Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
| 1,25-dihydroxyvitamin D | 1,25[OH] 2 D 3 |
| American College of Obstetricians and Gynecologists | ACOG |
| American Thyroid Association | ATA |
| Antithyroglobulin antibody | TgAb |
| Antithyroid drug | ATD |
| Carbimazole | CMZ |
| Endocrine Society | ES |
| Familial hypocalciuric hypercalcemia | FHH |
| Familial isolated primary hyperparathyroidism | FIHPT |
| Fine-needle aspiration biopsy | FNAB |
| Free thyroxine | FT 4 |
| Free thyroxine index | FT 4 I |
| Free triiodothyronine | FT 3 |
| Free triiodothyronine index | FT 3 I |
| Human chorionic gonadotropin | hCG |
| Hyperemesis gravidarum | HG |
| Immunoglobulin G | IgG |
| Intelligence quotient | IQ |
| Intrauterine growth restriction | IUGR |
| Levothyroxine | l -thyroxine |
| Methimazole | MMI |
| Neonatal severe primary hyperparathyroidism | NSPHPT |
| Parathyroid hormone | PTH |
| Parathyroid hormone–related protein | PTHrP |
| Postpartum thyroiditis | PPT |
| Pregnancy and lactation–associated osteoporosis | PLO |
| Primary hyperparathyroidism | PHPT |
| Propylthiouracil | PTU |
| Subclinical hypothyroidism | SCH |
| Thyroid-binding inhibitor | TBI |
| Thyroid-binding inhibitor immunoglobulin | TBII |
| Thyroid-receptor antibody | TRAb |
| Thyroid function test | TFT |
| Thyroid peroxidase | TPO |
| Thyroid peroxidase antibody | TPOAb |
| Thyroid-stimulating hormone | TSH |
| Thyroid-stimulating immunoglobulin | TSI |
| Thyrotropin-releasing hormone | TRH |
| Thyroxine | T 4 |
| Thyroxine-binding globulin | TBG |
| Total triiodothyronine | TT 3 |
| Total thyroxine | TT 4 |
| Triiodothyronine | T 3 |
| Thyroid-stimulating hormone receptor antibody | TRAb, TSHRAb |
Along with diabetes mellitus, thyroid diseases are the most frequent endocrine pathology seen in pregnancy. They are a risk for maternal, fetal, and neonatal pathology and may present a diagnostic and therapeutic challenge. The obstetrician should be aware of the symptoms and signs of the particular disease, the effect of pregnancy on the interpretation of endocrine tests, and the transfer of hormones and medications across the placenta with potential complications for the fetus and neonate. It is imperative that a team approach be used in the management of these conditions; multidisciplinary care including, ideally, the obstetrician, maternal-fetal medicine specialist, endocrinologist, neonatologist, pediatric endocrinologist, and anesthesiologist in advance of the delivery time will offer the patient the best maternal and perinatal outcomes.
Although uncommon in pregnancy, parathyroid diseases may produce significant perinatal and maternal morbidity and mortality if not diagnosed and properly managed.
Parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D (1,25[OH 2 ]D 3 ) are responsible for maintaining calcium homeostasis. Approximately 50% of serum calcium is protein bound, mostly to albumin; 10% is complexed to anions; and 40% circulates free as ionized calcium. During pregnancy, active transfer of maternal calcium to the fetus occurs. A full-term infant requires 25 to 30 g of calcium during the course of pregnancy for new bone mineralization, most of it occurring in the third trimester.
Total serum calcium during gestation is 8% below postpartum levels. Ionized calcium levels, however, remain unchanged throughout gestation. Serum phosphate and renal tubular reabsorption of phosphorus also remain normal throughout pregnancy. Maternal serum PTH levels are decreased in the first half of pregnancy (approximately 20% of the mean nonpregnant values) but return to normal by midgestation.
Blood levels of 1,25(OH)D (calcitriol), the active metabolite of vitamin D, increase early in gestation to twice the nonpregnancy level in the third trimester. This increase is caused by the stimulation of maternal renal 1α-hydroxylase activity by estrogen, placental lactogen, and PTH, as well as synthesis of calcitriol by the placenta. Also, 24-hour urinary calcium excretion increases with each trimester of gestation and decreases in the postpartum period, reflecting the increased intestinal calcium absorption induced by higher levels of 1,25-hydroxyvitamin D during gestation.
Parathyroid hormone–related protein (PTHrP), a peptide responsible for the hypercalcemia found in many malignant tumors, increases in early pregnancy. Maternal serum PTHrP is likely from both fetal and maternal sources (placenta, myometrium, amnion, decidua, fetal parathyroid glands, breast, umbilical cord). PTHrP increases 1α-hydroxylase activity with an increase in 1,25(OH)D; in addition, PTHrP plays a role in placental calcium transport and may also help protect the maternal skeleton during pregnancy.
Serum calcitonin levels are higher during pregnancy and in the postpartum period compared with nonpregnant controls.
Osteocalcin is a bone-specific protein released by osteoblasts into the circulation, proportional to the rate of new bone formation. Markers of bone resorption increase during pregnancy and values in the last trimester reach up to twice the normal level. These changes are consistent with the increase in bone turnover at the time of maximal transfer of maternal calcium to the fetus.
After delivery, urinary calcium excretion is reduced; ionized serum calcium remains within normal limits; and total calcium, 1,25-hydroxyvitamin D, and serum PTH return to prepregnancy levels. Intestinal absorption of calcium decreases to the nonpregnant rate as a result of the previously mentioned return to normal levels of 1,25(OH)D. Early concern for calcium loss in lactating mothers, with the development of osteopenia, has not been confirmed, and extra calcium supplementation during breastfeeding appears to be unnecessary because calcium supplementation above normal does not significantly reduce the amount of lost bone during gestation. The alteration in calcium and bone metabolism that accompanies human lactation represents a physiologic response that is independent of calcium intake, and the osteopenia appears to be reversible.
The prevalence of primary hyperparathyroidism (PHPT) is 0.5%. The incidence of the disease in pregnancy is not definitively known, but it is thought to be rare. With the introduction of routine, automated techniques in clinical medicine and with early diagnosis, most patients with PHPT are symptom free, and their serum calcium elevation is mild. In nonpregnant PHPT patients, indications for surgery include (1) serum calcium greater than 1 mg/dL (>0.25 mmol/L) above the upper limit of normal; (2) bone densitometry with a T score of −2.5 or greater at the lumbar spine, total hip, femoral neck, or distal third of the radius; (3) vertebral fracture; (4) creatinine clearance below 60 mL/min; or (5) clinical signs and symptoms of a renal stone or visualization of a significant renal stone on imaging. It is estimated that 10% of PHPT patients have a mutation in one of 11 genes. They may occur as part of complex disorders, such as multiple endocrine neoplasia (MEN) syndromes 1 through 4; as a familial disorder, familial isolated primary hyperparathyroidism (FIHPT); in the syndrome of familial hypocalciuric hypercalcemia (FHH); and in the syndrome of neonatal severe primary hyperparathyroidism (NSPHPT). In practice, genetic testing is indicated in cases of PHPT: (1) in the presence of multigland disease, (2) in the very young (before pregnancy), (3) with parathyroid carcinoma or atypical adenoma, and (4) with a family history of hypercalcemia in first-degree relatives. Patients should be referred to genetic counselors, and appropriate tests should be performed by accredited centers.
