shown to be more beneficial than shallow suctioning and should not be performed routinely because it might damage the mucosal epithelium and lead to an increase in granulation tissue.
A number of suction catheter tips have been designed to maximize removal of secretions without causing mucosal injury. Tips may have a single or multiple side ports proximal to the distal tip. Directional or Coude tip catheters are available for selective suctioning of the main stem bronchi.
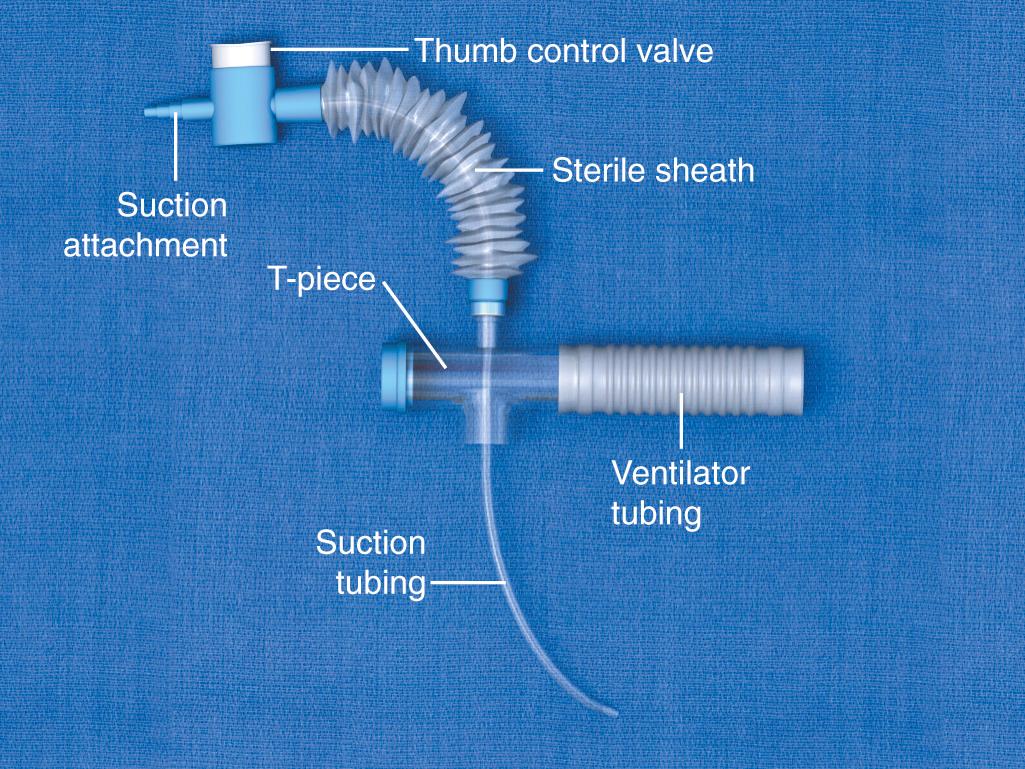
A closed-system airway encases a suction catheter in a sterile sheath attached to ventilator tubing. This prevents the suction catheter from being contaminated by contact with the outside environment and allows tracheal suctioning to be performed without interrupting ventilatory support. The vacuum should be set to the lowest possible pressure to reduce atelectasis. Vacuum pressure should not exceed 80 mm Hg in infants or 150 mm Hg in adults.
Before tracheal suctioning, continuous pulse oximetry, cardiac monitoring, and continuous capnography, if available, should be initiated ( Fig. 7.6 , step 1 ). Awake and alert patients should be sitting upright with their head in a neutral position. For mechanically ventilated patients, the head of the bed should be elevated to 30 degrees to improve respiratory mechanics. Aseptic technique should be used throughout all suctioning procedures to prevent the introduction of bacteria. Backup airway equipment should be readily available (see Fig. 7.2 ). Preoxygenate the patient for at least 30 to 60 seconds. For mechanically ventilated patients, increase the fraction of inspired oxygen (Fi o 2 ) to 100%. For nonventilated patients, provide 10 to 15 L of high-flow oxygen. Humidify the air before suctioning to reduce the viscosity of respiratory secretions. Routine instillation of normal saline has not been shown to provide regular clinical benefit and is no longer recommended.
