Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The dilatation of arteries was first recognized as a disease of the cardiovascular system in Egypt in the 1550s bc , and the first to describe an abdominal aortic aneurysm was the Flemish physician Vesalius about three millennia later in 1555. Two centuries later, in 1785, the English surgeon John Hunter began to treat aneurysms of the peripheral vessels by ligation. Hunter ligated the superficial femoral artery, causing a patient’s popliteal aneurysm to slowly decrease in size over several weeks. Before this procedure, the options for treating such a popliteal aneurysm were to either wait for rupture or to prophylactically amputate the extremity. The first attempt at percutaneous endovascular aneurysm repair was commenced by Moore and Muchison in 1864, in which a patient with a thoracic aortic aneurysm had an aneurysm cannulated and packed with a couple dozen yards of wire coils in hopes of inducing thrombosis. While the patient died, the aneurysm was noted to have partially thrombosed. Accompanied by the advancement of other therapeutic modalities, in 1948, Dr. Rudolph Nissen in New York used a cellophane wrap to treat aortic aneurysms. One of the greatest scientific minds of the 20th century experienced a thoracic aortic aneurysm and was treated by Nissen: in December 1948, Albert Einstein underwent surgery for a large thoracic aortic aneurysm, and Nissen performed the procedure. He was, however, only able to wrap the anterior portion of the aneurysm with cellophane, as aortic mobilization was generally considered to be forbidden at the time. The treatment was partially effective, but despite Nissen’s best efforts, Einstein died of a ruptured aneurysm 6 years later at the age of 76 years.
In 1951, Dr. Charles Dubost in Paris performed the first successful resection of an abdominal aortic aneurysm, using a 15 cm long cadaver aortic homograft. After this development, a new era in the surgical treatment of aortic aneurysms was launched along with the introduction and clinical application of cardiopulmonary bypass (CPB) by John Gibbon in 1953. These developments facilitated the application of various surgical techniques required for the reconstruction of the ascending aorta, including the use of aortic allografts as an aortic replacement. In the mid-1950s, other major milestones in open heart surgery were also taking place, such as the first open-heart repairs of ventricular septal defect, atrioventricular communis, and tetralogy of Fallot. Such procedures used extracorporeal cross-circulation.
Later, a combined operation to replace the ascending thoracic aortic aneurysm, as well as the aortic valve along with the reimplantation of the coronary arteries was performed by Hugh Bentall and Antony De Bono in 1968, , and around this time, Dacron was introduced by Dr. Michael DeBakey. Interestingly, DeBakey, himself a pioneering cardiac surgeon, was affected by an aortic aneurysm when he was 97 years old. He was at home while preparing a medicine lecture when he experienced symptoms that he himself recognized as those of an acute thoracic aortic aneurysm dissection. He initially refused anesthesia and surgery, but his situation deteriorated, and his wife demanded that her husband have surgery. The Ethics Committee at Methodist Hospital in Houston, Texas approved the planned surgery. His postoperative recovery was prolonged and complex. In retrospect, Dr. DeBakey publicly acknowledged that he was happy that he underwent the surgical procedure. He died of natural causes shortly before his 100th birthday in July, 2008.
In the decades following DeBakey’s accomplishments, developments in the realm of thoracic aortic aneurysm repair commenced. The use of one-lung ventilation (OLV) was first described by Dr. Eric Carlens, who introduced the double-lumen endobronchial tube. Dr. Irving Sarot, a thoracic surgeon at the Mount Sinai Hospital, started to use the same technique in 1971.