The first case of PHPT during pregnancy was reported in 1931. Shortly thereafter, the first case of neonatal hypocalcemia to cause tetany in a mother with undiagnosed hypercalcemia due to hyperparathyroidism was described by Friderichsen. The most common cause of PHPT in pregnancy is a single parathyroid adenoma, which is present in approximately 80% of all cases. Primary hyperplasia of the four parathyroid glands accounts for approximately 15% of the cases reported: 3% are due to multiple adenomas, and only a few cases due to parathyroid carcinoma have been reported in the English literature. In contrast to previously reported increased neonatal morbidity and mortality, Kelly found only two perinatal deaths (5%) among 37 infants born of hyperparathyroid mothers. Two additional cases of perinatal deaths were reported in mothers with hypercalcemic crisis.
In the nonpregnant state, almost 70% of patients are symptom free, and the diagnosis is made through the routine use of biochemical screening. In pregnancy, calcium determinations are not routinely performed, resulting in clinical manifestations of the disease being present in almost 70% of the diagnosed patients. Common symptoms may include gastrointestinal symptoms such as nausea, vomiting, and anorexia. Weakness and fatigue, headaches, lethargy, anxiety, emotional lability, and confusion may also occur. There is some overlap with normal pregnancy symptoms. More severe findings may include nephrolithiasis, bone disease, acute renal failure, acute pancreatitis and hypertension. A minority of patients may be completely symptom free.
Parathyroid cancer is a rare cause of hyperparathyroidism, with very few cases documented in pregnancy. Serum calcium levels are significantly higher than in other cases of PHPT, and perinatal mortality and morbidity are significant. Hypercalcemia with values above 13 mg/dL, in the presence of a palpable neck mass, should raise a strong suspicion of parathyroid carcinoma. On the contrary, in the presence of mild hypercalcemia and a neck mass, the most common cause of the neck lesion is a thyroid nodule. One other clinical feature of parathyroid carcinoma is poor response to the usual clinical therapeutic measures such as intensive hydration and loop diuretics. Surgery is the only effective therapy.
Hyperparathyroidism should be considered in the differential diagnosis of acute pancreatitis during pregnancy, which has been reported in 13% of pregnant women with PHPT. The incidence of acute pancreatitis in nonpregnant hyperparathyroid women is approximately 1.5% and is less than 1% in normal pregnancy. It is mostly likely to occur during the last trimester of pregnancy or the postpartum period. It has also been reported in the first trimester of pregnancy, mimicking hyperemesis gravidarum (HG). Serum calcium should be obtained in any pregnant woman with persistent, significant nausea, vomiting, and abdominal pain.
Hyperparathyroid crisis, a serious complication of PHPT, has been reported during gestation and the postpartum period and is characterized by severe nausea and vomiting, generalized weakness, changes in mental status, and severe dehydration. Hypertension may be present and should be differentiated from preeclampsia. The serum calcium level is frequently higher than 14 mg/dL; hypokalemia and elevation in serum creatinine are routinely seen. If not recognized and treated promptly, hyperparathyroid crisis may progress to uremia, coma, and death. It may occur at any time during pregnancy, including the postpartum period. Patients presented with severe nausea, vomiting, and elevation in serum creatinine caused by dehydration. Serum calcium levels higher than 20 mg/dL were reported in three cases, and three patients died. In addition, six cases have been associated with pancreatitis, and four fetal deaths have also been reported.
Bone disease in patients with PHPT is now unusual , but in early series, it was a common complication. Radiologic evaluation of the bones showed diffuse demineralization, subperiosteal resorption of the phalanges, and in severe cases, single or multiple cystic lesions and generalized osteoporosis.
Shani and associates reported five cases of excessive amniotic fluid in mothers with PHPT plus serum calcium levels between 11.3 and 14 mg/dL. The authors suggested that the fetal polyuria was similar to adult polyuria, a common finding in patients with hyperparathyroidism.
The two most common causes of neonatal morbidity are prematurity and neonatal hypocalcemia, and the latter is related to levels of maternal hypercalcemia. In early reports, it was often the only clue of maternal hyperparathyroidism. Neonatal hypocalcemia develops between the second and fourteenth day of life and lasts for a few days.
Preeclampsia has been reported in some cases of PHPT. Hultin and coworkers examined whether parathyroid adenoma, the main cause of hyperparathyroidism, which is diagnosed and treated before pregnancy is associated with preeclampsia. They reviewed the records of 52 women between 1973 and 1997 with the diagnosis of parathyroid adenoma confirmed by surgery and compared them with 519 women without the disease, all of whom had a subsequent singleton pregnancy. They concluded that PHPT caused by a single adenoma diagnosed and treated before delivery is significantly associated with subsequent preeclampsia (adjusted odds ratio [aOR], 6.89; 95% confidence interval [CI], 2.30 to 20.58; P < .0001). Therefore treated PHPT should be considered a risk factor for preeclampsia in future pregnancies.
The clinical manifestations of PHPT and pregnancy complications—maternal, fetal, and neonatal—are directly related to the serum calcium level. Dochez and Ducarme reviewed 34 published articles in English and French on the characteristics of PHPT, clinical presentations, pregnancy complications, birth outcomes, and management of PHPT during pregnancy. They emphasized the need to rule out FHH and hereditary syndromes such as multiple endocrine neoplasia syndrome (MEN-1 or MEN-2) and familial parathyroid hyperplasia. Urinary calcium excretion is low or low normal in the syndrome of FHH, another cause of hypercalcemia that must be included in the differential diagnosis. Nephrolithiasis was the most common finding in symptomatic patients during pregnancy; other maternal complications include depression, constipation, bone fracture, maternal heart rhythm disorders, pancreatitis, parathyroid crisis, and HG. Maternal hypertension and preeclampsia were observed in 25% of these patients.
The diagnosis of PHPT is based on persistent hypercalcemia in the presence of increased PTH or a PTH level inappropriate for the level of serum calcium. In pregnancy, because of the presence of hypoalbuminemia, a persistent total serum calcium value higher than 9.5 mg/dL is suspicious for hypercalcemia. A determination of 24-hour urinary calcium excretion is helpful in the diagnosis because most women with PHPT have an increase in urinary calcium excretion above the usual hypercalciuria of normal pregnancy. Ultrasonography of the neck is the current first-line evaluation strategy during pregnancy for localization of parathyroid diseases, with a sensitivity of 69% and a specificity of 94% in experienced hands. CT and MRI are alternative options. Parathyroid contrast imaging studies are contraindicated in pregnancy.