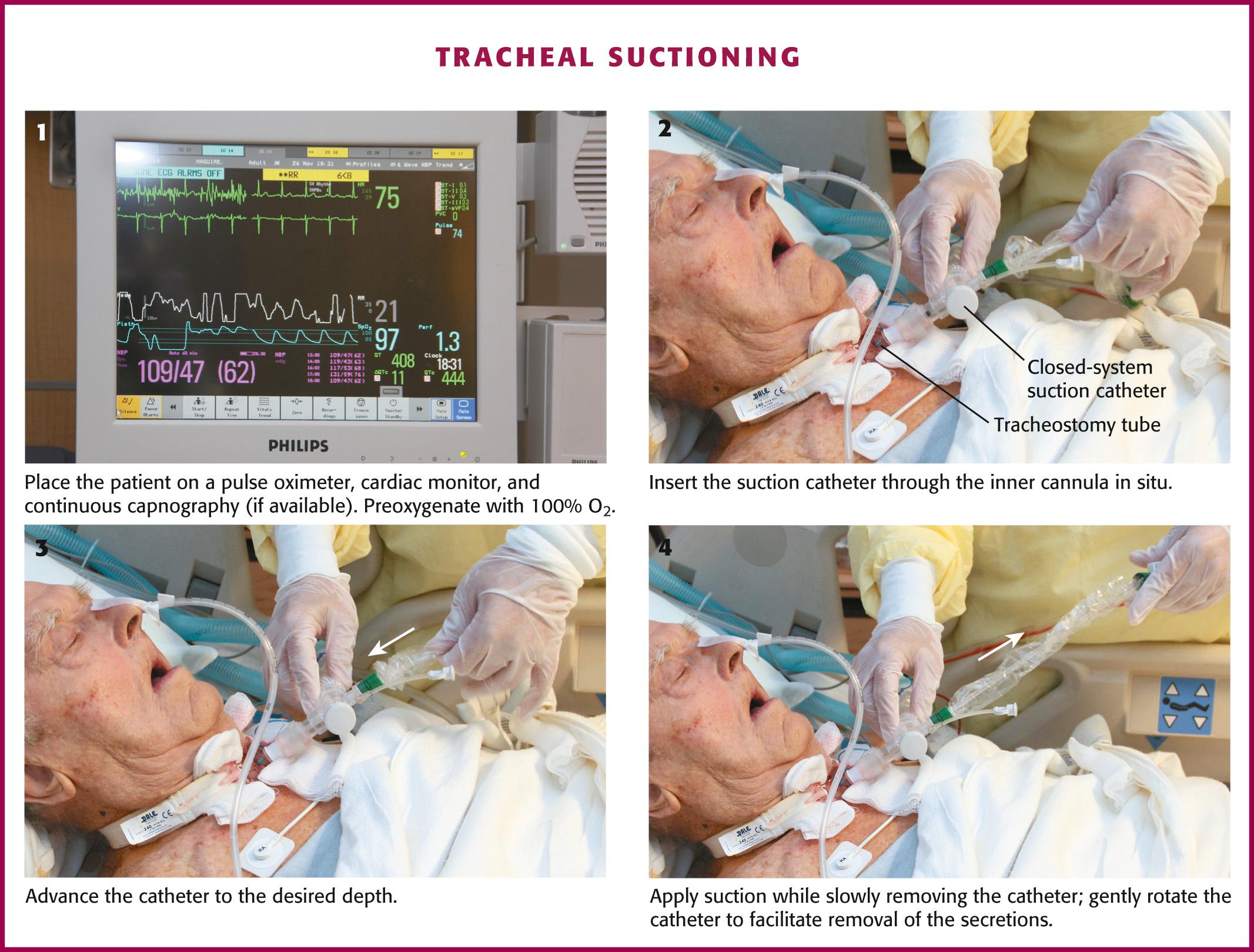
Once the patient has been adequately preoxygenated, insert the suction catheter through the inner cannula in situ (see Fig. 7.6 , step 2 ). If the carina is irritated during deep suctioning, a vigorous cough reflex will be activated. After reaching the desired depth, withdraw the suction catheter 1 to 2 cm and then apply suction while slowly removing the catheter (see Fig. 7.6 , steps 3 and 4 ). Gently rotate the catheter as it is withdrawn to facilitate removal of secretions. The duration of suctioning should not exceed 10 to 15 seconds.
