Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ticks are the most competent and versatile of all arthropod vectors of zoonotic infectious diseases for several reasons. First, ticks are not afflicted by most of the microorganisms that they may transmit or the paralytic salivary toxins that they may transfer during blood-feeding. Second, and unlike mosquitoes, ticks can transmit the broadest range of infectious microbes among all arthropods, including bacteria, viruses, and parasites. In addition, tick-transmitted coinfections appear to be increasing and complicate differential diagnosis and antimicrobial treatment. Third, ticks can vertically transmit infectious microorganisms congenitally to their offspring of both genders (transovarian transmission) and then disseminate carrier state infections among all generational growth stages (transstadial transmission). Tick-borne infectious diseases can also be transmitted to humans by blood transfusions and organ transplants, and babesiosis, a tick-borne infection caused by malaria-related parasites, can be transmitted congenitally. Fourth, ticks have capitalized on many competitive advantages afforded them by evolving changes in climate and human lifestyle, including the following: (1) wider geographic distributions and longer active breeding and blood-feeding seasons as a result of increases in global mean temperatures and humidity; (2) greater abundance of wild animal reservoir hosts no longer effectively controlled, especially deer, rabbits, and rodents; (3) greater residential construction in recently cleared woodlands adjacent to pastures and yards frequented by wildlife, domestic animals, and humans; and (4) more vacation and leisure-time activities enjoyed by humans and their pets during prolonged tick host-questing and blood-feeding seasons from earlier springs through later falls and milder winters. In short, ticks of all ages and both genders may remain infectious for generations without having to reacquire infections from host reservoirs, and environmental and behavioral changes now place humans and ticks together outdoors for longer periods for tick breeding, blood-feeding, and infectious disease transmission.
With the exception of toothed hypostomes for blood-feeding and clawless palps, adult ticks resemble large mites with eight legs and disk-shaped bodies. There are four stages in the tick life cycle: egg, six-legged larva, nymph, and adult. Ticks are classified into three families: the Ixodidae, or hard ticks; the Argasidae, or soft ticks; and the Nuttalliellidae, a much lesser known family with characteristics of both hard and soft ticks. Ixodid ticks have a hard dorsal plate or scutum, which is absent in the soft-bodied, argasid ticks. Ixodid ticks also exhibit more sexual dimorphism than argasid ticks, with both genders looking alike. However, all blood-fed ticks, especially females, are capable of enormous expansion, and engorged ixodid females are often confused with engorged argasid females. Although ticks from all families may serve as disease vectors, the ixodid or hard ticks are responsible for most tick-borne diseases in the United States.
Ixodid ticks have mouth parts that are attached anteriorly and visible dorsally. They live in open exposed environments, such as woodlands, grasslands, meadows, and scrub brush areas. Argasid ticks are leathery and have subterminally attached mouthparts that are not visible dorsally. Argasid ticks prefer to live in more sheltered environments, including animal nests, caves, crevices, woodpiles, and uninhabited rural cabins. All ticks feed by cutting a small hole in the host's epidermis with their chelicerae and then inserting their hypostomes into the cut, with blood flow maintained by salivary anticoagulants. Ticks are attracted to warm-blooded hosts by vibration and exhaled carbon dioxide. Ixodid ticks actually “quest” for hosts by climbing onto vegetation with their forelegs outstretched, waiting to embrace passing hosts ( Fig. 296.1 ). Ticks spend relatively short periods of their lives mating and blood-feeding on hosts. Soft ticks feed rapidly for hours and then drop off, whereas hard ticks blood-feed for days (6–12) before dropping off for egg laying.

Tick-borne infectious diseases have challenged researchers and physicians since Dr. Howard T. Ricketts identified the wood tick, Dermacentor andersoni, as the vector of Rocky Mountain spotted fever (RMSF) in 1906 and firmly established the insect vector theory of infectious disease transmission (see Fig. 296.1 ). The emergence and recognition of Lyme disease in the early 1970s in the United States, whose causative agent, the spirochete Borrelia burgdorferi, was not identified until 1982, sparked renewed interest in tick-borne diseases in the United States and Europe ( Fig. 296.2 ).

By the early 1990s, Lyme borreliosis (LB) had become the most common arthropod-borne infectious disease in the United States and Europe. Since the 1970s, every decade now describes emerging or rediscovered tick-borne infectious disease and new vectors for previously described tick-borne diseases, such as RMSF. These latest discoveries have been spawned by new immunodiagnostic technologies, especially by nucleic acid identification technologies, particularly the polymerase chain reaction (PCR) assay.
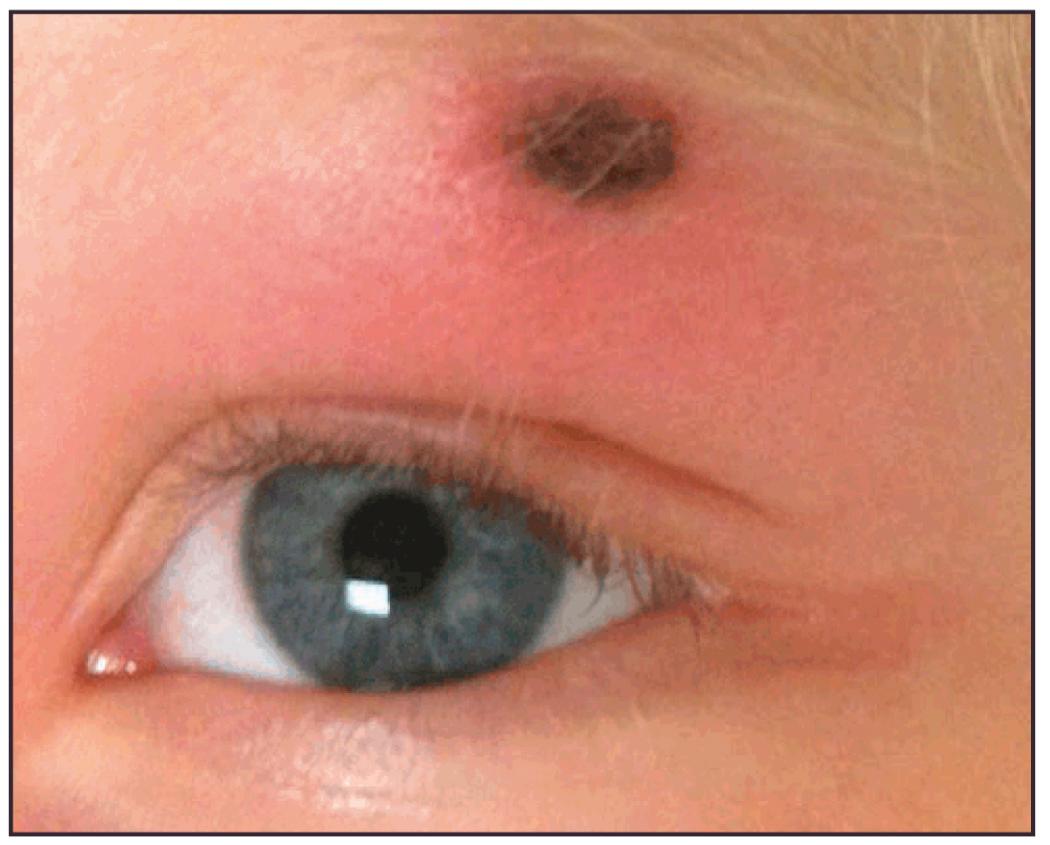
By the 1980s and 1990s, the causative agents of the ehrlichioses were stratified as newly emerging, Rickettsia- like species, and later (2001) they were completely reorganized into separate genera, Ehrlichia and Anaplasma. In 1997, Kirkland and colleagues described a new erythema migrans–like rash illness in North Carolina, a nonendemic region for Lyme disease, transmitted by the Lone Star tick, Amblyomma americanum ( Fig. 296.3 ). This new borreliosis would soon be named the southern tick-associated rash illness (STARI) or Masters disease, but its causative agent, Borrelia lonestari, a new Borrelia species, would not be identified until 2004.