Although most young women with hypercalcemia have PHPT, other unusual causes should be ruled out, mainly endocrine disorders, vitamin D or A overdose, the use of thiazide diuretics, or granulomatous diseases ( Box 47.1 ). A brief discussion of three uncommon syndromes associated with hypercalcemia during pregnancy follows.
Hyperparathyroidism (most common)
Rare causes related to pregnancy
Familial hypocalciuric hypercalcemia a
a Different expression with significant neonatal manifestations.
Postpartum hypercalcemia in hypoparathyroidism
Parathyroid hormone–related protein induced hypercalcemia
Other causes not related to pregnancy
Malignancy
Endocrine
Thyrotoxicosis
Adrenal insufficiency
Vitamin overdose
Vitamin D
Vitamin A
Drugs
Thiazide diuretics
Lithium
Granulomatous disease
Sarcoidosis
Tuberculosis
Histoplasmosis
Coccidioidomycosis
Milk alkali syndrome
Acute and chronic renal failure
Total parenteral nutrition
FHH is an autosomal-dominant condition with a high penetrance for hypercalcemia. The disorder is associated with an inactivating mutation in the gene for the calcium-sensing receptor. Mild hypercalcemia, a slight elevation in serum PTH, mild hypermagnesemia, and low urinary calcium excretion are the typical findings. Infants born to mothers with FHH may present with different clinical manifestations: (1) asymptomatic hypercalcemia can develop in an affected offspring if the mother is a carrier for FHH, (2) severe neonatal hypocalcemia can return to normal a few weeks after delivery, or (3) severe neonatal hypercalcemia can occur in infants homozygous for the FHH gene defect.
Postpartum hypercalcemia can occur in women with treated hypoparathyroidism, and the mechanism for hypercalcemia is not well understood. Nausea and vomiting develop a few days after delivery; therefore patients with treated hypoparathyroidism should be followed postpartum with serum calcium determinations, and vitamin D should be discontinued if hypercalcemia occurs. In severe cases, intravenous fluids and glucocorticoid therapy are required ( Fig. 47.1 ).

Hypercalcemia mediated by PTHrP during pregnancy and postpartum is rare. In one case, hypercalcemia developed in two successive pregnancies. In the second pregnancy, serum PTHrP levels were elevated to three times normal, and the infant was born with mild hypercalcemia that returned to normal within 24 hours after delivery. In a second case, a 25-year-old woman had massive bilateral breast enlargement at 24 weeks’ gestation. Her serum calcium level was 14.3 mg/dL, but her serum PTH level was undetectable. She underwent bilateral mastectomy during pregnancy. The immunohistochemical studies demonstrated PTHrP antigenic activity in breast tissue.
Surgery is the only definitive treatment for PHPT, and the procedure is safe when performed by a surgeon with experience in neck surgery. The cure rate is excellent, and complications caused by surgery are low, particularly in the presence of a single lesion. Improvements in the outcome of surgery and avoidance of intraoperative and postoperative complications include: (1) preoperative parathyroid adenoma localization by ultrasonography, (2) minimally invasive parathyroidectomy techniques, (3) intraoperative PHPT monitoring to confirm successful surgery, and (4) detection and management of postoperative hypocalcemia.
Although guidelines for the management of PHPT in nonpregnant individuals have been suggested, the proper medical management of PHPT in pregnancy has not been uniformly agreed. For asymptomatic pregnant women in whom serum calcium is not greater than 1 mg above normal range, close follow-up with proper hydration and avoidance of medications that could elevate calcium, such as thiazide diuretics, is reasonable. Because most of the neonatal complications have been reported in patients with symptomatic disease, a surgical approach is indicated in such patients and in those with complications such as nephrolithiasis, bone disease, and persistent hypercalcemia (>1 mg above the normal range). It is preferable to perform the surgery in the second trimester of pregnancy when possible.
In a series reported by Carella and Gossain, 38 women underwent parathyroidectomy during pregnancy, 7 during the first trimester and 18 in the second trimester. In the total group of 25, there was only 1 fetal loss. In 12 women in whom surgery was performed during the third trimester of pregnancy, the incidence of perinatal complications was 58%. For women with PHP first diagnosed after 28 weeks’ gestation, the optimal treatment strategy is unclear, and the decision in such a situation should be based on the general condition of the patient, severity of hypercalcemia, and other complicating circumstances. A significantly lower incidence of complications, both maternal and fetal, was reported in a review of 16 published cases of patients operated on after 27 weeks’ gestation.
Medical therapy is reserved for patients with significant hypercalcemia who are not surgical candidates. Oral phosphate therapy of 1.5 to 2.5 g/day has been shown to be effective in controlling hypercalcemia. Good hydration, early treatment of urinary tract infections, and avoidance of supplements and medications known to cause elevations in serum calcium—such as vitamin D, vitamin A, aminophylline, and thiazide diuretics—are all important therapeutic measures. Serum calcium should be determined on a regular basis. Cinacalcet hydrochloride, a novel oral agent that acts directly on the calcium sensor, has been used in isolated cases because it reduces serum calcium in combination with calcitonin.
In patients undergoing surgical treatment, hypocalcemia—albeit transient—may occur after surgery in some cases. Therefore serum calcium should be checked every 6 hours, and if the patient develops hypocalcemic symptoms, intravenous (IV) calcium in the form of calcium gluconate (1 to 2 g of calcium-gluconate, equivalent to 90 to 180 mg elemental calcium, in 50 mL of 5% dextrose) can be infused over 10 to 20 minutes. Oral calcitriol 0.5 mg every 8 to 12 hours and oral calcium should be started when oral feeding is tolerated. In patients with bone disease, postsurgical hypocalcemia may be profound; therefore aggressive treatment is needed. These patients may benefit from vitamin D supplementation in the form of calcitriol, 0.25 to 0.5 µg/day, for a few days before operative intervention.
The most common etiology of hypoparathyroidism is damage to or removal of the parathyroid glands in the course of surgery for thyroid gland pathology. The incidence of permanent hypoparathyroidism after thyroid surgery has been estimated to be between 0.2% and 3.5%. In many cases, hypocalcemia in the immediate postoperative period is only transitory. Idiopathic hypoparathyroidism is much less common and is frequently associated with other autoimmune endocrinopathies as part of the polyglandular autoimmune syndrome type 1.
The requirement for calcium supplementation and vitamin D may decrease in some women with hypoparathyroidism during the second half of pregnancy and lactation. In a few cases, hypocalcemic symptoms ameliorate with progression of pregnancy. The explanation for these findings is not clear but may be related to the increased intestinal absorption of calcium and/or the production of vitamin D by the placenta.
