Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The thyroid anlage appears in the embryo as a midline structure at the site corresponding to the foramen cecum of the adult tongue. From here, it descends as a component of the thyroglossal duct along the midline to reach its final position in the mid neck. Later, the hyoid bone is formed from the second branchial arch. The thyroglossal duct is usually situated anteriorly to this bone and is divided by it into a suprahyoid and an infrahyoid portion. In the normal course of events, the thyroglossal duct is obliterated and disappears, leaving as a vestige the pyramidal lobe in approximately 40% of normal individuals. At the same time, the thyroid anlage expands laterally to form the thyroid lobes. Microscopically, cords and plates of follicular cells are formed by the 9th week, small follicular lumina appear by the 10th week, and colloid secretion becomes evident by the 12th week. By the 14th week, the gland consists of well-developed follicles lined by follicular cells and containing thyroglobulin-positive colloid in the lumen.
The development of the thyroid gland is controlled by the coordinated action of specific transcription factors such as TTF-1 (Nkx2-1), TTF-2 (FoxE1), PAX8, HHEX, and NKX2-5. Functional maturation requires the activity of thyrotropin (TSH), of the TSH receptor (TSHr) and of the TSHr downstream effector Gsα. Genetic alterations associated with thyroid dysgenesis (a term referring to the spectrum of thyroid organogenetic defects) include those of the TSH receptor gene (TSHR) in non-syndromic cases, while the Gsα gene (GNAS) and the thyroid transcription factors genes PAX8, TTF-1 (Nkx2-1), TTF-2 (FoxE1), and NKX2-5 are typically associated with syndromes that include thyroid dysgenesis as one of the features.
The normal adult thyroid gland is composed of two lobes joined by the isthmus, which lies across the trachea anteriorly, below the level of the cricoid cartilage. It is endowed with a rich lymphatic network, not always evident in hematoxylin and eosin (H&E) sections but clearly evident with the D2-40 immunostain. The lymph vessels coalesce in the subcapsular region to give rise to collecting trunks that drain into the following nodes: (1) pericapsular; (2) internal jugular chain; (3) pretracheal, paratracheal, and prelaryngeal (the pretracheal node located near the isthmus is sometimes referred to as the Delphian node); (4) recurrent laryngeal nerve chain; and (5) retropharyngeal and retroesophageal. The anterosuperior mediastinal nodes are secondary to the recurrent laryngeal nerve chain and pretracheal groups, but studies have shown that dye injected into the thyroid isthmus can also drain directly into them.
Microscopically, the thyroid gland is made up of round or oval follicles that vary considerably in size, with an average diameter of 200 µm. They are lined by a single layer of follicular cells whose shape ranges from flattened to low columnar, depending on their degree of activity. The cytoplasm has a pale acidophilic or amphophilic staining quality; the greater the activity of the cell, the greater its amount. Follicular cells with abundant granular acidophilic cytoplasm are referred to as Hürthle cells (a misnomer), Askanazy cells, oxyphilic cells, or oncocytes. Ultrastructurally, this granularity is due to the accumulation of mitochondria. They can be detected immunohistochemically with antibodies directed against mitochondrial enzymes.
The proliferative activity of follicular cells is related to age, being highest in the prenatal group and lowest in adults.
The main ultrastructural features of follicular cells are abundant granular endoplasmic reticulum, a well-developed Golgi apparatus, lysosomes (particularly numerous in actively secreting cells, and mainly located toward the apical side, not to be confused with secretory granules), and numerous microvilli in the luminal border.
The intraluminal colloid is pale staining, with scalloped borders in follicles with active secretory function and densely eosinophilic in inactive ones. In old age, it tends to be broken up in globular formations. It is variably periodic acid–Schiff (PAS) positive and alcianophilic, depending on the types and relative amounts of carbohydrate components present. Birefringent calcium oxalate crystals may be found, always intraluminally, their number increasing with age. Their presence is of practical utility in distinguishing thyroid from parathyroid tissue (which lacks them), particularly at the time of frozen section. They occur in the normal gland and in many pathologic conditions, particularly benign ones. They are thought to represent a sign of functional inactivity on the part of the follicles containing them.
Collections of small follicles protruding into the lumen of larger follicles are commonly seen in actively secreting glands; they are referred to as Sanderson polsters and become prominent in hyperplastic conditions.
Immunohistochemically, many markers have been documented in normal follicular cells, most of them being also expressed in well-differentiated tumors of these cells:
Reactivity for thyroglobulin, thyroid peroxidase (TPO), triiodothyronine (T3), and thyroxine (T4) is found both in the colloid and in the cytoplasm of the follicular cells. Thyroglobulin is the most widely used of these markers. Less degree of reactivity is found in oncocytic (Hürthle) cells.
Immunoreactivity for the sodium iodide symporter (NIS), the ion channel protein that mediates active iodide uptake, is localized to the basolateral plasma membrane of follicular cells. NIS is expressed at higher level in the normal thyroid gland and in benign conditions than in carcinomas, where the localization of the protein is redirected to the intracellular compartment. Pendrin and Anoctamin-1 (ANO1) (also known as Transmembrane Member 16A-TMEM16A or Discovered on GIST1-DOG1), the ion channel proteins that mediate the passive iodide efflux towards the follicular lumen are localized by immunohistochemistry to the apical plasma membrane of follicular cells.
Thyroid transcription factor-1 (TTF-1) is expressed in normal follicular and parafollicular cells. It is an extremely useful marker for all types of primary and metastatic thyroid tumors (including medullary carcinoma), except for anaplastic carcinoma. TTF-1 is not expressed in normal, hyperplastic, or adenomatous parathyroid tissue. It is expressed in the normal lung (bronchial cells and pneumocytes) and in most cases of lung carcinoma (including the small cell neuroendocrine type). TTF-1 is occasionally expressed in other sites, such as the normal and neoplastic female genital tract, Wilms tumor, and Merkel cell tumor. Like TTF-1, PAX8 and TTF-2 (FoxE1) are also detectable by routine immunohistochemical methods in thyrocytes and tumors derived from them. PAX8 may be the best to demonstrate the thyroid origin of anaplastic carcinoma.
Low-molecular-weight keratins: CK7, CK18, and (to less degree) CK8 and CK19.
EMA (epithelial membrane antigen, MUC1).
Vimentin.
Estrogen and progesterone receptors.
Blood group antigens.
Metal-binding proteins: ceruloplasmin, lactoferrin, transferrin, metallothionein.
Follicular cells are joined by tight junctions containing the known components of these structures, including occludin and the various claudins.
The follicles rest on a basement membrane that is immunoreactive for laminin and type IV collagen.
Many of the markers listed previously have been evaluated in normal tissues, benign tumors, and malignant tumors with the hope of finding significantly different values among these groups that could be exploited diagnostically, but the results have generally been those to be expected, that is, disappointing.
The other major epithelial component of the thyroid gland is represented by neuroendocrine cells known as C cells or parafollicular cells. The latter term is a misnomer because immunohistochemical and ultrastructural studies have shown that they occupy a predominantly and perhaps exclusively intrafollicular position. It has been generally believed that mammalian, like avian C cells, derive from the neural crest. Lineage tracing experiments have now provided substantial evidence that they develop under the influence of specific transcription factors (including TTF-1) from the pharyngeal endoderm of the IV pouch and ultimobranchial body (see Solid cell nests next). Because of this fact, C cells are largely restricted to the middle and upper thirds of the lateral lobes along their central axes. The number of C cells varies according to age; they are more numerous in infancy and old age than in adults. Most adult glands contain no more than 10 C cells in a low-power microscopic field. In old age, they may form nodular aggregates.
Ultrastructurally, C cells contain numerous dense-core granules of neurosecretory type. They are argyrophilic with the Grimelius reaction; metachromatic with toluidine blue; positive for lead hematoxylin; and immunoreactive for calcitonin, katacalcin, calcitonin gene–related peptide, neuron-specific enolase (NSE), chromogranins A and B, secretogranin II, and synaptophysin ( Fig. 8.1 ). They are also immunoreactive for carcinoembryonic antigen (CEA), although less so than their neoplastic counterparts in medullary carcinoma.
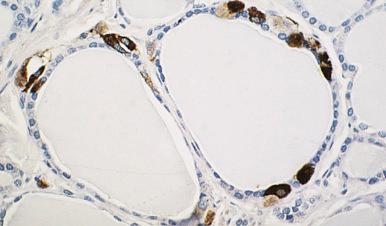
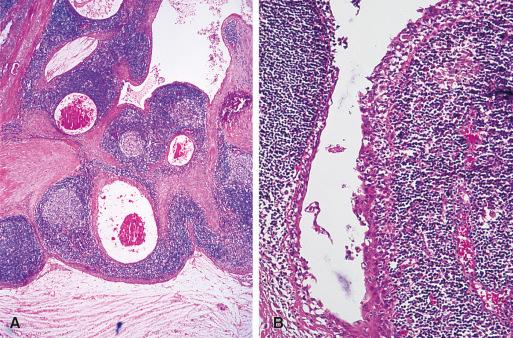
Solid cell nests (rests) are believed to represent remnants of the ultimobranchial body, which in turn is derived from the branchial cleft pouch complex IV–V. They measure 0.1 mm on average and can be detected in almost 90% of neonatal thyroid glands ( Fig. 8.2 ). Exceptionally they reach a large and even a “giant” (tumorlike) size. They are mainly composed of polygonal or oval cells admixed with occasional clear cells. Small glandular lumina containing a mucinous secretion are often present, the nests thus acquiring a combined solid and cystic appearance. A substantial number of C cells may be seen around the nests, in keeping with the well-known relationship that these cells have with the ultimobranchial body. Ultrastructurally, there are microfollicular structures, intracytoplasmic microvacuoles, and ciliated cells, in addition to lymphoid cells. Immunohistochemically, solid cell nests are positive for high-molecular-weight keratin (recognized by the antibody 34bE12) and for CEA. Their strong immunoreactivity for p63, telomerase expression, and proliferative activity suggest a stem cell origin. Solid cell nests need to be distinguished from metaplastic follicles, nodular C-cell hyperplasia, and papillary microcarcinoma. Carcinoma of the thyroid showing thymus-like elements (CASTLE) may originate from solid cell nests.
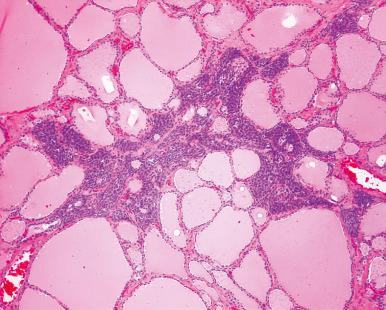
Focal collections of lymphocytes are seen at autopsy in the thyroid of approximately one-half of females and one-fourth of males; this finding is regarded as a subclinical manifestation of focal lymphocytic thyroiditis. Microscopic manifestations of nodular hyperplasia have been reported at autopsy in one-fourth of the cases, but their frequency varies greatly depending on the geographic location of the population being investigated.
Morphologic variations in the anatomy of the thyroid , several of them being the expression of branchial pouch remnants and having no clinical significance, include:
Adipose metaplasia of the interfollicular stroma. Only exceptionally will this result in a clinically noticeable mass (“localized adiposity”), to be distinguished from lipoadenoma (see p. 295 ).
Intrathyroidal islands of mature cartilage, presumably of branchial pouch derivation.
Intrathyroidal islands of ectopic thymus.
Intrathyroidal parathyroid.
Intrathyroidal bundles of skeletal muscle.
Intrathyroidal salivary gland tissue.
Perithyroidal pancreatic tissue, presumably a foregut remnant (see later).
Accumulation of melanin-like pigment in the cytoplasm of follicular cells occurs in old age and may become massive after the administration of some medications, such as minocycline. When intense, it is appreciable grossly and is referred to as melanosis thyroidi or—less pompously—as black thyroid ( Fig. 8.3 ). Some of the granules are of classic lipofuscin type, but most of them also contain colloid, thus becoming ambilysosomes.
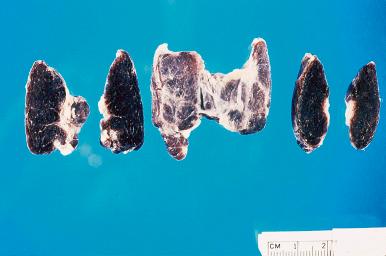
Thyroglossal duct anomalies are the result of a localized persistence of the thyroglossal duct. This may be in the form of a sinus tract connected either to the foramen cecum or to the suprasternal notch, or in the form of a blind tubular structure. It may be accompanied by a cystic dilation, as the result of secretion from the lining cells. When these cystic changes are prominent, the abnormality is designated as a thyroglossal duct cyst. Its usual location is the midline of the neck, in the region of the hyoid bone ( Fig. 8.4 ). Microscopically, the cyst is lined by pseudostratified ciliated or squamous epithelium which is often TTF-1 positive but thyroglobulin negative. Mucous glands and thyroid follicles are commonly seen in the subjacent stroma. Secondary inflammation is common, particularly in cases accompanied by a sinus tract. As a result, the epithelial lining may be partially absent, and the stroma may be infiltrated by inflammatory cells. Most cases are clinically evident during childhood, but others become apparent only later in life. The treatment is surgical removal. To minimize recurrence, it is important to include in the excision the middle third of the hyoid bone and the entire length of any sinus tract that might be present.
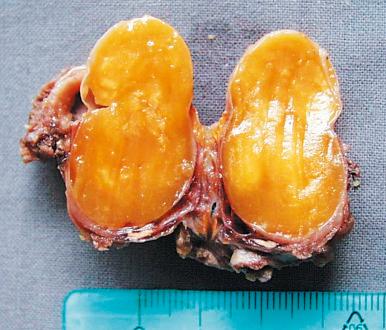
The thyroid tissue present in these anomalies can undergo malignant transformation, usually in the form of papillary carcinoma. The prognosis is excellent after surgical removal, which does not need to include the thyroid gland. Other tumor types have been described, including oncocytic (Hürthle cell) carcinoma and undifferentiated (anaplastic) carcinoma.
Heterotopic thyroid tissue can be found not only as a component of thyroglossal duct cyst but anywhere along the course of the thyroglossal duct, sometimes as the sole abnormality ( Fig. 8.5 ). The most frequent location is the base of the tongue, where it may result in difficulty in swallowing and respiratory obstruction, which in a few cases has proven fatal. At a microscopic (subclinical) level, lingual thyroid is found in 10% of normal individuals. This heterotopic thyroid does not differ microscopically from that seen in the main gland. Sometimes a capsule is formed around it; in other instances, the follicles grow between the skeletal muscle of the tongue, a feature that may simulate invasion by tumor. Significantly, in 70% of patients with grossly evident lingual thyroid there is absence of the normal thyroid gland. Therefore, removal of the heterotopic thyroid tissue will lead to hypothyroidism, requiring subsequent medical replacement therapy.
