Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hemorrhage continues to be the leading cause of death for traumatically injured patients despite all the advances that have been made in the past 20 years. Time to hemorrhage control is one of the most, if not the most, critical factor in saving the life of a traumatically injured patient. Delays in identifying and treating the fatal triad of hypothermia, acidosis, and trauma-induced coagulopathy (TIC) portend poor outcomes for trauma patients. The coagulopathy of trauma, even with the myriad of advances in prehospital resuscitation and interventions for early hemorrhage control, continues to increase the morbidity and mortality of civilian and military trauma casualties. Conventional coagulation studies including international normalized ratio (INR), prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen level, and platelet count were the focus of resuscitation strategies aimed at identifying and treating TIC in for decades. There are, however, more comprehensive and rapid testing strategies available to reduce time to identifying and treating TIC. The purpose of this chapter will be to discuss the history, techniques, and uses of thromboelastography (TEG) and thromboelastometry. These studies are helping revolutionize the identification and treatment of TIC through a set of principles known as damage control resuscitation ( Fig. 1 ).
Hellmut Hartert developed and first described TEG at the University of Heidelberg (Germany) in 1948. While initially only utilized for research purposes, TEG gained traction for clinical implementation with liver transplantation during the 1960s. TEG was specifically used to help identify hyperfibrinolysis, or excessive clot breakdown, after the placement of the donor liver in the recipient body. Over the next several decades, while techniques for liver transplantation improved and the number of transplants continued to rise, TEG did not gain traction in other areas of surgery. The 1980s, however, were the beginning of the increase in utilization of TEG specifically in cardiac surgery. Several case studies and small case series were written describing the management of bleeding and anticoagulation in these rapidly advancing fields of surgery. In 1997, Kaufmann et al were the first to show the utility of TEG in trauma patients. They performed TEG as a part of the initial evaluation of 69 adult trauma patients all with blunt injuries and found that 45 patients were hypercoagulable, 7 were hypocoagulable, and the other 17 had normal TEG tracings despite their injuries. In retrospective analysis, TEG was an early predictor of transfusion in blunt injury patients and was broadly able to define coagulation abnormalities in a timely fashion. The military conflicts since 2001 have led to a rapid expansion in the knowledge base of trauma and the resuscitation of the injured soldier. This knowledge has been transferred to the civilian sector where the implementation of resuscitation strategies and adoption of viscoelastic testing has led to a dramatic improvement in our understanding of both TEG and rotational thromboelastometry (ROTEM) and their application to the care of the acutely injured patient.
ROTEM is a proprietary product of TEM International (GmbH, Munich, Germany) and functions as a viscoelastic point-of-care analyzer testing the hemostatic profile of whole blood. ROTEM uses whole blood samples to produce five unique tracings that evaluate the ability of the sample to make clot in different environments. The five unique tracings (INTEM, EXTEM, HEPTEM, FIBTEM, and APTEM) are produced simultaneously via different activators and outlined in Table 1 . With INTEM assay, activation occurs by contact with ellagic acid. This assay is particularly sensitive to the intrinsic pathway factors and evaluates factors I, II, VII to XII, and von Willebrand. The EXTEM assay is activated by thromboplastin/tissue and is most sensitive to fibrinolysis. This test evaluates the extrinsic pathway components (II, VII, IX, X). Other adjunctive assays of ROTEM include FIBTEM (EXTEM with addition of cytochalasin D to platelet function, allowing isolated assessment of clot formation dependent solely on polymerization of fibrin), HEPTEM (INTEM with addition of heparinase to correct the clotting time in the presence of heparin, demonstrating “heparinized blood”), and APTEM (EXTEM with aprotinin added to inhibit fibrinolysis, demonstrating true hyperfibrinolysis when maximal lysis value on EXTEM is corrected).
