Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The name “thyroid” is derived from the Greek word for shield due to the gland's shape and relationship with the laryngeal thyroid cartilage. The human thyroid gland has a dual embryonic origin. The two thyroid cell types, follicular cells (thyrocytes) and parafollicular (C-cells), are derived from all three germ cell layers. The most abundant cells, the follicular cells, arise from the thyroid anlage. The development of the thyroid gland begins when a median epithelial proliferation appears in the floor of the primitive pharynx between the developing tuberculum impar and copula of the tongue anlage, which is first visible as a bud around 24 days gestation. This thickening, known as the median or thyroid anlage, soon forms a ventral outgrowth known as the thyroid diverticulum. The diverticulum is initially hollow but later becomes solid. The progenitor follicular cells continue to proliferate distally and then begin to proliferate laterally, which leads to the characteristic bilobed appearance of the gland connected by an isthmus. While the thyroid cells continue to proliferate, the embryo elongates and the tongue grows. As this happens, the developing thyroid gland descends anterior to the hyoid bone and larynx forming the thyroglossal duct. Because of the close association of the developing thyroid gland and embryonic heart, it is thought that the descent of the heart results in the thyroid gland being pulled. The thyroid gland remains connected to the tongue by the thyroglossal duct. At approximately 7 weeks gestation, the thyroid gland has reached its final site in front of the trachea and the thyroglossal duct has disappeared. The original opening of the thyroglossal duct persists as a vestigial pit called the foramen cecum . 15% to 75% of people have a pyramidal lobe, which is derived from the lower part of the thyroglossal duct and extends upwards from the isthmus. At about the time the thyroid gland reaches its final position, it merges with the two lateral anlagen or ultimobranchial bodies, resulting in the incorporation of the C-cells (parafollicular cells) into the thyroid gland. The ultimobranchial bodies are a pair of transient embryonic structures derived from the endoderm of the fourth pharyngeal pouch and the ectoderm of the fifth pharyngeal pouch. The C-cell precursors migrate from the neural crest bilaterally to the fourth pharyngeal pouches and become localized in the ultimobranchial bodies. As the cells of the thyroid anlage and the ultimobranchial bodies migrate from their respective sites of origin and complete the merging process, they disappear as individual structures and the cells contained within them disperse in the thyroid gland. The follicular cells continue to organize the thyroid follicles, where the C-cells scatter within the interfollicular space. Remnants of the ultimobranchial bodies, or solid cell nests, can be seen postnatally and are usually located in the middle third of the thyroid lateral lobes.
The thyroid gland's primary function is to produce hormones that play a vital role in regulating many cellular and physiologic activities such as growth, development, and metabolism.
The thyroid gland synthesizes and secretes two hormones, thyroxine (T 4 ) and triiodothyronine (T 3 ). The synthesis and secretion of these hormones is closely regulated through a complex feedback mechanism known as the hypothalamic-pituitary-thyroid-axis.
The hypothalamus synthesizes and secretes thyrotropin-releasing hormone (TRH), which is carried to the pituitary gland by the hypothalamic-pituitary portal venous system. Once in the pituitary gland, TRH stimulates the synthesis and secretion of thyrotropin (thyroid-stimulating hormone, TSH) by the thyrotrophs in the anterior pituitary gland. TSH binds to its receptor in the thyroid gland, stimulating the production and secretion of T 4 and T 3 within the thyroid follicular cells. Thyroid secretion and serum concentrations of T 4 and T 3 are maintained by a negative feedback loop involving inhibition of TSH and TRH secretion by T 4 and T 3 .
Iodide is actively transported into the follicular cells from the circulating plasma by the sodium-iodide symporter (NIS) at the basolateral membrane. Thyroid peroxidase (TPO) oxidizes iodide into its chemically active form. Thyroglobulin in the follicular lumen serves as a matrix for the synthesis of T 4 and T 3 . First, TPO catalyzes the iodination of selected tyrosyl residues in thyroglobulin in a process known as iodination and organification. This results in the formation of mono- and diiodotyrosines (MIT, DIT). TPO then catalyzes a coupling reaction in which two iodotyrosines are coupled to form T 4 or T 3 . Iodinated thyroglobulin is stored as colloid in the follicular lumen. When needed, thyroglobulin is internalized into the follicular cell by micro- and macropinocytosis and digested in lysosomes. Subsequently, T 4 (80%) and T 3 (20%) are released into the bloodstream. MIT and DIT are deiodinated, and released iodide is recycled for hormone synthesis.