By 2004, ticks were recognized as the most common vectors of all arthropod-borne infectious diseases in Europe, five new spotted fever (SF)–causing rickettsiae were described, four new subspecies of the Lyme disease–causing B. burgdorferi complex were identified, a new relapsing fever Borrelia species was isolated, and anaplasmosis was exported to Europe from the United States. In a seemingly unending era of new discoveries in tick-transmitted diseases, another new and unanticipated vector for RMSF— Rhipicephalus sanguineus, the brown dog tick—was identified in the United States in 2005 ( Fig. 296.4 ).

In 2011, the first human cases of relapsing fever caused by tick-transmitted Borrelia miyamotoi were reported from Russia. In 2013, 1% to 3% of surveyed residents of New England states where Lyme disease is endemic were seropositive for prior B. miyamotoi infection. Since 2011, more than 50 patients with acute febrile illnesses resembling Lyme disease without erythema migrans have been described as having B. miyamotoi infections. In most cases, patients present with fever, malaise, fatigue, headache, myalgias, and arthralgias, and they do not have recurrent fevers. This initial clinical presentation more closely resembles other hard tick–transmitted diseases, especially anaplasmosis and Lyme disease without a significant rash, rather than soft tick–transmitted classic relapsing fever. In two cases, immunocompromised patients developed meningoencephalitis. B. miyamotoi infections are transmitted by the same ixodid ticks that carry Lyme disease: Ixodes scapularis, the eastern black-legged tick ( Fig. 296.5 ), and Ixodes pacificus, the western black-legged tick ( Fig. 296.6 ). Uncomplicated cases may be effectively treated with oral doxycycline, and the cases complicated by meningoencephalitis responded to intravenous therapy with ceftriaxone or penicillin G.


In 2009, a new pathogenic Ehrlichia species in addition to endemic Ehrlichia chaffeensis and Ehrlichia ewingii was identified in four febrile patients in Minnesota and Wisconsin and presumed to be related to Ehrlichia muris.
In July 2008, an 80-year-old man with a tick-bite–appearing eschar, lymphadenitis, and regional lymphadenopathy, and three additional patients with similar presenting manifestations, were reported in northern California. An afebrile, “spotless” rickettsial disease was suspected initially in all cases. Convalescent sera from all four patients exhibited cross-reacting immunoglobulin antibody titers to the RMSF agent Rickettsia rickettsii, and also to a newly recognized SF group agent, Rickettsia 364D ( R. philipii [proposed]), later detected in the Pacific Coast tick Dermacentor occidentalis ( Figs. 296.7 and 296.8 ). All patients were effectively treated without fatalities with oral doxycycline over a 2-week course.

The Heartland virus, a novel phlebovirus (family Bunyaviridae) transmitted by the Lone Star tick A. americanum (see Fig. 296.3 ), was first described in 2009 in two elderly Missouri farmers believed to have ehrlichiosis and who presented with fever, fatigue, headache, confusion, myalgia, and laboratory evidence of leukopenia and thrombocytopenia. In 2013, a fatal case of Heartland virus disease was reported in an 80-year-old man in Tennessee who presented with similar clinical and laboratory findings, but he could not recall a tick bite. Other than supportive care, there are no specific drug treatments for Heartland virus disease.
In June 2014, a previously healthy man in eastern Kansas with a history of tick bite, fever, and fatigue was treated with doxycycline for a presumed tick-borne illness. He later died from a critical illness characterized by high fever, thrombocytopenia, leukopenia, and multiorgan failure. Given the proximity to the Heartland virus cases in adjacent Missouri, blood specimens were analyzed for antibodies against Heartland virus. Subsequently, a series of serologic, culture-based, and molecular tests demonstrated the presence of a novel thogotovirus (the first representative of the family was isolated in the Thogoto forest of Kenya) phylogenetically distinct from Heartland virus and named Bourbon virus after the patient's county of residence.
Another novel, tick-transmitted phlebovirus (family Bunyaviridae), the severe fever with thrombocytopenia syndrome virus (SFTSV), was first described in 2009 in China and has now been detected in Haemaphysalis species ticks in Korea and Japan. SFTSV is transmitted by at least two species of Haemaphysalis ticks, H. longicornis and H. concinna, and is characterized by the abrupt onset of high fever, abdominal pain, nausea, vomiting, leukocytopenia, and thrombocytopenia with hemorrhage. The zoonotic reservoir for SFTSV is in domestic animals, especially goats. Since 2010, about 2500 cases of severe fever with thrombocytopenia syndrome (SFTS) have been reported from central and eastern China, mostly in hospitalized patients, with a median case-fatality rate of 7.3%. Other than supportive care, there are no specific drug treatments for SFTS.
In each case of these newly described tick-transmitted diseases, another tick-borne disease was initially suspected. Ehrlichiosis was suspected in the Heartland virus disease cases, Lyme disease in the B. miyamotoi cases, and “spotless” RMSF with eschar in the Rickettsia 364D cases.
Because most tick-borne diseases are caused by obligate intracellular organisms, many of which infect erythrocytes, monocytes, granulocytes, or vascular endothelial lining cells, many tick-borne infections may also be transmitted congenitally (e.g., babesiosis) and by blood product transfusions and organ transplants. Blood product–transmitted infections have now been described for the tick-borne rickettsial diseases (including Q fever), babesiosis, and ehrlichiosis. In 2008, the Centers for Disease Control and Prevention (CDC) reported the first case in which transfusion transmission of Anaplasma phagocytophilum, the tick-borne causative agent of anaplasmosis (formerly, human granulocytic ehrlichiosis) was confirmed microscopically and serologically by testing of both the recipient and the donor.
In 2016, Moritz and coinvestigators from the American Red Cross reported the results of their screening intervention designed to reduce the risk of blood transfusion-transmitted babesiosis caused by Babesia microti in highly endemic regions of the United States, including Connecticut, Massachusetts, Minnesota, and Wisconsin. Babesia microti is an intraerythrocytic parasite, related to the malaria parasite, that, like malaria, can be transmitted by blood transfusion. Babesiosis is a potentially fatal disease as a result of splenic rupture. The investigators screened over 89,000 blood donation samples for B. microti antibodies (with arrayed fluorescence immunoassays) and for B. microti DNA (with PCR assays) and confirmed 335 samples (0.38%) as serologically positive and 67 (20%) of these as PCR-positive. In Connecticut and Massachusetts from June 2012 through September 2014, no reported cases of transfusion-transmitted babesiosis were associated with the screened donations (0 cases/75,331 screened blood donations), compared with 14 cases per 253,031 unscreened donations (1 case/18,074 donations; odds ratio, = 8.6; 95% confidence interval, 0.51–144; P = .05). Over the reporting period, 29 cases of transfusion-transmitted babesiosis were linked to unscreened blood from infected donors. Although no currently licensed tests exist to screen for B. microti in donated blood, the authors concluded that screening for antibodies to and DNA from B. microti could significantly decrease the rate of transfusion-transmitted babesiosis in highly endemic US states.