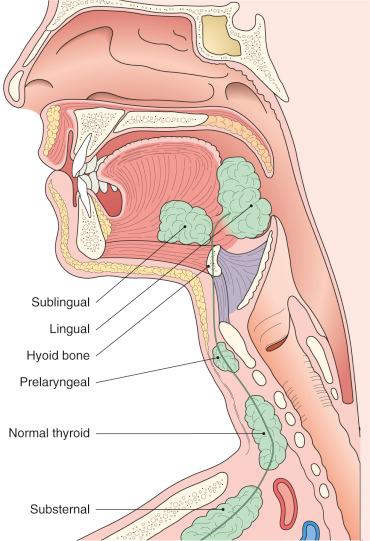
Other sites of heterotopic thyroid tissue are the anterior tongue, submandibular region, larynx, trachea, mediastinum (usually superior but sometimes posterior), and heart. The common denominator in all these sites is their location in or close to the midline, the large majority being found in the so-called Wölfler area , described by anatomists as an isosceles triangle with the edge of the mandible at its base and the concavity of the aortic arch at its apex. The possible occurrence of heterotopic thyroid tissue in cervical nodes is discussed elsewhere.
More distant locations in which heterotopic thyroid tissue has been reported include the wall of the duodenum, gallbladder, region of the porta hepatis, adrenal gland, fallopian tube, vagina, and—last but not least—the ovary and other sites as a component of a teratoma ( struma ovarii being the most spectacular example) (see Chapter 35 ). Heterotopic thyroid tissue in any of these locations is subject to the same diseases that can affect the main gland, including inflammation, hyperplasia, and tumors. Thus, several cases of follicular carcinoma arising from lingual thyroid have been reported, as well as numerous cases of carcinomas of various types arising in struma ovarii (see Chapter 35 ).
Branchial pouch anomalies are not directly related to the thyroid but are discussed here because of their close proximity to the gland. They may present as a patent fistula, a simple sinus, a blind cyst, or an island of cartilage ( Fig. 8.6 ). They are located in the anterolateral region of the neck, their exact position depending on the specific branchial cleft involved. Those related to the first pouch appear in the preauricular area or beneath the posterior half of the mandible and may be connected to the external auditory canal. Those related to the second pouch appear just anterior to the sternocleidomastoid muscle in the mid neck and may have an open tract communicating with the pharynx near the superior fold of the tonsil. Those related to the third and fourth pouches are typically found in the lower neck, in a suprasternal or supraclavicular location. Some of these have been mislabeled as bronchogenic cysts in the past.
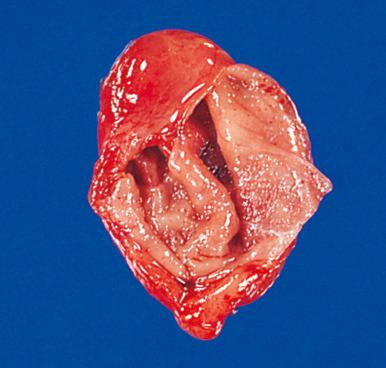
The lining of these cysts and fistulous tracts is usually squamous epithelium, but columnar ciliated epithelium is also common. Abundant lymphoid tissue, often with germinal centers, is observed beneath. Cysts located in the lower neck may also contain mucinous, seromucinous, and sebaceous glands. Secondary infection may complicate the microscopic picture. Squamous cell carcinomas arising in a branchial cleft cyst probably exist, but they are exceptionally rare. Any cystic mass in the lateral neck containing squamous cell carcinoma must be considered a lymph node metastasis with cystic degeneration until proved otherwise, and a search for the primary tumor should be instituted, particularly in the upper aerodigestive tract. Sites notorious for harboring small occult primary tumors include tonsil and posterior tonsillar pillar, retromolar trigone, tongue, and nasopharynx.
Similar comments pertain to the relationship of branchial cleft cysts and papillary carcinomas of thyroid type. Perhaps there exist cases of this tumor type arising in the ectopic thyroid tissue of branchial cleft cysts, but there is no question that the overwhelming majority of the cystic lesions in the lateral neck containing a recognizable focus of papillary thyroid carcinoma represent lymph node metastases from a thyroid primary that have undergone secondary cystic change.
Branchial pouch–related structures are found within the thyroid in the form of the already mentioned solid cell nests (a remnant of the ultimobranchial body or branchial pouch complex IV–V) and—less commonly—in the form of heterotopic cartilage (sharply outlined and often kidney shaped), thymic tissue, parathyroid glands, or epithelial-lined cysts with or without an associated lymphoid component. Most of the latter—sometimes designated as lymphoepithelial cysts —are seen in glands affected by Hashimoto thyroiditis, but they can also be found in its absence. Exceptionally, perithyroidal epithelial cysts have been found to contain pancreatic tissue in their wall, as representatives of foregut remnants.
Parenthetically, the presence of normal, cystic, or neoplastic thymic tissue in the neck should also be viewed as the expression of branchial pouch–derived anomalies (see Chapter 12 ).
Acute thyroiditis is usually of infectious nature. It may be associated with acute infections of the upper aerodigestive tract (e.g., pharyngitis, tonsillitis), generalized sepsis, or major trauma to the neck with an open wound. It tends to affect the malnourished infant, the debilitated elderly, and the immunocompromised patient. Streptococcus haemolyticus , Staphylococcus aureus , and Pneumococcus are the organisms most commonly responsible. Other causes include gram-negative bacteria, fungi (particularly Candida ), and Pneumocystis . Viral infection is rare, but several cases of cytomegalovirus infection of the thyroid in patients with AIDS have been documented.
Morphologically, the main changes in acute thyroiditis are neutrophilic infiltration and tissue necrosis. Nonsuppurative and suppurative forms exist, the latter sometimes evolving into an abscess . The interesting observation has been made that a large number of cases of recurrent acute suppurative thyroiditis (especially when left sided) are secondary to the presence of a piriform sinus fistula, a structure of presumably ultimobranchial body derivation.
The best method to confirm the diagnosis of acute thyroiditis is a fine-needle biopsy with smear cytologic examination and cultures. The presence of a pyriform sinus fistula can be established by barium meal.
Medical treatment of acute thyroiditis is usually effective, but abscesses need to be drained surgically. Cases associated with pyriform sinus fistula are best treated by fistulectomy.
Granulomatous thyroiditis, also known as de Quervain thyroiditis, giant-cell thyroiditis, subacute thyroiditis, subacute granulomatous thyroiditis, or painful subacute thyroiditis, typically presents in middle-aged women with sore throat, painful deglutition, and marked tenderness on palpation in the thyroid region, often associated with fever and malaise. Once the initial process has subsided, pressure symptoms and/or mild hypothyroidism may develop. However, in the majority of cases there is complete resolution. Because of the sometimes asymmetric involvement of the gland, the disease may be clinically confused with carcinoma. Thyrotoxicosis with elevated serum levels of T4 and T3 in combination with marked suppression of radioactive iodide uptake are typical of the initial phase of this disease, which has been found to be associated with the HLA-B35 haplotype.
Grossly, the process usually involves the entire gland, but the enlargement is often asymmetric. In a typical case, the gland is enlarged to approximately twice its normal size. In the advanced stage, the involved areas are firm. In contrast to Riedel thyroiditis, there is usually little or no adherence to the surrounding structures.
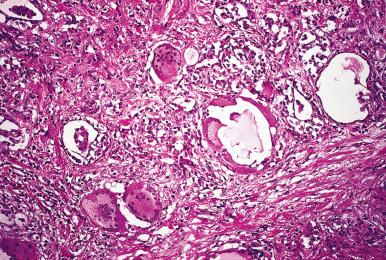
Microscopically, areas of marked inflammation and granulomas containing foreign body giant cells are present. It is characteristic for these granulomas to surround follicles and for the multinucleated giant cells (most of which are of histiocytic nature) to engulf colloid ( Fig. 8.7 ). The granulomas are not very distinct, and caseation necrosis is consistently absent. Areas of fibrosis are also seen, usually in a patchy distribution. Different stages of the same process may be seen in the same gland. Strong immunoreactivity for CA19-9 is found in the late stage of the disease, whereas positivity for CEA in the center of the granuloma is a feature of the acute stage.
The etiology is not known. Although the disease often follows an infection of the upper aerodigestive tract, the thyroiditis itself is of nonbacterial nature. A viral etiology has been often suggested on clinical and epidemiologic grounds but not conclusively proven.
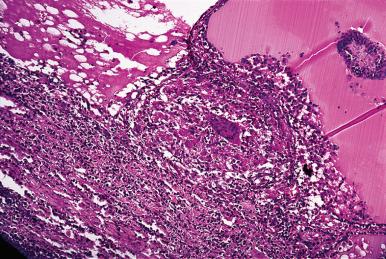
Palpation thyroiditis is the term that has been proposed for a relatively common, clinically insignificant, and grossly inconspicuous thyroid process (also known as multifocal granulomatous folliculitis) in which collections of histiocytes (some of them foamy), lymphocytes, and a few multinucleated giant cells are seen within the lumen of scattered thyroid follicles ( Fig. 8.8 ). In some of these follicles, the inflammatory infiltrate disrupts the epithelium and extends into the perifollicular region. These changes bear no relationship to granulomatous (de Quervain) thyroiditis. Rather, they seem to be the result of minor trauma to the gland, sometimes spontaneous and sometimes thought to be induced by vigorous palpation on physical examination, hence the term palpation thyroiditis.
Tuberculosis as a primary clinical manifestation within the thyroid gland is a pathologic curiosity. In disseminated miliary tuberculosis, it is common for an occasional tubercle to occur within the gland. It is also possible for tuberculosis of cervical lymph nodes or larynx to involve the thyroid gland secondarily. Many of the cases diagnosed in the past as tuberculosis of the thyroid were actually examples of granulomatous (de Quervain) thyroiditis.
Sarcoidosis may involve the thyroid in the form of interstitial (rather than follicle-centered) noncaseating granulomas in patients with systemic disease. Occasionally it manifests initially as a thyroid mass. Sarcoidosis has been associated with autoimmune thyroid disease (Graves disease and Hashimoto thyroiditis).
Mycoses of various types have been described, most of them occurring in immunocompromised hosts. In many of these cases, the tissue changes are characterized by necrosis and acute inflammation rather than granuloma formation.
Postoperative necrotizing granulomas , vaguely simulating rheumatoid nodules and morphologically similar to those more commonly seen in the prostate and bladder, have been observed within the thyroid.
It is now widely accepted that the thyroid diseases traditionally known as lymphocytic thyroiditis and Hashimoto thyroiditis represent different phases or manifestations of an organ-specific, immune-mediated inflammatory disorder generically designated as autoimmune thyroid disease. The disorder is common, affects predominantly women, and it is characterized by the production of autoantibodies that alter thyroid function. It is further believed that autoimmune thyroiditis is one of the two major forms in which autoimmune thyroid disease may manifest, the other being Graves disease. In favor of this interpretation is the existence of cases sharing features of the two diseases (sometimes designated by the picturesque term hashitoxicosis ), suggesting that one may evolve into the other. According to this interpretation, there is an immune-mediated insult that leads initially to diffuse or nodular hyperactivity of the gland and eventually to exhaustion atrophy, manifested by diffuse oxyphilia of the follicular epithelium. Rarely the sequence is reversed, in the sense that Hashimoto disease with hypothyroidism is followed by Graves disease.
The mechanisms leading to autoimmune thyroid disease are of both humoral and cellular nature. Circulating autoantibodies exist against thyroglobulin and other follicular cell antigens, notably TSHr. The leading pathogenetic event is the breakdown of immune tolerance caused by a combination of immunologic, genetic, and environmental factors. Available evidence indicates that autoimmunity is triggered by inflammatory events in the gland likely related to infection (viral or bacterial), or injury to the thyroid cells from toxins. The injured thyrocytes may exhibit new epitopes (or unmask hidden ones), causing the arrival of major histocompatibility complex (MHC) class II–positive antigen presenting cells (dendritic cells and different subclasses of macrophages). These cells present thyroid-specific antigens to naive lymphocytes within the regional lymph nodes, resulting in clonal expansion of CD4+ T cells, CD8+ cytotoxic T cells, and immunoglobulin (IgG) antibody–producing B cells that are autoreactive. These cells accumulate in the thyroid, resulting in thyroiditis. The intrathyroidal T and B cells, as well as antigen-presenting cells, produce numerous cytokines that alter the behavior of the follicular cells. These start expressing MHC class II, enabling the interaction with antigen-specific T cells that maintains and contributes to the autoimmune inflammatory process. Genome-wide association studies (GWASs) have identified several susceptibility loci for autoimmune thyroid disease, some of which have been specifically linked to Graves disease (the TSHR single nucleotide polymorphism rs2268458) and Hashimoto thyroiditis (e.g., the FoxE1 single nucleotide polymorphism rs965513).
Morphologically, the common denominator of autoimmune thyroid disease in general and autoimmune thyroiditis in particular is the presence of lymphocytic infiltration of the gland associated with germinal center formation . Histologically, it is the appearance of the intervening follicles that determines the category to which a given case belongs : Graves disease when the follicles are diffusely hyperplastic, lymphocytic thyroiditis (in various forms) when they are relatively normal, and classic Hashimoto thyroiditis when follicles are lined by severely damaged cells showing extensive oncocytic change. Considerable confusion exists in the use of terminology, depending on whether the emphasis is on tissue inflammation or the clinical findings. Some authors use the term Hashimoto thyroiditis to include any autoimmune thyroid disease characterized by marked lymphocytic infiltration. For practical purposes, autoimmune thyroiditis can be grouped histologically into “lymphocytic thyroiditis,” when the evidence of parenchymal damage is limited, and “Hashimoto thyroiditis” when there is marked parenchymal damage, following the original description of the disease by Hakaru Hashimoto in 1912. The practice of using the terms lymphocytic and Hashimoto thyroiditis synonymously is not recommended, one of the reasons being that a good correlation exists between the morphologic appearance of the follicular epithelium and thyroid function.
Lymphocytic thyroiditis , thus defined, is characterized by lymphoid infiltration with limited evidence of parenchymal damage. As these forms of thyroiditis rarely require thyroidectomy, few studies have accurately described the histologic alterations following needle biopsy procedures. Germinal centers are few, oncocytic changes are focal, follicular atrophy is absent (there may actually be signs of follicular hyperplasia), and fibrosis is mild or altogether absent. Several clinical entities fall into this category: painless (or silent, or subacute lymphocytic) thyroiditis that can be sporadic or postpartum , the “ juvenile form ” of lymphocytic/autoimmune thyroiditis, and the initial phase in cases of Hashitoxicosis . In patients with painless (silent) thyroiditis, sporadic or postpartum, a transient hyperthyroid phase is followed by hypothyroidism and then, in the majority of cases, by resolution with return to a euthyroid condition. The course of the juvenile form of autoimmune thyroiditis is variable. Drug-induced thyroiditis after treatment with amiodarone and lithium has been related to autoimmunity. Immunomodulatory drugs, cancer immunotherapy, and cancer vaccines have immune-related side effects that include endocrinopathies and thyroiditis.
The presence of focal lymphoid infiltration in the thyroid parenchyma is referred to as focal lymphocytic thyroiditis (or nonspecific thyroiditis, or focal autoimmune thyroiditis) . Most patients are euthyroid, but a minority has latent/subclinical hypothyroidism. Collections of lymphocytes are common in thyroid glands removed for a variety of reasons and can be found around thyroid tumors (particularly papillary carcinoma), in thyroid glands that have been irradiated (radioactive iodine ablation or external beam therapy), and in autopsy series. Autopsy studies indicate that the finding is more frequent in women, increases with age, and shows racial variation (more common in whites than in black or Japanese individuals). White females have the highest prevalence and the most pronounced increase with aging: focal lymphoid infiltration is found in approximately 55% of white women aged 80 years or older.