C-cells produce thyrocalcitonin, which is important in calcium homeostatis.
The thyroid gland is composed of a right and left lobe usually joined by an isthmus anteriorly. The thyroid gland extends superiorly to the level of the thyroid cartilage and inferiorly to the level of the fifth or sixth tracheal ring. Occasionally, there is an extra lobe called the pyramidal lobe. This lobe extends superiorly from the isthmus at the midline.
The thyroid gland is located in the visceral space of the infrahyoid neck, anterior and lateral to the trachea and posterior to the infrahyoid strap muscles. Anterolaterally to the thyroid gland are the sternocleidomastoid muscles. The carotid space, containing the carotid arteries and jugular veins, is located posterolaterally. Posteromedially to the thyroid gland are the tracheoesophageal grooves containing the recurrent laryngeal nerves, paratracheal lymph nodes, and parathyroid glands.
The middle layer of the deep cervical fascia surrounds the visceral space and ensheaths the thyroid gland. The fascia condenses to form the suspensory ligament of Berry, affixing the thyroid gland to the trachea and larynx, causing the thyroid gland to move with the larynx during deglutition. A thin fibrous capsule also covers the thyroid gland. From this true capsule, septae extend into the gland dividing the gland into lobes and lobules. The lobules are each made up of multiple follicles. The follicles consist of an outer layer of follicular cells, which enclose a lumen that contains thyroglobulin-rich colloid. Each thyroid follicle is surrounded by a basement membrane. C-cells are found within the basement membrane and are in contact with the follicular cells, but they do not abut the lumen. Follicular cells secrete thyroid hormones and C-cells secrete thyrocalcitionin.
The thyroid gland is highly vascular being supplied by paired superior thyroidal arteries (first anterior branches of the external carotid arteries) and inferior thyroidal arteries (branches of the thyrocervical trunks that originate from the subclavian arteries). The thyroidea ima is an inconstant single vessel that has a variable origin but usually arises directly from the aortic arch or innominate artery and helps supply the inferior thyroid gland. Venous drainage of the thyroid gland is via the superior and middle thyroid veins that drain to the internal jugular veins and the inferior thyroid veins that often join to form a single trunk draining to the left brachiocephalic vein. Lymphatic drainage is extensive and multidirectional. The thyroid gland is innervated by the vagus nerve and the cervical sympathetic neural plexus.
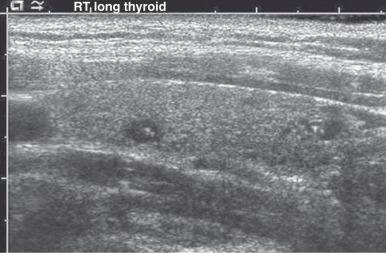
Ultrasonography (US) is a usually one of the first choices of imaging in pediatrics because it is noninvasive, readily available, and does not utilize radiation. A normal thyroid gland will have homogenous echotexture that is slightly hyperechoic relative to adjacent neck muscles. Colloid follicles are commonly seen as small (less than 3 mm in diameter) anechoic cystic areas. Occasionally, the follicles contain inspisated colloid, which appear as punctate echogenic foci ( Fig. 17.1 ).
Morphologic and functional information about the thyroid gland can be achieved with nuclear scintigraphy. Thyroid scintigraphy is performed using intravenous Tc-99m pertechnetate ( 99m TcO4) or oral Na I-123 (I 123 ) ( Table 17.1 ). Because of the large radiation dose to the thyroid gland (approximately 1–3 rads per uCi administered), I 131 is not used for routine diagnostic imaging. The normal thyroid gland shows homogenous radiopharmaceutical uptake and distribution in both lobes. The isthmus of the thyroid gland often demonstrates slightly less activity than the right and left thyroid lobes. Normal I 123 24-hour uptake ranges from 10% to 30%.