Monitor the patient throughout the procedure for signs of cardiac dysrhythmia, hypoxia, or a rise in end-tidal CO 2 . Immediately stop suctioning if any of these signs develop (see section on Complications of Suctioning ). If marked respiratory distress is presumed to be secondary to significant tracheal obstruction, continue with expeditious suctioning to remove the obstruction (see section on Obstruction and Complications from Tube Changes ).
Complications that occur during or after suctioning are relatively common and can result in significant morbidity. Most complications can be anticipated and simple maneuvers can reduce their incidence and severity.
Hypoxemia from suctioning may cause increased ICP, dysrhythmias, or even death. In neonates, hypoxia during suctioning may contribute to spontaneous intracerebral hemorrhage. A number of factors contribute to suctioning-related hypoxia, including interruption of mechanical ventilation, aspiration of air from the respiratory tract, and suctioning-related atelectasis. Use in-line suction catheters for ventilator-dependent patients to allow continuous oxygen delivery and positive pressure ventilation. Select the catheter size carefully to reduce the evacuation of airway gases during suctioning and help prevent atelectasis. Limit the duration of suctioning to 10 to 15 seconds and perform no more than three passes in succession. Measurement of arterial oxygen saturation may not be sufficient to assess hypoxia after suctioning. Oxygen consumption increases during suctioning despite insignificant changes in oxygen saturation. This increase in oxygen consumption is more prevalent in patients who possess a vigorous cough, are agitated, or resist suctioning.
Dysrhythmias associated with suctioning may be caused by hypoxia, increased myocardial oxygen consumption, vagal stimulation, hypoventilation, or catecholamine release. Vagal stimulation caused by suctioning can cause bradycardia and hypotension. Bradycardia in the setting of hypoxia potentiates ventricular dysrhythmias, including ventricular fibrillation. Nebulized or intravenous atropine is recommended for bradycardia and can be used as pretreatment in patients at risk for bradycardia, particularly infants. Digoxin enhances vagal activity and may potentiate the vagal stimulation of ET suctioning. Sympathetic stimulation may occur as a result of hypoxia, pain, or stress of the procedure. Pain medications, anxiolytics, and preparation of the patient for the procedure may blunt the sympathetic response. Suctioning should be stopped immediately if a dysrhythmia develops.
Increases in ICP during suctioning are well documented. ET suctioning can induce a strong cough reflex, which is thought to contribute to the increased ICP by raising intrathoracic pressure, reducing cerebral perfusion pressure, and increasing systemic blood pressure. In susceptible patients, increases in ICP can lead to devastating outcomes. If there is concern that increases in ICP could harm the patient, several preventive steps should be taken before initiating ET suctioning. The most important interventions are providing adequate sedation and maximizing oxygenation. Hyperventilation before and between passes of the suction catheter can transiently lower ICP by reducing systemic CO 2 . One minute before suctioning, hyperventilate the patient by increasing the respiratory rate to approximately 30 breaths/min. If not heavily sedated, most patients will cough vigorously when suctioned. Lidocaine can be instilled into the trachea to blunt the cough reflex, thereby preventing an increase in ICP. To anesthetize the trachea locally, instill 1 to 1.5 mg/kg of 2% lidocaine into the tracheal tube. After administering the medication, prepare the patient for suctioning by ensuring that sedation and oxygenation are adequate. After 10 minutes to allow the lidocaine to produce its local effect, begin tracheal suctioning. This method has been shown to prevent increases in ICP and changes in cerebral hemodynamics.
Atelectasis can occur when airway gases are suctioned too rapidly. To reduce this complication, choose a suction catheter that is less than half the inner diameter of the tracheostomy tube and minimize the duration and suction pressure. Atelectasis can be minimized by using a closed suction system and providing positive end-expiratory pressure after suctioning. Hyperventilation should not be performed routinely to resolve suction-related atelectasis.
Mucosal injury is a common complication of tracheal suctioning. Invagination of the mucosa into the side ports of the catheter occurs during suctioning and causes the tracheal mucosa to become denuded, edematous, and predisposed to bleeding. Mucosal damage also interferes with mucociliary transport. Tracheitis can occur as a result of frequent or improperly performed suctioning.
The minitracheostomy ( minitrach ) was designed to improve tracheal hygiene in patients with intact cough reflexes, normal ventilatory function, and vocalization. The minitrach serves as a small port solely for suctioning secretions. Commonly, a 4-mm indwelling cuffless cannula is inserted through the cricothyroid membrane into the trachea. Patients who are suctioned through a minitrach are at lower risk for gagging and aspiration because they are able to maintain laryngeal and glottic function. The minitrach device is seldom used in children because they have smaller airway diameters.
