Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thrombolytic therapy is an important modality in the treatment of patients with peripheral arterial and venous thrombosis. Randomized clinical trials have compared thrombolytic therapy with traditional surgical options, thus providing guidelines for patient selection. As a therapeutic intervention, lytic therapy may be the best alternative in certain clinical situations. In many other cases, it is just one aspect of the overall care of patients with thrombotic complications of peripheral vascular disease.
This chapter provides an overview of the fibrinolytic system and the available agents. This information can be translated into guidelines to help clinicians select patients who may benefit from thrombolytic therapy. Methods, dosages, complications, and promising new areas are also discussed.
The fluidity of blood post mortem is an observation that dates to the Hippocratic school in the 4th century BC. Almost 2000 years later, it was rediscovered by the Italian anatomist Malpighi. In 1761 Morgagni noted that blood does not retain its liquid state after death but frequently forms clots. This is followed by partial or complete reliquefaction.
In 1906 Morawitz observed that postmortem blood destroys fibrinogen and fibrin in normal blood. Thus the presence of an active fibrinolysin was postulated. The term fibrinolysis had been coined by Dastre in 1893 to describe the disappearance of fibrin in unclottable blood obtained from dogs subjected to repeated hemorrhage. From the latter part of the 19th century until the present, intense investigation has been undertaken to elucidate the complex and vital functions of the fibrinolytic system. Charles Dotter was the first to describe intraarterial drug delivery into thrombus for thrombolysis. The role of the fibrinolytic system from a homeostatic point of view is fully appreciated. The interplay of components, activators, and inhibitors are becoming more appreciated. The potential to harness the fibrinolytic system for therapeutic means has emerged in the past few decades, and results from prospective clinical trials are now available, providing guidelines to patient selection and therapy. For the vascular specialist, thrombolytic therapy represents one of the most promising therapeutic modalities for arterial and venous thrombosis, but careful dosing and monitoring of thrombolytic therapy are required. In addition, careful attention and observation of the patient, renal dysfunction, systemic effects, and often the limb undergoing treatment are required.
The complex and intricate relationships among all components of the fibrinolytic system are only partially understood. However, much progress has been made, mostly owing to recognition of the importance of the fibrinolytic system as a homeostatic system. This same process has been harnessed for a therapeutic effect in cases of clinical thrombosis. The concept of dynamic equilibrium was proposed by Astrup in 1958. In a delicate balance, fibrinolysis breaks down fibrin, which is continuously being deposited throughout the cardiovascular system. This is the result of limited activation of the coagulation system. This baseline fibrinolytic activity is probably under local and central control mechanisms. The feedback loop that prevents systemic fibrinolysis involves both inhibitors at the activator level and specific inhibitors of the proteolytic enzyme plasmin.
The final common pathway in the fibrinolytic system is the conversion of the proenzyme plasminogen to the active enzyme plasmin. Plasminogen is a glycoprotein produced by the liver. Full-sized plasminogen can be divided into a heavy N-terminal region that consists of five homologous but distinct triple-disulfide–bonded domains (kringles) fused to a lighter catalytic C-terminal domain. At least four forms occur in plasma, based on variations in the N-terminal and the degree of glycosylation. The two main forms are Glu-plasminogen and Lys-plasminogen. Glu-plasminogen contains glutamic acid and exists in high concentrations in plasma. Lys-plasminogen, containing mostly lysin in the N-terminal, results from limited proteolysis of the Glu form; it has a shorter half-life and is found in higher concentrations in thrombus, most likely secondary to its higher affinity for fibrin. A schematic view of the fibrinolytic system is presented in Fig. 35.1 .