| Assay | Activator/Inhibitor | Role |
|---|---|---|
| INTEM | Ellagic acid (contact activator) | Assessment of clot formation via intrinsic pathway |
| EXTEM | Tissue factor | Assessment of clot formation via extrinsic pathway |
| HEPTEM | Ellagic acid + heparinase | Assessment of clot formation in heparinized patients; INTEM assay performed in the presence of heparinase |
| FIBTEM | Tissue factor + platelet antagonist | Allows detection of fibrinogen deficiency or fibrin polymerization disorders |
| APTEM | Tissue factor + aprotinin (fibrinolysis inhibitor) | In vitro fibrinolysis inhibition: fast detection of lysis when compared with EXTEM |
When running a ROTEM, a whole blood sample is added to a sample cup using an electronic pipette. A disposable sensor pin is attached to the shaft, which relates to a thin spring, and slowly oscillates back and forth suspended in the blood sample. This signal from the pin is then transmitted via an optical detector system, and once the blood starts clotting, the clot restricts the rotation of the pin. The subsequent increase in the firmness of the clot restricts the rotation of the pin and the translated output is manifested as a graphical display with numerical parameters.
Each of the ROTEM tracings that is produced will provide numerical values as well as graphical representation of clot formation once the clot begins to form on the pin ( Fig. 2 ). It is the ability with both ROTEM and TEG to visualize these tracings as they are being produced that make these tests so much more valuable. The clinician need not wait for these values to be reported in the electronic medical record. Rather, they are able to view them in a real-time fashion by remote viewing or at the bedside, thereby cutting down delays in interpretation and intervention. The clotting time (CT) is the time from the addition of reagent until the blood starts to clot; when this is prolonged, there is abnormal clot formation. The clot formation time (CFT) is the time from CT until a clot firmness of 20 mm is reached and an alpha-angle, which is the angle of tangent between it and the curve. These numbers represent the speed at which the clot is forming and are mainly influenced by platelet function, as well as the fibrinogen and coagulation factors. Amplitude 10 minutes after CT (A10) is used to predict the maximum clot firmness at an earlier stage to allow earlier therapeutic decisions. Maximum clot firmness (MCF) is the greatest vertical amplitude of the trace. Low MCF is usually consistent with decreased platelet numbers or function decreased fibrinogen levels or low factor XIII activity. Maximum lysis (ML) is the fibrinolysis of >15% as identified relative to the MCF.