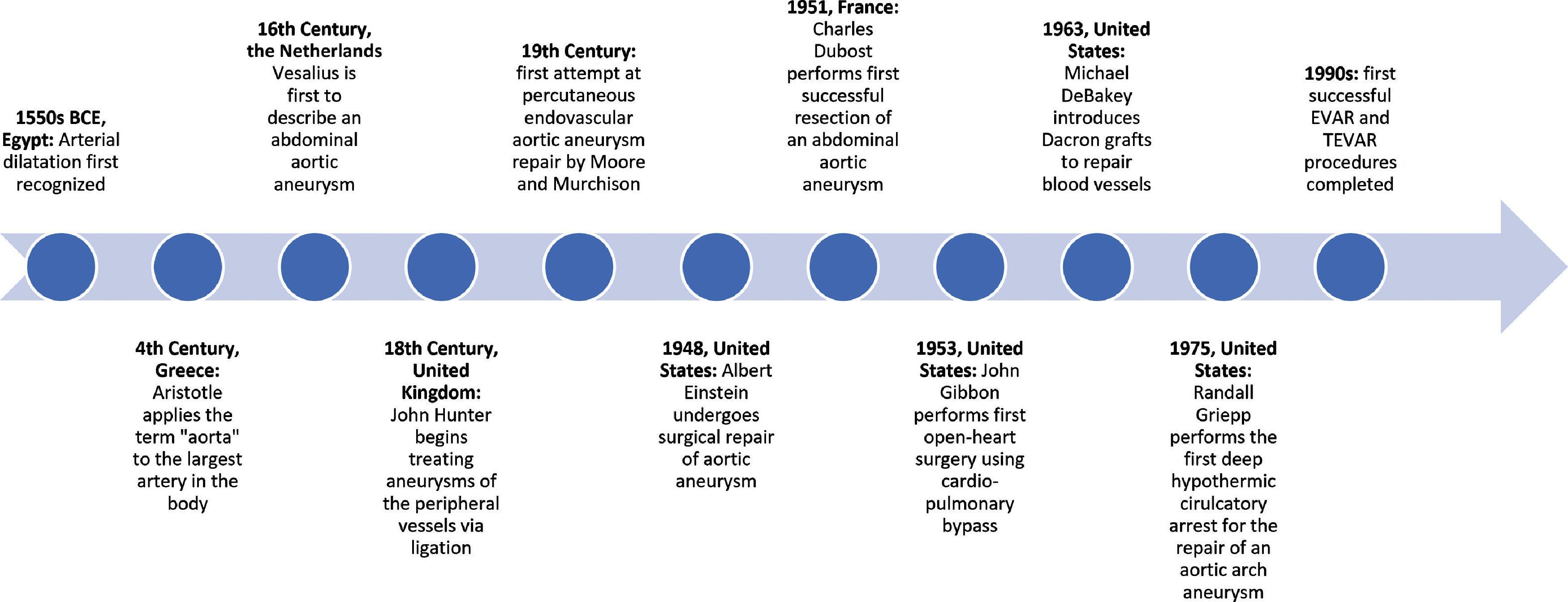
Dr. Randall Griepp, who later became the chairman of cardiothoracic surgery at the Mount Sinai Hospital, developed the use of deep hypothermic circulatory arrest in the repair of ascending thoracic aortic aneurysms while at Stanford University Hospital. Griepp explained that “a combination of surface cooling and CPB was used to produce total body hypothermia,” and subsequently “CPB was then used to warm the patient and resuscitate the heart.” A timeline incorporating some of the most significant events pertaining to aortic aneurysms can be seen in Fig. 39.1 , including the first successful endovascular aneurysm repair, which occurred in 1990, and the first successful thoracic endovascular aortic repair, which took place in 1992.
The repair of a thoracic aortic aneurysm is now a daily practice in cardiac surgery, and management of such cases presents challenges in the domains of anesthesia, analgesia, ventilation, perfusion, monitoring, and the surgical procedure itself. , Major milestones in the development of the contemporary clinical paradigm of the management of thoracic aortic aneurysms have included OLV, CPB, hemodilution, hypothermic circulatory arrest, cerebral perfusion, as well as advances in anesthesia, pharmacology, and monitoring, including echocardiography and cerebral oximetry. These multiple advances in surgery and anesthesia facilitated the development and practice of techniques for the reconstruction of the ascending aorta and aortic arch following a dissection. , ,
Marfan syndrome is a common genetic disorder that holds implications for thoracic aortic disease. The condition affects around one in 5000 people and will most likely be seen by almost all anesthesiologists during their careers. The specific mutation in Marfan syndrome is in the gene encoding the fibrillin-1 protein, a significant component of the extracellular matrix. It was previously believed that an abnormal fibrillin-1 protein decreased the strength of the extracellular matrix in tissue, such as the aortic wall, causing patients to have abnormal elasticity in the aortic wall and predisposing to dilatation. Interestingly, newer evidence suggests instead that aberrant signaling pathways lead to the degeneration of the aortic wall matrix. It is thought that transforming growth factor beta (TGF-β) is particularly affected by abnormal fibrillin. In fact, the inhibition of TGF-β in these mouse models largely reverses phenotypic and pathologic disease manifestations. The physical manifestations of Marfan syndrome are well-documented and include joint hypermobility, a tall, lean stature, high palate, ocular disorders, mitral valve prolapse, and aortic root dilatation. The most common cause of death in patients with Marfan syndrome is complications involving the aorta. It is not uncommon for patients to be totally unaware that they have Marfan syndrome until they present with an acute aortic event.