Today, the seroprevalence of tick-borne diseases is increasing significantly among blood and organ donors in the United States, combined tick-transmitted coinfections have been described in regional US populations, and an unexplained increase in the virulence of tick-borne infectious diseases has been described in the United States (RMSF), Europe, and North Africa (Mediterranean spotted fever [MSF]) and Australia (Queensland tick typhus [QTT]). Several tick-borne infectious diseases have now been reclassified by the CDC as potential biological terrorism agents, including the following: Francisella tularensis (tularemia), a category A agent (highly likely microorganism to be weaponized); Coxiella burnetii (Q fever), a category B agent (less likely to be weaponized); and the tick-borne encephalitis and hemorrhagic fever viruses, category C agents (least likely to be weaponized). In the future, the tick-transmitted infectious diseases will increase in prevalence over wider distributions at higher altitudes in a warmer world. Unexpected tick vectors of emerging infections caused by obligate intracellular microorganisms will continue to be discovered as people spend more leisure time outdoors in temperate climates in tick-preferred ecosystems.
The borrelioses are a large group of tick-borne spirochetal diseases caused by several species of Borrelia, with unique geographic distributions, tick vectors, and host animal reservoirs ( Table 296.1 ). The borrelioses are stratified into three separate epidemiologic and clinical presentations: LB, STARI, and the tick-borne relapsing fevers (TBRFs) ( Table 296.2 ).
| BORRELIA SPECIES | TICKBORNE DISEASES | GEOGRAPHIC DISTRIBUTION | TICK VECTORS | WILD ANIMAL RESERVOIRS |
|---|---|---|---|---|
| B. afzelii | European Lyme borreliosis (LB) or Lyme disease | Europe, Scandinavia | Ixodes ricinus | Mammals: deer, rodents |
| B. burgdorferi | American LB or Lyme disease | North America, specifically US Northeast, Midwest, Pacific Northwest; Europe | Ixodes scapularis (eastern United States), Ixodes pacificus (western United States) | Mammals: deer, rodents (preferred by nymphs) |
| B. crocidurae | North African tick-borne relapsing fever (TBRF) | North Africa, Mediterranean Basin | Carios erraticus | Mammals: rodents, birds |
| B. duttonii | East African TBRF | East, central, and South Africa | Ornithodoros moubata | Humans (main reservoir) |
| B. garinii | European LB | Northern Europe, Russia, Asia | I. ricinus (Europe), Ixodes persulcatus (Asia) | Mammals: rodents, birds |
| B. hermsii | American TBRF | Western United States and Canada | Ornithodoros hermsii | Mammals: rodents, chipmunks, squirrels |
| B. hispanica | Hispano-African TBRF | Iberian peninsula: Spain, Portugal; northwestern Africa: Algeria, Morocco, Tunisia | Ornithodoros marocanus | Mammals: rodents |
| B. latyschewii | White TBRF | Russian Caucasus regions (Tajikistan, Uzbekistan), Central Asia | Ornithodoros tartakovskyi | Mammals: rodents |
| B. lonestari | Southern tick-associated rash illness (STARI) or Masters disease | Southeastern United States from southeastern Atlantic Coast west to Central Texas, Oklahoma, Missouri | Amblyomma americanum | Mammals: rodents, cattle, other domestic animals; some reptiles, especially lizards |
| B. mazzottii | Southern TBRF | Southern United States, Mexico, Central America, South America | Ornithodoros talaje | Mammals: rodents |
| B. miyamotoi | Russian TBRF | Japan, Eastern Europe, US Northeast | Ixodes scapularis | Mammals: rodents |
| B. parkeri | Western TBRF | Southwest and south central United States, Mexico | Ornithodoros parkeri | Mammals: rodents |
| B. persica | Asiatic-African TBRF | Middle East (Egypt, Iran), central Asia, western China, northern India | Ornithodoros tholozani | Mammals: rodents |
| B. turicatae | American Southwestern TBRF | Southwest and south central United States, Mexico, Central America | Ornithodoros turicata | Mammals: rodents, armadillos, opossums, pigs, and monkeys (Panama) |
| B. venezuelensis | Venezuelan TBRF | Central and South America | Ornithodoros rudis | Mammals: rodents, opossums, armadillos, monkeys (Panama, Colombia) |
| INFECTIOUS DISEASE CHARACTERISTICS | LYME BORRELIOSIS | SOUTHERN TICK-ASSOCIATED RASH ILLNESS | TICK-BORNE RELAPSING FEVER |
|---|---|---|---|
| Microbial agents | Borrelia burgdorferi (United States, Europe), B. afzelii (Europe, Asia), B. garinii (Europe, Asia) | Borrelia lonestari has now been isolated from a skin biopsy of a patient with STARI and cultured in vitro from infected Amblyomma americanum ticks | Many Ornithodoros species of soft ticks (see Table 296.1 ) |
| Preferred tick vectors | Ixodes spp. hard ticks | A. americanum | Ornithodoros spp. soft ticks |
| Preferred animal reservoirs | Rodents (nymphs); deer, birds (adults) | Lizards | Rodents (nymphs); humans ( B. duttonii only); deer, birds (adults) |
| Endemicity | Highly endemic in United States and Europe | Southeastern United States | Highly endemic among vector-populated regions worldwide |
| Fever ≤39°C (102.2°F) | Very uncommon | Absent; low-grade fever may occur rarely | Present in relapsing episodes 1–3 days each; may reach 43°C (109.4°F) |
| Relapsing fevers | Not present | Not present | Present |
| Erythema migrans, or other rash | Present as annular or target-like maculopapular rash (mean diameter, 7 cm); more common on extremities | Present and mimics that of Lyme disease but with a smaller mean diameter of 4.5 cm; more common on trunk | Absent |
| Arthritis | May be present in untreated (up to 60%) late, or “chronic” infections, manifesting as oligoarthritis | Arthralgias, myalgias, and neck stiffness may occur less commonly than with Lyme disease; no chronic arthritic complications | Neck stiffness, arthralgias, myalgias common, not arthritis |
| Neurologic manifestations | May be present in up to 15% of cases; include headache, neuritis of cranial nerve (CN) VII (Bell palsy) | Dizziness, headache, memory loss, concentration difficulty may occur; no chronic neurologic complications | Common: meningitis, meningoencephalitis; neuritis of CN VII (Bell palsy); neuritis of CN VIII (deafness, myelitis, radiculopathy) |
| Other presenting clinical manifestations | Myocarditis, conduction defects in up to 8% of late-onset and “chronic” cases | Regional lymphadenopathy may occur; chronic complications have not been described | Splenomegaly in most, hepatomegaly in 10% of cases; myocarditis manifesting as prolonged QTc interval |
| Best screening serodiagnostics | Giemsa- or Wright-stained peripheral smear, phase-contrast, or darkfield microscopy for spirochetes; ELISA, IFA | Epidemiologic and clinical presentation; no screening serodiagnostics available at present; Lyme disease ruled out by ELISA, IFA, Western immunoblot | Giemsa- or Wright-stained peripheral smear, phase-contrast, or darkfield microscopy for spirochetes; ELISA, IFA |
| Best confirmatory diagnostics | In vitro cultivation, Western immunoblot, PCR assay | PCR assay on skin biopsy; in vitro cultivation | In vitro cultivation (not recommended; Biosafety Level 3 laboratory required), rodent inoculation, PCR assay |
| Recommended antibiotic therapy | Doxycycline, 100 mg PO bid, or amoxicillin, 500 mg PO tid, for 14–21 d; parenteral therapy for CNS involvement | Doxycycline, 100 mg PO bid, or amoxicillin, 500 mg PO tid, for 14–21 d | Tetracycline, 500 mg or 12.5 mg/kg PO qid, or doxycycline, 100 mg PO bid, or erythromycin, 500 mg or 12.5 mg/kg PO qid for 10 d; parenteral therapy with penicillin G or ceftriaxone recommended for CNS involvement |
LB, or Lyme disease (see Chapter 241 ), is now the most common tick-borne infectious disease in the Northern Hemisphere and the most common arthropod-borne infectious disease in the United States. In the United States, LB is caused by Borrelia burgdorferi (sensu stricto), first identified as a novel bacterial spirochete in 1982, and transmitted to humans by Ixodes spp. hard ticks in US regional pockets, specifically the Northeast (I. scapularis), upper Midwest (I. scapularis), and Pacific Coast ( I. pacificus; see Figs. 296.2, 296.5, and 296.6 ). Although B. burgdorferi is the sole agent of LB in the United States, most cases of LB in Europe and northern Asia are caused by Borrelia afzelii and Borrelia garinii (see Table 296.1 ). Collectively, the three Borrelia species are often referred to as B. burgdorferi (sensu lato). Ticks usually acquire Borrelia infections as larvae or nymphs by blood-feeding on small reservoir hosts, most commonly birds and rodents, and may transmit LB to humans during blood-feeding, which may go unnoticed (see Fig. 296.2 ). Borrelia organisms are further maintained in nature as infected adult Ixodes ticks blood-feed on larger mammals, especially deer.