The technique used to suction through a minitrach is the same as that for tracheal suctioning. The smaller port size may require that smaller catheters be used. Most patients with minitrachs are decannulated before discharge from the ICU and are rarely seen in EDs.
Maturation of the tracheostomy tract is generally completed by postoperative day 7. Most ED patients with a tracheostomy are seen after the stomal tract has matured, so routine changes of the tracheostomy tube can be done safely in the ED. Indications for exchange of the tracheostomy tube include cuff rupture or leak, leakage around the tube caused by tracheomalacia, other changes in tracheal anatomy, complete or partial tube occlusion, and conversion to an alternative tube style. There are no absolute contraindications to exchanging a tracheostomy tube in the ED as long as the stomal tract has matured. Before undertaking the exchange, consider whether further tissue trauma or hemorrhage might occur as a result of it or whether anatomic abnormalities could make the exchange difficult.
There is conflicting evidence on how frequently tracheostomy tubes should be changed. Do not perform tube changes on a predetermined schedule, but rather as the patient's clinical condition dictates. There is some evidence that tubes left in place longer than 3 months are at higher risk for infection. Most manufacturers recommend that tracheostomy tubes be changed approximately 30 days after placement.
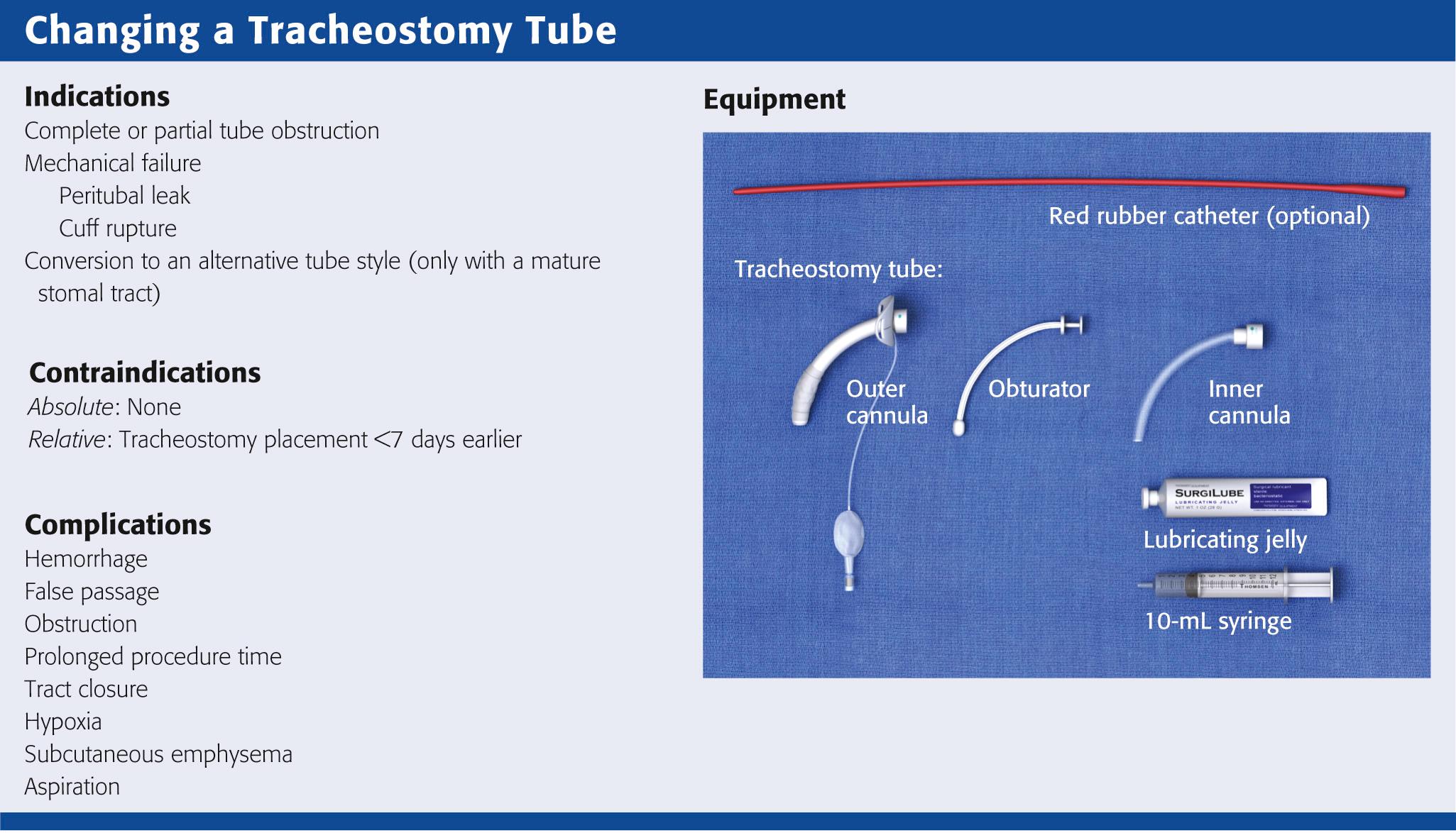
When changing a tracheostomy tube, familiarize yourself with the resources and equipment available in the ED. Keep the equipment readily available, if not at the patient's bedside. The necessary equipment is depicted in Fig. 7.2 .
