Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Access the accompanying videos for this chapter online. Available on ExpertConsult.com .
Access the accompanying videos for this chapter online. Available on ExpertConsult.com .
Esophageal atresia (EA), with or without a tracheoesophageal fistula (TEF), is one of the more uncommon congenital anomalies, occurring in 1 in 5000 births. These newborns present shortly after birth because of an inability to pass an orogastric tube, respiratory distress, or an inability to tolerate feeding. The condition may be associated with other major congenital anomalies (such as those seen in the VACTERL association, which include Vertebra, Anorectal atresia, Cardiac, Tracheo-Esophageal fistula, Renal, and Limb anomalies), or it may be an isolated defect. Improvements in maternal–fetal ultrasound have resulted in a prenatal diagnosis in a number of babies. Patients with EA/TEF require relatively emergent surgical intervention to prevent aspiration of gastric acid and overdistention of the stomach and intestines. Those with pure atresia can be managed in a more leisurely fashion, as long as the infant’s oral secretions are controlled by continuous or intermittent suction.
In 2000, the first successful thoracoscopic repair of an EA/TEF in a newborn was reported (Rothenberg 2000). The first large multi-institutional review of over 100 cases was published by Holcomb et al. (2005), and there are now many series that have been published. These papers have shown the safety and efficacy of thoracoscopic repair for this condition. This chapter will focus primarily on type III or C EA, but we will also briefly discuss thoracoscopic repair of H-type TEF and long-gap EA.
All patients with EA/TEF can be considered for thoracoscopic repair. The decision to approach these patients thoracoscopically depends on a number of different factors, the most important being the stability of the infant and the experience of the surgeon and anesthesiologist with thoracoscopy in neonates. The infant first needs to undergo a thorough evaluation to determine if there are any other congenital anomalies. The most relevant is an echocardiogram to determine the anatomy and function of the heart as well as the location of the aortic arch.
There are few absolute contraindications to the thoracoscopic approach for this condition. Currently, these include severe hemodynamic instability requiring significant vasopressor support, and significant prematurity (< 1000 g). Relative contraindications include significant congenital cardiac defects, smaller size (1000– 2000 g), or significant abdominal distention. Congenital heart defects are not an absolute contraindication to the thoracoscopic approach, and repairs have been successfully completed in patients with atrial septal defects, ventricular septal defects, or tetralogy of Fallot. However, patients with severe cardiac anomalies may not be able to tolerate even the short periods of single-lung ventilation that are required to ligate the fistula or complete the anastomosis.
It is also important to determine the side of the aortic arch as this determines the surgical approach. A right-sided arch implies that a left-sided approach is preferable. This can be done with minimal added difficulty. Anorectal atresia or cloacal anomalies are not a contraindication, and a thoracoscopic EA/TEF repair can be combined with a laparoscopic evaluation and management of these associated conditions. Other anomalies that may change the surgical approach include intestinal atresia. A proximal bowel atresia may result in significant gastric distention, which can cause immediate problems. The surgeon must evaluate each situation separately and determine the most appropriate approach.