Unlike argasid or soft ticks, Ixodes ticks prefer temperate ecotonal zones of canopied forests abutting cleared scrub or grasslands and transmit B. burgdorferi to humans during outdoor exposures in such habitats. Because Borrelia spirochetes must migrate from the tick's midgut to the salivary gland during blood-feeding, tick attachments for less than 24 hours rarely result in LB in humans. After an incubation period of 1 to 2 weeks, the hallmark of spirochete transmission manifests as solitary erythema migrans, a maculopapular erythematous rash with a bull's-eye pattern, at the site of tick attachment ( Fig. 296.9 ). Erythema migrans in LB results from the subcutaneous centrifugal movement of the spirochetes from the bite sites to the central circulation and target organs (see Figs. 296.3 and 296.9 ). An erythema migrans -like rash also occurs in STARI at the site of A. americanum (Lone Star tick) attachment, but the underlying mechanisms responsible for the rash in STARI are unclear.
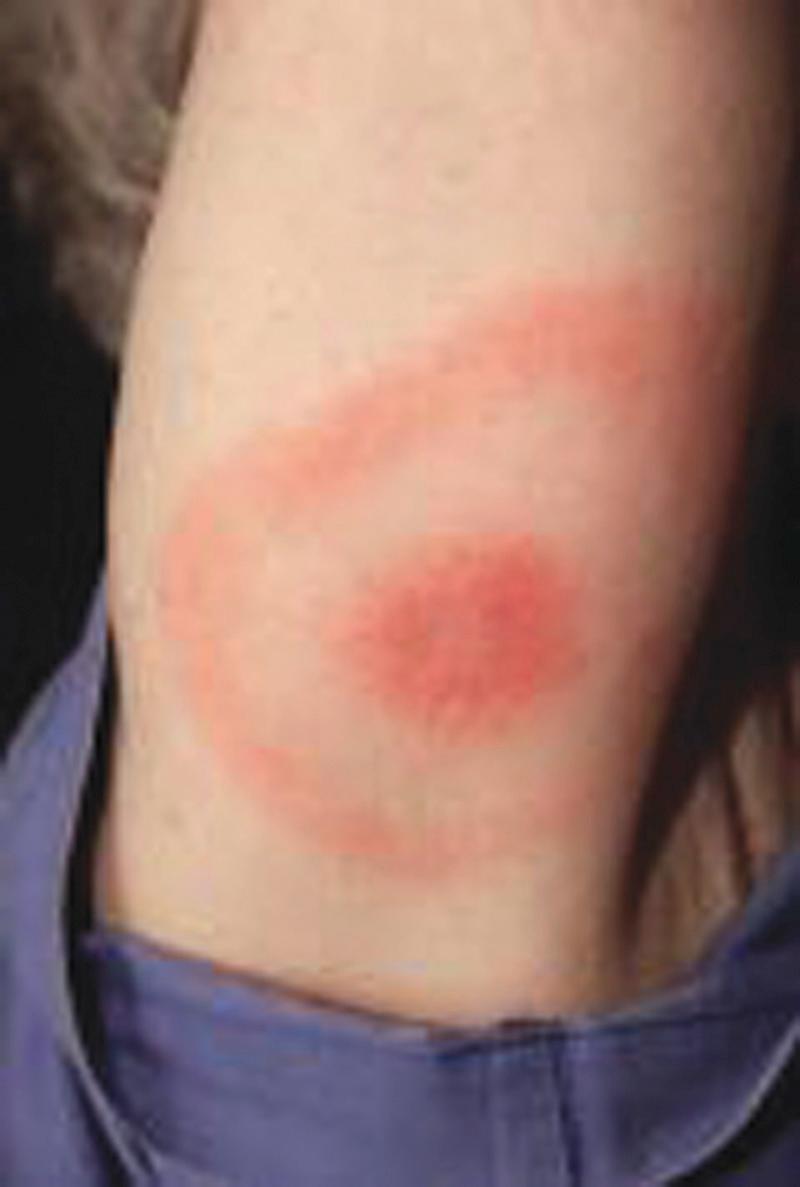
In a meta-analysis of 53 longitudinal studies of LB in the United States and Europe, Tibbles and Edlow reported that many patients did not recall a tick bite (74% in the United States, 36% in Europe), constitutional symptoms of low-grade fever (<39°C [102.2°F]) and headache were common but nausea and vomiting were rare, and a solitary erythema migrans lesion was the most common initial presentation of LB (81% in the United States, 88% in Europe). If LB is recognized and treated early in the erythema migrans stage, cure rates will exceed 90%, and outcomes will be excellent (see Table 296.2 ). Although deaths from LB are rare, 5% to 8% of patients may develop cardiac manifestations, and 15% to 20% of patients may develop neurologic manifestations.
In patients who previously had antibiotic-treated erythema migrans, reinfections with B. burgdorferi may occur in subsequent summers and will be again heralded by another erythema migrans lesion. Recent investigations by Nadelman and coworkers demonstrated that repeated episodes of pathognomonic erythema migrans in patients with previous appropriately treated early LB were due to reinfections and not to recurrences. However, persistent arthritis despite adequate antibiotic treatment of LB (now called post-infectious Lyme arthritis) may occur and was formerly referred to as chronic Lyme arthritis. Despite spirochetal elimination with antibiotic therapy, the proliferative synovitis of Lyme arthritis may extend for months to years and has been attributed to retained spirochetal fragments (such as B. burgdorferi peptoglycan) and/or autoimmune responses to these antigens and not to recurrent LB.
Unfortunately, some practitioners continue to diagnose patients who are suffering from a variety of generalized myofascial, arthritic, and neurologic pain conditions as having “chronic Lyme disease” and have prescribed long courses of oral and intravenous antibiotics despite evidence demonstrating that such therapies provide no improvement and can cause harm. In 2017, Marzec and coauthors reported a case series of five patients diagnosed with “chronic Lyme disease” who were then treated with protracted courses of antibiotics. The resulting complications from this unnecessary therapy included (1) septic shock from central venous catheter–associated bacteremia in two patients, with one fatality; (2) vertebral osteomyelitis in one patient; (3) intractable Clostridioides difficile (formerly Clostridium difficile ) colitis in one patient who later died from complications of amyotrophic lateral sclerosis (ALS); and (4) a paraspinal abscess that required surgical drainage in the last case.