The first description of Marfan syndrome was presented by French pediatrician Antonin-Bernard Jean Marfan at the Société Médicale des Hôpitaux de Paris on February 28, 1896 in a presentation entitled, “Un cas de deformation congénitale des quatre membres, plus prononcée aux extrémités, caractérisée par l’allongement des os avec un certain degré d’amincissement” (roughly translated as “a case of congenital deformation of the four limbs, more pronounced at the extremities, characterized by the elongation of the bones with a certain degree of thinning”). The presentation discussed multiple joint contractures, as well as scoliosis and abnormally long and thin limbs and fingers in a 5-year-old girl.
In 1901, Mery and Babonneix used the newly discovered tool of x-rays to further investigate this pathology. They presented newly observed skeletal features at the Société Médicale des Hôpitaux de Paris, documenting x-ray findings of the same patient described by Antonin-Bernard Jean Marfan, noting that the bones showed dévelopement hypertrophié des cartilages, or the development of hypertrophied cartilages. In 1938, the patient died of tuberculosis, and no cardiac or ocular findings were mentioned. Although it is unclear whether or not this small girl actually had what would now be referred to as Marfan syndrome, she indeed had congenital contractual arachnodactyly.
Descriptions further elucidating Marfan syndrome came into the literature over the next several years. A patient with hypogonadism and dolichostenomelia (i.e., lengthy limbs), in addition to mobile joints in the hands, was soon described, and his presentation was used to better characterize the condition. Also ectopia lentis (i.e., subluxation of the ocular lenses) was described in tandem with disproportionately lengthy limbs in 1914, and an association between these physical traits and hereditability was made in 1931, when Weve described distrophia mesodermalis congenita, typus Marfanis. , The following decade, dolichostenomelia was associated with mitral valve degeneration and aortic root dissection. The connection between aortic root dissection and skeletal abnormalities was made by Baer and colleagues in 1943, and the associations between skeletal, ocular, and aortic pathologies in Marfan syndrome have since been a cornerstone in its diagnosis.
It was not until 1986 that the connective tissue glycoprotein (fibrillin) was isolated from human fibroblast cell cultures. It was determined that this glycoprotein was ubiquitous throughout the human body, composing significant portions of the extracellular matrix in skin, tendons, cartilage, cardiac valves, lungs, kidneys, muscle, and corneas. In 1991, the gene linked to Marfan disease, fibrillin-1(FBN1), was ultimately uncovered by researchers at the Mount Sinai Medical Center in New York City. This gene structure was subsequently characterized, and these discoveries contributed to the currently available genetic testing for the diagnosis of Marfan syndrome.
Thoracic aortic aneurysms are oftentimes silent until catastrophe strikes, and almost 95% of patients with thoracic aortic aneurysms are undiagnosed and totally unaware of their condition. , The management of thoracic aortic aneurysms remains challenging in both the elective and emergent settings. The mortality of ruptured thoracic aortic aneurysms approaches 100%, and it can be a difficult decision whether or not to operate on a thoracic aortic dissection once it has been discovered. Clinician opinions differ on when to initiate aggressive surgical procedures, and these decisions hold tremendous consequences for patients. It is highly probable that reported incidences of thoracic aortic aneurysms are underestimates, as fatal thoracic aortic aneurysm ruptures can be misdiagnosed as myocardial infarctions. , No two thoracic aortic aneurysms are the same, and it is critical to understand the etiology and management of this phenomenon. Historically, the only treatment option for aortic arch disease has been open arch replacement under circulatory arrest conditions with or without selective cerebral perfusion. However, this open procedure has significant morbidity and mortality, especially in elderly patients with multiple comorbidities. To potentially mitigate the risks associated with open aortic arch replacement, endovascular arch repair has gained momentum as an alternative treatment option. Currently, efforts to stent the aortic arch are being trialed in numerous international healthcare facilities around the world. Patients selected for this procedure are considered high risk for conventional open arch replacement.