Tracheostomy tubes may be made from metal or other synthetic material such as plastic. Plastic tubes are either polyvinyl chloride, which softens at body temperature, or silicon, which is naturally soft and unaffected by temperature. Metal tubes are constructed of silver or stainless steel and lack both a cuff and a 15-mm connector for attachment to a ventilator or Ambu bag.
To determine the appropriate size of tube, consider the internal diameter (ID), outside diameter (OD), length, and curvature of the tube. The Chevalier Jackson sizing system indicates the length and tapering of the OD. This sizing method applies to metal tubes and most Shiley dual-cannula tracheostomy tubes. Table 7.1 lists common tracheostomy tubes and their dimensions. For dual-cannula tracheostomy tubes, the inner cannula has a 15-mm connection for a ventilator. Note that tracheostomy tubes with the same ID can have very different ODs and lengths. Consider the pretracheal distance before selecting a replacement tube of the appropriate size for obese patients (see section on Special Populations ).
| Portex Cuffed D.I.C. Tracheostomy Tubes | Shiley Tracheostomy Tubes a | ||||||
|---|---|---|---|---|---|---|---|
| TUBE SIZE (mm) AND COLOR CODE | INTERNAL DIAMETER (mm) | OUTER DIAMETER (mm) | LENGTH (mm) | TUBE SIZE (JACKSON) | INTERNAL DIAMETER (mm) | OUTER DIAMETER (mm) | LENGTH (mm) |
| 6.0 (orange) | 6.0 | 8.5 | 64.0 | 4 | 5.0 | 9.4 | 62 |
| 7.0 (green) | 7.0 | 9.9 | 70.0 | 6 | 6.4 | 10.8 | 74 |
| 8.0 (white) | 8.0 | 11.3 | 73.0 | 8 | 7.6 | 12.2 | 79 |
| 9.0 (blue) | 9.0 | 12.6 | 79.0 | 10 | 8.9 | 13.8 | 79 |
| 10.0 (yellow) | 10.0 | 14.0 | 79.0 | ||||
a Shiley also offers tracheostomy tubes with both distal and proximal (relative to the cuff) extended lengths for patients with large necks or other abnormal anatomy.
Single-cannula tracheostomy tubes are sized by the ID of the tube at its smallest dimension. The size of the tube is usually stamped on the flange. Before changing a tube, have the appropriate size of tube at the bedside along with tubes that are one or two sizes smaller. As a rule of thumb, most women can accommodate a tube with an OD of 10 mm, and most men can accommodate a tube with an OD of 11 mm. Table 7.1 lists the recommended sizes of tracheostomy tubes.
Most tracheostomy tubes have three standard components: an outer cannula, an obturator, and an inner cannula (see Fig. 7.3 A ) . The outer cannula is the permanent portion of the tracheostomy tube and should not be removed unless complications arise or a tube change is needed. Attached to the outer cannula is a flange on either side with eyelets used to tether the tube to the patient's neck. The obturator is a white rounded or cone-shaped object that is used to facilitate insertion of the tube. When inserted into the outer cannula, it extends several millimeters beyond the distal end of the tube.