Although the concept of chronic LB has been dispelled by carefully conducted clinical studies, lingering and unconfirmed associations continue to be made between LB cases and subsequent deaths due to four neurodegenerative disorders: ALS, Alzheimer disease (AD), multiple sclerosis (MS), and Parkinson disease (PD). If such associations truly existed, then the geographic distributions of LB cases and deaths from these four neurodegenerative disorders would be anticipated to significantly overlap. Forrester and coinvestigators compared LB incidence rates in each state from the National Notifiable Diseases Surveillance System during 2001–2010 with age-adjusted death rates for AD, ALS, MS, and PD obtained from the CDC Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) database over the same reporting period. LB incidence per US state was not correlated with rates of death due to ALS, MS, or PD. However, an inverse correlation was detected between LB and AD. The authors concluded that their failure to accurately confirm any positive correlations between the geographic distribution of LB and the geographic distribution of deaths due to AD, ALS, MS, and PD provided further evidence that LB was not associated with the development of these common neurodegenerative conditions in an aging population.
The Jarisch-Herxheimer reaction (JHR), an inflammatory cytokine-mediated reaction to dying spirochetes with a worsening of presenting symptoms, vasodilation, and myocardial dysfunction, may occur during antibiotic treatment for LB but is more common after antibiotic therapy forTBRFs. There have been no reported deaths from JHR during antibiotic therapy for LB, and the very rare case fatalities from LB have been attributed to cardiac conduction abnormalities from myocarditis in untreated cases.
First recognized in 1998, STARI manifests initially as erythema migrans, as in LB, but occurs in regions in which B. burgdorferi is not endemic and follows the prolonged attachment of blood-feeding Lone Star ticks (A. americanum), more abundant in the southeastern and south central United States (see Fig. 296.3 ). Patients who are bitten by Lone Star ticks may develop LB-like erythema migrans rashes and occasionally develop milder constitutional symptoms than in LB, including fever, headache, fatigue, and generalized myalgias. However, unlike LB, STARI is not a reportable infectious disease and has no diagnostic serologic tests, such as enzyme-linked immunosorbent assays (ELISAs), immunofluorescence assays, and Western immunoblot assays. In addition, a microbiologic analysis of skin biopsy specimens obtained from the rashes of 30 patients in Missouri with clinical diagnoses of STARI failed to detect B. lonestari, suggesting that STARI could be caused by other pathogens. Because some patients have recovered from STARI without antibiotic treatment and there have been no long-term sequelae reported in STARI cases, some have questioned whether antibiotic therapy is indicated in STARI. Because distinguishing STARI from LB may be difficult, Wormser and coworkers have recommended that the differential diagnosis rely on a combination of regional exposures, clinical presentations, serologic results, and potential for long-term sequelae based on their comparison of LB cases from New York and STARI cases from Missouri. The investigators noted that the timing of rash onset was shorter (6 days) in STARI compared with LB (10 days) and that STARI patients were less likely to be symptomatic than LB patients. In addition, the STARI rash was more often circular with central clearing than the LB rash. Most authorities recommend antibiotic therapy for STARI with oral doxycycline or amoxicillin, following the same regimen as for LB to cover any missed diagnoses of LB with the potential for chronic arthritic and cardiac sequelae (see Table 296.2 ).
The TBRFs (see Chapter 240 ) comprise a worldwide group of serious bacterial infections by Borrelia spirochetes after brief, painless, and usually unnoticed bites by Ornithodoros spp. argasid or soft ticks ( Fig. 296.10 ). Ornithodoros ticks prefer indoor living—in cabins, caves, and crevices—and quickly abandon warm-blooded rodent hosts for egg laying (see Table 296.1 ). Transovarian transmission of the TBRF spirochetes occurs commonly among all species and, unlike LB-causing Borrelia species, TBRF spirochetes are already present in the salivary glands at the onset of blood-feeding and do not need time to migrate from the gut to the mouthparts. The wild animal host reservoirs of TBRF are maintained in birds and several mammals, most commonly rodents. Adult ticks can live for as long as 15 to 20 years and survive without blood meals for several years.

The Ornithodoros species of soft ticks in the United States include Ornithodoros hermsii, Ornithodoros parkeri, and Ornithodoros turicatae (see Fig. 296.10 ). These soft ticks are widely distributed throughout the mountainous regions of the western half of the United States and southwestern Canada at elevations above 1500 m and transmit the spirochetes that cause TBRF ( Borrelia hermsii, Borrelia parkeri, and Borrelia turicatae ). Humans typically come into contact with Ornithodoros soft ticks when they stay in frequently unoccupied and rodent-infested mountain cabins. The ticks emerge at night and feed briefly and unnoticed while the person is sleeping. Unlike the ixodid ticks, Ornithodoros ticks feed very rapidly, usually for less than 30 minutes, and always at night. The bites are painless as a result of local anesthetic-like chemicals in the tick saliva. The bite site is marked after a few days by a small red to violaceous papule with a central eschar. One spirochete is sufficient to initiate TBRF, and the infection rate after a single bite by an infected tick is more than 50%. The incubation period from tick bite to onset of the first febrile episode is 3 to 12 days. Since most people are unaware that they have been bitten, the history of potential regional exposures in high-altitude mountainous regions is essential to the correct diagnosis.
TBRF is defined clinically by the sudden onset of two or more episodes of high fever (>39°C [102.2°F]) spaced by afebrile periods of 4 to 14 days, with the first febrile episode lasting 3 to 6 days and the relapsing episodes lasting 1 to 3 days each. The first episode ends with a 15- to 30-minute “crisis” with tachycardia, hypertension, hyperpyrexia (as high as 43°C [109.4°F]), and rigors, followed by diaphoresis and defervescence. All febrile episodes are accompanied by nausea, headache, neck stiffness, myalgia, and arthralgia. The relapsing febrile episodes result from the growth of new spirochete populations in the blood to replace those killed by macrophages and cytokines. Most patients have splenomegaly, and 10% will have hepatomegaly. Direct neurologic involvement is more common than in LB and may include cranial nerve neuritis (especially cranial nerves VII and VIII), radiculopathy, and myelopathy. Myocarditis is also more common than in LB; may be complicated by adult respiratory distress syndrome, pulmonary edema, and cardiomegaly; and is often fatal.
Laboratory diagnostics include identifying the spirochetes microscopically on peripheral blood smears and detecting their nucleic acids using PCR assays. Thrombocytopenia (platelets <150 µL) is a common finding on the complete blood count. Most patients will have elevated aminotransferase levels, unconjugated bilirubin, and prolonged prothrombin and partial thromboplastin times.
Treatment strategies include tetracycline (500 mg or 12.5 mg/kg PO four times daily), or doxycycline (100 mg PO twice daily), or erythromycin (500 mg or 12.5 mg/kg PO four times daily for 10 days). Parenteral therapy with penicillin G or ceftriaxone is recommended for central nervous system (CNS) involvement.
The JHR is much more common, although rarely fatal, during treatment of TBRF than during treatment of LB and occurs in 30% to 40% of patients with TBRF. At present, no prophylactic strategies to reduce the severity of the JHR have proved beneficial or have been adequately tested in multiple clinical trials, including therapy with antipyretics, corticosteroids, or naloxone. Antispirochetal therapy for TBRF with penicillin instead of tetracycline has a slightly lower risk of associated JHR.