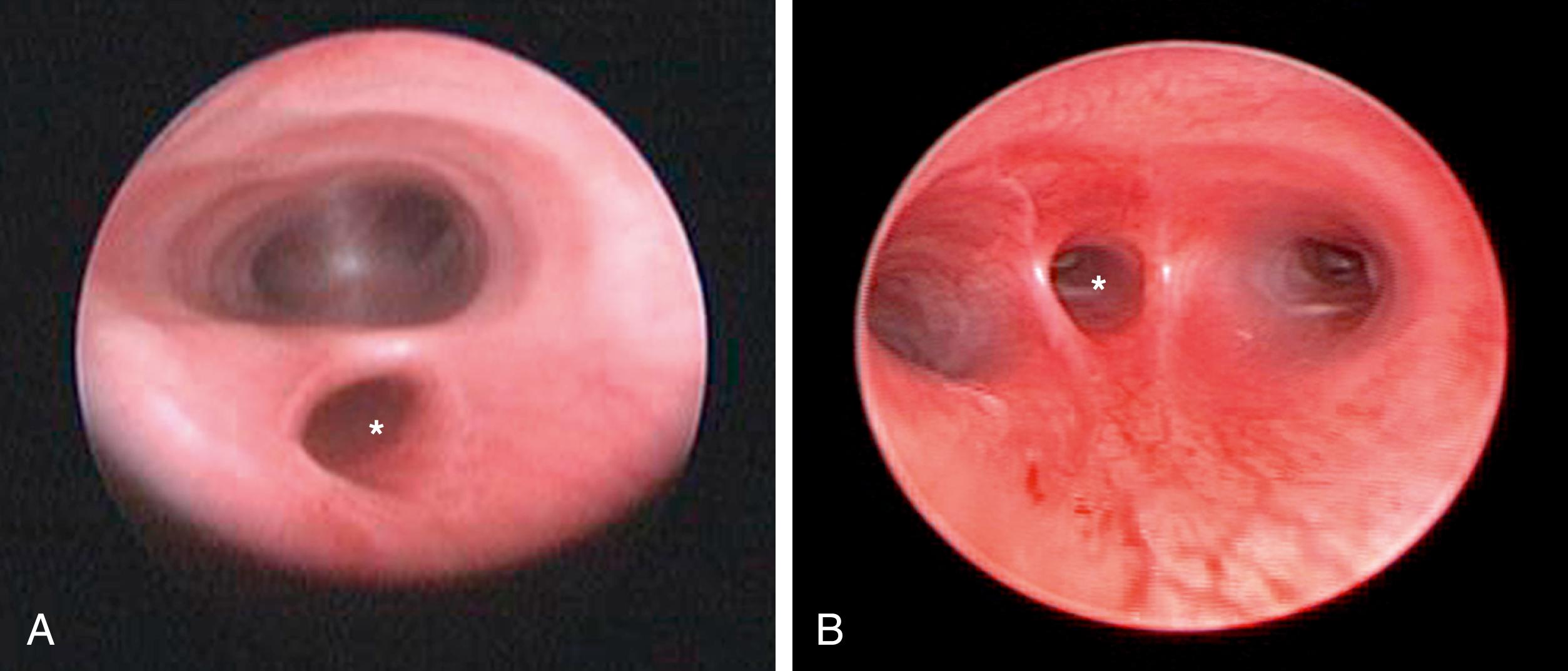
General endotracheal anesthesia is administered, but low peak pressures should be used until the fistula is ligated to prevent overdistention of the stomach and upper intestinal tract. A local anesthetic (0.25% bupivacaine) is instilled at the cannula sites. Single-lung ventilation is generally not required and adequate lung collapse can be achieved with CO 2 insufflation alone. Many prefer to perform a bronchoscopy at the time of the procedure to determine the site of the fistula as well as exclude an upper pouch fistula in type C patients ( Fig. 37-1 ). This may aid in placing the endotracheal tube above the carina but below the fistula. Significant extra time should not be wasted trying to accomplish this or to manipulate the tube down the left mainstem bronchus as effective collapse of the lung can be achieved with CO 2 insufflation alone and prolonged bronchoscopy before the fistula is ligated can cause the infant to decompensate. The decision to perform preoperative bronchoscopy remains controversial, but, in general, the surgeon should follow the guidelines he or she uses for open EA/TEF repair. A urinary catheter is also optional.
Once the endotracheal tube is secured, the patient is positioned in a modified prone position ( Fig. 37-2A ). This positioning gives the surgeon access to the area between the anterior and posterior axillary lines for port placement while allowing gravity to retract the lung anteriorly away from the posterior mediastinum. Generally, small towel rolls are sufficient to provide stabilization for positioning. A small bean bag can also be used. This positioning allows excellent visualization of the esophageal segments and fistula.