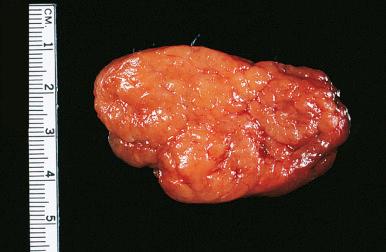
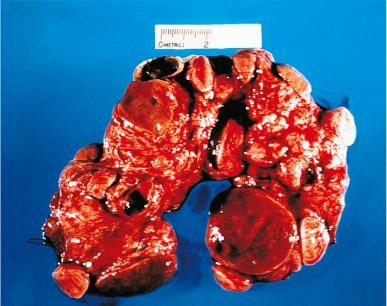
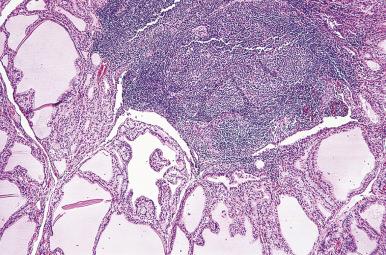
Hashimoto thyroiditis , originally described in 1912 by Hakaru Hashimoto as struma lymphomatosa, is characterized by extensive damage to the thyroid parenchyma. It is predominantly a disease of women over 40 years of age. It presents as diffuse firm thyroid enlargement, sometimes accompanied by signs of tracheal or esophageal compression. Initially the disease may be accompanied by mild hyperthyroidism and later by hypothyroidism. At surgery, the thyroid gland is easily separated from other structures. The fascial attachment between the thyroid gland and the tracheal wall is, at times, slightly thickened, but there is no strong fixation (unlike Riedel thyroiditis). Because of the firm character of the lesion, it may be confused with carcinoma, but the diffuse involvement without adherence to the surrounding structures should be strong evidence against it. Grossly, the typical case shows diffuse and symmetrical enlargement of the gland. However, in some instances, one lobe is more enlarged than the other, and in others the disease has a distinctly multinodular quality. The consistency is firm but not stony hard as in Riedel thyroiditis. There is no extension of the process outside the gland. The cut surface is friable, vaguely or distinctly nodular or micronodular, yellowish gray, and greatly resembles a hyperplastic lymph node ( Fig. 8.9 ). Colloid is not clearly discernible. Necrosis and calcification are absent. The texture of the parenchyma infiltrated by lymphoid cells results in a distinctive pattern on ultrasound examination characterized by reduced echogenicity.
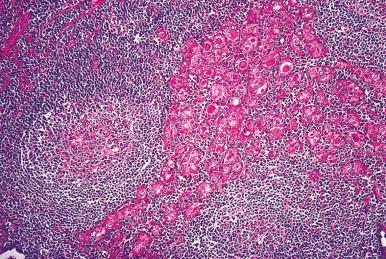
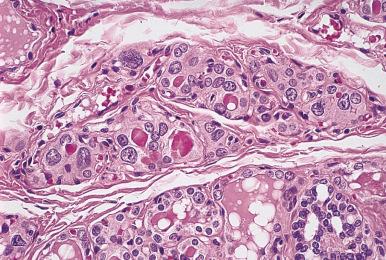
Microscopically, the two main abnormalities are lymphocytic infiltration of the stroma and oxyphilic change of the follicular epithelium ( Fig. 8.10 ). The lymphoid tissue is distributed within and around the lobules, and it invariably exhibits large follicles with prominent germinal centers. Lymphocytes predominate, T cells predominating over B cells. Plasma cells, histiocytes, and scattered intrafollicular multinucleated giant cells are also present. Ultrastructurally, some of these giant cells have been found to be of epithelial derivation, but most are of histiocytic nature. The lymphoplasmacytic population has been found to be polyclonal by immunohistochemical and gene rearrangement studies. Lymphatic vessels are increased in number and dilated, resulting in characteristic “cracking spaces” ( Fig. 8.11 ).
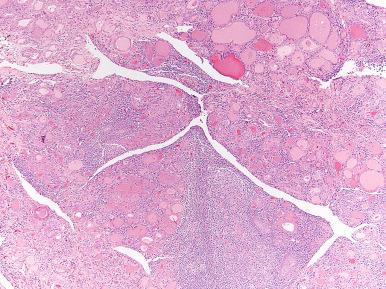
Most of the thyroid follicles are atrophic, but others show features consistent with regenerative hyperplasia. Most or all of these follicles are lined by variably sized oncocytic (Hürthle) cells. The nuclei of these cells may show enlargement and hyperchromasia or, conversely, an optically clear appearance and overlapping quality reminiscent of that seen in papillary carcinoma. Immunohistochemically, the follicular cells of autoimmune thyroiditis show greater reactivity for keratin (particularly the high molecular weight types), S-100 protein, HLA-DR, and N -acetyl-α- d -galactosamine than the corresponding normal cells, their immunohistochemical profile thus resembling that of the cells of papillary carcinoma. Biochemically, the oncocytic cells of Hashimoto thyroiditis have defects of cytochrome-c oxidase and the common 4977 base pairs deletion of mitochondrial DNA.
Squamous nests and duct-like structures, thought to arise from metaplasia of follicular cells as a response to the chronic inflammatory injury (and possibly representing a manifestation of phylogenetic regression), are common and can reach sizable proportions ( Fig. 8.12 ). Rarely, large cysts lined by squamous epithelium and bordered by a row of lymphoid follicles are seen, their appearance being highly reminiscent of branchial cleft cysts ( Fig. 8.13 ). Parenthetically, similar cysts can also be seen in the absence of thyroiditis.
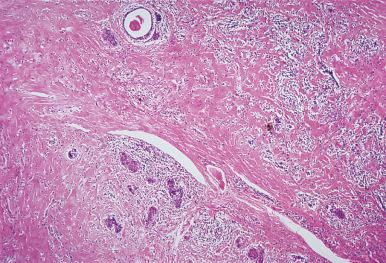
In the typical case of Hashimoto thyroiditis, connective tissue is scarce, with slight or moderate thickening of the interlobular septa. In the fibrous (or fibrosing) variant of the disease, described by Katz and Vickery in 1974 and comprising approximately 10% of all cases, fibrosis is more extensive. In contrast to Riedel thyroiditis, this fibrosis is of dense hyaline type (instead of the active proliferative fibrosis seen in the latter) and does not extend beyond the thyroid capsule. In this regard, it should be remembered that Hashimoto and Riedel thyroiditis can exceptionally coexist. The fibrous variant of Hashimoto thyroiditis can also be confused with carcinoma when the fibrosis is associated with epithelial islands showing squamous metaplasia. It typically presents in older women, with hypothyroidism requiring prompt hormone replacement, and may evolve into a severe form of thyroid atrophy . In these cases the thyroid gland is not palpable and the symptoms of hypothyroidism are difficult to identify since they overlap with those of aging.
A type of Hashimoto thyroiditis that shows partial overlap with the fibrous variant is the IgG4-related form. It is defined by an increased number of IgG4-positive plasma cells (greater than 20 IgG4-positive plasma/high-power field [×400] and IgG4/IgG ratios greater than 30% by immunohistochemistry). Compared with conventional Hashimoto thyroiditis the IgG4-related variant is associated with high titers of thyroid autoantibodies, runs a more aggressive course, shows greater ultrasound alterations with pronounced hypoechogenicity, develops at a younger age, has a lower female-to-male ratio. Histologically, the lympho-plasmacytic infiltration is extensive and there is a predominantly interfollicular pattern of fibrosis. Similar to the fibrous variant (and unlike Riedel thyroiditis) the inflammatory process does not extend to extrathyroidal tissues, nor is it typically associated with fibroinflammatory lesions in other sites.
Although Hashimoto thyroiditis typically exhibits a diffuse appearance both grossly and microscopically, cases do occur in which a distinct nodularity is evident, the epithelial component of the nodules having a hyperplastic quality. This could be interpreted as the combination of Hashimoto thyroiditis and nodular hyperplasia, but in all likelihood the two abnormalities are pathogenetically related. A term such as nodular Hashimoto thyroiditis would therefore seem more appropriate to designate this relatively common occurrence. A variation on the theme is represented by the Hashimoto thyroiditis in which one or more distinct hyperplastic (“dominant”) nodules exclusively composed of oncocytic (Hürthle) cells having either a follicular or a solid configuration are present. Some of these nodules are surrounded by a thick fibrous capsule and therefore fulfill the criteria for follicular, including oncocytic (Hürthle) cell, adenoma, even if their true neoplastic nature is debatable.
Hashimoto thyroiditis (and autoimmune thyroid disease in general) may be seen in association with lymphocytic inflammation of other organs, also on an autoimmune basis, as parts of autoimmune polyendocrine syndromes, namely Addison's disease (the combination being known as Schmidt syndrome ) and diabetes mellitus. Chronic autoimmune thyroiditis, Addison's disease, and type 1A diabetes are the distinctive features of autoimmune polyendocrine syndrome type II (the type I characterized by hypoparathyroidism and Addison's disease is not typically associated with thyroid disease). Hashimoto thyroiditis (and other autoimmune thyroid diseases) can also be associated with non-endocrine autoimmune disorders like vitiligo, pernicious anemia, myasthenia gravis, autoimmune gastritis, celiac disease, and hepatitis.
Complications of Hashimoto thyroiditis include thyroid lymphoma, oncocytic (Hürthle cell) neoplasms, and papillary carcinoma. Their identification at an early stage may be extremely difficult. This probably explains the remarkable difference in the reported figures for these complications, particularly the latter two. It is worth noting that follicular cells in Hashimoto thyroiditis can have loss of heterozygosity (LOH) at tumor suppressor gene loci and can become karyotypically abnormal when they form proliferative nodules. Also, the occurrence of sometimes striking nuclear clearing and overlapping in the follicular cells of Hashimoto thyroiditis is of interest; one wonders whether this might not represent a precursor (preneoplastic) stage of papillary carcinoma, as suggested by their similar phenotypic profile. Additional evidence suggests that RET/PTC may provide a link between Hashimoto thyroiditis and papillary carcinoma. As a matter of fact, patients exposed to the Chernobyl radioactive fallout not only develop RET/PTC -driven papillary cancer but also chronic autoimmune thyroiditis; transgenic mice expressing RET/PTC show chronic thyroiditis in addition to papillary carcinoma; and—more importantly—low level RET/PTC rearrangement is commonly detectable in non-neoplastic thyrocytes of Hashimoto thyroiditis, both in nodular and non-nodular areas of the inflamed gland. This finding is intriguing given the established oncogenic potential of RET/PTC . It has to be pointed out that the rearrangement is present in very few cells, that its identification requires highly sensitive methods (that need therefore to be avoided for the molecular diagnosis of papillary carcinoma), and that the very restriction of the rearrangement to rare follicular cells does not necessarily anticipate the development of papillary carcinoma. The finding of oncogenic events in non-neoplastic cells is certainly not unique, a good example being the occurrence of BCL2/JH rearrangement (the molecular signature of follicular lymphoma) in rare lymphocytes in hyperplastic follicles. Just as the identification of low-level BCL2/JH rearrangement does not establish per se a diagnosis of follicular lymphoma, the detection of low level RET/PTC does not establish papillary carcinoma. It rather supports the epidemiologic data showing that Hashimoto thyroiditis is associated with a small but real risk of developing papillary carcinoma, by providing a biologic link between the two. Incidentally, BRAF p.V600E mutations have not been detected in Hashimoto thyroiditis. Medullary carcinoma has also been described in thyroid glands with Hashimoto thyroiditis, but the occurrence is probably coincidental.
The treatment of Hashimoto thyroiditis depends on its severity. In mild cases no therapy is needed. In some instances, thyroid hormone is given to relieve hypothyroidism. In still others, surgery in the form of subtotal thyroidectomy is performed because of large size and/or pressure symptoms. Some cases of Hashimoto thyroiditis are treated surgically simply because they are clinically confused with a neoplastic process.
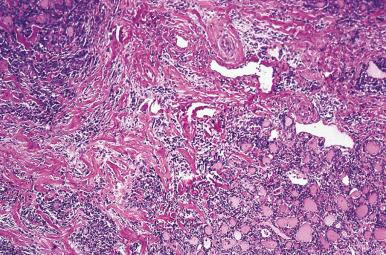
Riedel thyroiditis, also known as Riedel struma, fibrous thyroiditis, and invasive thyroiditis was originally reported by Bernhard Moritz Riedel in 1896. It is an extremely rare disorder that affects adult and elderly patients and shows a slight predilection for females. It presents with an ill-defined thyroid enlargement often associated with profound dyspnea. The lesion, which is extremely firm, binds the soft tissues of the neck in an iron collar (in the original description Riedel used the words eisenharte— iron hard — struma) and may compress the trachea to a slitlike state. Clinically, it is often thought to be carcinoma. In contrast to granulomatous thyroiditis, it is not preceded by an acute inflammatory process or by tenderness of the thyroid gland. The regional lymph nodes are not involved. Grossly, the process is asymmetric and involves only localized areas of the thyroid gland. The affected portion is stony hard and cuts with great resistance. Dense fibrous tracts extend from the thyroid capsule into adjacent muscle so that at surgery the tissue planes are obliterated. On cross section, areas with complete obliteration of the architecture alternate with others having a nearly normal appearance.
Microscopically, fibrous tissue completely replaces the area of the gland involved ( Fig. 8.14 ). The fibrosis is the result of an active proliferative process of fibroblasts and myofibroblasts showing areas of storiform growth, and dense hyaline collagenization in the later stages of the disease. In the resected thyroid glands, hyalinization is frequently extensive, and skeletal muscle cells in the immediate area are often directly infiltrated by fibrous connective tissue. Giant cells are absent. The inflammation present is patchy and of mononuclear type, with a predominance of lymphocytes and plasma cells. The plasma cells are polyclonal, with numerous IgA- and IgG4-producing cells. Collections of eosinophils may also be present. Medium-sized veins encased by the fibrosis typically show inflammation of their wall. This process of inflammatory phlebosclerosis is referred to as obliterative phlebitis and is important diagnostic feature ( Fig. 8.15 ). Elastic stain may be necessary to recognize fully obliterated veins. Arteries are less likely to be affected.
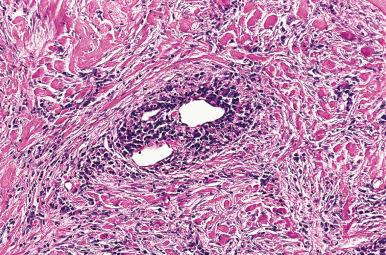
Riedel thyroiditis is the prototypical manifestation of IgG4-related disease in the thyroid gland. IgG4-related diseases are a group of tumefactive fibro-inflammatory disorders (generically known as inflammatory fibrosclerosis ) that can affect many organs and sites. The group includes retroperitoneal or mediastinal fibrosis, sclerosing cholangitis, and—in the head and neck region—inflammatory pseudotumor of the orbit, eosinophilic angiocentric fibrosis, IgG4-related disease submandibular disease, and Mikulicz's disease. IgG4 is a rare IgG idiotype , inefficient as antibody, with paradoxical anti-inflammatory properties. The diagnosis of IgG4-related diseases is based on distinctive histologic features (lymphoplasmacytic infiltrate, fibrosis—arranged at least focally in a storiform pattern, and obliterative phlebitis), and on the finding of numerous IgG4-positive plasma cells with an IgG4 to IgG ratio greater than 40% by immunohistochemistry. IgG4-related fibro-inflammatory disorders develop (synchronously or metachronously) in other sites (e.g., the retroperitoneum) in 30%–40% of patients with Riedel thyroiditis.