Tracheostomy tubes can be cuffed or uncuffed. Cuffed tracheostomy tubes are used for patients on long-term mechanical ventilation and those at risk for aspiration. Cuffed tubes also prevent loss of volume during positive pressure ventilation while preventing air leaks across the vocal cords. Speech is not possible for patients with a cuffed tube. Most tubes have a high-volume, low-pressure cuff that reduces mucosal injury and the risk for tracheal erosion or stenosis. Low-volume, low-pressure cuffs may be more effective in preventing aspiration. Inflate the tracheostomy cuff and deflate it by attaching a syringe to the Luer-Lok port at the proximal end of the pilot balloon and either injecting or removing approximately 10 mL of air. Determine cuff pressure by connecting the Luer-Lok port to a handheld manometer.
Uncuffed and metal tubes are used in patients with adequate ventilatory effort who are alert and at low risk for aspiration. Depending on the size of the tracheostomy tube and how much of the tracheal diameter is filled by the tube itself, the patient may be able to speak. Air must be able to bypass the tube and be transmitted across the vocal cords. Digital occlusion of the tracheostomy tube or the use of specialized speaking valves can occlude expired air from the tracheostomy and facilitate voice production. Fenestrated tubes also allow air to be transmitted across the vocal cords. The fenestrations are generally located at the superior, posterior arch of the tube but can also be found on the inner cannula in some models.
During weaning from tracheostomy, tracheal buttons can be used to maintain patency of the stoma in patients who do not require mechanical ventilation. They can be retained permanently if decannulation is not possible. Tracheal buttons have a hollow outer cannula and a solid inner cannula and extend from the outer skin into the tracheal lumen. Tracheal buttons can become displaced into the tracheal lumen if they are not tethered correctly and may become clogged with secretions. They may also have a speaking valve, such as the Passy-Muir (Passy-Muir, Inc., Irvine, CA) or the Shiley Phonate (Mallinckrodt Medical, St. Louis, MO). These devices generally clip or twist onto the 15-mm coupling of the tracheostomy tube or inner cannula. Remove the speaking valve before changing the tracheostomy tube.