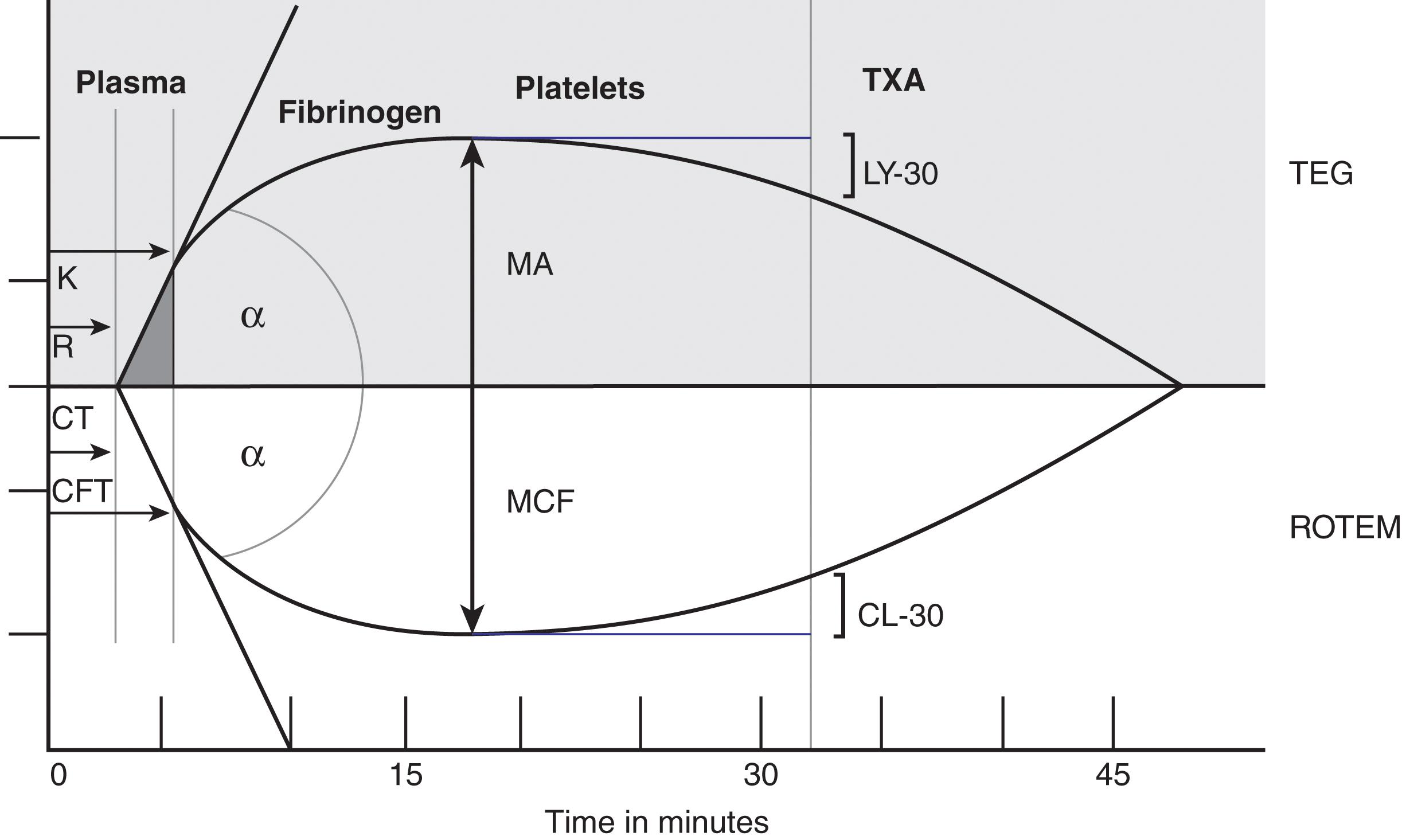
TEG is a proprietary product of Haemonetics Corporation (Niles, Illinois). The current TEG 5000 analyzer provides a graphical representation of the clotting process. A whole blood sample is incubated in a cup at 37° C. A stationary pin is attached to a wire that can monitor movements and is immersed into the sample. The cup oscillates six times per minute as kaolin is added to activate the coagulation cascade leading to thrombin formation. This thrombin formation leads to the conversion of fibrinogen to fibrin as a clot is formed and is considered a standard TEG. Rapid TEG (r-TEG) uses tissue factor in addition to standard kaolin to accelerate the activation of the clotting cascade. This allows for faster evaluation of clot formation. The clot strength influences the oscillation of the pin, and these dynamic changes are converted to a curve on a graphical display. The clot formation curve reflects the different phases of the clotting process and allows for qualitative evaluation of the individual steps that are required to create and breakdown clot. Figure 2 provides an overview of the graphic products of both TEG and ROTEM machines.
R (reaction) time is the period from the initiation of the test until the beginning of clot formation as perceived by the pin. ACT (activated clotting time) is the time in seconds between initiation of the test and the initial fibrin formation and is faster than the R time due to the addition of tissue factor in conjunction with kaolin to start the clotting cascade. This is available with the rapid TEG assay. K time is the period from the start of clot formation until the curve reaches amplitude of 20 mm, which is indicative of clot kinetics. Alpha-angle is the angle between the baseline and the tangent to the TEG curve through the starting point of the clot, immediately after the R time. This angle is associated with acceleration of clot formation and the kinetics of fibrin formation and cross-linking. Maximum amplitude (MA) is a direct measure of the apex on the TEG curve and represents overall clot strength. This amplitude is dependent on platelet concentration, platelet function, and the interactions of platelets and fibrin. LY30 is the ratio between the amplitude at a given time point and MA given as a percent of the MA. This ratio is reflective of the measurement of fibrinolysis at any one point in time.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here