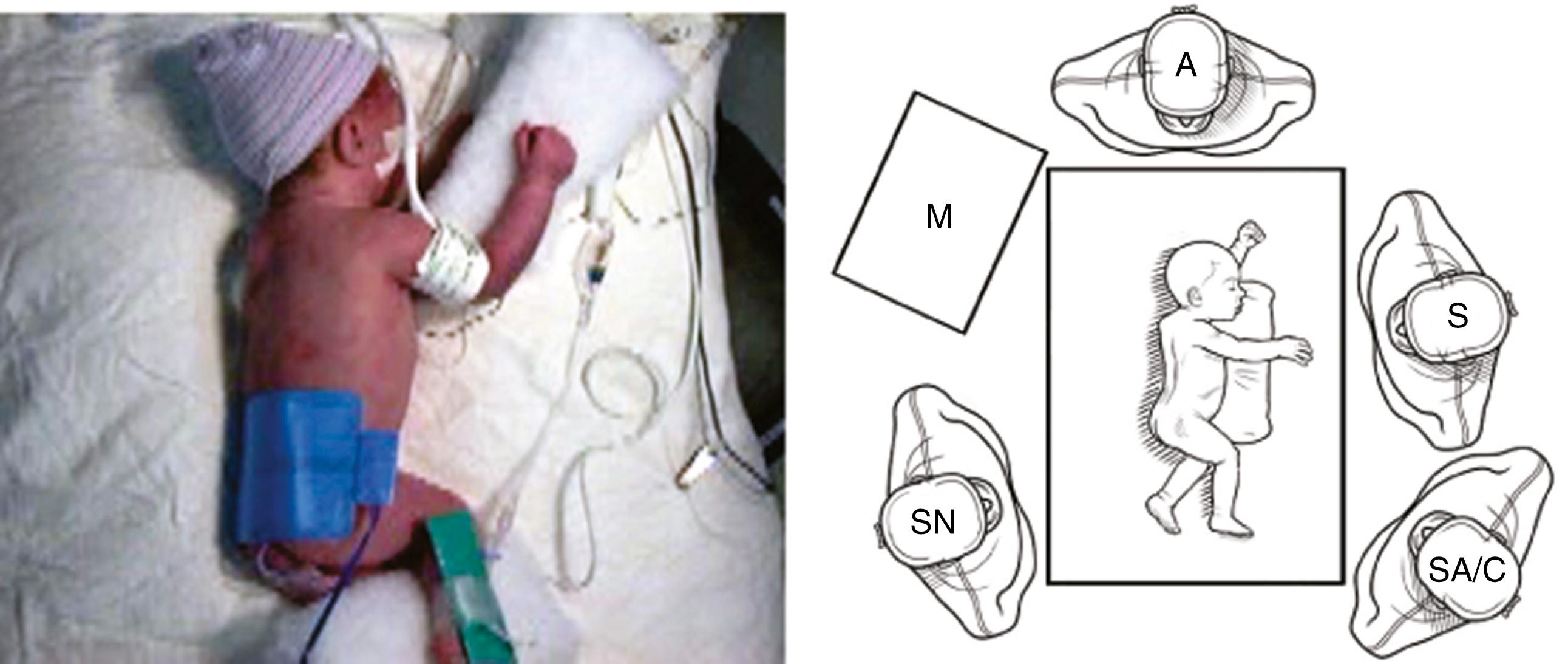
The surgeon and assistant stand at the front of the infant and the monitor is placed over the infant’s back ( Fig. 37-2B ). This arrangement allows the surgeon and the assistant to work in line with the monitor. The assistant should also be able to see the monitor. The scrub nurse is situated on either side of the patient or at the front of the table, depending on the room layout and surgeon preference. Because of the fine manipulations necessary during the operation, the surgeon and assistant should position themselves in the most ergonomic and comfortable position; usually, the assistant is to the surgeon’s left.