The family Rickettsiaceae contains two genera: the SF–causing genus Rickettsia and the scrub typhus–causing genus Orientia (see Chapters 186 and 191 ). The rickettsiae may be further stratified clinically into the tick-borne SF group and mouse mite–transmitted rickettsialpox caused by Rickettsia akari (see Chapter 187 ). The rickettsiae are obligate intracellular, gram-negative bacteria that thrive in ixodid tick salivary glands and are transmitted during blood-feeding. Once injected into the host, rickettsiae are initially distributed regionally via lymphatics, with some species causing marked regional lymphadenopathy (e.g., Rickettsia slovaca ). Within 2 to 14 days (mean, 7 days), rickettsiae are disseminated hematogenously to vascular endothelial lining cells of target organs, including the CNS, lungs, and myocardium ( Fig. 296.11 ).
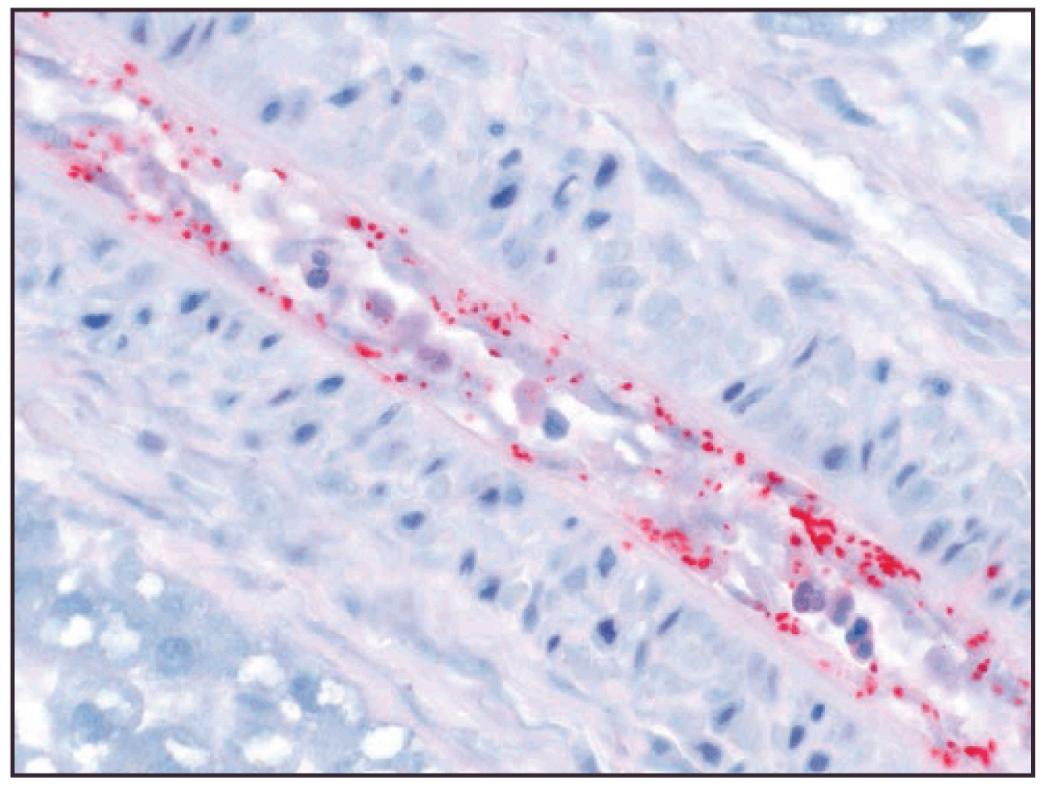
Rickettsiae gain entry into host endothelial cells in a Trojan horse–like manner by using their outer membrane proteins (OmpA and OmpB) to stimulate endocytosis. Once within phagosomes, rickettsiae escape to enter the cytosol or nucleus for rapid replication by binary fission, safe from host immune attack. The tick-borne rickettsial diseases that cause SFs are compared in a descending order of clinical severity of infection by preferred tick vectors and wild animal reservoirs in Table 296.3 .
| RICKETTSIA SPECIES | TICK-BORNE DISEASES | GEOGRAPHIC DISTRIBUTION | TICK VECTORS | WILD ANIMAL RESERVOIRS (MAMMALS) |
|---|---|---|---|---|
| R. rickettsii | Rocky Mountain spotted fever (SF), Brazilian SF | Continental United States, Central America (Costa Rica, Mexico, Panama), South America (Argentina, Brazil) | Amblyomma, Dermacentor, Rhipicephalus spp. | Ungulates, rodents |
| R. conorii | Boutonneuse fever: Mediterranean SF, Israeli SF, Astrakhan SF, Indian tick typhus, Kenyan tick typhus | Mediterranean Basin, Africa, Middle East, Asia | Rhipicephalus spp. | Ungulates, rodents |
| R. sibirica | North Asian tick typhus (Siberian tick typhus) | Africa (Niger, Mali, South Africa), Asia (Russia, China, Mongolia, Pakistan, Kazakhstan, Kirgizia, Tajikistan), Europe (France) | Dermacentor, Haemaphysalis, Hyalomma spp. | Ungulates, rodents |
| R. japonica | Japanese SF | Japan and China | Haemaphysalis spp., Ixodes ovatus | Ungulates, rodents |
| R. australis | Queensland tick typhus | Eastern Australian seaboard from Cairns, Queensland, to Gipps Island, Victoria | Ixodes spp., especially I. holocyclus and I. tasmania | Rodents, bandicoots, wombats, cattle, domestic dogs |
| R. honei | Flinders Island SF | Southern Australia, Thailand | Aponomma spp. | Rodents |
| R. honei subspecies marmionii | Australian SF | Cape York, Queensland | Haemaphysalis novaeguineae | Similar to R. australis: Rodents, bandicoots, wombats, cattle, domestic dogs |
| R. africae and R. parkeri | African tick-bite fever | Sub-Saharan Africa, North America, South America, Caribbean | Amblyomma spp. | Rodents |
| R. 364D ( R. philipii [proposed]) | R. 364D “spotless” rickettsiosis with tick-bite eschar | California | Dermacentor occidentalis | Rodents, other small mammals |
| R. slovaca | Tick-borne lymphadenopathy; Dermacentor -borne eschar, lymphadenopathy, or necrosis | Europe | Dermacentor spp. | Ungulates, rodents |
| R. aeschlimannii | Not named at present | Southern Europe, Africa | Hyalomma spp. | Ungulates, rodents |
The global epidemiology of the tick-borne SF–causing rickettsiae has dramatically evolved since the transmission cycle of RMSF was first described by Ricketts in 1906 with the following: emerging new strains and diseases ( R. slovaca –associated lymphadenopathy); greater understanding of the highly conserved genome of several related species ( Rickettsia africae–Rickettsia parkeri and the Rickettsia conorii subspecies); wider geographic distribution and greater virulence of existing strains ( R. rickettsii, R. conorii subspecies, Rickettsia australis ); unanticipated new tick vectors for some SFs ( Rhipicephalus sanguineus for RMSF in the United States); cluster outbreaks of tick-borne rickettsioses in returning travelers ( R. africae causing African tick-bite fever); and regional clusters and epidemic cycles of more severe SFs worldwide (RMSF in the United States, MSF in Europe, and QTT in Australia). The reasons for such changes in rickettsial SF epidemiology are unclear and may include warming temperatures and increasing humidity, more frequent drought-rain cycles, residential development in preferred tick ecosystems, more competent tick vectors given competitive advantages by environmental and genetic changes, more frequent contact between ticks and humans outdoors, and international trade and travel distributing tick vectors and their preferred animal hosts quickly and widely.