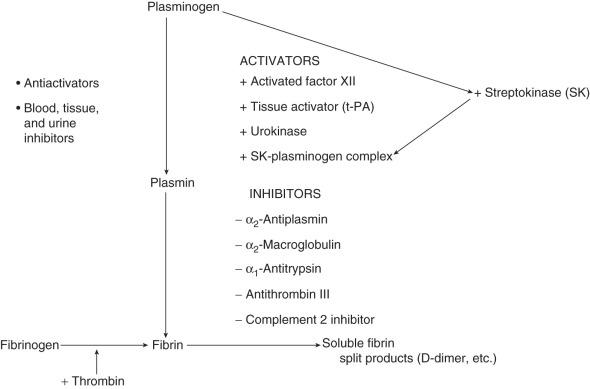
The kringle portion of plasminogen is a nonprotease, or heavy chain, consisting of five homologous domains. These domains exhibit a high degree of sequence homology with one another and with domains found in prothrombin, tissue plasminogen activator (t-PA), urinary plasminogen activator, and factor XII. Kringle-4 shares homology with apolipoprotein A. The function of these kringles is thought to be of paramount importance in the binding of plasminogen and plasmin to fibrin, α 2 -antiplasmin, and other macromolecules. In addition, the kringle portion of plasminogen has been implicated in mediating neutrophil adherence to endothelial cells. On binding, conformational changes occur that transform a closed structure into an open structure. ε-Aminocaproic acid and tranexamic acid induce this change from the closed structure to the open structure. Because of this change, plasminogen is far more readily cleaved to the active enzyme plasmin by plasminogen activators. The open conformation also binds more readily to exposed lysine residues on fibrin's surface. Concentrations of lysine analogs, such as tranexamic acid and aminocaproic acid, that actually promote the more active, open conformation of Glu-plasminogen also prevent its binding to fibrin and therefore exhibit an antifibrinolytic effect.
The primary substrates for the proteolytic activity of plasmin in circulation are fibrinogen and fibrin. Circulating fibrinogen is composed of three polypeptide chains known as the α, β, and γ chains. These chains are bonded together by disulfide bonds, which are also linked to a second identical chain, thus making fibrinogen a dimer of trimers. Thrombin, the common pathway of the coagulation cascade, removes several amino acid peptides from the end terminal of the α chain, the β chain (fibrinopeptide B), to form fibrin. As new sites are exposed, staggered polymerization is initiated. Through catalysis by factor XIIIa, the domains are brought together and chemically cross-linked. Plasmin catalyzes the hydrolysis of these bonds, producing peptides that can be assayed in circulation. Specifically, those produced after the cleavage of fibrinogen consist of truncated polypeptides collectively known as X fragments . X fragments can be incorporated into both newly forming and existing thrombi, causing them to be more fragile. This has been proposed as a possible explanation of why fibrin-specific fibrinolytic agents such as t-PA do not result in fewer bleeding complications compared with nonspecific agents. Because t-PA is such a potent fibrinolytic activator, the accumulation of these X fragments could make existing thrombi more susceptible to its fibrinolytic action. Several fragments are specifically produced by the action of plasmin on fibrin, as opposed to fibrinogen. Unique fragments such as D-dimers can be assayed, documenting fibrinolysis as opposed to fibrinogenolysis.
Plasmin is a relatively nonspecific protease and thus can hydrolyze many proteins found in plasma and extracellular spaces. Known targets of plasmin are factors V and VIII and von Willebrand factor. Prothrombotic activity can be shown with the initial administration of fibrinolytic agents (specifically t-PA and streptokinase, which may relate to the release of fibrinopeptide A). Plasminogen can also cause the release of kinin from high-molecular-weight kininogen. In addition, it can directly and indirectly activate prekallikrein, again inducing kinin formation. Plasmin can also attack protein components of the basement membrane, as well as other active proteases within the matrix, including fibronectin, collagen, and laminin.
Activation of factor XII by various stimuli results in initiation of the coagulation cascade, conversion of prekallikrein to kallikrein and kinin (inflammatory response), and formation of plasmin from plasminogen. This intrinsic mechanism of activation is complemented by a second intrinsic pathway that is not dependent on factor XII. The main pathway for plasminogen activation is known as the extrinsic system. Two activators are recognized in humans: urokinase-type plasminogen activator (u-PA) and t-PA. Their physiologic activity is controlled by inhibitors, mostly plasminogen activator inhibitor (PAI) type 1 (PAI-1) and type PAI-3. These inhibitors control the activity of the activators in plasma and possibly at the cellular level. PAI-1 is synthesized in the liver and vascular endothelial cells and is normally present in trace amounts in plasma. PAI-1 elevation leading to prothrombotic states is becoming increasingly recognized. When pharmacologic dosages of these agents are administered, the inhibitor activity is suppressed. It is estimated that one-third to one-half of the initial pharmacologic dosage of urokinase, for example, becomes inactivated shortly after administration. Once plasminogen has been converted to plasmin, inhibitors of plasmin come into play. The main physiologic inhibitor of plasmin is α 2 -antiplasmin. This protease inhibitor is a single-chain glycoprotein that inhibits plasminogen in two steps: a fast reversible binding step, followed by the formation of a covalent complex involving the active site of plasmin. The half-life of this complex is approximately 12 hours. Other inhibitors of plasmin include α 2 -macroglobulin, protease nexin, and aprotinin.