Clinical clues for the diagnosis of hypoparathyroidism include a previous history of thyroid or parathyroid surgery and clinical, radiologic, and laboratory information. Typical symptoms of hypocalcemia are numbness and tingling of the fingers and toes and around the lips. Patients may complain of carpopedal spasm, laryngeal stridor, and dyspnea. Seizures may be a manifestation of severe hypocalcemia. On physical examination, patients with idiopathic hypoparathyroidism demonstrate changes in the teeth, skin, nails, and hair as well as papilledema and cataracts. Chvostek sign, a twitch of the facial muscles—notably those of the upper lip—when a sharp tap is given over the facial nerve, is seen in many patients with hypocalcemia. Chvostek sign has also been described in 10% of normal adults. Trousseau sign is another manifestation of hypocalcemia. It is the induction of spasm of the hand and forearm by reducing the circulation in the arm with a blood pressure cuff. The constriction should be maintained above the systolic blood pressure for 2 minutes before the test is considered negative.
The diagnosis of hypoparathyroidism is confirmed by the presence of persistent low serum calcium and high serum phosphate levels. Serum PTH is low in primary hypoparathyroidism. The differential diagnosis of hypocalcemia includes rickets and osteomalacia.
Radiologic bone changes characterized by generalized skeletal demineralization may be present in the newborn as a consequence of transient intrauterine hyperparathyroidism, as well as subperiosteal bone resorption, bowing of the long bones, osteitis fibrosa cystica, and rib and limb deformities.
Treatment of hypoparathyroidism in pregnancy does not differ from that in the nonpregnant state, including a high-calcium diet and vitamin D supplementation. Normal calcium supplementation during pregnancy is approximately 1.2 g/day. Calcitriol, 1 to 3 µg/day, is used almost routinely in most patients affected with hypoparathyroidism. Calcitriol must be given in divided doses because its half-life is much shorter than that of vitamin D. If vitamin D is used, the dose is in the range of 50,000 to 150,000 IU/wk. Vitamin D requirements may decrease in some patients by the second half of gestation. The importance of compliance with medications should be strongly emphasized, particularly when calcitriol is prescribed, in view of its short half-life. The major problem in the treatment of hypoparathyroidism is the recurrence of both hypercalcemia and hypocalcemia; therefore serum calcium determinations should be performed at regular intervals. Care should be taken to continue monitoring maternal levels during the postpartum period and during lactation.
Pseudohypoparathyroidism encompasses several different disorders, having varying degrees of target-organ resistance to PTH as a common feature. In some forms of the syndrome, somatic changes are present that include short stature, obesity, a round face, brachydactyly, and mental retardation with brain calcifications. This variant is known as Albright syndrome type 1a. Most patients suffer from hypocalcemia due to a derangement of renal 1α-hydroxylase and production of calcitriol, and a few cases have been reported during pregnancy. Infants are at risk for intrauterine fetal hyperparathyroidism, perhaps because of the relative maternal hypocalcemia during pregnancy.
Classically, vitamin D deficiency was related to the development of rickets, with its subsequent impact on obstetric care. Deformities of weight-bearing bones, including the pelvis, prevented vaginal delivery in mothers affected by the disease. When first discovered in the early 20th century as a substance that could be ingested from food, it was designated as a vitamin. In reality, vitamin D is a hormone synthesized from cholesterol that affects hundreds of genes other than those involved in calcium metabolism. Specifically, vitamin D plays a major role as a regulator of immunity and the modulation of inflammation. There are potential associations between vitamin D deficiency and a variety of medical problems including autoimmune disease, inflammatory airway disease, and cancer. Obstetrically, vitamin D deficiency has also been linked with preterm birth, low birthweight, preeclampsia, and gestational diabetes. Vitamin D appears to function as part of a complex biochemical apparatus, which regulates possible maternal responses to immune or inflammatory stimuli.
The physiologic measure of vitamin D status is the serum 25-hydroxyvitamin D , the concentration of which captures the effect of multiple vitamin D input sources (food, sun, supplement) and makes provision for interindividual variability in dose response. The normal reference range values for serum vitamin D (25[OH 2 ]D 3 ), the active metabolite of vitamin D, are controversial, with values varying between 20 and 40 ng/mL, equivalent to 50 to 100 nmol/L.
Many studies in the past few years have linked low maternal 25(OH)D levels with maternal, fetal, and neonatal complications . In a report from Amsterdam, mothers with singleton pregnancies and serum 25(OH)D levels below 29.9 nmol/L (12 ng/mL) had infants with a lower birthweight and a higher incidence of growth restriction than mothers with values of more than 50 nmol/L (20 ng/mL). Hart and colleagues examined the relationship between maternal vitamin D deficiency at 18 weeks’ gestation and long-term health outcomes in 901 mother-offspring pairs in Perth, Western Australia. The incidence of serum 25(OH)D deficiency (<50 nmol/L [<20 ng/mL]) was present in 36% of the pregnant women. After adjusting for relevant covariates, women with vitamin D deficiency had children with impaired lung development at age 6, neurocognitive deficiencies at age 10, increased risk of eating disorders in adolescence, and lower peak bone mass at age 20.
A Cochrane review of data from three randomized controlled trials (RCTs) involving 477 pregnant women found that vitamin D supplementation reduced the risk of preterm birth by 36% (RR 0.36, 95% CI 0.14 to 0.93; moderate quality) and the risk of low birthweight (LBW) (<2500 g) was reduced by 40% (RR 0.44, 95% CI 0.24 to 0.67). Another study demonstrated that higher achieved 25(OH)D levels during pregnancy in a prospective Spanish cohort was associated with significantly improved mental and psychomotor development in infants.
Recent systemic reviews and meta-analyses of observational studies and RCTs have failed to corroborate earlier observations. Theodoratou and colleagues concluded that despite a few hundred systematic reviews and meta-analyses, highly convincing evidence for a clear role of vitamin D does not exist for any outcome, but associations with a selection of outcomes are probable. There may be several reasons for these discrepant findings. Common limitations include the failure to use doses of vitamin D sufficient to significantly change maternal vitamin D status. Clinical trials have demonstrated that a vitamin D supplement dose of 4000 IU/day was most effective in achieving a 25(OH)D concentration of at least 32 ng/mL by the early second trimester in a diverse cohort of women, and that the traditionally recommended supplement of 400 IU/day was ineffective in achieving an adequate circulating 25(OH)D level.
Given the differences in the many published studies to date and the legitimate methodologic concerns over the appropriate study design for nutrient supplementation trials, the practicing obstetrical care provider will be faced with making some nuanced patient care decisions. We anticipate the future completion of an increasing number of vitamin D trials involving both pregnancy and the preconceptional period, with a variety of different outcomes. Hopefully, these will help clarify the proper management course.
In the meantime, the practicing obstetrician will have to decide for each individual patient whether determination of 25(OH)D levels is indicated. If a patient is vitamin D deficient (<30 ng/mL, and especially if <20 ng/mL), then supplementation should be undertaken and dosages between 2000 and 4000 IU/day are typically required. If supplementation is undertaken, then follow-up testing is recommended because of significant interindividual differences in dose response and issues of compliance. Treatment goals are a 25(OH)D level of, ideally, >40 ng/mL but at least >30 ng/mL. In terms of safety, the Cochrane review found no adverse effects or toxicity from vitamin D supplementation in any of the reported studies.
