Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Long QT syndrome (LQTS) is an arrhythmogenic disorder that presents with QT interval prolongation and high risk for onset of ventricular arrhythmias and sudden death. It is a genetically heterogeneous condition involving at least 17 different genetic loci. , The focus of this chapter is the locus on chromosome 12p13.33 encompassing the CACNA1c gene that is the cause of Timothy syndrome (TS), also defined as LQT8 .
TS is a rare LQTS variant with a distinctive and mechanistically interesting spectrum of cardiac and extracardiac abnormalities that can include facial dysmorphisms, skeletal abnormalities, congenital cardiac defects, and central nervous system and metabolic abnormalities. The prognosis is often severe. After the initial anecdotal case reports, Katherine Timothy led an international collaborative effort that defined the clinical features of the disease and reported the first CACNA1c mutations. , CACNA1c encodes for the α-subunit of the voltage-dependent calcium channel (Ca V 1.2; also called L-type calcium channel) that is mainly active during the plateau of the cardiac action potential (AP). In recent years, molecular genetics in vitro and in vivo experimental studies and clinical reports have highlighted a complex pathophysiology and a heterogeneous spectrum of clinical phenotypes. Unfortunately, only limited advances have been achieved in the identification of effective therapies, and TS remains a disease with a high mortality.
Voltage-dependent calcium channels (Ca V ) are involved in several important physiologic functions, including excitation-contraction coupling of cardiac cells, electrical activation neurons, embryonic development, smooth muscle motility, osteogenesis, and insulin and hormone secretion. The Ca V family of channels includes several members (L, N, P, Q, R, N, and T), which are encoded by different genes and have different biophysical properties and physiologic functions.
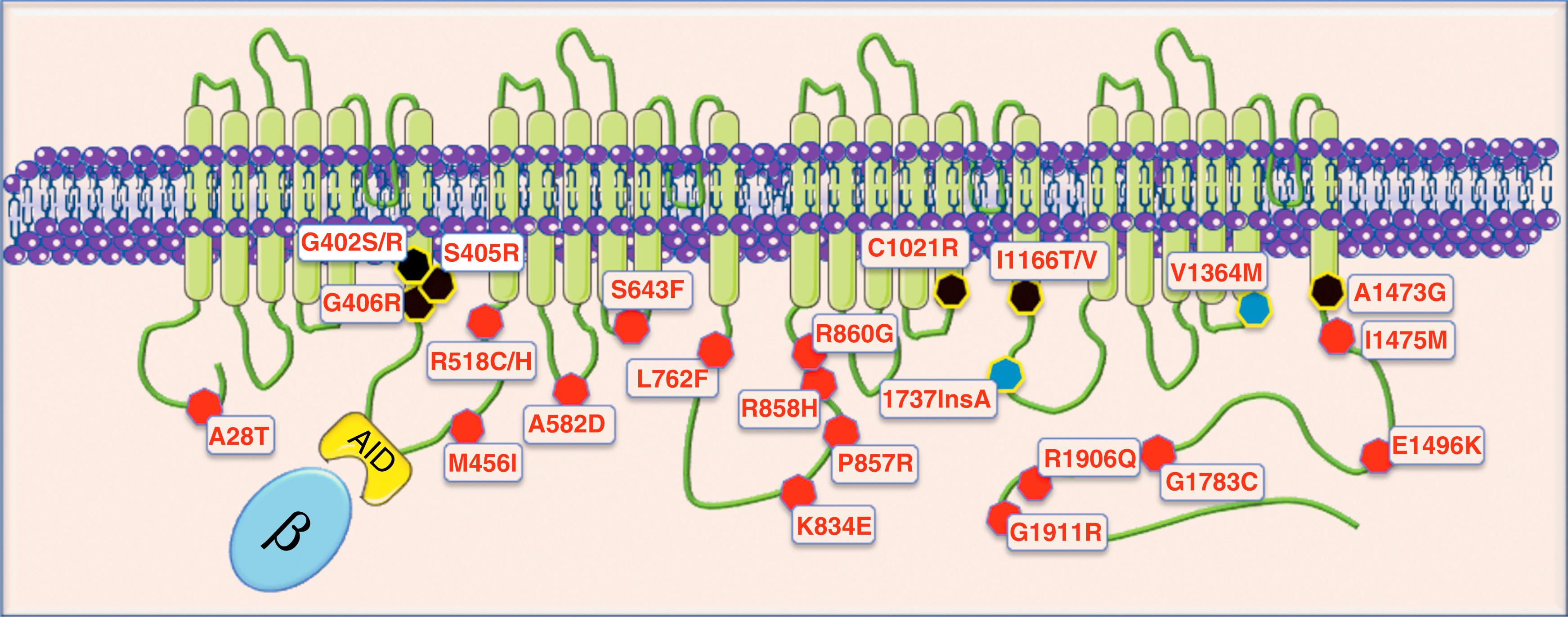
The L-type calcium channel, also called Ca V 1.2, is encoded by the CACNA1c gene on the short arm of chromosome 12. It is the predominant calcium channel in brain, heart, and vascular smooth muscle, but it is also present in the pancreas and other tissues at the lower level. Ca V 1.2 is the pore-forming protein (the α 1 -subunit) of a macromolecular complex that includes the α 2 δ, β 1-4 , and γ subunits that are required for the proper functioning and regulation of the channel. The α 1 -subunit consists of four homologous domains (I to IV), each one formed by six transmembrane-spanning segments (S1 to S6) and constitutes the channel pore forming protein. The current flowing through this channel is called I Ca . The other subunits modulate gating, trafficking, and the responses to neuro-hormonal stimuli. The β-subunit, which may be involved in TS pathophysiology, has an important role because it increases I Ca density by regulating the channel open probability and accelerating the rate of voltage-dependent inactivation. The β-subunit binds to the α 1 interaction domain (AID) in the DI-DII linker and interacts with the terminal part of the S6 transmembrane segments, which often harbor TS mutations.