It is essential to ensure that all airway equipment is at the patient's bedside before performing the tube exchange. In addition to having airway equipment ready, place the patient on continuous pulse oximetry, cardiac monitoring, and capnography, if available, to confirm tube placement. Identify the tracheostomy tube model and determine its size. Have this size and two other tubes that are one or two sizes smaller in the event that tube replacement is difficult. Inspect all equipment for proper function, including the replacement tube cuff for leaks and the obturator for ease of insertion and removal ( Fig. 7.7 , step 1 ). Coat the replacement tracheostomy tube with a water-based lubricant (see Fig. 7.7 , step 2 ).
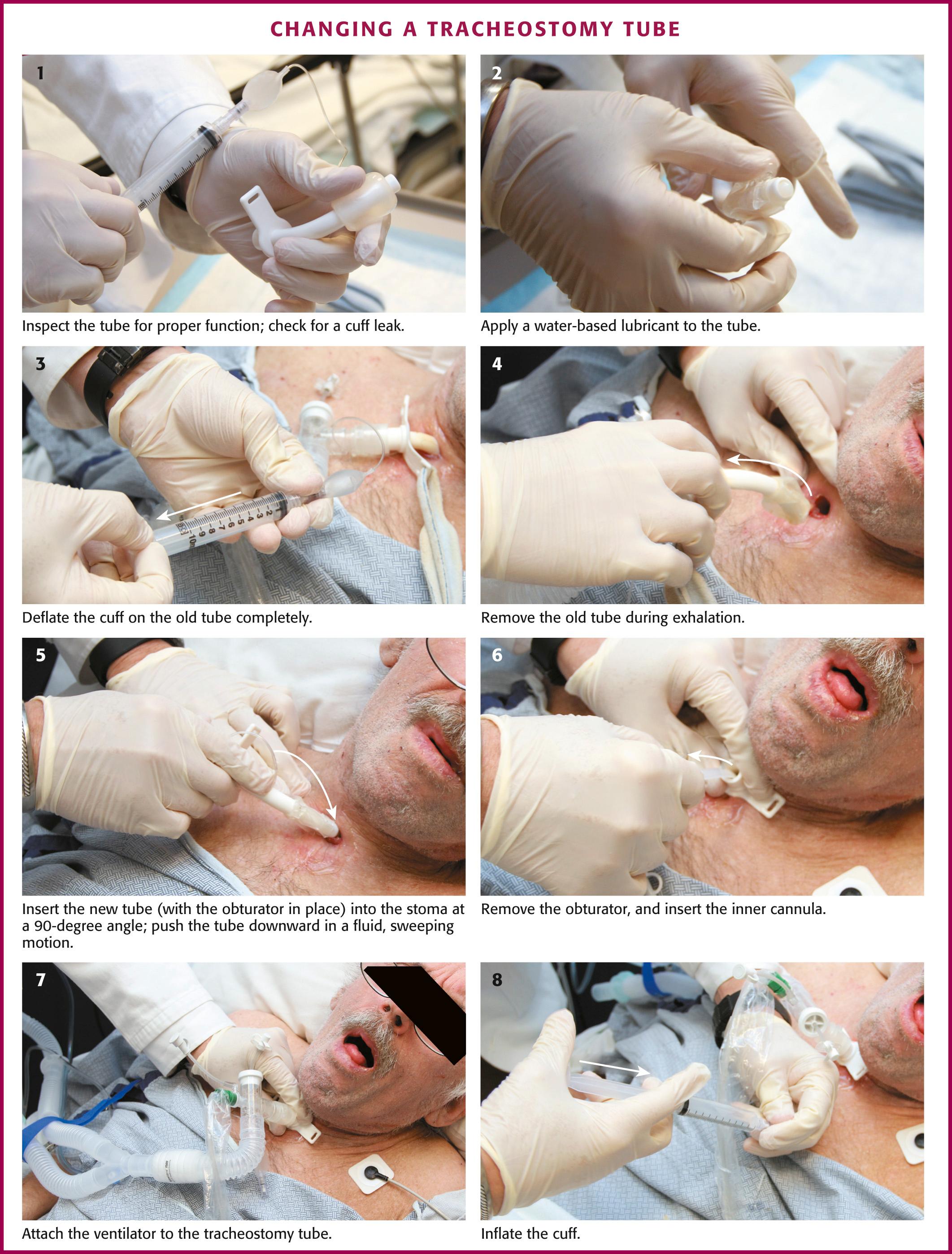
Place the patient in a semirecumbent position with the neck slightly extended to ensure proper alignment of the external stoma and the tracheostomy tract. Do not flex the neck because this may misalign the tissues and make tube replacement more difficult. Remove the old tracheostomy dressing and clean the stomal site. If awake, preoxygenate the patient by placing a non-rebreather oxygen mask over the tracheostomy site for 3 minutes and then suction the oropharynx. If the patient is ventilator dependent, increase the Fi o 2 to 100% for at least 1 minute before beginning the tube exchange to ensure adequate oxygenation. If needed, provide the patient with soft restraints or anxiolytic medication to improve compliance.
The tracheostomy tube can be changed by either of two methods. If the tracheostomy tract is well matured, the tube can be exchanged with an obturator. To remove the old tracheostomy tube, first deflate the cuff completely (if present) (see Fig. 7.7 , step 3 ). Remove the existing tube with an “out-then-down” movement while the patient exhales (see Fig. 7.7 , step 4 ). Next, with the obturator in place, insert the new tube into the stoma at a 90-degree angle to the cervical axis (see Fig. 7.7 , step 5 ). If two experienced providers are available, one can be responsible for deflating the cuff and removing the old tube while the other inserts the new device. Next, gently push the tube downward in a fluid, sweeping motion so the external flange is flush against the neck. If necessary, use a tracheal hook to hold the stoma open. Remove the solid obturator immediately, insert the hollow internal cannula, inflate the cuff, return the patient's head to the neutral position, and secure the external flange (see Fig. 7.7 , steps 6 to 8 ).