Riedel thyroiditis is different from granulomatous or Hashimoto thyroiditis, despite the existence of isolated instances in which the two diseases coexist. While many cases of fibrous and some cases of conventional Hashimoto thyroiditis are IgG4 related, their histologic and clinical features are different, and they lack the extraglandular infiltration and systemic involvement by IgG4-related disorders in other sites that is typical of Riedel thyroiditis. Riedel thyroiditis needs also to be distinguished from malignancies, particularly the paucicellular variant of anaplastic carcinoma (a challenging distinction in small biopsies), and from lymphoplasmacytic lymphoma.
Steroid therapy has been effective in some cases (particularly those diagnosed at early stages), but most patients need surgical intervention to relieve the compression symptoms and to rule out the presence of carcinoma. The resection is quite difficult because no plane of cleavage exists and aggressive surgery is not recommended due to the risk of complications.
An uncommon but very distinctive form of thyroiditis has been recognized and designated multifocal fibrosing (or sclerosing) thyroiditis by Dr. Rosai in the course of his long and extensive activity as consulting pathologist. As the name implies the condition is characterized by one or more foci of fibrosis throughout the gland (typically foci are multiple, sometimes very numerous), having a stellate, radial configuration, and in a minority of cases a band-like appearance ( Fig. 8.16 ). The size of the foci is quite variable, with a mean diameter of 3 mm. On low power, the appearance can be indistinguishable from that of papillary microcarcinoma, but on high power it is possible to appreciate the lack of a neoplastic glandular component. Although the sclerotic foci are heterogeneous, the individual lesions are similar to each other in composition, characterized by a fibrotic poorly cellular center that merges with a cellular peripheral zone. Some of the follicular structures present at the periphery of the sclerotic focus (i.e., at the interface between the fibrotic core and the normal tissue), and to less extent those entrapped inside it, undergo reactive/regenerative changes (so-called reactive atypia ) that simulates malignancy ( Fig. 8.17 ). The thyroid follicles entrapped in the more central sclerotic areas are minute, with scant colloid. Those at the periphery appear hyperplastic, are surrounded by stroma that can have a nodular fasciitis-like appearance, and are lined by crowded thyrocytes with overlapping nuclei that sometimes display mild clearing and irregularities of the nuclear membrane contour. There are no nuclear pseudoinclusions or psammoma bodies. BRAF p.V600E mutations have not been identified.

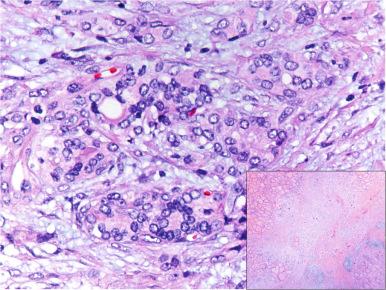
The pathogenesis of multifocal fibrosing thyroiditis is obscure. The condition probably represents a peculiar host response to injury that stimulates a localized tissue response followed by a process similar to that of a healing scar. The condition develops predominantly in women (F/M = 12.75) and is often associated with nodular hyperplasia, Hashimoto thyroiditis, and Graves disease. In approximately 30% of cases it coexists with papillary microcarcinoma, and it is possible that some cases may represent a pre-neoplastic condition. As Dr. Rosai has pointed out, an association between fibrosis and malignancy has been reported in other organs (e.g., radial scar leading to ductal breast carcinoma).
Hyperplastic disorders of the thyroid have been classified on the basis of their presumed pathogenesis, morphologic features, and clinical manifestations. In practice, three major forms of thyroid hyperplasia are recognized by combining these criteria ( Table 8.1 ).
| NAME | MECHANISM | PATHOLOGY | FUNCTIONAL STATUS |
|---|---|---|---|
| Dyshormonogenetic Goiter | Genetically determined error in thyroid hormone synthesis | Nodular or (less frequently) diffuse hyperplasia | Hypothyroid |
| Graves Disease | Autoimmune | Diffuse hyperplasia | Hyperthyroid |
| Nodular Hyperplasia | |||
| Endemic goiter | Iodine deficiency | Nodular hyperplasia (preceded by a transient phase of diffuse hyperplasia) | Usually euthyroid; sometimes hypothyroid |
| Sporadic goiter | Unknown | Nodular hyperplasia | Usually euthyroid; sometimes hyperthyroid or hypothyroid |
Dyshormonogenetic goiter is a condition in which enlargement of the thyroid gland is due to defects in the synthesis of thyroid hormones. Thyroid dyshormonogenesis (thyroid hormone deficiency resulting from inborn errors of thyroid hormone synthesis) accounts for approximately 20% of cases of primary congenital hypothyroidism, the other 80% being the result of thyroid dysgenesis. Some studies have shown that the incidence of primary congenital hypothyroidism with a normally located (eutopic) thyroid gland and milder dysfunction may be increasing, possibly due to changes in screening thresholds. Dyshormonogenetic goiter is linked to mutations in genes that encode for proteins involved in the steps of thyroid hormone synthesis. Mutations in genes that cause defective iodide transport ( NIS/SCL5A5 encoding the Na/Iodide symporter and SCL26A4/PDS encoding pendrin), defective iodide organification ( TPO encoding thyroid peroxidase , DUOX2 encoding dual oxidase 2, DUOXA2 encoding dual oxidase maturation factor 2), defects of thyroglobulin synthesis ( TG encoding thyroglobulin), or dehalogenation deficiency ( IYD/DEHAL1 encoding iodotyrosine deiodinase) are well characterized. Disease phenotypes are inherited in an autosomal recessive manner and are not associated with other defects, other than deafness in Pendred syndrome (due to SCL26A4/PDS mutations).
Grossly, the gland is enlarged and multinodular ( Fig. 8.18 ). Microscopically, the most common alteration consists of hypercellular nodules exhibiting a variety of architectural appearances, with a predominance of solid and microfollicular patterns ( Fig. 8.19 ). In some cases there are papillary and insular formations. Fibrosis is a common finding, in some instances resulting in irregularities at the edge of the nodules that can simulate capsular invasion. Other common features include marked nuclear atypia and minimal amounts of colloid. A diagnostically important feature is the fact that the nuclear atypia (in the form of bizarre hyperchromatic nuclei) is present mainly in the tissue between the hyperplastic nodules rather than in the nodules themselves. Mitotic figures are often seen, presumably as a result of the continuous TSH stimulation. The suggestion has been made that there is some correlation between the specific molecular defect and the morphologic appearance of the gland.
The histologic features of dyshormonogenetic goiter are characteristic enough that a pathologist may strongly suggest the diagnosis. This is important given the need for genetic counseling to assess the risk of inheritance and recurrence in the family. Cases of thyroid carcinoma have been reported in patients with dyshormonogenetic goiter, but the number of well-documented cases is very low. Most have been of follicular type, and others have been incidental papillary microcarcinomas. The reported follicular tumors have included adenomas (some developing after T4 replacement therapy) and carcinomas.
Because dyshormonogenetic goiter is commonly associated with pleomorphism, hypercellularity, and mitotic activity of the follicular epithelium, one should be particularly strict with the criteria for the diagnosis of follicular carcinoma in this setting. Specifically, nothing short of clear-cut capsular or blood vessel invasion should be accepted.
Graves disease (also known as Basedow disease, diffuse toxic goiter, and exophthalmic goiter) typically presents in young adult females with muscle weakness, weight loss, irritability, heat intolerance, fatigue, tachycardia, goiter, and often a great increase of appetite. It can also develop in children, in whom it constitutes the most common cause of hyperthyroidism. Atrial fibrillation may occur, particularly in older patients. The enlargement of the thyroid gland is typically diffuse, but in case of iodide deficiency Graves disease may coexist with multinodular goiter. The goiter is palpable in the majority of hyperthyroid patients who are younger than 60 years, as opposed to less than 50% of older patients.
Graves ophthalmopathy (or orbitopathy) develops in 25%–30% of patients. Cigarette smoking is a risk factor and has been associated with more aggressive ophthalmopathy. This is an autoimmune disorder of the periorbital tissues that results in increased volume of both adipose tissue and extraocular muscles due to inflammation, the accumulation of hydrophilic glycosaminoglycans (typically hyaluronic acid) and related edema, and tissue remodeling with activation of lipogenesis and fibrosis. Exophthalmos (or proptosis, i.e., protrusion of the eyeball) develops in approximately 60% of patients with ophthalmopathy, but imaging can identify subtle orbital connective tissue abnormalities in 70% of patients with Graves disease.
Late clinical manifestations of Graves disease are localized dermopathy (pretibial myxedema) and so-called thyroid acropachy ; the latter is characterized by swelling of the extremities and clubbing of fingers and toes resulting from periosteal new bone formation.
Laboratory abnormalities include elevation of bound T4, free T4, or bound T3 levels and increased radioactive iodine uptake in the presence of low TSH levels. A variant characterized by increased serum levels of T3 in the presence of therapy-induced low levels of T4 has been described as T3-predominant Graves disease .
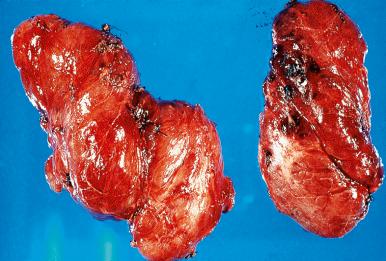
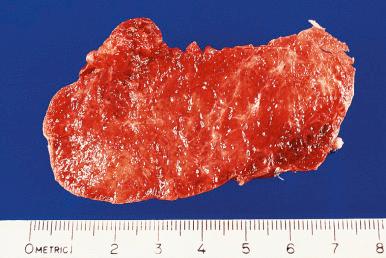
Grossly, the gland shows a mild to moderate symmetric diffuse enlargement. It is succulent and reddish and has the consistency of pancreatic tissue ( Fig. 8.20 ). The cut surface is uniformly gray or red, depending on the degree of vascularity ( Fig. 8.21 ). In longstanding cases, the gland appears friable and dull yellow.
Microscopically, the follicles are markedly hyperplastic, with prominent papillary infolding that may cause confusion with papillary carcinoma ( Fig. 8.22 ).
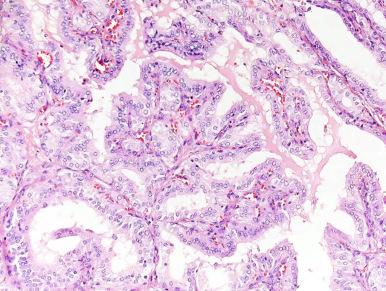
The lining epithelium is columnar, with basally located normochromatic or hyperchromatic nuclei and a clear, sometimes microvacuolated basophilic or amphophilic cytoplasm that may contain fat and glycogen. These features are said to be even more prominent in T3-predominant Graves disease. A variable number of oxyphilic cells may be present, suggesting a possible evolution toward Hashimoto thyroiditis. The colloid is pale and finely vacuolated, with prominent scalloping where it abuts the epithelium. The stroma contains aggregates of lymphoid tissue with germinal center formation ( Fig. 8.23 ). Immunohistochemically, most of these lymphocytes are of T-cell type, the cytotoxic-suppressor subtype predominating in the follicles and the helper-induced subtype in the interstitium. Mild fibrosis is present in longstanding cases. Hyperplastic follicles may be seen outside the thyroid gland, sometimes growing into the skeletal muscle of the neck. This may represent extrathyroid extension of the hyperplastic process or—more likely—the expression of hyperplasia in ectopic thyroid follicles, which in the normal situation are so small and scanty as to pass undetected. Whatever the mechanism for their development, they should not be interpreted as evidence of malignancy.
Controversy still exists regarding the incidence, as well as the prognosis, of thyroid cancer associated with Graves disease (and hyperthyroidism in general, e.g., in the case of toxic multinodular goiter), but the numbers are such as not to influence the selection of therapy for Graves disease in the absence of palpable thyroid nodules. Not surprisingly, incidental carcinomas have been found in the pathologic examination of thyroid glands removed for hyperthyroidism, their incidence varying from 1% to 10% in most reported series. These figures are no higher than those reported in otherwise normal glands. Nearly all of the cases have been small papillary carcinomas without clinical significance, although some studies have suggested that carcinomas arising in patients with Graves disease may potentially be more aggressive. The treatment of Graves disease may consist of antithyroid drugs such as propylthiouracil, methimazole, and carbimazole; ablation of the gland with radioactive iodine; or subtotal thyroidectomy. The latter operation provides the specimen most commonly seen by the pathologist in this disorder. It should be noted that the microscopic picture described previously is rarely seen in their pristine state in these organs because of the changes that supervene as a result of therapy with antithyroid drugs and iodine or beta-blockers, which are given routinely in the preoperative period. The glandular enlargement and the lymphoid infiltrate persist, but most of the hyperplastic changes in the follicles regress. However, thorough sampling will usually reveal residual foci of hyperplasia. After surgery the thyroid remnants regenerate, so that a euthyroid status can be maintained, especially if the amount of thyroid tissue left is approximately 5 g on each side. The greater the lymphocytic infiltration and the larger the number of oxyphilic cells in the operative specimen, the greater the chance that hypothyroidism will develop postoperatively.
Glands treated with radioactive iodine show initially nuclear abnormalities, dissolution of some of the follicles, and vascular alterations. In later stages, nodularity, oncocytic changes, follicular atrophy, and fibrosis occur. As a consequence, the long-term incidence of hypothyroidism is very high with this therapy. It should be noted that none of these treatments improves the ophthalmopathy associated with hyperthyroidism; rather, in some instances radioactive iodine treatment may provoke or worsen it.
Graves disease is one of the two major forms in which autoimmune thyroid disease may manifest, the other being Hashimoto thyroiditis.
In Graves disease, thyroid-stimulating immunoglobulins activate TSHr, causing the overproduction of thyroid hormones and hyperthyroidism. The T and B cells, as well as antigen-presenting cells infiltrating the thyroid parenchyma, produce numerous cytokines (including interleukins-1β, -6, and -12 and interferon-γ). These cytokines activate and sustain inflammation and alter the behavior of the follicular cells. Follicular cells start producing cytokines and expressing MHC class II molecules and CD40, allowing for productive interactions between thyroid epithelium and antigen-specific T cells that contribute to the autoimmune inflammatory process. Other antithyroid antibodies (e.g., anti-peroxidase) are also present but are not relevant for the development of the hyperthyroidism.
The occurrence of an immune attack on the thyroid follicle is also evident on electron microscopic examination, which demonstrates deposits consistent with immune complexes in the follicular basement membrane.
Genetic and environmental factors have been associated with the development of Graves disease, but the specific initiating events are unknown: infections and emotional stress are often reported in the patient history. An increased incidence of Graves disease after irradiation to the neck for lesions such as Hodgkin lymphoma has been observed, and cases have been reported in association with true thymic hyperplasia.