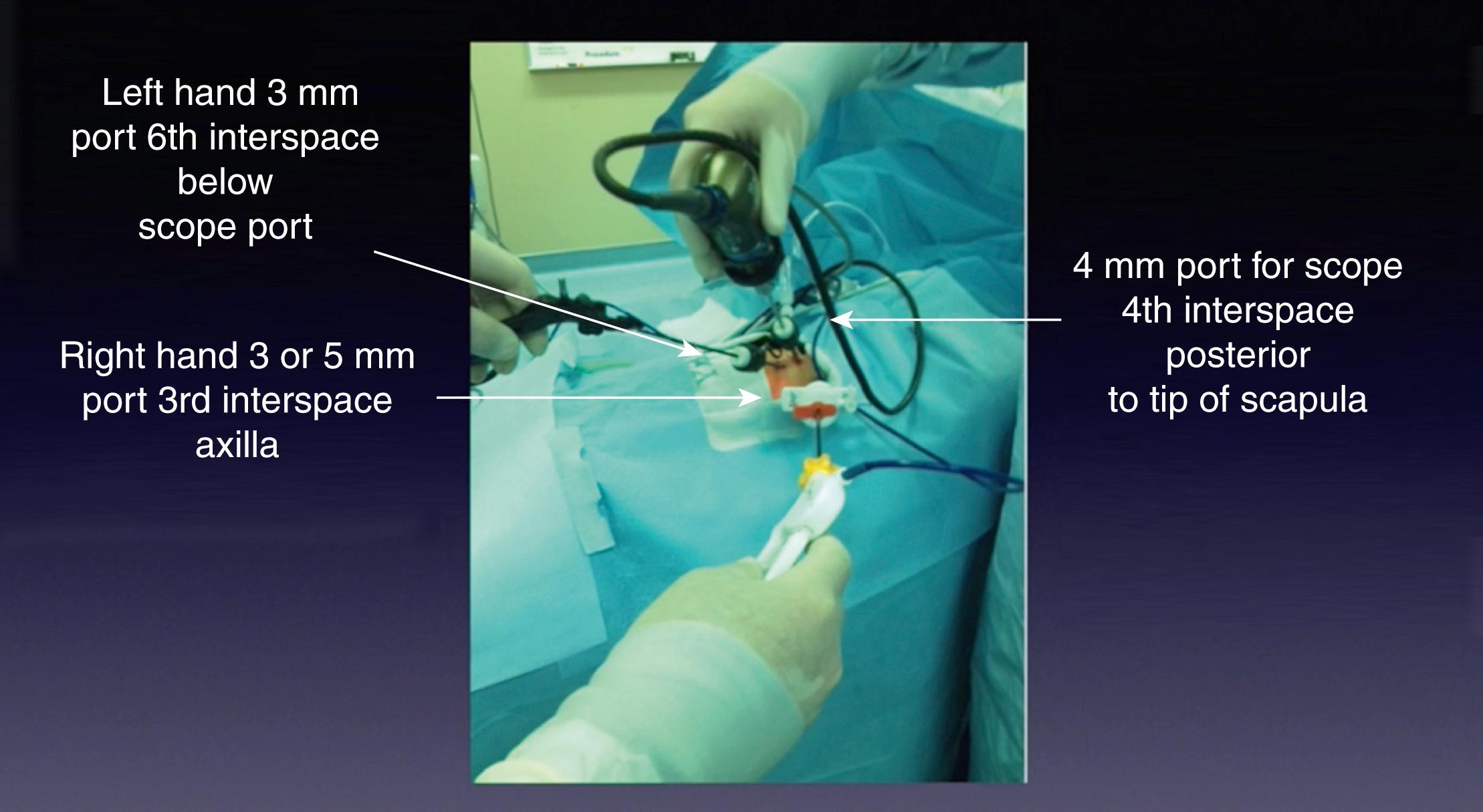
Port placement is extremely important because of the small chest cavity and the delicate nature of the dissection and reconstruction. The procedure can be performed with three ports, but occasionally a fourth port is necessary for lung retraction ( Fig. 37-3 ). Stab incisions can be used for insertion of 3-mm instruments if preferred. The initial cannula (4 or 5 mm) is introduced in the fourth or fifth intercostal space just behind the tip of the scapula. This is the camera port, and it allows excellent visualization of the posterior mediastinum in the area of the two esophageal segments. A 30- or 45-degree telescope is useful to allow the surgeon to look down on the area of dissection. In infants under 1500 g, a 3-mm telescope may be necessary but provides a smaller field of view. The two instrument ports are then inserted, the upper port in the midaxillary line, one to two interspaces above the camera, and the lower port slightly posterior two interspaces below. The upper port is usually a 5-mm port (with a 3-mm reducer—Step mini port) for introduction of a 5-mm clip applier and suture needles. The lower port is 3 mm. Ideally, these ports are situated so that the instrument tips approximate a right angle (90 degrees) at the level of the fistula. This positioning facilitates suturing in the small working space. A fourth cannula (3 mm) can be inserted lower in the thoracic cavity to help retract the lung but is often not needed. The operative principles are the same as those used for the open procedure.