| Sodium Iodide (Capsule or Liquid) (1-23I) | Tc99m-Pertechnatate (99mTcO4) | Perchlorate Discharge or Washout | |
|---|---|---|---|
| Advantages | Better visualization of retrosternal thyroid tissue Yields better images when uptake is low |
Less expensive More readily available More rapid examination Can be performed while the patient is on thionamides |
Can determine organification defects |
| Disadvantages | Higher cost Longer imaging times Patient must be off thionamides |
Trapped, but not organified Activity in esophagus or vascular structures may be misleading Poor image quality when uptake is low |
Cannot detect enzymatic defects beyond the point of organification |
| Dose | 1.5 µCi/kg Minimum dose = 25 µCi Maximum dose = 100 µCi |
0.1 mg/kg Minimum dose = 0.5 mCi Maximum dose = 1.0 mCi |
|
| Route of administration | Oral | Intravenous | Oral |
| Time until imaging | 4–6 hours 24 hours (for uptake determination) |
20–30 minutes |
|
| Radiation dosimetry (5 year old) Administered activity Critical organ Effective dose equivalent |
Assuming 25% uptake 0.1–0.3 MBq 0.003–0.01 mCi/kg Thyroid 16 mGy/MBq 59 rad/mCi 0.54 mSv/MBq 2.0 rem/mCi |
1.8–9.2 MBq/kg 0.05–0.25 mCi/kg Upper large intestine 0.21 mGy/MBq 0.78 rad/mCi 0.04 mSv/MBq 0.15 rem/mCi |
Same as I-123 |
| Notes | Approximate normal uptake values (may vary greatly) 4 hours = 10%–35% 24 hours = 6%–18% |
|
|
Computed tomography (CT) and magnetic resonance imaging (MRI) provide important adjunctive anatomic information by delineation of lesions within the thyroid gland, detection of lymph node metastases, and detection of thyroid disease extension into adjacent neck structures. The anatomic information provided by CT and MRI is also valuable in guiding the surgical approach. The normal thyroid gland (due to its iodide content) has a density of approximately 80 to 100 HU on CT. In fact, a well-visualized gland usually indicates a normally functioning thyroid. Conversely, a poorly seen gland correlates with poor thyroid function. The injection of iodinated contrast material diffusely and homogenously enhances the gland. Remember that the use of iodinated contrast agents will alter radioactive iodine uptake, whereas gadolinium contrast material used for MRI will not. The normal thyroid gland shows homogenous signal intensity slightly greater than muscle on precontrast T1-weighted images. On T2-weighted images, the thyroid gland is relatively hyperintense to muscle. Following contrast administration, the gland enhances diffusely and homogenously.
Hypothyroidism is the most common disturbance of thyroid function in children. It can be congenital ( Box 17.1 ) or acquired in childhood or adolescence ( Box 17.2 ). Whatever its cause, hypothyroidism can have deleterious effects. Untreated congenital hypothyroidism in early infancy results in profound retardation of growth and neurocognitive development (cretinism). Untreated hypothyroidism in older children leads to growth failure as well as slowed metabolism and impaired memory.
Thyroid dysgenesis (80%)
Ectopia (75%)
Aplasia or athyrosis (25%)
Hypoplasia (rare)
Dyshormonogenesis (15%–20%)
Peroxidase deficiency (most common)
Other deficiencies (less common)
Iodide transport defect
Iodotyrosine deiodinase defect
Pituitary and hypothalamic defects (uncommon)
Thyroid-stimulating hormone resistance
Transient congenital hypothyroidism
Functional immaturity-common in premature infants
Transplacental transfer of maternal medication
Transplacental transfer of maternal thyroid-blocking antibodies
Maternal antithyroid medications
Iodine deficiency
Iodine excess
Chronic autoimmune (Hashimoto) thyroiditis (most common cause in the United States, increased incidence in some chromosomal abnormalities and syndromes)
Iodine deficiency (most common cause worldwide)
Iodine excess
External radiation therapy
Radioactive iodine therapy
Thyroidectomy
Goitrogen foods
Medications (e.g., thionamides, lithium, anticonvulsants)
Late-onset congenital hypothyroidism
Thyroid dysgenesis
Inborn errors of thyroid metabolism
Central hypothyroidism caused by:
Craniopharyngioma and other tumors pressing on hypothalamus or pituitary
Septo-optic dysplasia
Infiltrative processes
Langerhans cell histiocytosis
Neurosurgery
Cranial irradiation
Head trauma
Thyroid hormone resistance
Infection (usually not permanent)
Hemangiomas of the liver
Hypothyroidism in the newborn can be permanent or transient. Congenital hypothyroidism is a condition in which lower than normal thyroxin (T 4 ) causes retardation of growth and neurocognitive development if left untreated. The incidence of congenital hypothyroidism in the United States has dramatically increased over the last 2 decades, from 2.9 cases per 10,000 births in 1991 to nearly 4 cases per 10,000 births in 2000.