In 2010, the Institute of Medicine reviewed nearly 1000 published studies that included reports of protection from cancer, autoimmune disease, heart disease, and diabetes from vitamin D supplementation. For women who are not pregnant, the authors recommended a daily vitamin D dietary allowance of 600 IU with an upper limit intake of 4000 IU; for calcium, they recommended a 1000 mg dietary allowance and an upper limit intake of 2500 mg a day. In the presence of vitamin D insufficiency/deficiency, it is reasonable to normalize serum levels as early as possible, preferably starting early in pregnancy.
Detection of women at risk for vitamin D deficiency by proper history seems reasonable until the results of an RCT become available. Vitamin D deficiency is common during pregnancy, especially among high-risk groups that include vegetarians, women with limited sun exposure (e.g., those who live in cold climates, reside in northern latitudes, or wear sun and winter protective clothing), and ethnic minorities, especially those with darker skin. For pregnant women thought to be at increased risk of vitamin D deficiency, maternal serum 25(OH)D levels can be considered and should be interpreted in the context of the individual clinical circumstance.
The American College of Obstetricians and Gynecologists (ACOG) has stated, “At this time there is insufficient evidence to support a recommendation for screening all pregnant women for vitamin D deficiency. For pregnant women thought to be at increased risk of vitamin D deficiency, maternal serum 25(OH)D levels can be considered and should be interpreted in the context of the individual clinical circumstance. When vitamin D deficiency is identified during pregnancy, most experts agree that 1000 to 2000 IU/day of vitamin D is safe.”
The condition of idiopathic osteoporosis related to pregnancy, pregnancy and lactation–associated osteoporosis (PLO), was recognized in the 1950s. In the past few years, interest has increased in several clinical aspects of osteoporosis in pregnancy and lactation. Although the prevalence is unknown, approximately 120 cases have been reported. Another form of rare pregnancy-associated osteoporosis is called transient osteoporosis of pregnancy. It usually presents in the third trimester of pregnancy—sometimes with very severe pain while walking or standing, usually localized in the hip—and it sometimes leads to hip fracture, with complete recovery a few months postpartum. Preexisting secondary causes of osteoporosis such as vitamin D deficiency, celiac disease, anorexia nervosa, mastocytosis, and hyperparathyroidism or hyperthyroidism should always be considered. Severe PLO may prompt screening for an underlying monogenetic bone disorder when associated with one of the following three features: (1) severely reduced bone density; (2) family history of osteoporosis or multiple fractures, joint hypermobility, blue sclerae, congenital blindness, or severely reduced vision; or (3) a history of fractures before pregnancy.
Although osteoporosis has been diagnosed during pregnancy, pregnancy unmasks low bone mass; it does not cause it . Postural changes during pregnancy, including increased lordosis, when superimposed on a small and transient decrease in bone mass, may lead to pain and instability. In a study of 24 women with symptoms of bone pain for many years, 18 complained of back pain, 5 complained of hip pain, and 1 complained of ankle pain in late pregnancy and up to 8 months after delivery; radiologic examination of the spine showed vertebral deformities in 17, bone mass was measured in 21, evidence of osteoporosis was found in 7, and 13 were osteopenic. The authors concluded that bone mass was probably low before pregnancy and that a transient and slight decrease in bone mass during pregnancy could have weakened the bone further.
A recent study identified 78 cases with exposure to bisphosphonates before conception or during pregnancy. Although it did not demonstrate serious adverse effects, cases of increased spontaneous abortions, shortened gestational age, low neonatal birthweight, and transient hypocalcemia of the newborn were reported. Etidronate appears to be the agent to use in women with severe disease who are planning pregnancy, although the drug should be stopped a few months before conception.
The impact of lactation on the progression of osteoporosis is controversial . It has been suggested that lactation by itself is not a determinant of bone mineral density. Although one investigation reported that lactation for more than 8 months was associated with greater bone mineral density at both the femoral neck and shaft, another study found that nursing for longer than 9 months produced a greater decrease in bone mass than observed during the first 6- to 9-month period of nursing. Given this controversy, the healthcare provider must decide whether cessation of lactation is advisable in the management of osteoporosis. Bolzetta and colleagues studied 752 women (mean age, 64.5 ± 9.3 years) of whom 23% reported vertebral osteoporotic fractures. The women with vertebral fractures had breastfed their infants for longer periods (11.8 ± 12.9 vs. 9.3 ± 11.2 months, P = .03) and had more pregnancies (2.6 ± 2.2 vs. 2.2 ± 1.3, P = .002). Breastfeeding for more than 18 months was associated with a twofold risk of developing vertebral fractures (OR, 2.12; 95% CI, 1.14 to 5.38; P = .04), particularly in those without current or past use of drugs that positively affect bone. The authors concluded that an association exists between long periods of breastfeeding and vertebral fractures, which supports a role for lengthy lactation as a risk factor for osteoporotic fractures after menopause.
Heparin-associated osteoporosis has been reported during pregnancy, which may be related to the total dose of heparin. The authors concluded that heparin adversely affected bone density in approximately one-third of exposed patients.
Thyroid disorders in pregnancy present an opportunity for health care professionals to use a similar team approach that has successfully improved the care of women with diabetes mellitus and other medical conditions. Because of changes in thyroid physiology that occur early in pregnancy, it is imperative to advise women with chronic thyroid diseases to plan their pregnancies and contact their health care professionals before or as soon as the diagnosis of pregnancy is confirmed. Autoimmune thyroid disease occurs five to eight times more often in women than in men, and its course could be affected by the immunologic changes that occur in pregnancy and in the postpartum period. A physical examination of the neck, thyroid, and adjacent structures is a standard and important element of any physical examination of a pregnant patient ( Fig. 47.2 ).

In early pregnancy, the maternal thyroid gland is challenged with an increased demand for thyroid hormone secretion, caused mainly by (1) the increase in thyroxine-binding globulin (TBG) secondary to the effect of estrogens on the liver; (2) the stimulatory effect of human chorionic gonadotropin (hCG) on the thyroid-stimulating hormone (TSH) receptor; (3) high concentrations of type 3 iodothyronine deiodinase (D3), which degrades thyroxine and triidothyronine to inactive compounds; and (4) the supply of iodine available to the thyroid gland. In the United States, the iodine content in the diet—although decreased in past decades—appears to be insufficient in only about 10% of pregnancies. The suggested total daily iodine ingestion for pregnant women is 229 µg /day, and for lactating women it is 289 µg /day; prenatal vitamins should contain 150 µg of iodine in the form of potassium iodine.