In the United States, the SF group rickettsioses are nationally notifiable infectious diseases that are primarily caused by the highly pathogenic R. rickettsii and less pathogenic rickettsial species such as R. parkeri and Rickettsia 364D ( Fig. 296.12 ; see also Fig. 296.8 ). In 2016, Drexler and coinvestigators reported the results of their summary analysis of all passive surveillance data regarding SF group rickettsioses reported to the CDC during the period 2008–2012 in order to reassess the epidemiology of reported cases and to analyze any significant trends in incidence rates. The incidence rate for SF group rickettsioses in the United States increased from 1.7 cases per million person-years in 2000 to 14.3 cases per million person-years in 2012. SF group rickettsiosis cases were more frequently reported among males and persons of white race and non-Hispanic ethnicity. Although the case-fatality rate (CFR) was low (0.4%), it was significantly higher for American Indian/Alaska Natives (relative risk = 5.4) and Asian/Pacific Islanders (relative risk = 5.7) and was highest overall in children under 10 years of age (CFR = 1.6%). The authors recommended the increased use of PCR and the improved reporting of clinical signs, such as eschars, to better delineate the risk factors, incidence rates, and outcomes of SF group rickettsioses. Following these recommendations, Herrick and coauthors utilized improved clinical reporting and molecular confirmation of R. parkeri infections by real-time PCR to establish a new geographic distribution and tick vector for R. parkeri infections in the United States (see Fig. 296.12 ). Although all prior reported cases of R. parkeri rickettsiosis were described in the coastal Southeast and linked to transmission by the Gulf Coast tick ( Amblyomma maculatum ), Herrick and coauthors described one confirmed and one probable case of R. parkeri rickettsiosis acquired in a mountainous region of Arizona, well outside of the distribution range of the Gulf Coast tick and likely transmitted by a newly recognized tick vector, Amblyomma triste (see Fig. 296.12 ).
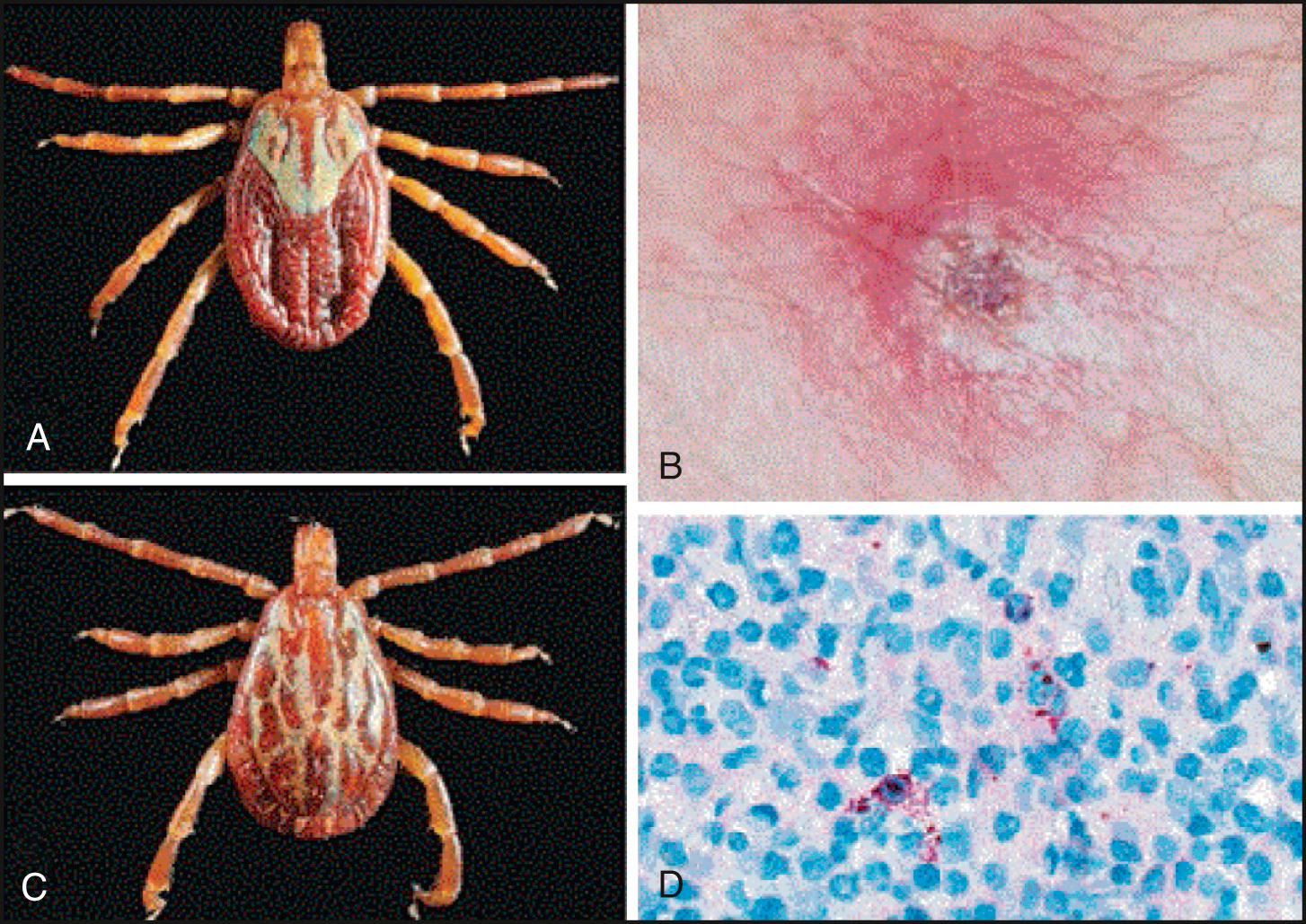
The tick-borne SF rickettsioses share many common presenting features, including: (1) incubation periods of about 1 week; (2) flulike prodromes of fever, headache, myalgia, nausea, vomiting, and abdominal pain (that may mimic acute appendicitis in RMSF); (3) spotty rashes within 3 to 5 days of fever onset ( Fig. 296.13 ); and (4) necrotic eschars at tick-bite sites (see Fig. 296.12B ). Some SF rickettsial diseases may be “spotless,” including RMSF in 10% to 15% of cases, complicating early differential diagnosis. The tick-borne rickettsial infections that can cause spotty rashes include R. rickettsii (RMSF), R. conorii (MSF), R. australis (QTT), and R. africae–R. parkeri (African–North American tick-bite fever) in about 50% of cases. The tick-borne rickettsial infections that are associated with one or more necrotic eschars at tick-bite sites include R. conorii, R. australis, R. africae–R. parkeri, R. helvetica, R. japonica, R. slovaca, R. aeschlimannii, R. honei, and R. 364D ( R. philipii [proposed]). The SF rickettsioses may vary in severity from causing multisystem organ failure (RMSF, MSF) to painful lymphadenopathy ( R. africae–R. parkeri, R. slovaca ) to mild to subclinical disease ( R. aeschlimannii ).