This complex system is capable of maintaining a balanced equilibrium between clotting and lysis, so that blood fluidity is ensured. It is important to recognize that although plasmin is highly selective for fibrin, it also digests fibrinogen and other plasma proteins. Circulating plasmin inhibitors prevent this otherwise disordered lytic action and preclude free circulating plasmin under normal conditions.
A link between lipoprotein metabolism and fibrinolytic function has been suggested by the demonstration of significant homology between the amino acid sequence of apolipoprotein A and the structure of plasminogen. Thus a prothrombotic function by virtue of interference with the numerous physiologic functions of plasminogen has been suggested in patients with increased levels of apolipoprotein A. Apolipoprotein A has also been found to competitively inhibit the binding of plasminogen to fibrinogen and to the plasminogen receptor on endothelial cells.
Plasma levels of both t-PA and PAI-1 exhibit circadian variations. For example, t-PA activity is lowest in the early morning and highest in the afternoon. Plasma PAI activity peaks in the early morning and passes through a trough in the afternoon. Thus overall there is decreased fibrinolytic activity in the morning. Differences in patterns have been observed between men and women, suggesting a hormonal influence. Furthermore, PAI activity has been noted to vary secondary to diet, with caffeine-containing beverages possibly enhancing fibrinolytic activity. Conversely, cigarette smoking induces an acute increase in t-PA; this increase in t-PA may deplete normal stores and thus paradoxically decrease fibrinolytic capacity.
From the foregoing discussion, it is evident that the fibrinolytic (plasminogen-plasmin) system plays a vital role in biological homeostasis. In addition, it has a pivotal role in certain disease states, ranging from atherosclerosis to carcinogenesis.
From a therapeutic standpoint, drugs capable of converting plasminogen to plasmin achieve their lytic effect to a great extent by overwhelming circulating plasmin inhibitors and generating an abundance of plasmin (exogenous fibrinolysis). Circulating plasmin not only produces the desired fibrinolysis but also proceeds to digest circulating fibrinogen. A more desirable situation results in the activation of thrombus-bound plasminogen (endogenous fibrinolysis) by these agents. Thrombus-bound plasminogen is, to a certain extent, protected from circulating inhibitors and thus proceeds with fibrin digestion much more effectively. Current investigations are concentrated on producing agents with a high affinity for thrombus-bound plasminogen and little activation of the circulating zymogen. Clinical experience to date has failed to demonstrate this theoretical benefit. With better understanding of the complexity of the fibrinolytic system, it is possible that these benefits can be realized.
The use of thrombolytic agents has clearly resulted in a significant improvement in the outcome of patients with acute cardiac ischemia, myocardial infarction, and cerebral infarction. It has also resulted in a modification in the treatment algorithm for patients with peripheral arterial occlusion. Thrombolytic therapy is an important treatment option for patients with vascular occlusive disease. Table 35.1 summarizes the characteristics of some of the common thrombolytic agents. Several schemes may be used to classify thrombolytic agents. The agents can be grouped by their mechanism of action—those that directly convert plasminogen to plasmin versus those that are inactive zymogens and require transformation to an active form before they can cleave plasminogen. Agents can be classified by their mode of production. In addition, thrombolytic agents can be classified by their pharmacologic actions—those that are fibrin specific (i.e., bind to fibrin but not to fibrinogen) versus those that are nonspecific, and those that have a great degree of fibrin affinity (i.e., bind avidly to fibrin) versus those that do not. Another useful classification of thrombolytic agents is groups based on their origin: the streptokinase compounds, the urokinase compounds, the t-PAs, and an additional group consisting of novel agents. In the following sections, we have divided thrombolytic drugs chronologically.