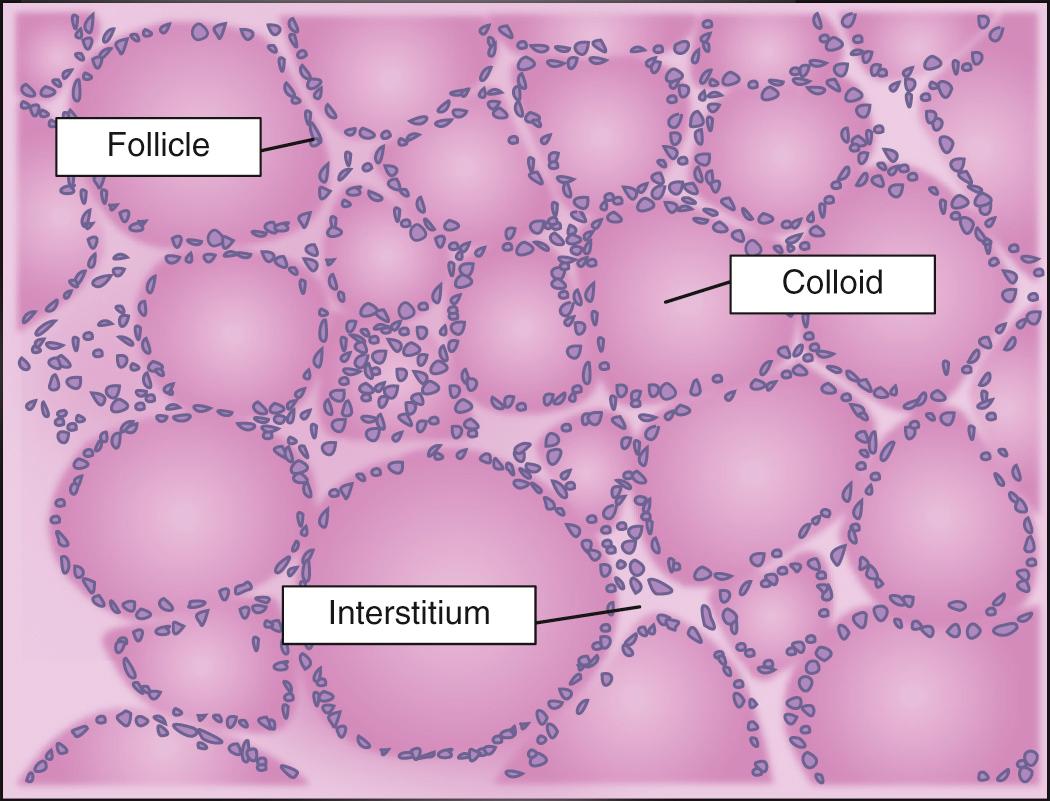
The normal thyroid gland is able to compensate for the increase in thyroid hormone demands by increasing its secretion of thyroid hormones stored as colloid ( Fig. 47.3 ) and maintaining them within normal limits throughout gestation. However, in those situations in which there is a subtle pathologic abnormality of the thyroid gland, such as chronic autoimmune thyroiditis, or in women on thyroid hormone replacement therapy, the normal increase in the production of thyroid hormones is not met. As a consequence, the pregnant woman could develop biochemical markers of hypothyroidism such as elevation in serum TSH.
Active secretion of thyroid hormones by the fetal thyroid gland commences at approximately 18 weeks’ gestation, although iodine uptake by the fetal gland occurs between 10 and 14 weeks. Transfer of thyroxine (T 4 ) from mother to embryo occurs from early pregnancy. Maternal T 4 has been demonstrated in coelomic fluid at 6 weeks and in the fetal brain at 9 weeks. Maternal transfer continues until delivery but only in significant amounts in the presence of fetal hypothyroidism. Thyroid hormone receptor gene expression has been shown in human fetal brain by 8 weeks’ gestation, which supports the important role of maternal thyroid hormone during the first trimester of human pregnancy in fetal brain development.
The levels of maternal thyroid hormone concentrations, both total thyroxine (TT 4 ) and total triiodothyronine (TT 3 ), increase from early pregnancy, caused by an elevation in TBG and a reduced peripheral TBG degradation rate. TBG reaches a plateau by 20 weeks’ gestation and remains unchanged until delivery. Despite these acute changes in total hormone concentration, the serum free fractions of both T 4 and T 3 remain within normal limits, unless supply of iodine to the mother is decreased or abnormalities of the thyroid gland are present.
hCG is a weak thyroid stimulator that acts on the maternal thyroid gland TSH receptor; peak hCG values are reached by 9 to 12 weeks’ gestation. A transient suppression of TSH in the first trimester is common and can be exacerbated by situations in which there is a high production of hCG, such as in cases of multiple pregnancies or hydatidiform mole. In these conditions, serum free T 4 (FT 4 ) concentrations may rise to levels seen in thyrotoxicosis.
Goiter is commonly seen in pregnancy in areas of iodine deficiency. However, in the United States and other areas of the world with sufficient iodine intake, the thyroid gland does not clinically increase in size during pregnancy. Therefore the detection of a goiter in pregnancy is an abnormal finding that needs careful evaluation. The most common cause of diffuse goiter is chronic autoimmune thyroiditis or Hashimoto thyroiditis. Most patients are euthyroid, and the diagnosis is made by the determination of thyroid function tests and antibodies, mainly thyroid peroxidase (TPO). Antibody concentration decreases during pregnancy and increases in the postpartum period. High values in the first trimester of pregnancy are also predictors of the syndrome of postpartum thyroid dysfunction.
Measurement of serum TSH is the most practical, simple, and economic screening test for thyroid dysfunction. Normal TSH concentrations, as well as serum FT 4 and TT4, are trimester specific and depend on iodine intake in a given population, ethnicity, and assay performance. Serum TSH is lower in the first trimester compared with prepregnancy values and then increases across the second and third trimester of pregnancy. Elevated serum TSH is consistent with the diagnosis of primary hypothyroidism in combination with suppressed free thyroxine index (FTI) , whereas a low one, with few exceptions, is a normal finding in the first trimester ( Fig. 47.4 ) secondary to the stimulatory effect of hCG on the thyroid gland TSH receptor. Significant clinical data support a serum TSH below 2.5 mIU/L in the first trimester, and less than 3.0 mIU/L in the second and third trimesters, as the upper limit of normal ; however, studies from China, the United Kingdom, and India, which have used a specific reference range for their populations, reported TSH reference ranges in the first trimester up to 4.5 mIU/L. Guidelines from the American Thyroid Association (ATA) and the Endocrine Society (ES) recommend the use of trimester-specific reference ranges for serum TSH and FT 4 in a given population, and when not available, they recommend a serum TSH upper limit of normal of 2.5 mIU/L in the first trimester and up to 3.0 mIU/L in the second and third trimesters, as noted above. Because many pregnant women present for their first obstetric visit after 6 weeks’ gestation, the upper serum TSH limit of 2.5 mIU/L is reasonable for detection of subclinical or overt hypothyroidism, depending on the value of FTI.