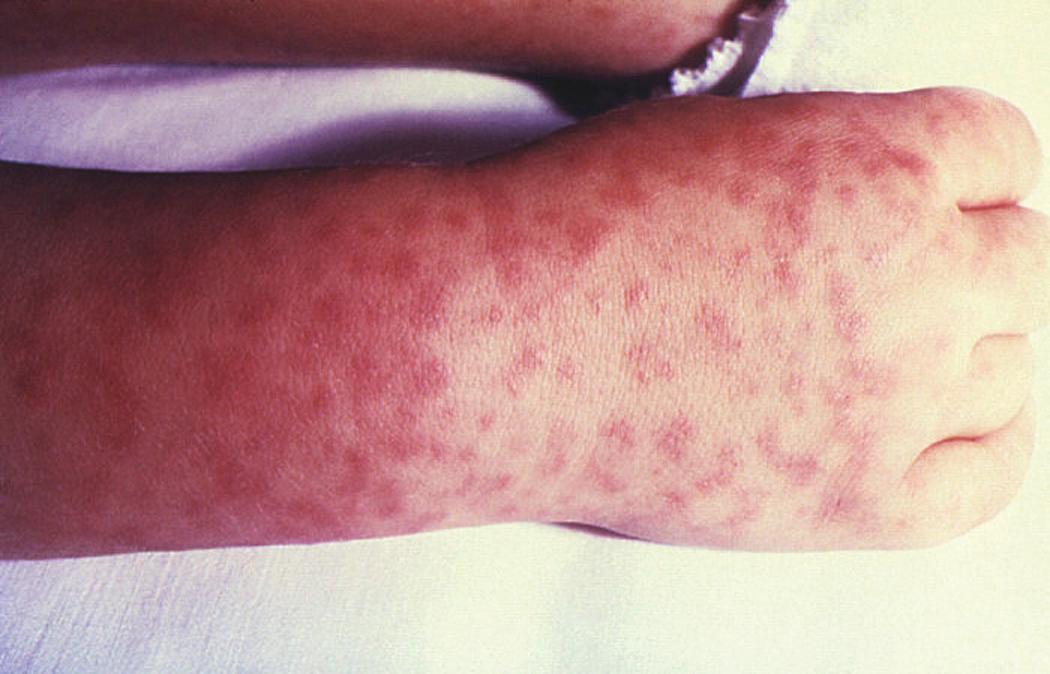
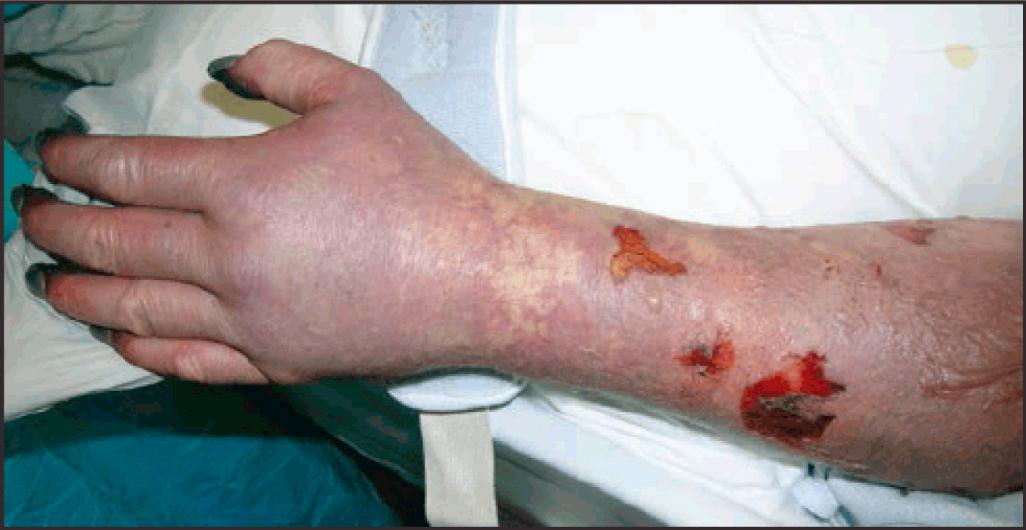
After an average incubation period of 1 week, RMSF starts with a flulike, febrile prodrome followed by a characteristic maculopapular evolving to petechial rash in 85% to 90% of cases in 3 to 5 days. The pathognomonic rash starts distally on the wrists and ankles and then spreads centripetally up the limbs (see Fig. 296.13 ). The pathophysiologic mechanisms of petechial rashes and target organ system damage (CNS, lungs, heart) in the SF rickettsioses include vascular endothelial cell damage by microbial replication, vascular inflammation (vasculitis), and increased widespread vascular permeability, which may result in hypovolemic shock, oliguric prerenal failure from acute tubular necrosis, cerebral edema, and noncardiogenic pulmonary edema (see Fig. 296.11 ). Distal, digital skin necrosis and gangrene of the digits and limbs may occur in severe cases of RMSF and QTT from hypoperfusion ( Fig. 296.14 ).
Cardiac vasculitis may manifest as myocarditis with intraventricular conduction blocks. Aside from petechial rash and thrombocytopenia, other hemorrhagic manifestations in RMSF and other SFs are rare. CNS complications in RMSF and other severe SF infections may include ataxia, photophobia, transient deafness, focal neurologic deficits, meningismus, meningoencephalitis, seizures, and coma. Pulmonary complications may include cough, alveolar infiltrates, interstitial pneumonitis, pleural effusions, pulmonary edema, and adult respiratory distress syndrome.
Initially, MSF caused by R. conorii was thought to be a more benign disease than RMSF. Severe cases of MSF with multiple eschars and multisystem disease similar to RMSF with CNS, renal, and pulmonary complications were first reported in 1981, and now appear to be increasing across Europe. In a 1997 outbreak of MSF in Portugal, CFRs of 32% were recorded and exceeded those of untreated RMSF (CFR of 23%). QTT, African tick-bite fever, and R. slovaca –associated lymphadenopathy are generally milder diseases than RMSF and MSF. However, severe cases of QTT with RMSF-like complications, including renal insufficiency and pulmonary infiltrates, were recently reported from Australia.
Although African tick-bite fever caused by R. africae, a similar tick-bite fever in North America caused by R. parkeri, and R. slovaca infections may all cause multiple necrotic eschars and painful regional lymphadenopathy, these SF infections are often spotless (≤50%) and follow typical rickettsial SF prodromes. A history of tick bites, eschars, and painful regional lymphadenopathy helps to establish the correct diagnosis, especially in the absence of adequate diagnostic laboratory services. The precise laboratory diagnosis of tick-borne rickettsial SFs may be established by microbiologic isolation of the causative organisms from skin biopsy specimens or blood cultures, nonspecific immunofluorescence antibody tests that cross react with many SF antigens, other immunocytologic techniques to demonstrate intracellular rickettsiae, and PCR assay to identify and speciate rickettsial DNA or RNA.
Antibiotic treatment mainstays for the tick-borne rickettsial SFs remain the tetracyclines for most cases and chloramphenicol for severe multisystem disease and during pregnancy. Although the quinolones, azithromycin, and clarithromycin may be as effective as tetracyclines and chloramphenicol for rapidly managing some SFs, they are not recommended for initial therapy at this time. Although short (1- to 2-day) courses of doxycycline have been reported to be as successful as 10-day courses in some SF infections (e.g., MSF), such treatment strategies have not been tested in randomized controlled trials in other SF infections and are also not recommended at this time. Most authorities now recommend that tetracycline, chloramphenicol, or ciprofloxacin for tetracycline-allergic patients be continued for a minimum of 7 days or until the patient has been afebrile for at least 48 hours and is improving clinically.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here