Graves disease is only one of the many disorders—although by far the most common (80% of cases in iodide sufficient areas) —that can lead to clinically evident hyperthyroidism, i.e., hyperfunction due high thyroid hormone levels caused by an increase in their synthesis and secretion by the thyroid parenchyma. Hyperthyroidism is not synonymous with thyrotoxicosis, the clinical hypermetabolic syndrome caused by increased free thyroxine-T4 and/or free triiodothyronine-T3 levels that may or may not be due to increased hormone synthesis and secretion by the thyroid. Other causes of hyperthyroidism include the following : (1) “Toxic” sporadic (nodular) goiter; (2) “Toxic” follicular adenoma and the rare hyperfunctioning thyroid carcinoma. In these instances the hyperthyroidism is due to activating mutations in the gene encoding for the TSH receptor or in that for its associated Gsα subunit protein (with or without associated EZH1 gene mutations); (3) TSH-secreting pituitary adenoma (hyperthyroidism due to stimulation by inappropriate TSH secretion); (4) Trophoblastic tumors (hydatidiform mole, choriocarcinoma) and human chorionic gonadotropin (hCG)-induced gestational hyperthyroidism. In these cases the hyperthyroidism is due to increased stimulation of the TSH receptor by hCG; (5) Struma ovarii (hyperthyroidism due to the autonomous function of thyroid tissue in the ovarian teratoma); (6) Iodine-induced hyperthyroidism (the Jod-Basedow effect— jod is the German word for iodine; hyperthyroidism after exposure to excessive amounts of iodide caused by increased hormone synthesis in patients with underlying thyroid disease, particularly those with multinodular goiter containing autonomously hyperfunctioning areas); (7) Familial and sporadic non-autoimmune hyperthyroidism due to gain-of-function germline mutations in the TSH receptor gene.
Thyrotoxicosis without hyperthyroidism is due to the release of stored, preformed iodothyronine hormones into the circulation (follicular cell damage with “hormone leakage”) as seen in various types of thyroiditis (e.g., de Quervain or drug-induced thyroiditides), or due to excessive intake of exogenous thyroid hormones (iatrogenic or factitious). Amiodarone-induced thyroid disease includes both thyrotoxicosis and hypothyroidism. Amiodarone is a cardiac antiarrhythmic and antianginal drug containing a large amount of iodine (37% by weight) that is in part released in the blood. Amiodarone-induced thyrotoxicosis type I is a form of iodine-induced hyperthyroidism caused by the exposure to high amounts of iodine; amiodarone-induced thyrotoxicosis type II is due to the direct toxic effect of the drug on thyrocytes, characterized histologically by degenerative and destructive follicular lesions causing the release of iodothyronines, involutional changes, and fibrosis. Amiodarone-induced hypothyroidism (due to iodine excess) occurs predominantly in patients with preexisting thyroid autoimmune disease.
Autoimmune thyroid diseases like Graves disease and Hashimoto thyroiditis can be components of autoimmune polyendocrine syndromes and can also be associated with non-endocrine autoimmune disorders (e.g., vitiligo, pernicious anemia, myasthenia gravis, autoimmune gastritis, celiac disease, hepatitis).
Nodular hyperplasia (multinodular hyperplasia, nodular or multinodular goiter, adenomatoid goiter, adenomatous hyperplasia) is the most common thyroid disease. Its development is influenced by environmental factors, the most important of which is iodine deficiency, and by genetic factors.
In the form traditionally known as endemic goiter , the disease is due to low iodine content of the water and soil, and it can be largely prevented by the addition of iodine to common salt. The deficiency in thyroid hormone production induced by iodine deficiency leads to increased TSH secretion, which results initially in a hyperactive thyroid with tall follicular epithelium and small amounts of colloid (so-called parenchymatous goiter ) and later in follicular atrophy with massive storage of colloid, with or without nodularity (so-called colloid goiter ). In these endemic areas, the frequency of the disease at post mortem examination is virtually 100%. Although endemic goiter is typically a disease of developing countries, iodine deficiency is a global public health problem and may cause goiter also in developed ones.
In the form known as sporadic (nodular) goiter , which is the one most commonly seen in the United States and other developed parts of the world, the pathogenesis remains unclear. Mild dietary deficiency of iodine, slight impairment of hormone synthesis, natural goitrogens, smoking, and lack of selenium and iron have been variously suggested. In most patients with nodular hyperplasia, the blood levels of TSH are not elevated. Some cases are associated with lymphocytic or Hashimoto thyroiditis and can be viewed as the nodular forms of these immune-mediated inflammatory diseases. Hereditary predisposition is polygenic and influenced by environmental factors. Single nucleotide polymorphisms at specific chromosomal loci (1p36, 15q21and 16q23) have been linked to thyroid volume by genomewide association studies (GWAS), while polymorphisms at both thyroid-specific genes (encoding for TPO, thyroglobulin, sodium iodine symporter, pendrin, and TSHr) and non thyroid-specific chromosomal loci (e.g., MNG-1 at 14q31, MNG-2 at Xp22, MNG-3 3q26.1-q26.3) have been associated with nodular goiter. Patients with DICER1 syndrome, a rare autosomal dominant cancer syndrome caused by inactivating germline mutations in the gene encoding DICER1, a ribonuclease (RNase) involved in the generation of mature miRNA species, develop pleuropulmonary blastoma, ovarian Sertoli-Leydig cell tumors, cystic nephroma of the kidney and multinodular goiter with adenomatous nodules, and sometimes thyroid carcinoma (often follicular variant papillary carcinoma).
The prevalence of goiter in the general adult population of iodide-sufficient regions is 10% in women and 2% in men, while thyroid nodularity is identified at autopsy in approximately 50% of cases. Clinically, most patients are euthyroid and when first seen have a multinodular gland that may become very large, cause tracheal obstruction, and produce considerable disfigurement. In cases with a single, firm, dominant nodule, the clinical distinction from a true neoplasm becomes impossible. Hemorrhage within a nodule can cause sudden enlargement and pain. A small proportion of patients initially have clinical signs of hyperthyroidism, but the exophthalmos of Graves disease does not occur.
Some cases of nodular hyperplasia are located substernally and enter in the differential diagnosis of superior mediastinal tumors (see Chapter 12 ). The morphologic changes are similar to those of the nodular form of endemic goiter. As in the latter, the degree of thyroid nodularity is directly related to the duration of the disease. On occasion, one or more of the hyperplastic nodules appear in the lateral neck, anatomically separate from the main gland, sometimes simulating metastatic thyroid carcinoma ; these are referred to as parasitic nodules .
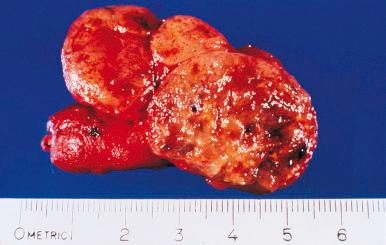
Grossly, the thyroid with nodular hyperplasia is enlarged and its shape distorted, one lobe being frequently larger than the other ( Fig. 8.24 ). The thyroid capsule may be stretched but is intact. On cross section, multiple nodules are seen; most are devoid of a capsule ( Fig. 8.25 ), but some may be partially or even completely encapsulated. Secondary changes in the form of hemorrhage, calcification, and cystic degeneration are common.
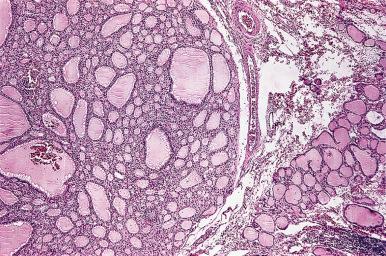
Microscopically, the appearance is very variable. Some nodules are composed of huge follicles lined by flattened epithelium, others are extremely cellular and hyperplastic, and still others are composed predominantly or exclusively of oncocytic (Hürthle) cells ( Fig. 8.25 ). Some of the dilated follicles have a conglomerate of small active follicles at one pole (so-called Sanderson polsters ) ( Fig. 8.26 ). Others have papillary projections facing the lumen of a cystic follicle, a feature that may lead to confusion with papillary carcinoma ( Fig. 8.27 ).
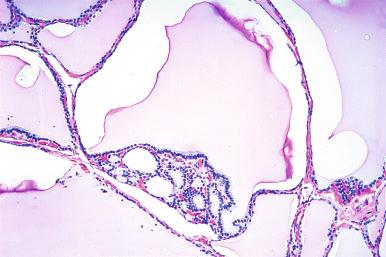
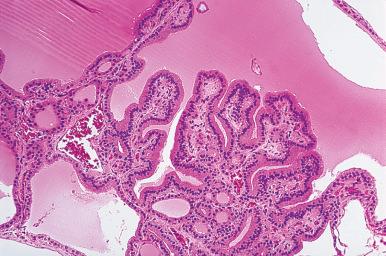
It is not unusual to find, within a nodule predominantly composed of large dilated follicles, sharply outlined solid or microfollicular clusters of follicular cells. It has been suggested that nodular goiters grow by episodic replication of these clusters, which have been referred to as foci of secondary proliferation . The proliferative activity in these clusters overlaps with that of neoplastic nodules (follicular adenoma, follicular carcinoma, papillary carcinoma).
Rupture of follicles leads to a granulomatous reaction to the colloid, with appearance of histiocytes and foreign body–type giant cells. Greatly thickened vessels with calcified media, areas of fresh and old hemorrhage, coarse fibrous trabeculation, foci of calcification, and osseous metaplasia may be seen as expression of regressive changes. Exceptionally, foci of extramedullary hematopoiesis are found in patients with known or occult primary myelofibrosis.
Vascularization inside the nodules is usually prominent, sometimes accompanied by reactive endothelial hyperplasia and exceptionally even by the presence of hyperplastic follicles within vascular lumina. Obviously, the latter feature raises the differential diagnosis with angioinvasive follicular carcinoma. Although the distinction can be very difficult, if the overall appearance of the lesion is typical throughout of nodular hyperplasia (including the intravascular component), a benign diagnosis is justified. Conversely, the multinodular pattern of invasion of some widely invasive carcinomas (particularly Hürthle cell carcinomas) may simulate nodular hyperplasia. A variable number of chronic inflammatory cells are present in the stroma in many of the cases, indicating the coexistence of chronic thyroiditis; the larger their number, the higher the incidence of postoperative hypothyroidism. The presence of highly atypical nuclei in a case of nodular hyperplasia should raise the possibility of previous exposure to radioactive substances if present in the nodule themselves, and of dyshormonogenetic goiter if present between the nodules. It is not possible to predict on the basis of the morphologic appearance whether the patient has clinical or laboratory evidence of hyperthyroidism.
The differential diagnosis between a dominant nodule from a case of nodular hyperplasia and a true adenoma is based on a set of admittedly arbitrary criteria. The adenoma is usually single, is totally surrounded by a capsule, is dissimilar from the remaining parenchyma, compresses the adjacent tissue, and is composed mainly of follicles that are smaller than those of the normal gland. The lesion of nodular hyperplasia is almost always one of many nodules, its encapsulation is incomplete, the follicular size is variable, some or all of the follicles are larger than those in the surrounding gland, and there is no compression of the adjacent parenchyma. In some cases, the distinction becomes impossible, inasmuch as lesions with the morphologic features of adenoma may be multiple and/or occur in a setting of nodular hyperplasia.
To address this, the clonality of hyperplastic nodules has been evaluated using X chromosome inactivation patterns: thyroid nodules, particularly the “dominant” adenomatous ones that develop in the background of nodular hyperplasia, may be monoclonal, even when they are partially or completely devoid of a fibrous capsule. These studies have limitations given the large size of the embryonal X chromosome inactivation patch in the thyroid gland (approximately 0.5–1 cm 2 ), but results consistent with a monoclonal cell population are identical to those obtained in many follicular adenomas (that are typically, but not always, clonal by X chromosome inactivation analysis).
Most hyperplastic nodules have a normal karyotype. However, chromosomal DNA abnormalities detectable by conventional karyotyping and ploidy analysis occur in a minority of hyperplastic nodules, particularly in those with adenomatous features that frequently have extra copies of chromosome 7. Activating mutations of the TSH receptor gene, typically associated with “toxic” adenomas, have been identified in hyperfunctioning, scintigraphically “hot” areas in multinodular goiter and in microscopic, autoradiographically “hot” foci in approximately 30% of euthyroid goiters. Activating RAS mutations occur in a few hyperplastic nodules. These observations (monoclonal origin of some hyperplastic nodules, the occurrence of cytogenetic abnormalities, of aneuploidy and of oncogenic mutations) have been used to argue that hyperplastic nodules are in fact neoplastic. They certainly reflect the difficulty in drawing the line between hyperplasia and neoplasia, a problem common to all endocrine proliferative lesions.
A longstanding and still unresolved issue is whether nodular hyperplasia is associated with an increased incidence of clinically significant carcinoma, particularly of the follicular type. Suffice it to say that if such an increase really exists, it is small enough to be disregarded for practical purposes.
Mild asymptomatic cases of nodular hyperplasia require no treatment. Suppressive medical therapy with exogenous thyroid hormones is only moderately effective. If the enlargement is such that disfigurement or pressure symptoms develop, bilateral subtotal thyroidectomy is performed.
The majority of clinically apparent thyroid neoplasms are primary and epithelial. Traditionally, they have been divided into adenomas and carcinomas, the latter group incorporating the medullary carcinomas together with the more common lesions composed of follicular cells. From a histogenetic/differentiation standpoint, it is preferable to divide thyroid neoplasms into three major categories, depending on the cell types involved, and subdivide them into the various benign and malignant categories:
Tumors exhibiting follicular cell differentiation
Tumors exhibiting C-cell differentiation
Tumors exhibiting follicular and C-cell differentiation.
Lesions in the first category comprise more than 95% of the cases, the remainder being largely made up by tumors in the second category.
Follicular adenoma is defined as a benign encapsulated tumor that shows evidence of follicular cell differentiation and lacks: (1) evidence of capsular, vascular, or any other type of invasion; and (2) the nuclear features of the papillary family of neoplasms. It is a common thyroid neoplasm. Most patients are euthyroid adults who initially have a thyroid lump, which on scan is usually “cold,” sometimes “cool” or “warm,” and only rarely “hot.” The large majority of “hot” nodules are benign, but exceptions have been documented.
By ultrasound, follicular adenomas are well circumscribed, usually solid, isoechoic or hypoechoic with a peripheral halo, similar to follicular carcinomas.
Few of the tumors are associated with clinical hyperthyroidism (so-called toxic or Plummer adenomas ). The thyroid tissue outside hyperfunctioning nodules often contains intraluminal crystals of calcium oxalate, thought to be a sign of hypofunction.
Adenomas are almost always solitary (except when occurring in genetically determined syndromes). They are characteristically surrounded by a generally thin capsule that is grossly and microscopically complete ( Figs. 8.28 and 8.29 ). The architectural and cytologic features are different from those of the surrounding gland, which usually shows signs of compression. Adenomas may exhibit a variety of patterns, singly or in combination: normofollicular (simple), macrofollicular (colloid), microfollicular (fetal), and solid/trabecular (embryonal) ( Fig. 8.30 ). The morphologic differences among these various patterns may be striking. They have no apparent clinical significance but elicit different differential diagnoses. Thus, as a general rule, the larger the follicles, the less likely that the lesion is a follicular adenoma or a follicular carcinoma (as opposed to a hyperplastic nodule or a papillary carcinoma with a predominantly follicular growth pattern). Instead, a predominance of solid/nesting/trabecular areas should alert one to the alternative possibilities of poorly differentiated or medullary carcinoma (which, however, are usually—but not always—clearly invasive).