A word of caution is required regarding the determination of FT 4 levels in the different trimesters of pregnancy. A significant inconsistency is found in FT 4 values in the second half of pregnancy among the different immunoassays as reported by commercial laboratories, because of both the methodology used and the variation in dietary iodine intake among the different populations studied. FT 4 values in the lower limits of normal, and even in the hypothyroid range, are not uncommonly seen in daily clinical practice, particularly in the third trimester of pregnancy. Lee and colleagues compared the diagnostic performances of two different immunoassays to traditional approaches for estimating FT 4 (total T 4 and FT 4 index [FT 4 I]) relative to the physiologic TSH changes known to occur throughout pregnancy. They studied euthyroid women who were negative for TPO antibody in the first, second, and third trimesters of gestation. Control women were premenopausal nonpregnant women matched for ethnicity. Serum TT 4 , as expected, was elevated in all three trimesters, and serum FT 4 I was elevated in the first trimester compared with controls ( P < .05), returning to the nonpregnant range in the second and third trimesters. In contrast, FT 4 values were either comparable or lower than controls as measured by two different immunoassays, and by the second and third trimesters, they were aapproximately 65% of controls. The authors concluded that TT 4 and FT 4 I retained an appropriate inverse relationship with serum TSH throughout pregnancy and appeared to provide a more reliable FT 4 estimate than the FT 4 test . Because the determination of FT 4 by the dialysis method—the gold standard for FT 4 assessment—or the use of tandem mass spectrometry are not routinely available, the determination of TT 4 adjusted by a factor of 1.5 for pregnant patients has been suggested. Therefore it is imperative for the practicing physician to be familiar with the interpretation and significance of the thyroid tests as reported by a given commercial laboratory.
A suppressed serum TSH value and high concentrations of FT 4 or FT 4 I is diagnostic of hyperthyroidism. However, in other situations, subclinical hyperthyroidism may be diagnosed in the presence of suppressed TSH and normal concentrations of FT 4 , such as in the rare case of an autonomous thyroid nodule. In such cases, a serum TT 3 or free triiodothyronine index (FT 3 I) determination should be obtained.
Graves disease is caused by direct stimulation of the thyroid epithelial cells by TSH receptor-stimulating antibodies (TSIs, or TRAbs). Highly sensitive and specific assays for detection of TRAbs are now commercially available and are very valuable in assessing fetal and neonatal risk in pregnancy, not only in women with active disease but also in those with a previous history of Graves hyperthyroidism, both in spontaneous remission and after ablation therapy. TRAbs are also used in the differential diagnosis of hyperthyroidism, when the etiology is not clinically evident. Two methods are used for measuring TRAbs, namely competition-based assays (thyroid-binding inhibitor [TBI]–thyroid-binding inhibitor immunoglobulin [TBII] assays) or bioassays that detect cyclic adenosine monophosphate production (TSI assays). The specificity of TBI-TBII methods is lower because positive tests may be obtained in patients with chronic autoimmune (Hashimoto) thyroiditis who may have TRAbs with TSH receptor (TSHR)-blocking activity. Like all immunoglobulin G (IgG) molecules, TRAb crosses the placenta during pregnancy, and in significant titers, more than three times the reference range, it can stimulate the fetal thyroid gland and produce hyperthyroidism (TRAbs with stimulating function) or, rarely, hypothyroidism (TRAbs with blocking activity). Recently, predictable levels for the development of fetal/neonatal hyperthyroidism using second-generation TBI-TBII methods have been reported. This topic is discussed in the section on fetal and neonatal hyperthyroidism in this chapter.
The physician may be faced with different clinical situations when counseling a woman suffering from thyroid disease who is contemplating pregnancy:
Hyperthyroidism diagnosed de novo or under antithyroid drug (ATD) treatment. A choice of the three classic therapeutic options for prepregnancy treatment of hyperthyroidism should be given: (1) long-term ATD therapy, (2) radioactive iodine-131 ( 131 I) ablation, or (3) near-total thyroidectomy. Potential side effects of ATDs on the fetus should be discussed with the future parents. 131 I ablation therapy should be avoided in patients with positive TRAb titers because serum levels increase following 131 I therapy, and the effect persists for several years. Fetuses whose mothers have titers above three times the normal level in the second half of pregnancy are at risk of fetal and neonatal hyperthyroidism. Thyroidectomy may be selected by some physicians and patients concerned about the potential side effects of ATDs or radioactive treatment. Regardless of the form of therapy chosen, it is important for the patient to be euthyroid at the time of conception.
Previous treatment with 131 I. It is reasonable for patients treated with ablative doses of 131 I to wait 6 months to 1 year after completion of treatment before conception. Data obtained from 2673 pregnancies in patients treated for thyroid carcinoma but without significant external radiation to the ovaries were analyzed by Garsi and colleagues, who found no evidence that exposure to radioiodine affects the outcome of subsequent pregnancies and offspring.
Treated hypothyroidism. Women under treatment with thyroid hormone usually require an increase in levothyroxine ( l -thyroxine) dose soon after conception. The increase in requirements is observed as early as the first 6 to 8 weeks after the last menstrual period. As soon as the diagnosis of pregnancy is made, thyroid function tests should be performed, and thyroid doses should be adjusted accordingly. It has been recommended to add two extra doses of l -thyroxine per week to the customary doses as soon as the diagnosis of pregnancy is confirmed until the results of thyroid function tests become available. Recently it was reported that in hypothyroid women (excluding those who underwent thyroidectomy because of thyroid cancer) on l -thyroxine replacement therapy, if the serum TSH was below 1.3 mIU/L before conception, only 17% of women needed an increase in l -thyroxine in the first trimester of pregnancy compared with 58% of those with a serum TSH above 1.3 mIU/L. After delivery, the dose should be reduced to prepregnancy levels in most women. Common medications may affect the absorption of l -thyroxine, such as ferrous sulfate and calcium, among others. Patients should take l -thyroxine at least 2 hours apart from other medications and 1 hour before or after food intake.
Euthyroid chronic thyroiditis. Patients with Hashimoto thyroiditis are at a greater risk for developing hypothyroidism de novo early in pregnancy, spontaneous abortions, prematurity, and postpartum thyroiditis (PPT).
Studies over the past few decades have shown an important role of maternal thyroid hormones in embryogenesis. T 3 receptors are found in most tissues and work directly to influence transcription ( Fig. 47.5 ). Maternal T 4 crosses the placenta in the first half of pregnancy at a time when the fetal thyroid gland is not functional, and maternal TSH does not cross the placenta. Thyrotropin-releasing hormone (TRH) does cross the placental barrier, but its physiologic significance is unknown.

Methimazole (MMI), propylthiouracil (PTU), and carbimazole (CMZ)—a drug that is metabolized to MMI—do cross the placenta, and if given in high doses may produce fetal hypothyroidism and goiter. There remains a concern about their association with congenital anomalies with first-trimester exposure. Preparations that contain iodine given in large doses or for prolonged periods are contraindicated in pregnancy because accumulation by the fetal thyroid may induce goiter and hypothyroidism.
As mentioned above, TRAb crosses the placenta, and serum concentrations decrease with progression of pregnancy. However, its titers increase significantly in Graves hyperthyroidism after therapy with 131 I.