The majority of cases of primary congenital hypothyroidism are caused by thyroid gland dysgenesis (see Table 17.2 ). Thyroid dysgenesis refers to a developmental defect of thyroid morphogenesis. There are three types: ectopia, aplasia (athyrosis), and hypoplasia.
| Name | Synonyms | Mechanism of Hyperthyroidism/Thyrotoxicosis | Laboratory Tests | RAIU Appearance on Scintigraphy | Imaging Appearance of Thyroid Gland |
|---|---|---|---|---|---|
| H yperthyroidism , T hyroid G land H yperfunction (I ncreased S ynthesis of T hyroid H ormone ) | |||||
| Graves disease | Hyperthyroid goiter von Basedow disease |
TRS-Ab | Low TSH Elevated free T 4 Elevated T 3 High thyroglobulin TSI positive TRS-Ab positive TPO-Ab positive or negative TBII positive |
Elevated, often >80% Diffuse increased uptake |
Ultrasonography: Enlarged gland; hypoechoic but may be normal; may be nodular; increased vascularity, “thyroid inferno” CT and MRI: Nonspecific findings; enlarged gland; avid, diffuse enhancement Decreased attenuation on noncontrast CT reflecting decrease in iodine concentration |
| Multinodular goiter | Toxic multinodular goiter Adenomatous goiter Nodular hyperplasia Adenomatous hyperplasia |
Autonomous overproduction of thyroid hormones by nodules | Low TSH Elevated free T 4 Elevated T 3 High thyroglobulin All thyroid antibodies negative |
Normal or elevated Heterogeneous uptake; multiple foci increased uptake |
Ultrasonography: Enlarged gland; multiple heterogeneous nodules; cystic changes |
| Autonomous nodule | Plummer disease Toxic nodule |
Uncommon in children; autonomous overproduction of thyroid hormones by solitary nodule; usually produce T 3 | Low TSH Normal or elevated free T 4 Elevated T 3 High thyroglobulin All thyroid antibodies negative |
Elevated Single hot focus; rest of gland suppressed |
|
| TSH-producing pituitary adenoma | Autonomous overproduction of TSH | Normal TSH Elevated free T 4 High serum TSH alpha subunit concentration All thyroid antibodies negative |
High | Pituitary adenoma on MRI | |
| Pituitary resistance to thyroid hormone | Autosomal-dominant; thyroid beta-receptor gene mutation ; overproduction of TSH | Normal or slightly elevated TSH Elevated free T 4 Elevated T 3 (less than T 4 ) All thyroid antibodies negative |
High Diffuse uptake |
||
| T hyrotoxicosis -E xcess S ecretion of P reformed T hyroid H ormones | |||||
| Thyrotoxic phase of chronic lymphocytic (Hashimoto) thyroiditis | Hashitoxicosis Lymphadenoid goiter |
Autoimmune; release of preformed hormones | Low TSH Elevated free T 4 Elevated T 3 High thyroglobulin TPO-Ab positive; Thyroglobulin positive; TRS-Ab positive or negative |
Elevated Diffuse increased uptake |
Ultrasonography: Findings are nonspecific; enlarged gland; hypoechoic, heterogeneous echotexture |
| Subacute lymphocytic thyroiditis | Painless sporadic thyroiditis Silent thyroiditis |
Autoimmune; release of preformed hormones May be associated with drugs (interferon-alpha, interleukin-2, lithium) |
Low TSH Elevated free T 4 Elevated T 3 High thyroglobulin TPO-Ab positive |
Very low Diffuse decreased uptake |
Ultrasonography: Findings are nonspecific; enlarged gland; hypoechoic, heterogeneous echotexture |
| Thyrotoxic phase of subacute granulomatous thyroiditis | Subacute thyroiditis Painful subacute thyroiditis de Quervain thyroiditis Granulomatous giant cell thyroiditis |
Viral; release of preformed hormone | Low TSH Elevated free T 4 Elevated T 3 High thyroglobulin TPO-Ab negative; elevated ESR |
Low Diffuse decreased uptake |
Ultrasonography: Findings are nonspecific; enlarged gland; hypoechoic, heterogeneous echotexture |
| Factitious thyroiditis | Thyrotoxicosis factitia | Intentional ingestion of too much thyroxine | Low TSH Elevated free T 4 Elevated T 3 Low thyroglobulin |
Low | |