Thoracic aortic aneurysms affect more than 15,000 people in the United States every year, and around 60% of all thoracic aortic aneurysms are in the ascending aorta. One of the principal causes of death because of thoracic aortic aneurysms is a dissection, or a tear in the wall of the aorta, as well as total rupture. Type A aortic dissection (TAAD), for example, is specified in the Stanford classification as a dissection of the ascending aorta, regardless of the distal extent of the tear, whereas type B dissection involves the lower aorta. Overall, pooled hospital mortality from a recent systematic review and metaanalysis demonstrated that hospital mortality for all surgical repairs of TAAD was 11.9%. , Etiologies of TAAD include hypertension, atherosclerosis, connective tissue disorders, trauma, infection, and previous cardiac or vascular surgery. The inherited disorders associated with TAAD include aortopathies associated with Marfan syndrome, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, and the bicuspid aortic valve. Examples of other such conditions that serve as a differential diagnosis for Marfan syndrome can be seen in Box 39.1 . The revised Ghent criteria for the diagnosis of Marfan syndrome in adults is found in Box 39.2 . An artistic illustration of a thoracic aortic aneurysm is presented in Fig. 39.2 . In addition, a computed tomography (CT) angiography of an aneurysmal thoracic aorta in Marfan syndrome is seen in Fig. 39.3 .
Hereditary Connective Tissue Disorders
Fibrillinopathies
MASS (mitral valve, aorta, skeleton, skin) phenotype
Familial mitral valve prolapse
Familial arachnodactyly
Familial ectopia lentis
Shprintzen-Goldberg syndrome
Nonfibrillinopathies
Loeys-Dietz syndrome
Ehlers-Danlos syndrome
Arterial tortuosity syndrome
Aneurysm-Osteoarthritis syndrome
Nonsyndromic familial aortic aneurysms
Bicuspid aortic valve with thoracic aortic aneurysm
Familial aortic aneurysms and dissection
Loeys-Dietz syndrome variants
From Cañadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome. Part 1: pathophysiology and diagnosis. Nat Rev Cardiol . 2010;7(5):256; Loeys B, Matthys D, De Paepe A. Genetic fibrillinopathies: new insights in molecular diagnosis and clinical management. Acta Clin Belg . 2003;58(1):3–11.
Criteria for the Diagnosis of Marfan Syndrome
In the absence of a family history of Marfan syndrome:
Aortic root dilatation Z score >2 and ectopia lentis
Aortic root dilatation Z score >2 and an FBN1 mutation
Aortic root dilatation Z score >2 and a systematic score >7
Ectopia lentis and an FBN1 mutation associated with aortic root dilatation
In the presence of a family history of Marfan syndrome (as defined in 1–4 earlier):
Ectopia lentis and a family history of Marfan syndrome (as defined earlier)
Systemic score > 7 and a family history of Marfan syndrome (as defined earlier)
Aortic root Z score >2 if above 20 years old, >3 if below 20 years old, and a family history of Marfan syndrome (as defined earlier)
Systemic points calculate as follows: wrist and thumb sign (3 points), wrist or thumb sign (1 point), pectus carinatum deformity (2 points), pectus excavatum or chest asymmetry (1 point), hindfoot deformity (2 points), plain flat foot (1 point), spontaneous pneumothorax (2 points), dural ectasia (2 points), protucio acetabulae (2 points), scoliosis or thoracolumbar kyphosis (1 point), reduced elbow extension (1 point), three of five facial features (1 point), skin striae (1 point), severe myopia (1 point), mitral valve prolapse (1 point).