Hyperthyroidism is relatively uncommon during pregnancy, affecting 0.1% to 0.4% of all pregnancies. The two most common etiologies of hyperthyroidism encountered during pregnancy are Graves disease and gestational transient thyrotoxicosis (GTT). Graves disease accounts for approximately 95% of cases of overt hyperthyroidism during pregnancy, while GTT is diagnosed in an estimated 1% to 3% of pregnancies and tends to occur in the latter half of the first trimester. The majority of pregnant patients with Graves disease will enter pregnancy with a known history of thyroid disease. New diagnoses during pregnancy are not common. The remaining etiologies of hyperthyroidism in pregnancy are all relatively uncommon ( Box 47.2 ). The symptoms of hyperthyroidism are similar between the nonpregnant and pregnant patients and include palpitations, insomnia, weight loss, heat intolerance, goiter, nervousness, tremors, tachycardia, loose stools, and sweating.
Graves disease
Chronic thyroiditis
Sporadic silent thyroiditis
Multinodular goiter
Toxic adenoma
Subacute thyroiditis
Multiple gestations
Nausea and vomiting
Hyperemesis gravidarum
Trophoblastic tumor
Hydatidiform mole
Choriocarcinoma
Excessive levothyroxine intake
Overtreatment
Factitious
Iodine induced
Also known as gestational thyrotoxicosis, transient hyperthyroidism of HG, and transient nonautoimmune hyperthyroidism of early pregnancy, this condition is defined as transient hyperthyroidism in the first trimester of pregnancy due, with few exceptions, to high titers of hCG secretion that stimulate the TSH receptor. The most common causes of gestational hyperthyroidism are HG, multiple gestation, and hydatidiform mole. Other isolated reports included hyperplacentosis.
HG is characterized by severe nausea and vomiting with onset between 4 and 8 weeks’ gestation that requires frequent visits to the emergency department and sometimes repeated hospitalizations for IV hydration. Weight loss of at least 5 kg, ketonuria, abnormal liver function tests, and hypokalemia are common findings, depending on the severity of vomiting and dehydration. FT 4 and TT 4 levels are elevated, sometimes up to four to six times the normal values, whereas TT 3 and free triiodothyronine (FT 3 ) values are elevated in up to 40% of affected women, although FT 3 values are not as high as those of serum FT 4 . The TT 3 /TT 4 ratio is less than 20, compared with Graves hyperthyroidism, in which the ratio is more than 20. Serum TSH measured by a sensitive assay is consistently undetected or suppressed. Despite the significant biochemical hyperthyroidism, signs and symptoms of hypermetabolism are mild or absent. Patients may complain of mild palpitations and heat intolerance, but perspiration, proximal muscle weakness, and frequent bowel movements are rare. On physical examination, ophthalmopathy and goiter are absent, a mild tremor of the outstretched fingers is occasionally seen, and tachycardia may be present due in part to dehydration. Significant in the medical history is the lack of hyperthyroid symptoms before conception because most patients with Graves disease diagnosed for the first time during gestation give a history of hypermetabolic symptoms that antedate conception. Spontaneous normalization of hyperthyroxinemia parallels the improvement in vomiting and weight gain, and most cases resolve spontaneously between 14 and 20 weeks’ gestation, although persistence of hyperthyroidism beyond 20 weeks’ gestation has been reported in 15% to 25% of cases. Suppressed serum TSH may lag for a few more weeks after normalization of free thyroid hormone levels ( Fig. 47.6 ). Antithyroid medications are not needed. In one series in which antithyroid medication was used, pregnancy outcome was not significantly different from that of a similar group of patients who received no therapy. Routine monitoring of thyroid function tests is not required because the symptoms tend to be transient. Severe or persistent vomiting and hyperthyroidism may require parenteral nutrition.

The degree of thyroid abnormalities is directly related to the severity of vomiting and weight loss. In 67 patients studied by Goodwin and colleagues, liver and electrolyte abnormalities were routinely found in women with worse symptoms, including severe vomiting, weight loss of at least 5 kg, and significant dehydration. They also presented with more significant elevations in FT 4 levels and suppression of serum TSH values; indeed, in 30% of their patients, serum TSH was undetectable (<0.04 mIU/L). Those with a lesser degree of hyperemesis had a less severe disorder of thyroid function.
Transient hyperthyroidism due to HG should be suspected in women who present in the first few weeks after conception with sudden onset of severe nausea and vomiting and thyroid tests in the hyperthyroid range. These patients do not complain of hypermetabolic symptoms that antedate pregnancy, goiter is not detected by palpation, and symptoms or signs of tissue thyrotoxicosis are mild or absent. In addition, screens for thyroid anti-TPO antibodies and TRAbs, markers of autoimmune thyroid disease, are negative. The differential diagnosis may also be difficult because vomiting can also be a presenting symptom of the hyperthyroidism of Graves disease ( Table 47.1 ).
| Graves Hyperthyroidism | Gestational Thyrotoxicosis | |
|---|---|---|
| Symptoms before pregnancy | ++ | − |
| Symptoms during pregnancy | +/++ | −/+ |
| Nausea, vomiting | −/+ | +++ |
| Goiter, ophthalmopathy | + | − |
| Thyroid receptor antibodies | + | − |
| Thyroid ultrasound | Vascularity | Normal |
The cause of the elevations of thyroid hormones in patients with HG is the endocrine effect of hCG. Most likely, high levels of hCG—a known stimulator of the TSH receptor—play an important role, as does the prolongation in its biologic activity seen in twin pregnancies. A significant, albeit weak, correlation has been found between the degree of thyroid stimulation and total hCG levels in normal women and in those with hyperemesis. Titers of more than 200,000 mIU/mL of hCG consistently bring serum TSH to low or undetectable values.
As noted previously, the diagnosis of transient hyperthyroidism of HG should be considered in women with severe vomiting, no clinical manifestations of Graves disease, biochemical evidence of hyperthyroidism in early pregnancy, suppressed or undetectable serum TSH values, and elevated serum FT 4 . Normal serum TSH in early pregnancy may be as low as 0.01 mIU/L; therefore the presence of elevated serum FT 4 is required for the diagnosis. The treatment of transient hyperthyroidism of HG is primarily supportive and aimed at the medical management of nausea, vomiting, and dehydration as well as correction of the electrolyte or acid-base abnormalities associated with HG. Symptoms of hyperemesis resolve in 60% by the end of the first trimester and in 91% by 20 weeks of gestation.
HG may also occur in women with Graves hyperthyroidism. However, while symptoms of heat intolerance, anxiety, tremor, and palpitations are similar between both GTT and Graves disease, the lack of symptoms prior to pregnancy or having symptoms of nausea and vomiting favor the diagnosis of GTT, whereas having had symptoms prior to pregnancy and/or the presence of TRAbs favors Graves disease.
Obstetric outcome is not affected by gestational hyperthyroidism. Birthweight may be slightly lower, but not significantly different, compared with fetuses of control mothers and is related to maternal weight loss.
Gestational trophoblastic diseases, partial and complete hydatidiform moles, and choriocarcinoma are other causes of hyperthyroidism early in pregnancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here