| Characteristics | Streptokinase | Urokinase | Tissue Plasminogen Activator | Reteplase |
|---|---|---|---|---|
| Source | β-Hemolytic Streptococcus | Fetal renal cell culture | Recombinant DNA technology | Plasminogen activator |
| Metabolism | Liver | Liver | Liver | Liver |
| Advantages | Low cost | Direct activator; no allergic reaction | Fibrin-selective direct activator | Plasminogen activator in presence of fibrin |
| Disadvantages | Allergic reactions; complex mechanism of action | High cost | High cost | High cost |
| Regional infusion dosage | Low dose: 5000–10,000 units/h High dose: 30,000–60,000 units/h |
30,000–50,000 units/h 2000–4000 units/min for 1–2 h, then 1000–2000 units/min |
0.05–0.1 units/kg per hour | 0.5 units/h IV; no bolus Rate may be adjusted up to 0.75–1.0 units/h or down to 0.25 units/h |
First-generation thrombolytic agents—namely, streptokinase and urokinase—are highly effective at thrombolysis, but their potency is limited by the fact that they are not fibrin specific. They also convert circulating plasminogen to plasmin, but because circulating plasminogen and the plasminogen in thrombus are in equilibrium, the plasminogen in thrombus would be depleted, thus limiting the efficacy of the agent. This has been termed plasminogen steal and is thought to reduce clot lysis.
Streptokinase is a single-chain nonenzymatic protein produced by β-hemolytic streptococci. Its discovery by Tillett and Garner in 1933 revived an interest in fibrinolysis that has spanned the past 7 decades. Early clinical experience was complicated by a multitude of pyogenic and allergic reactions. This prompted manufacturers to refine the drug, achieving the currently purified product and a marked reduction in febrile and allergic reactions.
The mechanism of action of streptokinase is complex. It initially forms an equimolar complex with plasminogen to form a plasminogen activator. Thus it requires plasminogen as a cofactor and a substrate. Once the activator complex is formed, it is an excellent activator of all mammalian plasminogen. Besides converting uncomplexed plasminogen to plasmin, plasminogen within the activator complex is converted to plasmin, and during this conversion, streptokinase undergoes rapid progressive degradation.
The kinetics of these reactions have been studied in vitro. In vivo a more complicated series of reactions occurs. Infusion of streptokinase is followed initially by neutralization by circulating antistreptococcal antibodies. The remaining drug then combines with circulating plasminogen to form the activator complex. This then converts uncomplexed plasminogen to plasmin, which combines with any excess free streptokinase, is neutralized by circulating antiplasmins, or binds to preformed fibrin. The last produces the desired effect of thrombolysis. However, when activity is measured, two half-lives are detected—16 minutes and 83 minutes—indicating that these complex interactions have a significant effect on the concentration and activity of the drug.
From the foregoing discussion, it is evident that precise control of thrombolysis is not possible because the dose-response relationship of streptokinase varies from patient to patient. Initially, clinical use was guided by titers of antistreptococcal antibodies and measurement of the various components or products of the system. This proved impractical, and current practice relies on standardized dosages that achieve the desired effect in the great majority of cases. A potential drawback of this approach is that excess amounts of drug could use most of the circulating plasminogen to form activator complex; this might leave inadequate amounts of zymogen to convert to plasmin. This problem of exceeding plasminogen availability may be important during regional, rather than systemic, administration. Some investigators have combined streptokinase with plasmin administration, resulting in improvements in measured parameters such as plasminogen level, fibrinogen level, and potential fibrinolytic capacity. Similar results are obtained by intermittent rather than continuous infusion of the drug. Unfortunately, the results of these noncontrolled trials have raised doubts about the effectiveness of such regimens. Streptokinase has largely fallen out of favor, partly because of the immunogenicity of even the refined form, which can result in fever, allergic reactions, and acquired drug resistance.
Streptokinase is the only thrombolytic agent approved by the US Food and Drug Administration (FDA) for use in peripheral arterial and venous thrombolysis, but it is rare to find it being used for that purpose, for the reasons stated earlier. The newer thrombolytic agents have much better safety profiles and better therapeutic efficacy; therefore off-label use of these alternative thrombolytic agents is the norm.
Urokinase is a serine protease with direct activator activity; it is normally present in urine as a product of renal tubular cells. It was originally isolated by MacFarlane and Pilling in 1947. Urokinase is present in varying molecular weights, with variable activity. Original purification was done from urine, yielding small amounts of the enzyme at a considerable cost. Newer production methods use human fetal kidney cell culture.