CACNA1c spans over 500 kb in the human genome and undergoes extensive alternative splicing. , The splicing of this gene is complex and only partially understood. Nonetheless, it may have a role in the definition of the variability of the clinical manifestations of the disease. There are at least 42 splicing variants (also called isoforms) and there is tissue-specificity of splicing control; for example, the isoforms found in the heart are different from those found in the brain. , Furthermore, there is interindividual variability of isoform abundance. Wang et al., by analyzing 65 left ventricular (LV) human heart tissues, detected possible individual variability of inclusion of two key alternative exons, 8 and 8A, that harbor the typical TS mutations.
No experimental data directly address the clinical relevance of such individual splicing variability in TS, but experimental studies show that alternative splicing may affect the channel function. Bartels et al. has shown that the alternatively spliced exons 1 and 8 affect the current density with increased current densities when channels are produced with 1/8A or 1/8 variants compared with the 1A/8A and 1A/8 variants. This behavior is likely because of the increased number of channels expressed in the plasma membrane rather than biophysical differences because the single channel properties were not different. It is therefore tempting to speculate that individual variability of splicing machinery or environmental factors affecting this process can have a role in determining the clinical manifestations of TS.
Ca V 1.2 alternative splicing leading to isoforms with different biophysical properties is also influenced by the presence of pathologic conditions such as heart failure and hypertension. Less is known about the splicing profile of Ca V 1.2 in the brain, and even less is known about other tissues. The available evidence supports the presence of regional differences of Ca V 1.2 isoform expression in the brain that are possibly associated with psychiatric disorders and, interestingly, autism spectrum disorder (ASD), often observed in TS patients.
Upon square- or AP-shaped voltage pulses, I Ca activates rapidly (2–3 ms), which is then followed by a slow decay. The I Ca decay (inactivation) shows a biphasic behavior that has been linked to the separate processes of Ca 2+ (fast decay) and voltage (slow decay) dependent inactivation. The Ca 2+ mediated component of inactivation (CDI) is mainly modulated by the intracellular (cytosolic) concentration of calcium ([Ca 2+ ]i) and by the Ca 2+ /calmodulin complex that binds to the C-terminus of the channel. , The majority of Ca V 1.2 mutations associated with TS impair the voltage-dependent inactivation (VDI) process. To take place, VDI requires a sequence of events triggered by the membrane voltage shift during depolarization that induces a movement of the positively charged S4 transmembrane segments and of the DI-DII loop leading to pore closure. The pore-forming transmembrane segments S6, the intracellular linkers between the other domains, and the carboxyl terminus also participate in the VDI process. , Furthermore, the binding of the β 1 -subunit to the DI-II linker speeds VDI by moving it toward the pore upon cell depolarization. All these events that control VDI have a role in the pathophysiology of TS mutations.
Among the various components of the calcium channel macromolecular complex, only α 1 -subunit mutations have been causally associated with TS. In 2004, as a result of an international collaborative study, we identified 17 cases with a distinguishing phenotype (see clinical session later) and demonstrated the presence of a single CACNA1c mutation in all of them. This mutation, G406R , was present in the alternatively spliced exon 8A located at the end of transmembrane segment S6 of domain I. A few months later Igor Splawski identified two CACNA1c mutations in the alternatively spliced exon 8: G406R (homologous to the mutation identified in exon 8A) and G402S. Interestingly both patients displayed the entire spectrum of clinical manifestations (see clinical session later) but without syndactyly. For this reason, the disorder was classified as a new variant known as TS2.