| Iodine-induced hyperthyroidism | Underlying multinodular goiter; thyroid hormone release triggered by exposure to iodine (contrast agents), amiodarone | Low TSH Elevated free T 4 Elevated T 3 |
Increased | ||
Dyshormonogenesis is the second largest cause of primary congenital hypothyroidism which is often inherited as an autosomal recessive trait. Dyshormonogenesis is an abnormality of one or more of the enzymes involved in the pathway of thyroid hormone synthesis and secretion. Most inborn errors of thyroid hormone synthesis are caused by defects in iodide organification with the most common defect being TPO deficiency. This results in a failure of iodide oxidation which is necessary for organification. A small percentage of infants with congenital hypothyroidism will have hypothalamic-pituitary hypothyroidism or thyroid-stimulating hormone resistance, whereas the remainder will have a transient form of congenital hypothyroidism.
Less than 5% of congenital hypothyroidism patients are diagnosed clinically at birth; most are identified by newborn screening. Newborn screening involves measuring TSH and T 4 concentrations. T 4 concentrations are decreased and TSH concentrations are elevated in patients with congenital hypothyroidism, except in central hypothyroidism where the TSH is not elevated. The laboratory abnormalities tend to be more marked in cases of thyroid aplasia than in thyroid ectopia. Patients with transient hypothyroidism will also have elevated TSH concentrations and low or normal T 4 concentrations that subsequently normalize.
Imaging is not routinely used to diagnose congenital hypothyroidism. The most recent recommendations discuss thyroid imaging in congenital hypothyroidism as optional because of controversy regarding the risk-benefit ratio of early thyroid scanning of infants with suspected hypothyroidism. There are also clinicians who do not order imaging studies because they believe the results would not alter their management of congenital hypothyroidism.
Diagnostic studies for a patient with congenital hypothyroidism can include US and thyroid scintigraphy with 123 1 or 99m TcO4. Use of both US and thyroid scintigraphy has been shown to provide a more complete depiction of congenital hypothyroidism in the newborn than either study performed alone.
The role of US is to identify the presence or absence of thyroid tissue, distinguish rudimentary glands from anatomically normal thyroid glands, and identify an enlarged gland or goiter.
99m TcO4 or 123 I can be used to help determine if the cause of hypothyroidism may be due to thyroid dysgenesis (see Table 17.1 ). In patients with thyroid agenesis, the examination fails to demonstrate functional thyroid tissue. It is important that the images include the oropharynx and upper neck as well as the upper portion of the chest so that an ectopic location of the thyroid gland can be excluded.
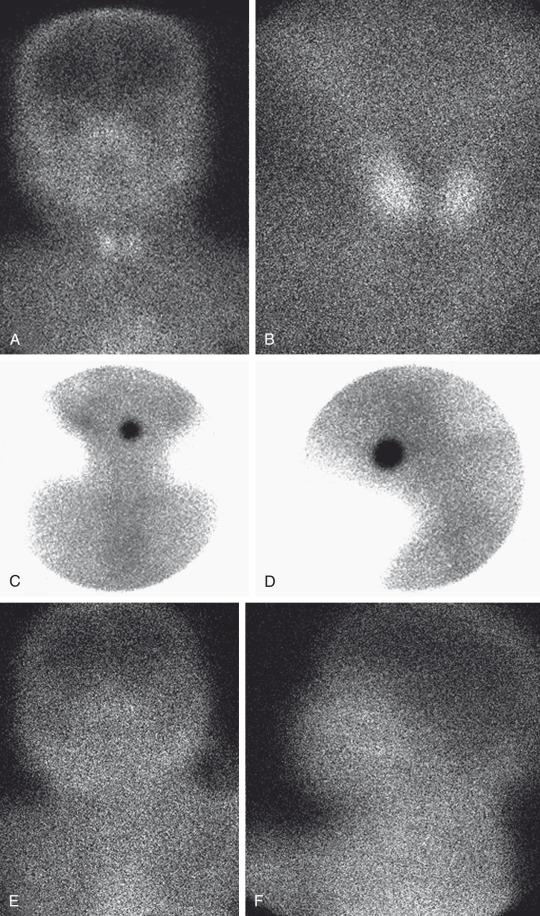
99m TcO4 scintigraphy demonstrates a round or oval area of uptake in the midline of the upper neck in most cases of ectopia ( Fig. 17.2 ). The ectopic gland may occupy a lingual (most common), sublingual, or prelaryngeal location. Mediastinal and lateral locations are rare. Functional thyroid tissue may be identified in more than one location, most commonly in the lingual and sublingual regions. It is unusual to identify thyroid tissue in its normal location at the base of the neck in the presence of an ectopic gland. Usually patients with an ectopic thyroid gland will have hypothyroidism. Unusual cases do occur in which the ectopic gland is capable of secreting sufficient thyroid hormone such that hypothyroidism is not apparent on neonatal screening. These patients often present with signs of ectopia later in life when the hyperstimulated gland enlarges and causes local symptoms.