Urokinase is nonantigenic, and its mechanism of action is much more direct compared with that of streptokinase. Urokinase cleaves plasminogen (its only known protein substrate), by first-order reaction kinetics, to plasmin. It is pH and temperature stable. The lack of circulating neutralizing antibodies and its direct mechanism of action allow for a predictable dose-response relationship. Although allergic reactions are rare, over the past few years a febrile response to drug administration has become more common. It has been suggested that this may be related to interleukins that are still present in recently manufactured drug batches. In the past, when the use of urokinase was less common, aging of the drug actually allowed the interleukins to become inactive. These febrile reactions respond readily to antipyretics. Interestingly, urokinase does not contain any lysine-binding sites and therefore does not have any fibrin-binding properties. However, high-affinity receptors for urokinase have been demonstrated in several cell types and have been postulated as a mechanism by which cells can invade the intracellular matrix and play a role in other physiologic and pathologic processes.
Urokinase is a serine protease and hydrolyzes synthetic esters containing arginine and lysine. Unlike streptokinase, urokinase directly activates plasminogen by cleaving the Arg560-Val561 activation bond. The activation of plasminogen by urokinase occurs by proteolysis. When administered intravenously, urokinase is rapidly removed from the circulation, mainly via hepatic clearance. It has been estimated that the half-life of urokinase in humans is on the order of 14 minutes. Urokinase also reacts with other proteins, including fibrinogen. Urokinase is much more effective in cleaving the susceptible site in plasminogen when it is in the Lys form than in the Glu form. However, the activation reaction of the latter by urokinase may be enhanced by the presence of fibrin. Administration of exogenous plasminogen may also accelerate thrombolysis by urokinase.
Controversy exists regarding the actual thrombolytic effect of urokinase when administered in vivo. Experimental studies have suggested exogenous fibrinolysis as the main pathway, with limited activation of plasminogen within the thrombus (endogenous fibrinolysis). In clinical practice, the results of urokinase therapy have paralleled those achieved with streptokinase, with a decreased incidence of bleeding complications suggested by several investigators. Although major bleeding complications are seen in 15% to 20% of patients treated with streptokinase, such complications have been reported in only 5% to 10% of patients treated with urokinase. Thus the benefits observed in laboratory results and the reduced incidence of significant plasminemia with urokinase seem to translate into a decreased incidence of bleeding complications in clinical practice. Although the cost of urokinase remains high compared with that of streptokinase, when complications are considered, the cost of therapy for streptokinase and urokinase is comparable.
Residual thromboplastic activity was detected in the early urokinase preparations, and this may account for the initial hypercoagulable state reported by Kakkar and Scully. At present, this does not appear to be a clinically significant problem.
Despite the drug's record of safety and efficacy accrued over the years, the FDA halted the release and use of urokinase, manufactured by Abbott Laboratories (Chicago, IL), on the grounds of deviations from the FDA's current good manufacturing practices guidelines, developed to prevent the manufacture of unsafe products. The FDA inspection of Abbott's manufacturing facility in North Chicago in 1998 raised concerns about the neonatal kidney cells that were being used as a source of urokinase. The cells originated from Cali, Colombia, and were obtained through a separate company. The source population was thought to be at high risk for various diseases, including tropical ones, although there were no documented cases of infectious transmission resulting from urokinase administration. The FDA stated that any connection between the drug and such cases might have gone unrecognized.
In October 2002 the FDA approved the reintroduction of urokinase to the market after Abbott made significant changes in its quality control and manufacturing practices. Urokinase was approved for use in the treatment of pulmonary embolism (PE). Although it has not been approved for use in the peripheral arterial and venous systems, many practitioners have experience with off-label use in the periphery. During the time that urokinase was unavailable, increased experience and familiarity were gained with other agents, such as recombinant tissue plasminogen activator (rt-PA) and reteplase. Now, the use of urokinase in the United States has dwindled as most practitioners are using other agents.
Unlike first-generation thrombolytic drugs, second-generation agents are supposed to be fibrin selective. These agents were developed to avoid systemic depletion of circulating fibrinogen and plasminogen and the consequent systemic thrombolytic state; these agents are represented by t-PA, or alteplase, and single-chain u-PA, or pro-urokinase. There is considerable evidence that the plasminogen activator agents are not appreciably fibrin or thrombus specific and that they activate the complement system and damage the cell membranes of platelets and endothelial cells.
t-PA is a naturally occurring enzyme present in all human tissues. Its concentration is variable, with high levels detected in the uterus and moderate amounts in the heart, skeletal muscles, kidneys, ovaries, lungs, thyroid, pituitary, and lymph nodes. Scant amounts of t-PA are found in the liver, spleen, brain, and testes. It is thought to originate from vascular endothelium, and, with the exception of the liver and spleen, tissue concentration correlates with vascularity.