In the subsequent years the number of mutations associated with typical TS and atypical phenotypes has progressively grown ( Table 98.1 ). Currently, among the known Ca V 1.2 mutations, seven are associated with typical TS: G402S (exon 8), G402R (exon 8), S405R (exon 8), G406R (exon 8), G406R (exon 8A), I1166T , and A1473G . The G406R in exon 8A is the mutation identified in the large majority (>90%) of patients. It is very interesting to observe that these mutants are located in similar positions at the terminal portion of transmembrane segments lying close to the inner pore , and particularly the S6 transmembrane segments ( Fig. 98.1 ). Mutations at these positions may induce conformational abnormalities of the interdomain linkers that are thought to be relevant for VDI because they control the channel closure with a “ball and chain” mechanism. , Interestingly, the other Ca V 1.2 mutations that cause QT prolongation (gain of function) are invariably located in the intracellular portion of the protein (see Table 98.1 ) even when they do not recapitulate the full picture of TS. Other reported mutations cause complex multiorgan phenotypes, including growth failure, developmental delay, joint contractures, seizures, and primary pulmonary hypertension in the absence of QT prolongation. On the bases of the current evidence, the intracellular position of LQT8 mutations is remarkably constant even if robust structure-function explanations are lacking.
| Mutation | QTc a (ms) | CM/CONG | Syndactyly | ASD | Immuno | Hypog | SK | Arrhythmias and Other Systemic Manifestations | In Vitro | References |
|---|---|---|---|---|---|---|---|---|---|---|
| A28T | 450 | Yes | No | No | No | N/A | N/A | Asymptomatic | ↑ Density; ↓ inactivation | 64 |
| G402S | 650 | No | No | Yes | Yes | No | Yes | CA | ↓ VDI | 3, 45, 53, 65 |
| G402R(8) | 650 | No | No | Yes | No | No | No | CA | N/A | 49 |
| S405R(8) | 566 | No | Yes | Yes | No | No | No | TdP, anesthesia | N/A | 49 |
| G406R (8A) | 570 | Yes | Yes | Yes | Yes | Yes | Yes | CA/SCD | ↓ VDI | 3, 66 |
| G406R (8) | >700 | No | No | Yes | No | Yes | Yes | CA /SCD | ↓ VDI | 4, 65, 67 |
| M456I | 568 | No | No | No | No | No | No | Asymptomatic | No effect b | 68 |
| R518C/H | 520 | Yes | No | No | No | No | No | Asymptomatic | ↓ Inactivation; ↓ trafficking; ↓ density; ↑ window current | 47 |
| A582D | 560 | No | No | No | No | No | No | Asymptomatic | ↓ VDI | 68 |
| S643F | 480 | No | No | Yes | No | No | No | CA | ↓ VDI; ↓ density; | 25 |
| L762F | 530 | No | No | No | No | No | No | Syncope | ↓ Inactivation; ↑ window current | 69 |
| P857R | 500 | No | No | No | No | No | No | Asymptomatic | ↑ Density | 47 |
| R858H | 567 | No | No | No | No | No | No | CA/bradycardia | ↑ Density | 68 |
| R860G | 498 | No | No | No | No | No | No | CA | ↑ Density; ↓ inactivation | 64 |
| C1021R | 590 | No | Yes | Yes | No | N/a | Yes | Asymptomatic | N/A | 49 |
| R1024G | 450 | No | Yes | Yes | Yes | Yes | Yes | No arrhythmias, PH; joint contracture | N/A | 19 |
| I1166V | 450 | No | No | No | N/a | N/a | No | Syncope | ↑ VDA; ↓ inactivation | 64 |
| I1166T | 550 | Yes | Yes | Yes | Yes | N/a | Yes | SCD | ↑ Density; ↓ SSA neg shift; ↑ window current | 50, 64, 70 |
| c.3717InsA | c | Yes | No | Yes | Yes | Yes | Yes | LVNC, TOF | N/A | 48 |
| V1363M | c | No | Yes | Yes | No | Yes | Yes | Hypoglycemia, malignant seizures | N/A | 48 |
| A1473G | 640 | Yes | Yes | Yes | No | Yes | Yes | CA | N/A | 46 |
| I1475M | 473 | No | No | No | N/A | No | No | CA | ↑ VDA; ↓ inactivation | 64 |
| E1496K | 480 | No | No | No | N/A | No | No | CA | ↓ VDI; ↑ VDA | 64 |
| G1783C | 720 | No | No | No | No | No | No | CA | = | 68 |
| R1906Q | 513 | No | No | No | No | No | No | Syncope | N/A | 47 |
| G1911R d | 520 | No | No | Yes | Yes | No | Yes | Polymorphic VTs | ↑ Window current; ↓ VDI | 71 |
b Not different from wild type but cosegregation with QT prolongation in the family.
c No values given but reported as normal.
d Also reported in Brugada syndrome, minor allele frequency 0.01: unclear causative role.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here