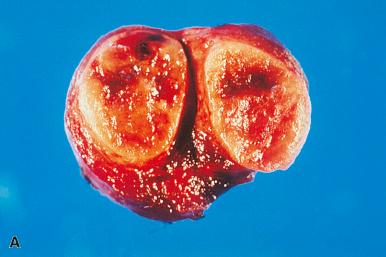
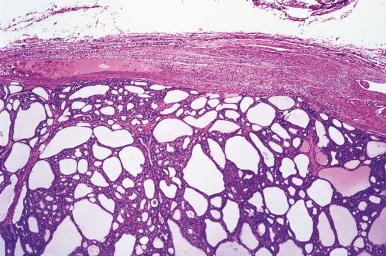
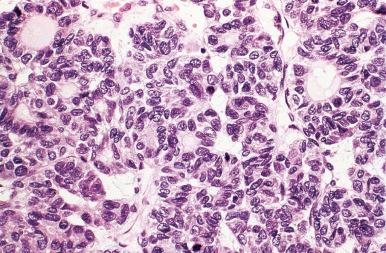
Mitoses are rare or absent in the follicular adenomas. They are not necessarily indicators of malignancy, but when they are present in appreciable numbers one should sample and examine the specimen with particular care. Secondary degenerative changes such as hemorrhage, edema, fibrosis, calcification, bone formation, and cystic degeneration are common, especially among the larger tumors. Sometimes the vessels in the capsule of adenomas (and also at the periphery of hyperplastic nodules) show prominent focal thickenings of their wall, referred to as muscular cushions . Adenomas may exhibit papillary or pseudopapillary structures, which may prompt confusion with papillary carcinoma. Some authors have referred to these tumors as “papillary adenomas,” a term that should be replaced with follicular adenomas with papillary hyperplasia . The differential diagnosis, which includes hyperplastic nodules and encapsulated papillary carcinomas, is discussed later.
Some correlation exists between the microscopic appearance of a follicular adenoma and its activity as judged by the scan appearance. In general, hyperfunctioning (hot) adenomas are more cellular and their cells have more abundant cytoplasm (and therefore a decreased nucleocytoplasmic ratio) than nonfunctioning (cold) tumors. However, the range of morphologic appearances that hot thyroid nodules can exhibit is wide, including less well-differentiated and oncocytic patterns. Hyperfunctioning follicular adenomas that are scintigraphically “hot” (and sometimes associated with overt hyperthyroidism, so-called toxic or Plummer adenomas ) typically have activating mutations in the gene encoding the TSH receptor ( TSHR ) or in that encoding its associated Gsα subunit protein (GNAS). TSHR mutations are more common (~50%–80%) than those of GNAS (~5%). EZH1 gene mutations have been identified in approximately 20%–30% of cases, nearly always in combination with TSHR or GNAS mutations, or with other presumed alterations in cAMP pathway genes. It should also be remembered that hot thyroid nodules are usually but not always benign.
The enzyme histochemical and immunohistochemical profile of adenomas mirrors that of the normal follicle. There is reactivity for low-molecular-weight keratin, TPO, and thyroglobulin in the cytoplasm, for TTF-1 and PAX8 in the nuclei, and for laminin and other basement membrane components around the follicles. The use of immunohistochemical markers in the differential diagnosis is discussed later. To date, no molecular test can reliably distinguish between follicular adenoma and follicular carcinoma, since genetic alterations (e.g., chromosomal abnormalities, activating RAS mutations, and PAX8/PPARG rearrangement) found in the former are also found, albeit with a higher prevalence, in the latter ( Table 8.2 ). This points to a tumorigenic pathway common to all follicular neoplasms (adenomas and carcinomas) as opposed to papillary carcinoma. Reasoning along this line, it may be argued that follicular adenomas with PAX8/PPARG are in fact noninvasive or preinvasive follicular carcinomas, or that the tumor has not been adequately sampled and that invasion of the capsule or blood vessel was missed. This is supported by the more common occurrence of the rearrangement in follicular carcinomas and also by the high cellularity and atypical features of some follicular adenomas with PAX8/PPARG . A similar argument can be made for RAS mutations, since RAS mutated follicular adenomas may progress to RAS mutated follicular carcinomas and undergo further dedifferentiation.
| FOLLICULAR ADENOMA | FOLLICULAR CARCINOMA | PAPILLARY CARCINOMA (CONVENTIONAL TYPES) | ENCAPSULATED FOLLICULAR VARIANT OF PAPILLARY CARCINOMA | POORLY DIFFERENTIATED THYROID CARCINOMA | ANAPLASTIC THYROID CARCINOMA |
|---|---|---|---|---|---|
a The main molecular alterations are reported; in parentheses the mutation prevalence percentage range, as estimated from the literature quoted in the text.
b Mutations in N-, H, KRAS ; NRAS is the gene most frequently mutated.
c Hyperfunctioning follicular adenomas and adenomatous nodules have mutations of the TSH gene receptor ( TSHR ) (~50%–80% of cases), of the GNAS gene (~5% of cases), of the EZH1 gene (~20%–30% of cases, nearly always in combination with TSHR or GNAS mutations, or with other presumed alterations in cAMP pathway genes).
d According to conventional cytogenetics, cytometric measurement of DNA content, comparative genomic hybridization, loss of heterozygosity and somatic copy-number alterations studies.
e The prevalence of RET/PTC is higher in children and much higher in radiation-associated papillary carcinomas (~50%–80%).
f The prevalence of NTRK rearrangements is variably reported between 0% and 5% for NTRK1 and NTRK3 in most series from nonradiation-associated papillary carcinoma in adult patients; the prevalence is higher in children and young patients, and in radiation-associated papillary carcinomas (up to ~15% for NTRK3/ETV6 ).
g EIF1AX mutations often co-exist with RAS mutations; they have been reported in follicular adenomas and rarely in hyperplastic nodules.
h The prevalence of BRAF p.V600E is approximately 40% in aggressive, radioiodine-refractory and FDG-PET positive tumors
i CTNNB1 is the gene encoding β-catenin (effector of the wnt pathway); previous studies reported a higher prevalence of CTNNB1 mutations in poorly and undifferentiated carcinomas.
Follicular adenomas are typically (but not always) clonal according to X chromosome inactivation studies. Conventional karyotyping identifies aberrations in 35%–65% of cases. Numerical changes begin with an extra copy of chromosome 7, followed by the acquisition of additional copies of other chromosomes, a sequence that parallels the transition from hyperplastic to adenomatous nodules. Structural abnormalities overlap with those of follicular carcinomas. Chromosomal translocations that involve 19q13, with the breakpoint corresponding to the ZNF331 gene locus, and chromosome 2p21, with the breakpoint corresponding to the thyroid adenoma associated ( THADA ) gene locus, have been identified in approximately 10% of cases but are apparently rare in follicular carcinoma. Fusion of THADA with the LOC389473 pseudo gene (at 7p15.3), leading to increased insulin-like growth factor receptor signaling, has been reported in approximately 5% of papillary carcinomas (follicular variant papillary carcinomas or NIFTP). The PI3K/PTEN/AKT pathway is rarely altered in sporadic follicular adenomas, due to activating mutations of the PIK3CA gene that have been identified in a few cases (~5%); PTEN gene mutations are also uncommon. This is somewhat surprising, considering that loss of function germline PTEN mutations are responsible for the Cowden syndrome (one of the PTEN-hamartoma tumor syndrome, PHTS) that is characterized by multicentric and bilateral thyroid follicular adenomas and hyperplastic/adenomatous nodules. Follicular adenomas and adenomatous nodules also occur in the Carney complex type I ( PRKARIA germline mutations) and in the McCune–Albright syndrome (sporadic, postzygotic activating mutations of GNAS ), in which case they are typically hyperfunctioning.
Several variants of follicular adenoma have been described. Oncocytic (Hürthle cell) adenoma is discussed later and hyalinizing trabecular adenoma in the section that follows. The term atypical adenoma has been proposed for adenomas with pronounced cellular proliferation and less regular cytoarchitectural patterns but still lacking evidence of capsular or blood vessel invasion.
Adenoma with bizarre nuclei is characterized by the presence of huge hyperchromatic nuclei, usually in clusters, unaccompanied by other features of malignancy ( Fig. 8.31 ). This phenomenon is analogous to that seen in parathyroid adenomas and other endocrine neoplasms.
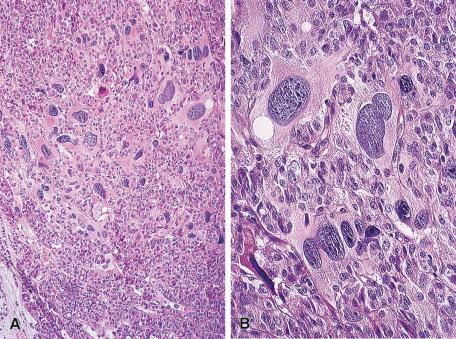
Other rare types of follicular adenoma are those with clear cell changes (including the signet ring, mucin-producing, and lipid-rich types), adenomas with adipose metaplasia of the stroma (so-called lipoadenomas or adenolipomas ), adenomas with cartilaginous metaplasia (so-called adenochondromas ), spindle cell adenomas (some vaguely resembling meningiomas), and adenomas with massive deposition of cytoplasmic black pigment following minocycline therapy (so-called black adenomas ).
The differential diagnosis of follicular adenoma includes a dominant nodule of nodular hyperplasia, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), minimally invasive carcinomas (follicular carcinoma or follicular variant papillary carcinoma), medullary carcinoma, and intrathyroidal parathyroid adenoma. Some follicular adenomas may also be confused with vascular tumors because of their high vascularization.
The lack of immunoreactivity for galectin-3, HBME1, and CITED1 and the retention of CD56 helps to distinguish adenomas from NIFTP and follicular-variant papillary carcinoma ( Table 8.3 ); immunoreactivity for thyroglobulin and lack of calcitonin and CEA distinguishes adenomas with solid/trabecular growth from medullary carcinoma; immunoreactivity for thyroglobulin and TTF-1 and lack of chromogranin and parathyroid hormone distinguishes follicular adenomas from intrathyroidal parathyroid adenomas.
| HBME-1 | GALECTIN-3 | CK19 | CITED1 | CD56 | Ki-67 LABELING INDEX (% RANGE) | |
|---|---|---|---|---|---|---|
| Normal thyroid | ~0 | ~0 | 0–40 | ~0 | ~100 | ~0.2 (0–0.5) |
| Nodular hyperplasia | 0–20 | 0–30 | 0–40 | 0–10 | 90–100 | ~0.7 (0–3) |
| Follicular adenoma | 5–30 | 10–50 | 10–50 | 5–10 | 90–100 | ~1.5 (0–5) |
| Follicular carcinoma | 60–80 | 20–100 | 20–80 | 10–20 | 80–100 | ~5.0 (1–10) |
| Papillary carcinoma, follicular variant | 80–100 | 60–100 | 50–100 | 50–80 | 0–30 | ~2 (0–5) |
| Papillary carcinoma, conventional | 90–100 | 90–100 | 80–100 | 50–90 | 0–10 | ~3 (0–5) |
| Anaplastic carcinoma | 0–80 | 0–100 | 0–80 | ~0 | ~0 | ~35 (20–70) |
a Percentage range of positive cases estimated from the literature and proliferative activity (Ki-67 labeling index)
The standard therapy for follicular adenoma is removal by lobectomy. Enucleation of the adenoma should not be attempted. Suppression of the nodule with levothyroxine and treatment of the toxic adenoma with radioactive iodine have been used, but the results have generally been less than satisfactory.
Hyalinizing trabecular adenoma was the term given by Dr. J.A. Carney in 1987 to a peculiar type of follicular cell neoplasm (apparently first identified by Langhans). Because of its unclear relationship with malignancy and because rare cases may show invasion (of the tumor capsule or vascular spaces) or metastases (see later) the term hyalinizing trabecular tumor (HTT) is currently preferred. The lesion has a very distinctive histologic appearance, characterized by a prominent trabecular arrangement and an equally prominent hyaline appearance ( Fig. 8.32 ). The latter is present both in the cytoplasm of the tumor cells (as the result of the accumulation of intermediate filaments) and in the extracellular space (as the result of heavy deposition of hyalinized collagen fibers and basement membrane material). The trabeculae can be straight or curved, resulting in curious organoid formations ( Fig. 8.33 ). The pattern of growth may simulate that of paraganglioma and medullary carcinoma, whereas the presence of perinucleolar clearing, nuclear grooves, and psammoma bodies may suggest papillary carcinoma, particularly in material from fine-needle aspiration (FNA) ( Fig. 8.34 ). By ultrasound, HTT is typically hypoechoic with a pattern most similar to that of follicular adenoma and the follicular variant papillary carcinoma. Another distinct (but not specific) morphologic feature of HTT is the so-called cytoplasmic yellow body , which is a round, 2–5 µm, pale yellow cytoplasmic inclusion body in a paranuclear location having a refractile quality and detectable both in tissue sections and in FNA smears. These bodies correspond to giant secondary lysosomes and can be found in other thyroid tumors (including papillary and follicular carcinoma), where they are however numerically rare. Immunohistochemically, HTT is positive for thyroglobulin and TTF-1 but not for calcitonin. HBME-1, galectin-3, and cytokeratin 19 are expressed inconsistently. Focal and inconstant signs of neuroendocrine differentiation have been shown by immunohistochemistry (reactivity for NSE and chromogranin A) and confirmed by the finding of neurosecretory granules by electron microscopy.
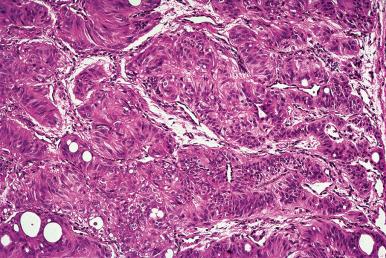
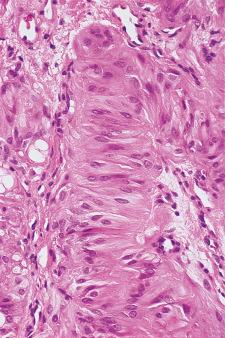
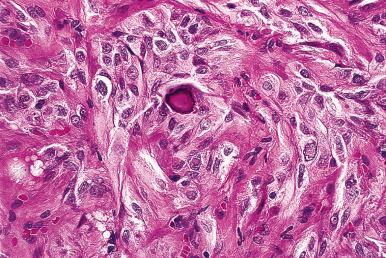
HTT also shows a peculiar cell membrane and cytoplasmic (instead of nuclear) immunostaining with the monoclonal antibody MIB1 to the proliferation marker Ki-67. The peculiar pattern (likely due to epitope cross reactivity of the MIB1 antibody) is not seen with automated immunostainers which operate at 37°C but only when the reaction is performed at room temperature. Immunostains for type IV collagen and laminin are consistent with heavy deposition of basement membrane material around the tumor cells (partially explaining their “hyaline” appearance) and—surprisingly—in the nuclear pseudoinclusions.
Considerable controversy has arisen as to whether HTT is a distinct entity or an unusual pattern of growth that can be present in a variety of thyroid lesions. It is generally accepted that the “HTT pattern” can be seen as a microscopic incidental finding in nodular hyperplasia, and in rare neoplasms with HTT features accompanied by capsular and/or vascular invasion, which have therefore been designated as hyalinizing trabecular carcinomas. Intriguing is the apparent link between HTT and papillary carcinoma, which manifests itself by the presence in HTT of morphologic features traditionally associated with papillary carcinoma, such as nuclear alterations (grooves and pseudoinclusions) and the finding of psammoma bodies; by the occurrence of papillary carcinoma merging with HTT ( Fig. 8.35 ) ; by the association of both conditions with Hashimoto thyroiditis and possibly with radiation exposure.