Isolation and purification of t-PA were initially hampered by inadequate sources and procedures. In 1979 Rijken and associates were successful in obtaining 1 mg of t-PA from 5 kg of human uterine tissue. Recognizing the potential of this drug, investigators have concentrated on other sources.
At present, there are two main sources of t-PA. The Bowes melanoma cell line is uniquely efficient in producing large quantities of t-PA, which was subsequently proved to be identical to uterine t-PA. Another source has emerged from the use of recombinant DNA technology, and efforts in the cloning and expression of the t-PA gene from the melanoma cell line have been successful. Since 1987, when rt-PA was approved for the treatment of acute myocardial infarction, it has been used for peripheral thrombolysis as an alternative to urokinase.
In general, plasminogen activators do not cause clot dissolution directly; they must first find and activate molecules of plasminogen in the vasculature at the site of the clot. t-PA is a direct plasminogen activator. Its main advantage is its high affinity for thrombus-bound fibrin. The agent exhibits significant fibrin specificity. t-PA is a poor enzyme in the absence of fibrin. However, the presence of fibrin strikingly enhances the activation rate of plasminogen by t-PA. Two types of t-PA are recognized, with a commercial preparation being a mixture of both types. A single-chain form is cleaved by plasminogen to yield two-chain t-PA. The one- and two-chain forms of t-PA are comparable in activity, with the one-chain form being quickly converted to the two-chain type as lysis proceeds. Most of the circulating t-PA is in the single-chain form. Its selective action promises to produce fewer systemic effects when compared with streptokinase or urokinase. The half-life of t-PA has been estimated to be between 4 and 7 minutes in vivo. With its presumed nonantigenicity and high affinity for fibrin, t-PA theoretically should produce improved clinical results.
When fibrin-selective agents are used for regional infusion, most of the thrombolytic effect is secondary to fibrin-bound plasminogen. However, the importance of a fresh supply of plasminogen to maintain the fibrin-bound plasminogen pool has been emphasized. Experimental studies have suggested that clot lysis induced by the activation of plasminogen is dependent on clot-associated plasminogen, which in turn depends on the concentration of plasminogen in plasma. Depletion of both contributes to less frequent and less rapid recanalization, which is more noticeable with non–fibrin-selective agents than with fibrin-selective ones, likely the result of the depletion of plasminogen induced by the nonselective agents.
Trials comparing rt-PA with streptokinase in patients with acute coronary thrombosis have failed to establish that this more specific drug is a better thrombolytic agent. Systemic bleeding complications have been similar, despite a milder homeostatic defect by laboratory evaluation in the rt-PA groups. Questions still exist regarding proper dosage to achieve effective local lysis with minimal systemic effects.
t-PA may also bind and be activated on platelet surfaces. Owing to this binding to platelet receptors, platelets can direct t-PA action on their surface, leading to rapid cleavage of glycoprotein Ib and the loss of platelet binding to von Willebrand factor. This may explain why concentrations of t-PA achieved early in therapy may inhibit.
In a study in which 17 patients were infused with rt-PA at a rate of 0.1 mg/kg per hour, all patients demonstrated thrombolysis, with 16 showing clinical improvement. More important, there were no systemic complications, with a mean fibrinogen drop of 42% of baseline. The infusion time was 1 to 6 hours, compared with the usual 48 to 72 hours necessary for streptokinase infusion. One patient died from an intracranial hemorrhage during postinfusion heparin therapy. Experience in randomized trials has suggested that a lower dosage is just as effective, with a decreased risk of bleeding. The recommended lower dose is 0.05 mg/kg per hour.
Systemic complications may be more related to dosage and method of administration with t-PA than with urokinase or streptokinase. It appears that t-PA is more potent and faster than the older agents, perhaps because of its high fibrin affinity. In this regard, t-PA may be ideally suited for intraarterial administration, because a 4- to 6-hour trial could be followed by timely surgical intervention. In addition, intraoperative use could be a welcome adjunct to surgical embolectomy.