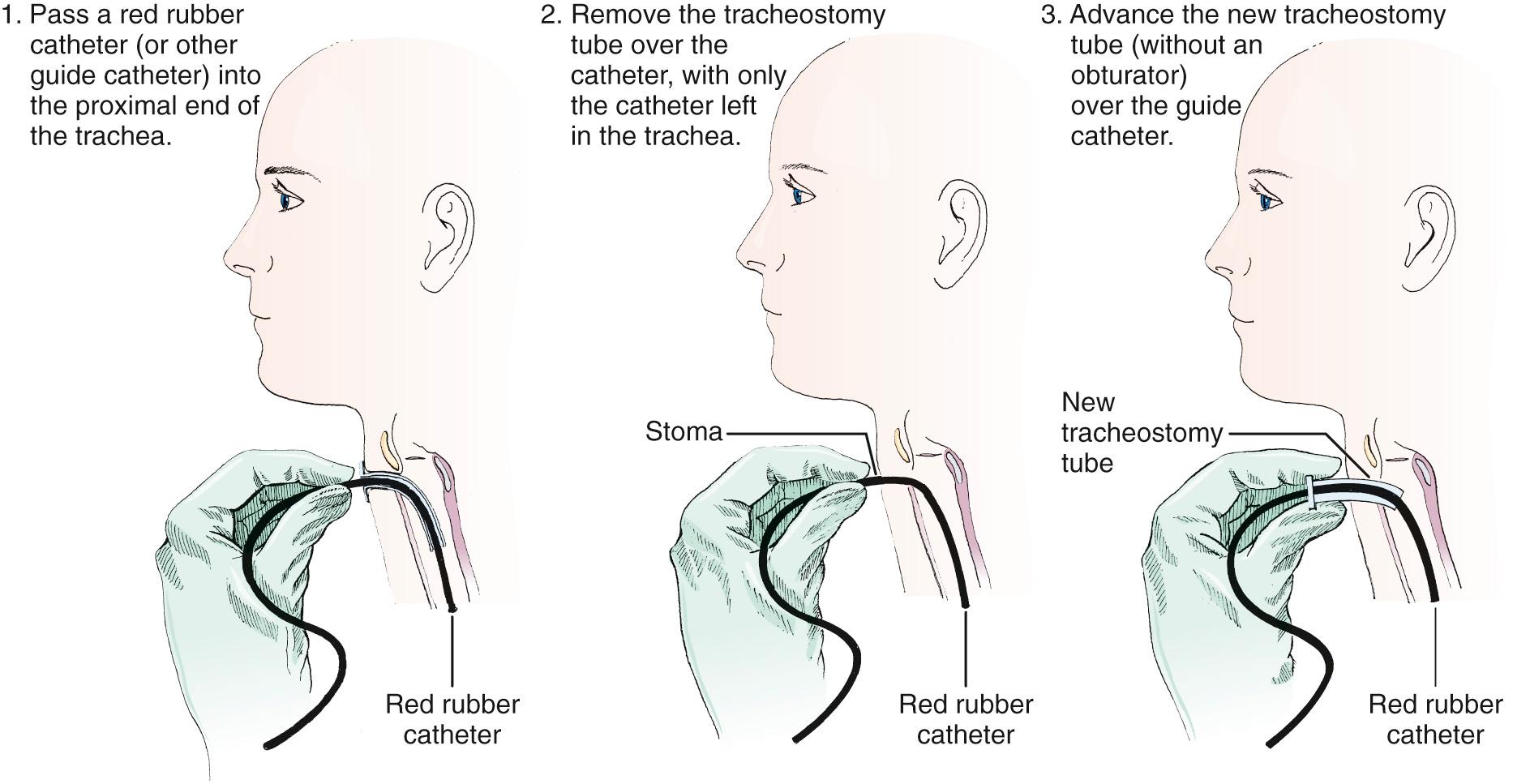
The second technique involves exchanging the tube with a modified Seldinger technique using a red rubber catheter, nasogastric tube, gum elastic bougie, or fiberoptic nasopharyngeal scope ( Fig. 7.8 ). This technique is preferable if the tracheostomy tract is not well defined or if there is concern that tube exchange could be difficult. Using a nasopharyngeal scope can also confirm placement by way of direct visualization. To exchange tubes with this method, first premeasure the distance needed to extend the guidewire device beyond the distal tip of the old tracheostomy tube. Advance the guide to the premeasured distance, deflate the cuff, and remove the tube as described previously. Next, without the obturator in place, advance the new device over the guide until it is seated securely in the trachea. Remove the guide and secure the new tube. Weinmann and Bander developed a modified Seldinger technique involving the use of an airway exchange catheter to allow jet or bag ventilation into the trachea during tube exchange. This adjunct delivers intratracheal oxygen and is helpful if hypoxemia is likely to occur.
Confirm proper placement of the tube within the trachea with one of several possible techniques. Traditionally, the patient is ventilated and correct tube placement confirmed by observation of equal chest rise and auscultation of bilateral breath sounds. Although both signs are important to confirm correct placement, quantitative waveform capnography is now a class I American Heart Association recommendation for confirmation of ET intubation. It should be used for tracheostomy tube placement, if available. Multiple studies have shown quantitative capnography to be a highly sensitive tool for confirming correct placement of airway devices. Correct tube placement can also be confirmed by direct visualization of the tracheal rings with a fiberoptic scope.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here