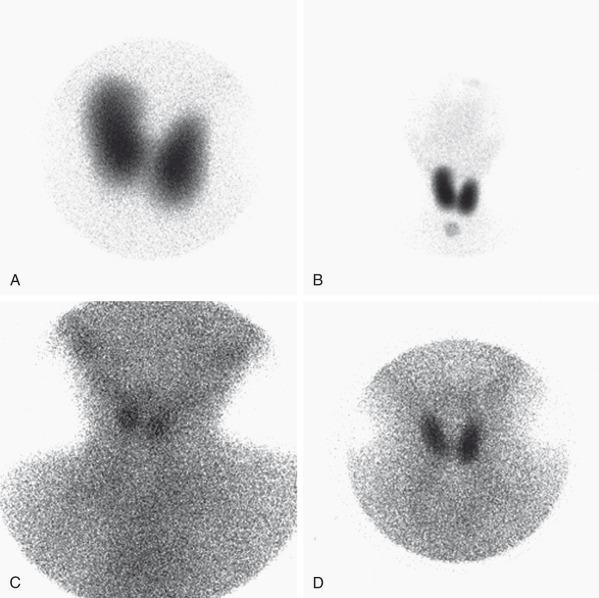
99m TcO4 scintigraphy in cases of dyshormonogenesis will demonstrate a normally positioned thyroid gland that may or may not be enlarged ( e-Fig. 17.3 ). If dyshormonogenesis is suspected, a perchlorate washout test may be performed. Perchlorate is actively transported into the thyroid gland similar to iodide but with a greater affinity for the transporter and is therefore a competitive inhibitor of the thyroid iodide trap. During unimpaired thyroid hormonogenesis, iodide entering the thyroid gland is rapidly oxidized and iodinates tyrosine, forming MIT and DIT, with subsequent coupling of MIT and DIT to generate T 4 and T 3 . Intrathyroid deiodination of the iodinated tyrosines and thyronines results in a very small pool of thyroidal inorganic iodide. Any congenital or acquired condition associated with a defect in iodide organification may yield a higher intrathyroidal inorganic iodide concentration. The perchlorate discharge/washout test is a means of estimating the size of this intrathyroidal “free” iodide pool, thereby detecting and roughly quantifying disturbances in iodide organification. The perchlorate discharge/washout test is performed by giving the patient an oral dose of 123 I followed by a dose of perchlorate and measuring the “washout” (see Table 17.1 ). The perchlorate test will be negative in patients who do not have an organification defect, but also when enzymatic defects are present in the synthetic pathway beyond the point of organification.
Levothyroxine (T 4 ) is the treatment of choice for children with hypothyroidism. The goals of the treatment are to restore normal growth and development.
There are many causes of acquired hypothyroidism in the pediatric population (see Table 17.2 ) Chronic autoimmune (Hashimoto) thyroiditis is by far the most common cause of acquired primary hypothyroidism in children and adolescents in iodine sufficient areas. It is more common in girls than boys and increases in frequency with age during childhood and adolescence. Euthyroid goiter is more common than hypothyroidism.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here