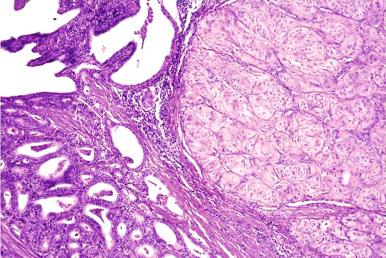
Regardless of this link, in the absence of capsular or vascular invasion there are no well-documented examples of HTT that have metastasized, and the only tumor that developed distant metastases (in the lung) out of a series of 119 cases had both.
A small number of cases are aneuploid by flow cytometric DNA analysis or show simple karyotypic alterations. Reports of RET/PTC rearrangements are in all likelihood the result of subclonal events that are not diagnostically relevant. BRAF p.V600E or RAS mutations do not occur, and the miRNA profile is different from that of papillary carcinoma.
Papillary carcinoma is defined by the presence of a distinctive set of alterations of nuclear morphology, the PTC-type nuclei (see later), although the presence of either papillae or invasion of the surrounding thyroid parenchyma is generally required for the diagnosis. It is the most common type of thyroid carcinoma and the most common endocrine malignancy.
Females are more affected than males. It can present in any age group, the mean age at the time of initial diagnosis being approximately 40–50 years. Papillary carcinoma accounts for more than 90% of thyroid malignancies in children. In approximately 5% of cases, there is a history of irradiation exposure to the neck, and the non-neoplastic gland may show nuclear aberrations as a result. There is convincing evidence for an increase in the incidence of papillary carcinoma in Hashimoto thyroiditis, but the wide variation in the figures quoted suggests that the diagnostic criteria vary just as widely. Whether the frequency of papillary carcinoma is increased in Graves disease remains a controversial subject.
The prevalence of thyroid carcinoma has been increasing globally, mostly due to the increased detection of small papillary carcinomas through neck ultrasound. By ultrasound, papillary carcinoma is usually solid, hypoechoic, with infiltrative or microlobulated margins, microcalcifications, and a “taller than wide” shape. Before the widespread use of ultrasound screening the large majority of patients had clinically evident disease in the neck when they were first seen, localized to the thyroid gland in 67% of cases, thyroid and lymph nodes in 13%, and lymph nodes alone in 20%.
The size of the primary tumor ranges from microscopic to very large. A very high proportion of thyroid cancers measuring less than 1 cm in diameter are of papillary type. Grossly, most cases are solid, whitish, firm, and clearly invasive; 10%–20% are surrounded by a complete capsule ( Figs. 8.36 and 8.37 ). Marked cystic changes are seen in approximately 10% of cases. Sometimes the papillary formations are evident to the naked eye.
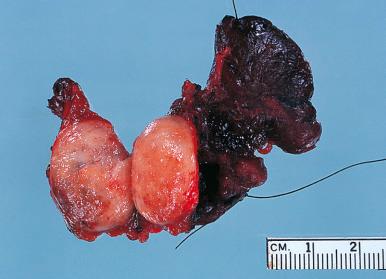
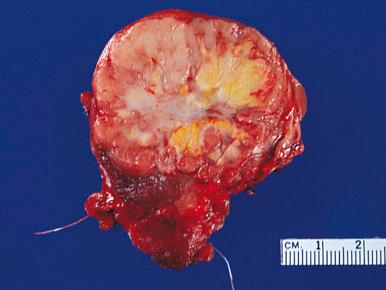
Microscopically, the typical papillary carcinoma contains numerous true papillae, an easily recognizable feature which—as one would have deduced—was the one originally chosen to name the tumor. The papillae are usually complex, branching, and randomly oriented, with a central fibrovascular core and a single or stratified lining of cuboidal cells, some of which may have hobnail features ( Fig. 8.38 ). The stroma of the papillae may be edematous or hyaline and may contain lymphocytes, foamy macrophages, hemosiderin, or—exceptionally—adipose tissue. These papillae are nearly always associated with the formation of follicles, the ratio between the two components varying greatly from case to case. The follicles tend to be irregularly shaped, often tubular and branching. Tumors with a combination of papillary and follicular structures have the biologic behavior of papillary carcinoma and should therefore be classified as such instead of as mixed carcinomas.
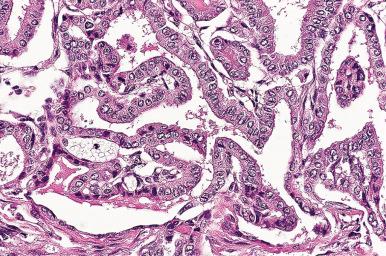
The cells of papillary carcinoma have characteristic nuclear features. These have acquired so much relevance that currently the diagnosis of papillary carcinoma is more dependent upon their presence than on the papillary architecture, which may be minor or altogether absent (see under Variants).
These nuclear features (PTC-type nuclei) consist of:
Ground-glass (optically clear) nuclei, which often have a large size and an overlapping quality ( Fig. 8.39A ). The nucleolus is usually inconspicuous and pushed against the nuclear membrane, which appears thickened. This change is present in sections obtained from paraffin-embedded material regardless of the fixative used, but is less apparent or altogether absent in frozen sections or cytology material, and is particularly prominent in tissue fixed in high concentrations of formalin. The mechanism of its formation has not yet been ascertained.
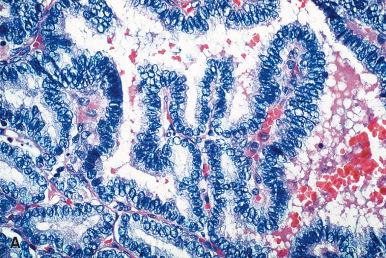
Nuclear pseudoinclusions. These represent invaginations of the cytoplasm and appear as sharply outlined (nuclear membrane–bound) round acidophilic vacuoles (see Fig. 8.39B ). In contrast to the ground-glass feature, the pseudoinclusions are also readily apparent in specimens from frozen section and aspirations. They should be distinguished from the nuclear “bubbles” that can be seen in a variety of lesions and organs and which are in all likelihood a processing artifact. These “bubbles,” when present, tend to affect most nuclei and to be multiple within a single nucleus and have been related to the process of histology section heating at 60°C in the oven before deparafinization (they are absent when sections are dried overnight or with a fan).
Nuclear grooves. These tend to occur in oval or spindle nuclei, are usually arranged along the longest nuclear axis, and represent—like the pseudoinclusions—the morphologic expression of infoldings of a redundant nuclear membrane as confirmed by confocal microscope analysis and tridimensional reconstruction. These nuclear membrane irregularities are seen to better advantage with markers specific for emerin, a nuclear membrane protein.
Nuclear microfilaments. A few cases have been described in which the nuclear clearing is due to the accumulation of fine threadlike fibrils.
Mitoses are usually very scanty or absent in papillary carcinoma. Over half of the cases show extensive fibrosis, usually in the form of sharply outlined bands traversing the tumor; the appearance of this fibrosis may range from sclerohyaline to highly cellular ( Fig. 8.40 ). Elastic tissue is often abundant in the tumor stroma.
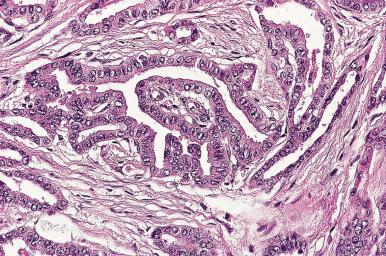
Psammoma bodies are seen in approximately half of the cases. These basophilic structures showing concentric laminations stain for mucin, calcium, and iron and appear to arise from necrosis of individual tumor cells, which occasionally may be seen at their very center, and which represent the nidus for their formation, very much the way a grain of sand acts as the nidus for the formation of a pearl in an oyster. They may be located in the papillary stalk, in the fibrous stroma, or between tumor cells in solid foci ( Fig. 8.41A ). Their presence in a thyroid gland strongly suggests the diagnosis of papillary carcinoma, inasmuch as their occurrence in other thyroid lesions is exceptional ; Klinck and Winship found them only once in a review of 2153 benign thyroids. Thus they represent a very important clue to the diagnosis not only in paraffin sections, but also in frozen sections and cytology preparations. If numerous enough they are visible on ultrasound scans.
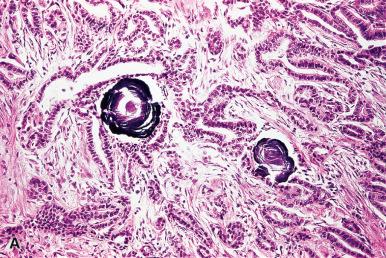
When psammoma bodies are found in otherwise normal thyroid tissue or lymph nodes from the neck, the chances are high that a papillary carcinoma is present in the immediate vicinity (see Fig. 8.41B ). Psammoma bodies should be distinguished from other forms of calcification and from the intraluminal foci of inspissated secretion often seen in oncocytic (Hürthle cell) tumors.
Areas with a solid/trabecular pattern of growth are present in 20% of the cases and foci of squamous metaplasia in a similar number; these two patterns often merge and are probably related. The presence of such foci is not per se an indication that the tumor is poorly differentiated unless accompanied by other alterations.
Occasionally, papillary carcinomas are seen to contain a bland-looking spindle cell component; this clinically insignificant metaplastic change (recognizable because of its blending with more typical epithelial cells and its immunoreactivity for thyroglobulin and TTF-1) should not be confused with a mesenchymal neoplasm or with the anaplastic transformation of a papillary carcinoma.
Papillary carcinoma has a rich microvasculature, with high microvessel density. On the other hand, lymphatic vessels are very few inside the tumor, but their density is very high at the interface with the surrounding non-neoplastic thyroid. Blood vessel invasion is found in only 5% of cases, a much lower proportion compared to follicular carcinoma. Lymphocytic infiltration of the stroma is seen in a fourth of cases; it is not clear whether this represents a reaction to the tumor or the expression of preexisting thyroiditis. Many tumors also exhibit a heavy infiltrate of S-100 protein–positive dendritic/Langerhans cells and macrophages. Scattered multinucleated giant cells may be present, often in the lumen of the neoplastic follicles and probably representing a response to leakage of colloid.
Multiple microscopic foci of tumor are found in approximately 20% of cases if a few random sections are taken and in over 75% if step sections of the entire gland are examined. It is not entirely clear whether this represents multicentricity (multiple independent primary tumors) as opposed to intrathyroidal lymphatic permeation ( multifocality due to spread of a single primary tumor). Both mechanisms likely operate: mutation analysis indicates the independent origin of these multiple foci in 30%–60% of cases.
Ultrastructurally, the most distinctive feature of the cells of papillary carcinoma is the highly indented nuclear membrane, with formation of pseudoinclusions and multilobation. Changes in distribution of chromatin and ribonucleoprotein have been observed. The cytoplasm is rich in mitochondria, lysosomes, and intermediate filaments. The apical surface exhibits microvillous differentiation. The abnormal intracellular and extracellular deposition of basement membrane material (of less degree but otherwise similar to that seen in hyalinizing trabecular carcinoma) is very evident at the ultrastructural level.
Papillary carcinoma retains immunoreactivity for pan-follicular cell markers, although these are generally downregulated compared with normal follicular cells. Thus, TTF-1, and the other two transcription factors TTF-2 and PAX8 that are required to maintain the differentiated phenotype of follicular cells show consistent nuclear immunoreactivity in neoplastic cell nuclei. Thyroglobulin is also consistently positive (although less than in well-differentiated follicular neoplasms), while TPO and NIS show reduced expression (in the case of NIS localization is redirected from the membrane to the cytoplasm). Similar to normal follicular cells, those of papillary carcinoma are immunoreactive for keratins (detectable with pan-keratin, CAM 5.2, AE1/3 antibodies), with a CK7 positive/CK20 negative profile. Unlike normal follicular cells, papillary carcinoma cells are often positive for the low-molecular-weight CK19, and in keeping with their tendency to undergo squamous metaplasia they express high-molecular-weight keratins recognized by the antibody 34βE12. Unlike normal follicular cells, that infrequently coexpress vimentin and keratin, papillary carcinoma cells are often vimentin positive, although coexpression of both markers does not appear to be diagnostically relevant.
The diagnosis of papillary carcinoma does not routinely require immunohistochemistry. When this is performed it is usually to establish the thyroid origin of a tumor at metastatic sites. Thyroglobulin and TTF-1 are very specific markers, and PAX8 can be used to differentiate papillary carcinoma (positive for both TTF-1 and PAX8) from lung adenocarcinoma and other tumors that are positive for TTF-1 but negative for PAX8.
Another situation, considerably more complex, is the use of immunochemistry for the differential diagnosis of papillary carcinoma from other benign and malignant thyroid lesions. HBME-1 (membrane immunoreactivity), galectin-3 (both nuclear and cytoplasmic immunoreactivity), cytokeratin 19 (cytoplasmic immunoreactivity), and CITED1 (both nuclear and cytoplasmic immunoreactivity) are the markers more frequently overexpressed in papillary carcinoma (HBME-1 followed by galectin-3 are considered the most specific), while CD56 is typically negative (see Table 8.3 ). While the sensitivity of these markers is high, their specificity is relatively low, thus they are more effective when used in combination (usually HBME-1, galectin-3, cytokeratin 19). Since results vary from one institution to another, in order to diagnose papillary carcinoma the performance characteristics of the panel need to be established in individual laboratories. Unfortunately, in problematic cases immunohistochemical results tend to be difficult to interpret, even if a panel is utilized, and this is particularly true for the abnormal follicular epithelium of Hashimoto thyroiditis.
Traditional mucin stains are positive in many papillary carcinomas whether in the colloid, luminal border, or cytoplasm. This correlates with the immunohistochemical demonstration of EMA (MUC1), mucins, simple mucin antigens, and histo-blood group antigens in papillary carcinoma cells (but also in those of follicular carcinoma). EMA and alcian blue stain the apical membrane of the neoplastic cells lining the papillae but not those of the benign papillae of diffuse or nodular hyperplasia.
A variety of other markers have been shown by immunohistochemistry in papillary carcinoma, including estrogen receptors, S-100 protein, S-100C, HER2/neu, cMet/hepatocyte growth factor receptor, and involucrin, while laminin and type IV collagen are demonstrable immunohistochemically at the interface of the tumor with the stroma. Mutation-specific antibodies are available to detect BRAF p.V600E, although molecular analysis is necessary in those cases with equivocal results. Immunohistochemistry with the D5F3ALK antibody may be used as a surrogate marker to identify those rare papillary carcinomas with ALK rearrangement.
Activation of the mitogen-activated protein kinase (MAPK) pathway is the defining molecular genetic feature of papillary carcinoma. This pathway regulates important cellular functions (e.g., proliferation, differentiation, and survival) and is frequently altered in human cancers.
Since the oncogenic events that cause MAPK signaling act sequentially in the same pathway, they are usually mutually exclusive. One can be identified in the vast majority (~90%) of papillary carcinomas: in three out of four cases the MAPK pathway is activated by point mutations of BRAF (~60%) or of RAS (~15%), and in ~15% by chromosomal rearrangements that cause aberrant expression of receptor tyrosine kinases (RET, NTRK, and ALK) or of the kinase domain of BRAF. Copy number gene variations likely play a tumorigenic role in approximately two-thirds of the remaining ~10% of cases, leaving less than 5% of papillary carcinoma without a known genetic defect.