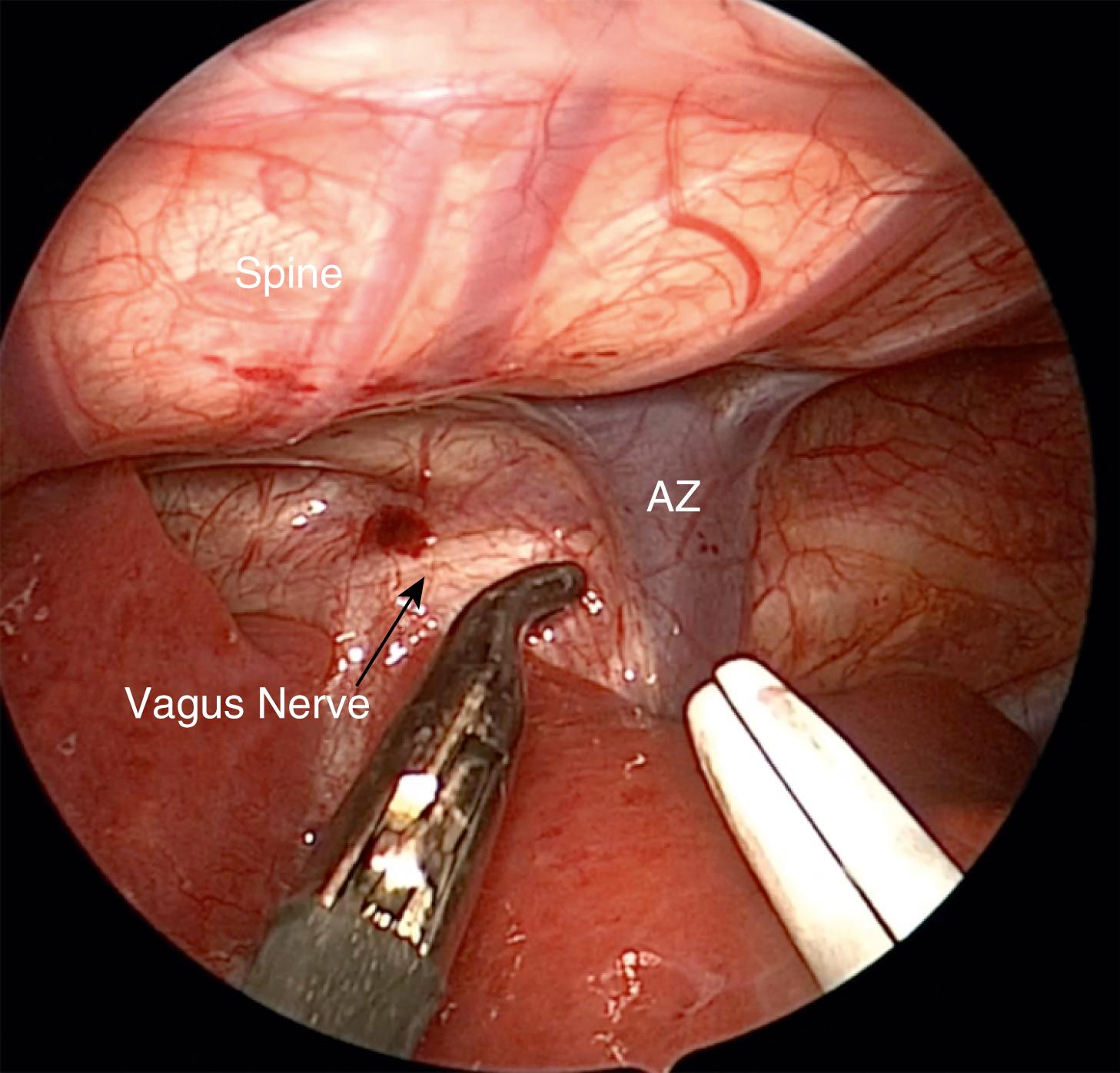
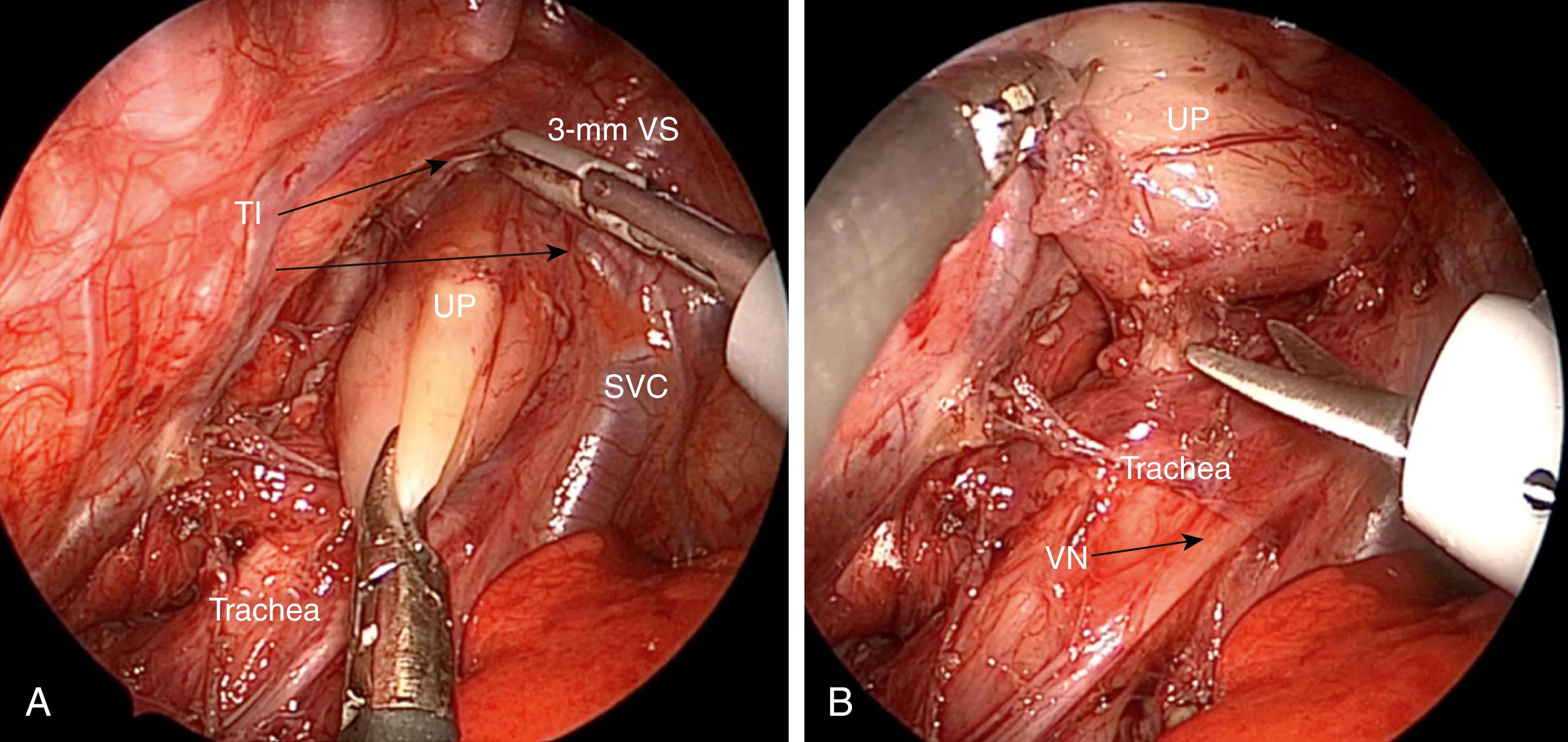
Once the chest cavity has been insufflated and the lung collapsed, the surgeon should first identify the TEF. In most cases, the fistula enters the membranous portion of the trachea just above the carina. This level is usually demarcated by the azygos vein ( Fig. 37-4 ). After the azygos is identified, it should be mobilized for a short segment using a curved dissector or scissors. The vein is sealed with the 3-mm vessel sealer (Bolder Surgical, Louisville, CO) and then divided, or it can be cauterized with a 3-mm hook if the sealer is not available.
With the vein divided, the lower esophageal segment is identified and followed proximally to the trachea ( Fig. 37-5A ). Because of the magnification afforded by the thoracoscopic approach, it is easy to visualize where the fistula enters the back wall of the trachea. Endoscopic clips can then be applied across the fistula flush with the membranous wall of the trachea ( Figs. 37-5B and 37-5C ). If the surgeon prefers, the fistula can be suture ligated ( Fig. 37-5D ). In general, a 3 or 4-0 PDS suture ligature is used.