Saruplase, also known as recombinant single-chain u-PA, or pro-urokinase, is a prodrug produced from a naturally occurring physiologic protease. Pro-urokinase is a single-chain polypeptide of 411 amino acids that is converted by plasmin into an active, low-molecular-weight form of urokinase with 276 amino acids. Pro-urokinase functions as a potent plasminogen activator of fibrin-bound plasminogen without requiring extensive systemic conversion to two-chain urokinase. Thus the entire thrombolytic process is confined to the fibrin clot itself. Administration of pro-urokinase causes decreases in α 2 -antiplasmin and fibrinogen and an increase in fibrinogen degradation products. Pro-urokinase is highly effective in the conversion of Lys-plasminogen to plasmin. In contrast, it has little or no activity in the conversion of Glu-plasminogen to plasmin. Because Lys-plasminogen is present in high concentrations in thrombus, this gives pro-urokinase fibrin-specific properties. In addition, plasminogen that is absorbed in thrombus changes its configuration to a pseudo–Lys-plasminogen, which is also attacked by pro-urokinase, converting it to Lys-plasmin. Circulating pro-urokinase is very stable in plasma because of its resistance to plasma inhibitors and ionized calcium. The fibrin specificities of t-PA and pro-urokinase appear to rely on different mechanisms. Although t-PA is fibrin clot binding, the fibrin-selective properties of pro-urokinase are thought to be secondary to its preference for activation of Lys-plasminogen or Lys-like–plasminogen substrate found in thrombus. This effect prolongs half-life, which has been estimated to be several days. Such a prolonged half-life has theoretical advantages in clinical situations in which prolonged activity is desired. However, in peripheral arterial occlusions, if the regional infusion fails to produce the desired result and the patient must go to the operating room shortly after discontinuation of the infusion, this prolonged effect may be undesirable.
A recombinant form of pro-urokinase is Prolyse (Abbott Laboratories). This urokinase compound has the advantage of not originating in a human cell source. Many of the clinical trials using this drug have been studies of patients with myocardial infarction, where the notable finding was an increased incidence of intracranial hemorrhage (0.9%). The most recent trial was the Prolyse in Acute Cerebral Thromboembolism II (PROACT II) study, which evaluated intraarterial prourokinase for acute ischemic stroke. Early intracranial hemorrhage with neurologic deterioration within 24 hours occurred in 10% of pro-urokinase patients and 2% of control patients. Although pro-urokinase is effective at thrombolysis, the increased bleeding risk has limited its widespread use. A phase II trial evaluating pro-urokinase versus urokinase for thrombolysis of acute peripheral arterial occlusion showed that pro-urokinase had a greater efficacy but an increased risk of bleeding complications at a dose of 8 mg/h; with a dose of 2 mg/h, there was a slightly lower rate of thrombolysis, combined with a lower incidence of bleeding complications and fibrinogenolysis.
The last few years have seen the development of a new generation of thrombolytic drugs, including mutant molecules of single-chain u-PA and t-PA; chimeric plasminogen activators; conjugates of plasminogen activators with monoclonal antibodies against fibrin, platelets, or thrombomodulin; and plasminogen activators of animal and bacterial origin.
Reteplase is a single-chain deletion mutant of alteplase, consisting of just the kringle-2 and protease domains. Reteplase is produced by recombinant genetic technology in Escherichia coli cells. It has a fivefold decrease in fibrin binding and a half-life of 14 to 18 minutes because of the aforementioned structural differences. Reteplase has less binding to endothelium and monocytes compared with t-PA, and this reduced binding results in increased circulating levels in the bloodstream. Reteplase catalyzes the cleavage of endogenous plasminogen to generate plasmin. The activation of plasminogen is stimulated in the presence of fibrin and is mediated by the kringle-2 domain. Plasmin then degrades the fibrin matrix of the thrombus, thus exerting its fibrinolytic action.
The fact that plasminogen activators generally activate plasminogen molecules in or near the clot allows efficient lysis in small clot burdens, such as the coronary circulation. The absolute dependence on a sufficient amount of available plasminogen limits the dose-related efficacy when the clot burden is large. Long, retracted (i.e., organized) clots, such as those in the peripheral arterial circulation, are often deficient in plasminogen. Despite this deficiency, plasminogen activators such as reteplase and t-PA are efficacious when delivered through a catheter directly into the thrombus rather than systemically.