The prevalence of the main molecular alterations is shown in Table 8.2 . The Cancer Genome Atlas (TCGA) Research Network has published in 2014 an integrated genomic characterization of 496 papillary carcinomas. The work, while confirming previous observations, has provided a clear picture of their molecular landscape. The genome of papillary carcinoma is stable when compared to that of tumors from other organs, with approximately 0.4 non-synonymous somatic mutations per megabase of DNA. Papillary carcinomas typically have a diploid karyotype with low rates of LOH, although in ~7% of cases gene copy number variations likely play an important tumorigenic role. They are a heterogeneous group that includes histopathologic variants defined on the basis of size (papillary microcarcinoma), growth pattern (follicular, macrofollicular, cribriform-morular, solid), or cellular characteristics (tall cell, hobnail/micropapillary, columnar cell, oncocytic), each with distinctive sets of molecular alterations (see later).
A general and schematic view that takes into account the four basic morphologic features used to diagnose differentiated thyroid carcinoma—that is, papillary growth pattern, follicular growth pattern, presence of a tumor capsule (with or without invasion, of the capsule itself or of vessels), PTC-type nuclei—identifies the following scenario of diagnostic categories : classic PTC → follicular predominant classic PTC → infiltrative follicular variant PTC → encapsulated follicular variant papillary carcinoma (PTC-EFV, when invasion is present; noninvasive follicular thyroid neoplasm with papillary-like nuclear features, NIFTP, when there is no invasion) → follicular neoplasm (FTC, when invasion is present; FTA, when there is no invasion) ( Fig. 8.42 ). In this schematic scenario, a decrease in the extent of papillary growth and PTC-type nuclei is paralleled by increased follicular growth pattern, the appearance of a defined tumor capsule, and a shift from a BRAF p.V600E to a RAS -like molecular signature (see Fig. 8.42 ). The unequivocal recognition of these two broad molecular families of papillary carcinoma has been one important result of the TCGA's study. Tumors with a BRAF p.V600E-like signature have high levels of MAPK pathway signaling, a low thyroid differentiation score (based on the expression of 16 genes involved with follicular cell function and metabolism), a heterogeneous molecular profile (due to the molecular alterations specific to the different histopathologic papillary carcinoma variants), and represent the group of conventional papillary carcinoma. Tumors with a RAS -like molecular signature have low levels of MAPK pathway signaling, a high thyroid differentiation score, a relatively homogeneous molecular profile, and are follicular variant papillary carcinomas, reclassified as NIFTP when noninvasive (see “Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features,” later).
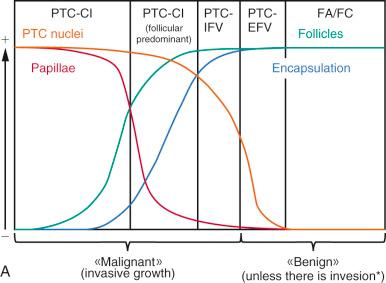
The genetic changes that characterize conventional papillary carcinoma are BRAF p.V600E, and RET/PTC ( Fig. 8.43 ), NTRK, or ALK rearrangements (fusion oncogene papillary carcinomas).
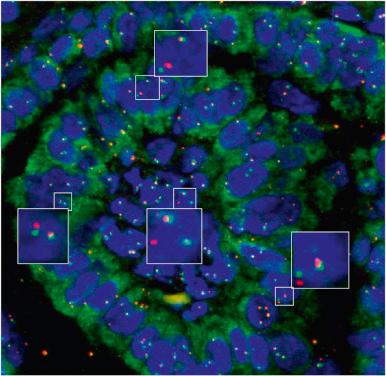
BRAF p.V600E is the most common alteration of papillary carcinoma and a very powerful diagnostic marker for those tumors characterized by papillary growth pattern (classic/conventional papillary carcinomas) that carry the mutation in ~40%–80% of cases. The mutation is highly specific and has been identified in papillary carcinomas arising within struma ovarii. The strongest association is with the tall cell variant that carries BRAF p.V600E in more than 90% of cases. BRAF p.V600E is an early molecular alteration, in most cases clonally distributed in the majority of neoplastic cells. Point mutations other than p.V600E (e.g., p.K601E) and other rare alterations are typically found in the follicular variant and have an oncogenic potential lower than the p.V600E mutation. BRAF rearrangements, found in up to approximately 10% of radiation-associated papillary carcinomas are AKAP9/BRAF and AGK/BRAF. BRAF p.V600E–specific antibodies are available, but sequencing is the gold standard for molecular diagnosis. The mutation is detectable in the peripheral blood of papillary carcinoma patients, and its identification correlates with the status of disease. The prognostic relevance of BRAF p.V600E is controversial. Several studies have associated the p.V600E mutation with poor outcome, while other have not confirmed the association. BRAF p.V600E has been linked to extrathyroid tumor extension and to an increased risk of recurrence (also in patients with low risk tumors), but the overall prognostic relevance of the mutation appears limited when other factors (e.g., stage, papillary carcinoma variant) are considered. Clinical response to kinase inhibitors that target the BRAF p.V600E mutation (such as dabrafenib and vemurafenib ) has been demonstrated in patients with advanced papillary carcinoma.
RET/PTCs represent the prototypical fusion oncogenes of papillary carcinoma. Gene rearrangements occur in up to ~30% of papillary carcinomas, and the majority of them involve RET , a transmembrane tyrosine kinase receptor. This oncogene is thus uniquely related to thyroid tumors, with activating RET point mutations in medullary carcinoma, and somatic RET rearrangements in papillary carcinoma. The latter, called RET/PTC (for p apillary t hyroid c arcinoma), are chimeric genes generated by the in-frame fusion of the entire RET tyrosine-kinase domain with other genes, that distinguish the different RET/PTC isoforms. The heterologous genes drive the expression of RET (not present at any significant level in normal follicular cells); by causing the chimeric proteins to dimerize, they also induce ligand independent, constitutive activation of the RET tyrosine kinase.
Sixteen different oncogenic RET rearrangements have been identified so far: RET/PTC fusion proteins RET/PTC1 to PTC9, RET/PCM1, RFP/RET, HOOK3/RET, ELKS/RET, DLG5-RET, AFAP1L2/RET, and PPFIBP2/RET (the latter two found in carcinomas of young patients from the Fukushima area). The most common are RET/PTC1 ( CCDC6/RET ), which accounts for approximately 60% of RET -rearranged tumors, RET/PTC3 (NCOA4/RET) representing approximately 30%, and RET/PTC2 (PRKAR1A/RET) representing approximately 5%; the other RET rearrangements have been described in a few papillary carcinomas that have characteristically arisen following radiation exposure. RET rearrangements (as well as rearrangements of other tyrosine kinase genes e.g., NTRK3 ) typically occur in tumors that develop in children and young patients and in those related to radiation exposure (therapeutic or environmental). In one large study RET/PTC was identified in 62.3% of 191 papillary carcinomas diagnosed after the 1986 Chernobyl nuclear disaster. In the study, RET/PTC3 was particularly common in tumors that developed after exposure to high radioactivity levels that were of short latency, more aggressive, and classified as solid variants of papillary carcinoma. RET -rearranged carcinomas have in general classic papillary histology, with typical alterations of nuclear morphology and psammoma bodies. The rearrangement is considered an early molecular alteration, and highly sensitive methods detect low-level RET/PTC recombination in a variable proportion of papillary with or without other mutational events (e.g., BRAF ) due to genetic heterogeneity. Clonal RET/PTC expression is specific, but low-level RET/PTC recombination has been shown in hyperplastic thyroid nodules and in Hashimoto thyroiditis. While very sensitive methods can detect RET/PTC in the peripheral blood of patients with papillary carcinoma, they must thus be avoided for routine molecular diagnosis. With appropriate methods, RET/PTC is now detected in ~5%–25% percent of papillary carcinomas, with a decreasing prevalence among non–radiation-associated tumors over the years. Several studies have failed to correlate RET/PTC with increased morbidity and mortality, although RET rearranged papillary carcinoma in young patients may present with lymph node metastases and aggressive clinicopathologic features. The rearrangement, in particular RET/PTC1 that largely predominates in papillary carcinomas not associated with radiation exposure, is often found in indolent tumors showing little propensity to progress to poorly differentiated and undifferentiated carcinomas.
In addition to RET, other tyrosine kinases are rearranged in papillary carcinoma. These include the NTRK and ALK kinases. The NTRK1 gene is rearranged with TPM3, TPR and TFG , NTRK3 with ETV6 , or less frequently with other genes. Some fusion partners of both NTRK1 and NTRK3 are still uncharacterized. NTRK rearrangements have been reported in up to approximately 10% of unselected papillary carcinomas. The general clinicopathologic features of papillary carcinomas with NTRK rearrangement are similar to those of RET/PTC -positive cases. As in the case of RET/PTC , NTRK rearrangements have a higher prevalence in children and young adults and in cases associated with radiation exposure. ETV6/NTRK3 has been identified in approximately 15% of radiation-induced papillary carcinomas and after RET/PTC is one of the most common forms of oncogenic alteration in radiation-associated tumors.
ALK is rearranged in approximately 1%–5% of papillary carcinomas. The most common ALK recombination is the STRN/ALK rearrangement, EML4/ALK , typically found in non–small cell lung carcinoma, has been identified in a few cases, including radiation-associated tumors from atomic bomb survivors. ALK -rearranged carcinomas have a predominantly follicular, infiltrative pattern, often with areas of solid growth, or have the histologic features of diffuse sclerosing papillary carcinoma. ALK rearrangement can be identified using the same fluorescent in situ hybridization probes and antibodies used to detect ALK rearrangement in non–small cell lung carcinoma. ALK -rearranged tumors may have extrathyroidal extension and lymph node metastases and may develop in papillary carcinomas undergoing dedifferentiation. However, one large series has failed to demonstrate a statistical association with aggressive clinicopathologic features.
Patients with ALK- rearranged tumors may respond to targeted therapies with ALK inhibitors (e.g., crizotinib).
The encapsulated follicular variant of papillary carcinoma, regardless of whether the tumor has capsular or vascular invasion, or is noninvasive and now reclassified as NIFTP (see “Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features,” later), has a RAS -like molecular signature with a profile of genetic alterations overlapping that of follicular adenomas and carcinomas. As in the case of follicular adenomas and carcinomas, the most common genetic alterations are point mutations in NRAS , followed by HRAS , with most mutations in codon 61. Up to approximately 30% of follicular variant papillary carcinomas carry PPARG rearrangements ( PAX8/PPARG or, much less frequently, CREB3L2/PPARG) . Most of the EIF1AX mutations identified by the TCGA study in a small subset of papillary carcinoma were of the follicular variant. Fusion of THADA (at 2p21) with the LOC389473 pseudo gene (at 7p15.3), 12 kb upstream of IGF2BP3 leading to overexpression of the full-length IGF2BP3 mRNA and protein, and to increased insulin-like growth factor receptor signaling, has been reported in approximately 5% of papillary carcinomas. All THADA -rearranged cases were classified follicular-variant papillary carcinomas or NIFTP.
TERT promoter mutations represent an important marker of poor prognosis in differentiated thyroid carcinoma. They are identified with a prevalence of approximately 5%–15% in most series of papillary carcinomas, and the association with aggressive behavior appears stronger in case of a coexisting BRAF p.V600E mutation.
MED12 -G44C substitutions, consistent with gain- or change-of-function and RBM10 mutations (truncating and missense mutations), have been identified in fatal forms of papillary carcinomas (and in poorly differentiated thyroid carcinoma).
MicroRNA expression is dysregulated in papillary carcinoma, with high levels of miRs-146b, -221, and -222. A large meta-analysis has found that expression levels of miRs-21, −34b, −130b, −135b, −146b, −151, −181b, −199b-5p, −221, −222, −451, −623, −1271, −2861, and let-7e show a significant association with at least one aggressive feature (large tumor size, extrathyroidal extension, multifocality, lymphovascular invasion, lymph node metastases, distant metastasis, advanced stage, BRAF p.V600E). Patients with DICER1 syndrome, a rare autosomal dominant cancer syndrome caused by inactivating germline mutations of DICER1 , encoding the DICER1 ribonuclease (RNase) necessary to generate mature miRNA species, develop pleuropulmonary blastoma, ovarian Sertoli-Leydig cell tumors, cystic nephroma of the kidney and multinodular goiter with adenomatous nodules. Patients with the syndrome have an increased risk of developing thyroid carcinoma, often follicular-variant papillary carcinoma. Reduced DICER1 expression has been associated with aggressive clinicopathologic features in non-familial papillary carcinomas.
The following morphologic variants of papillary carcinoma have been described.
This is defined as a papillary carcinoma measuring 1 cm or less in diameter ( Figs. 8.44 and 8.45 ). Most cases have a stellate configuration and correspond to the lesions formerly known as occult sclerosing carcinoma or nonencapsulated sclerosing tumor, whereas others show partial or near total encapsulation, with or without tumor outside the capsule. Sometimes the fibrous capsule is extremely thick and may be calcified. The microscopic features and the mutational profile are not different from those of their larger counterpart, and both BRAF p.V600E and RET/PTC have been identified.
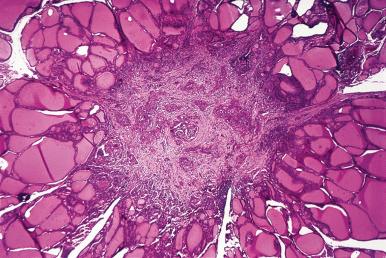
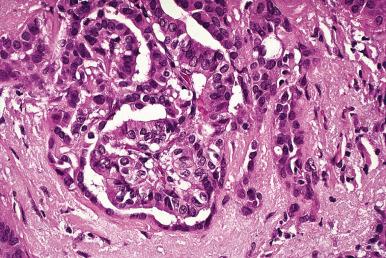
Papillary microcarcinoma is an extremely common incidental finding depending on the thoroughness of the examination, with figures as high as 35.6% in one autopsy series. A meta-analysis reviewing approximately 1000 microcarcinomas from 15 autopsy series from different parts of the world has found an overall prevalence of 11.5%, compatible with the 12%–13% rate for microcarcinomas diagnosed incidentally in thyroid glands surgically removed for goiter or other reasons. In contrast to its clinically evident counterpart, incidental papillary microcarcinoma seems to be relatively more common in males than females. Despite its small size, the tumor can be identified in FNA specimens, and the increased detection of small papillary carcinomas is largely responsible for the considerable increase of thyroid carcinoma in developed countries. It may be associated with cervical node metastases. However, distant metastases are exceptionally rare and the prognosis is generally excellent. Thus a proposal has been made to replace microcarcinoma with the term “ papillary microtumor ” when occurring in adults and in its typical form. Observational studies are showing that active surveillance is equally effective as immediate surgery to manage patients with low-risk microcarcinoma (no high-grade cytology, extrathyroidal extension, or metastases), while sparing the patients the side effects of surgery. However, a few microcarcinomas can exhibit malignant behavior, particularly if the tumors carry the BRAF p.V600E mutation. Rare, but well-documented fatal cases with evidence of tumor progression in cervical lymph node metastases have been reported.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here