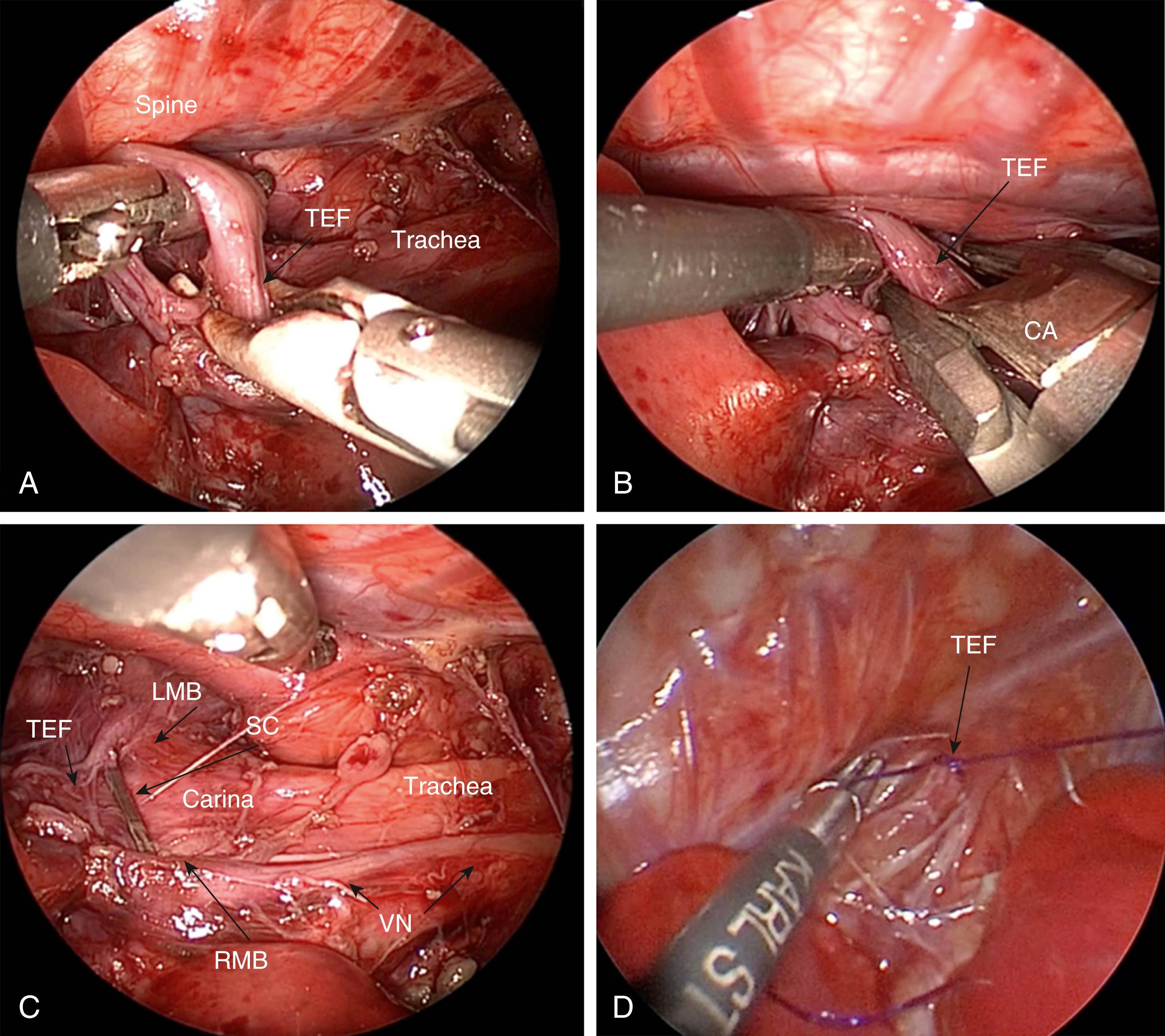
Care should be taken to identify and avoid injuring the vagus nerve as well as the right or left mainstem bronchus. The fistula can then be safely divided distal to the clip with scissors. The distal esophageal segment may retract, making it difficult to visualize. Therefore, after ligating the fistula, it is preferable to wait until the upper pouch is dissected before completely dividing the fistula.