Reteplase has increasingly been used in the treatment of peripheral vascular occlusion, given the unavailability of urokinase for a few years. Nevertheless, published studies regarding its use in controlled trials are relatively few in number. There are two pilot studies that evaluated the dosing regimen of reteplase in the treatment of myocardial infarction. These studies demonstrated that reteplase produced significantly higher TIMI-3 (Thromboembolism in Myocardial Infarction) flow rates at 60 and 90 minutes than did front-loaded alteplase. However, in two subsequent trials—the International Joint Efficacy Comparison of Thrombolytics (INJECT) and Global Use of Strategies to Open Occulated Coronary Arteries (GUSTO III) trials—despite the higher TIMI-3 flow rates, this did not translate into a lower mortality in the reteplase-treated patients (7.5% for reteplase vs. 7.2% for alteplase). Reteplase penetrates the clots and activates plasminogen inside the clot, whereas t-PA binds tightly to the fibrin matrix and accumulates on the surface of the clot. This difference is possibly due to the lower affinity of reteplase for fibrin compared with t-PA, resulting in faster clot lysis. Pharmacodynamic differences and pharmacokinetic differences between reteplase and t-PA may account for the higher initial clot lysis rates reported with reteplase.
Thrombolytics may also cause platelet activation, and this may have been responsible for some of the previously noted lack of efficacy. The addition of glycoprotein IIb/IIIa inhibitors appears to increase the efficacy of thrombolytic agents, as well as speed the lysis.
Tenecteplase (TNK-t-PA) is a t-PA mutant in which a threonine molecule ( 100 Thr) is replaced by Asn, and the sequence Lys-His-Arg-Arg is changed to Ala-Ala-Ala-Ala. This change confers high fibrin selectivity and prolongs the half-life to 15 to 19 minutes. TNK-t-PA is highly effective in arterial, platelet-rich thrombi and is more resistant to PAI. Although most of the published data regarding TNK-t-PA have been related to acute coronary syndromes, there is increasing experience in peripheral arterial thrombolysis. One group published its experience with continuous tenecteplase infusion in conjunction with glycoprotein IIb/IIIa inhibition with tirofiban for peripheral arterial thrombolysis. The dose of TNK-t-PA infusion was 0.25 to 0.50 mg/h, with a mean infusion time of 7.5 hours. Of 48 patients with iliofemoral arterial thrombosis, complete lysis was achieved in 35 patients (73%). There were no deaths, no intracranial bleeding, and no embolic events. It appears, at least from this study, that lysis time is shorter; however, the longer half-life has implications for surgical intervention, as addressed earlier.
Staphylokinase is a plasminogen activator produced by certain strains of Staphylococcus aureus and was first described as having fibrinolytic properties in 1948. The gene has been cloned from genomic DNA of a lysogenic strain of S. aureus . When exposed to a fibrin clot in human plasma, staphylokinase reacts with plasmin at the clot-plasma interface; this staphylokinase-plasmin complex activates thrombus-bound plasminogen and exerts its fibrinolytic activity. Any plasmin that is liberated from the clot is rapidly inactivated by α 2 -antiplasmin. In this manner, plasminogen activation by staphylokinase is confined to the thrombus, and the collateral effects of fibrinogen depletion and serum plasminogen activation are minimized. However, patients treated with staphylokinase do develop neutralizing antibodies, the titers of which can remain elevated for several months.
In an attempt to develop fibrin-specific agents, monoclonal antifibrin antibodies have been bonded to urokinase or streptokinase, rendering these agents fibrin selective. These monoclonal antibodies do not appear to cross-react with fibrinogen and thus show a marked increase in in vitro fibrinolysis compared with unmodified activator. The clinical applicability of these agents remains to be determined; they may significantly alter the current approach to the management of thrombotic disease. Nevertheless, repeated therapy would require different monoclonal antibodies to prevent adverse immunologic reactions.
Fibrolase is a direct-acting fibrinolytic enzyme. It is a metalloprotease isolated from the venom of the southern copperhead snake, which dissolves fibrin through rapid hydrolysis. There are some data to suggest that fibrolase dissolves thrombi much quicker than the plasminogen activators. An added advantage of fibrolase is the rapid inactivation by α 2 -macroglobulin, which is relatively abundant in the systemic circulation. Alfimeprase (Nuvelo, Inc., San Cerlos, CA), a recombinant variant of fibrolase, underwent evaluation in clinical trials of peripheral arterial occlusion, with inconclusive results. In addition, amediplase (Menarini Group, Florence, Italy) is undergoing evaluation in clinical trials. Amediplase is a chimeric protein that combines part of the t-PA and part of the single-chain urokinase plasminogen activator (sc-UPA). In animal models, amediplase is a more potent and longer-lasting thrombolytic than alteplase.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here