Attention is now turned to the thoracic inlet. The anesthesiologist exerts pressure on the oral-esophageal tube to help identify the upper pouch. The pleura overlying the upper pouch is incised sharply and the pouch is mobilized with blunt and sharp dissection. This dissection is greatly facilitated with the 3-mm sealer ( Fig. 37-6A ). If not available, monopolar cautery should be used sparingly so as to avoid injury to the trachea and recurrent laryngeal nerves. In some cases, it is helpful to place a stay suture in the tip of the pouch to aid in this dissection. The plane between the esophagus and the trachea is usually seen well ( Fig. 37-6B ), and the two can usually be separated using sharp dissection. Mobilization of the upper pouch is extended into the thoracic inlet until adequate length is achieved to allow as tension free an anastomosis as possible. It is often necessary to mobilize the lower pouch as well. This can safely be done down to the diaphragm. However, care must be taken to avoid breeching the esophageal hiatus or damaging the phrenoesophageal ligament as this can result in a hiatal hernia and increase the incidence of gastroesophageal reflux (GER).
Once adequate mobilization is achieved, the distal tip of the upper pouch is resected. An adequate opening is needed to prevent later stricture formation. With the two esophageal ends mobilized, the anastomosis is performed usinga 4-0 or 5-0 suture (PDS or Vicryl) on a small tapered needle. The sutures are placed one at a time in an interrupted fashion. The back wall is secured first (three to five sutures), with the knots being intraluminal ( Fig. 37-7A ). A small silastic tube is then passed under visualization through the anastomosis and into the lower esophagus. The anterior wall is then completed with the nasogastric tube acting as a guide to prevent incorporation of the posterior wall and to ensure patency of the anastomosis ( Fig. 37-7B ). Adequate bites of tissue are needed to prevent the sutures from tearing the esophagus. Also, it is imperative to incorporate the mucosa in every stitch. The anastomosis usually requires 8 to 10 sutures.
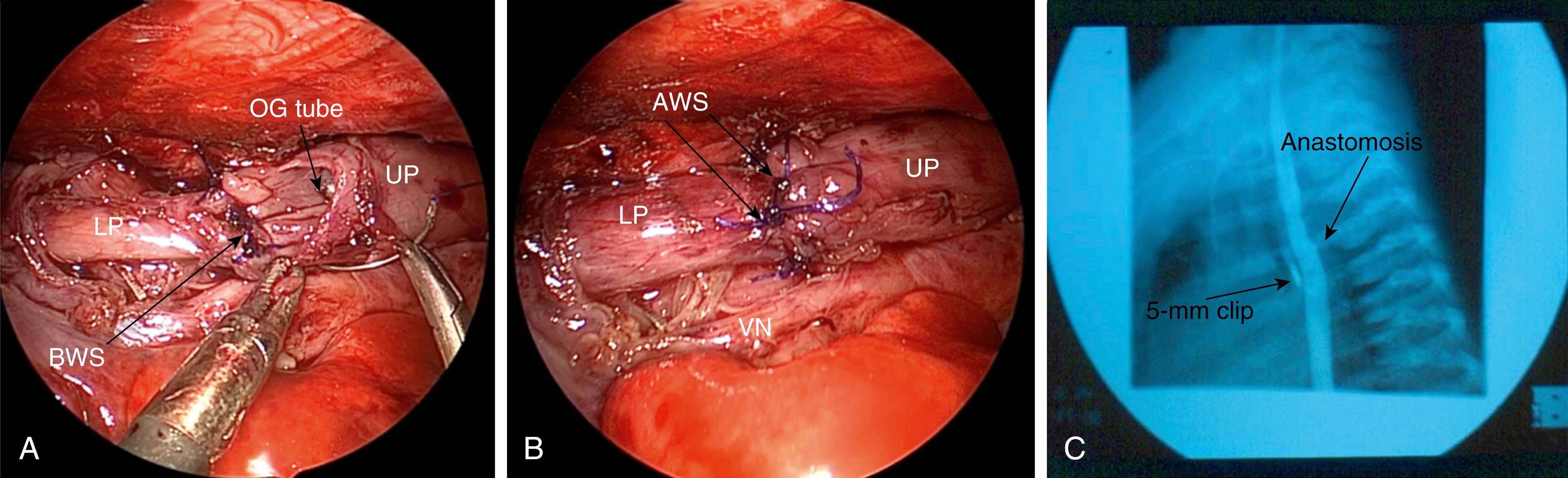
Once the anastomosis is complete, the orogastric tube is removed, in most cases, as it was shown to be associated with a higher risk of stricture formation in a retrospective study. A chest tube or drain can be inserted, if desired, through the lower cannula site, and the tip is positioned under visualization near the anastomosis, although this is also controversial. The other ports are then removed and the sites are closed with absorbable sutures.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here