From Von Kodolitsch Y, De Backer J, Schüler H, et al. Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome. Appl Clin Genet. 2015;8:137.
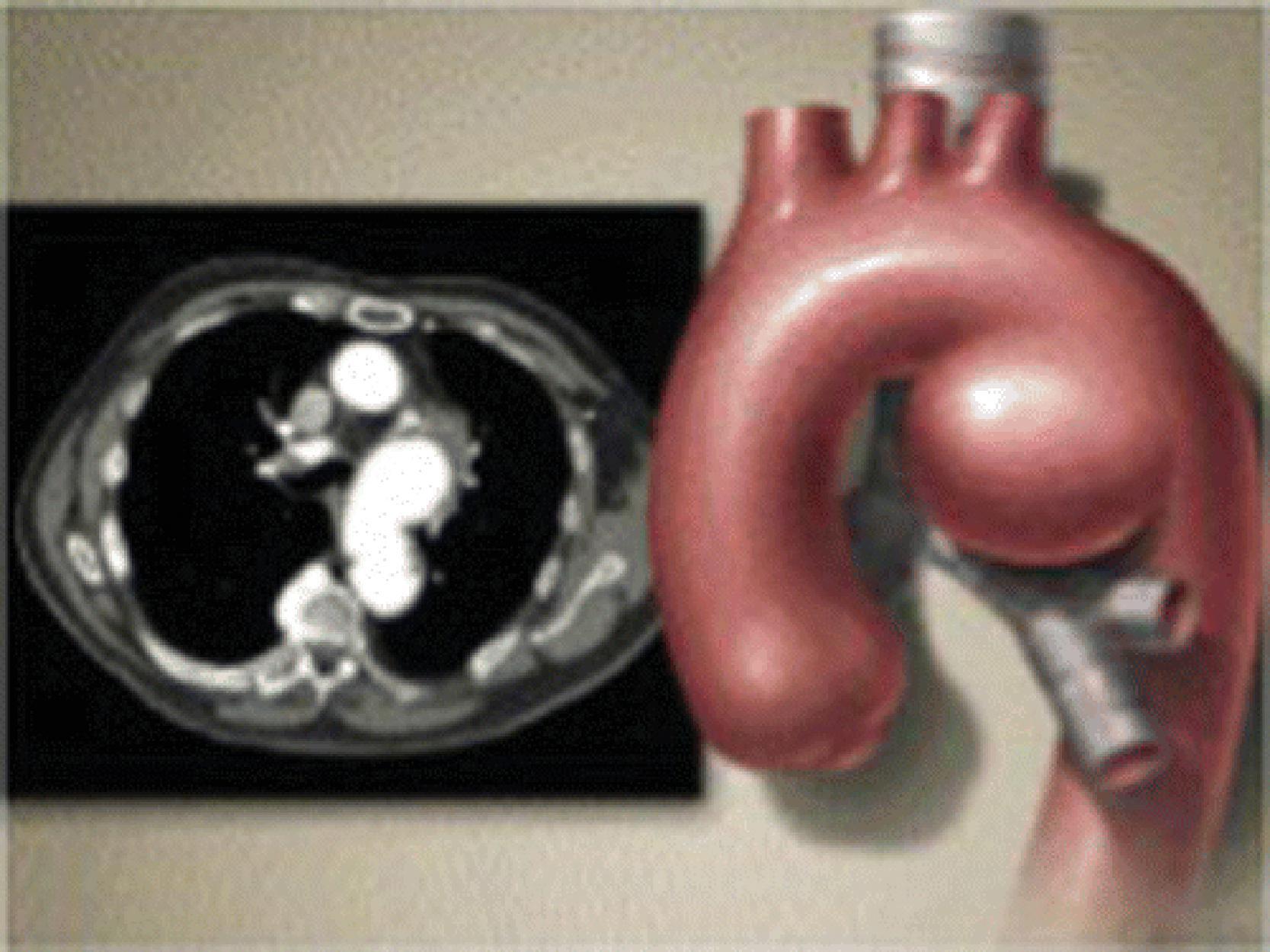
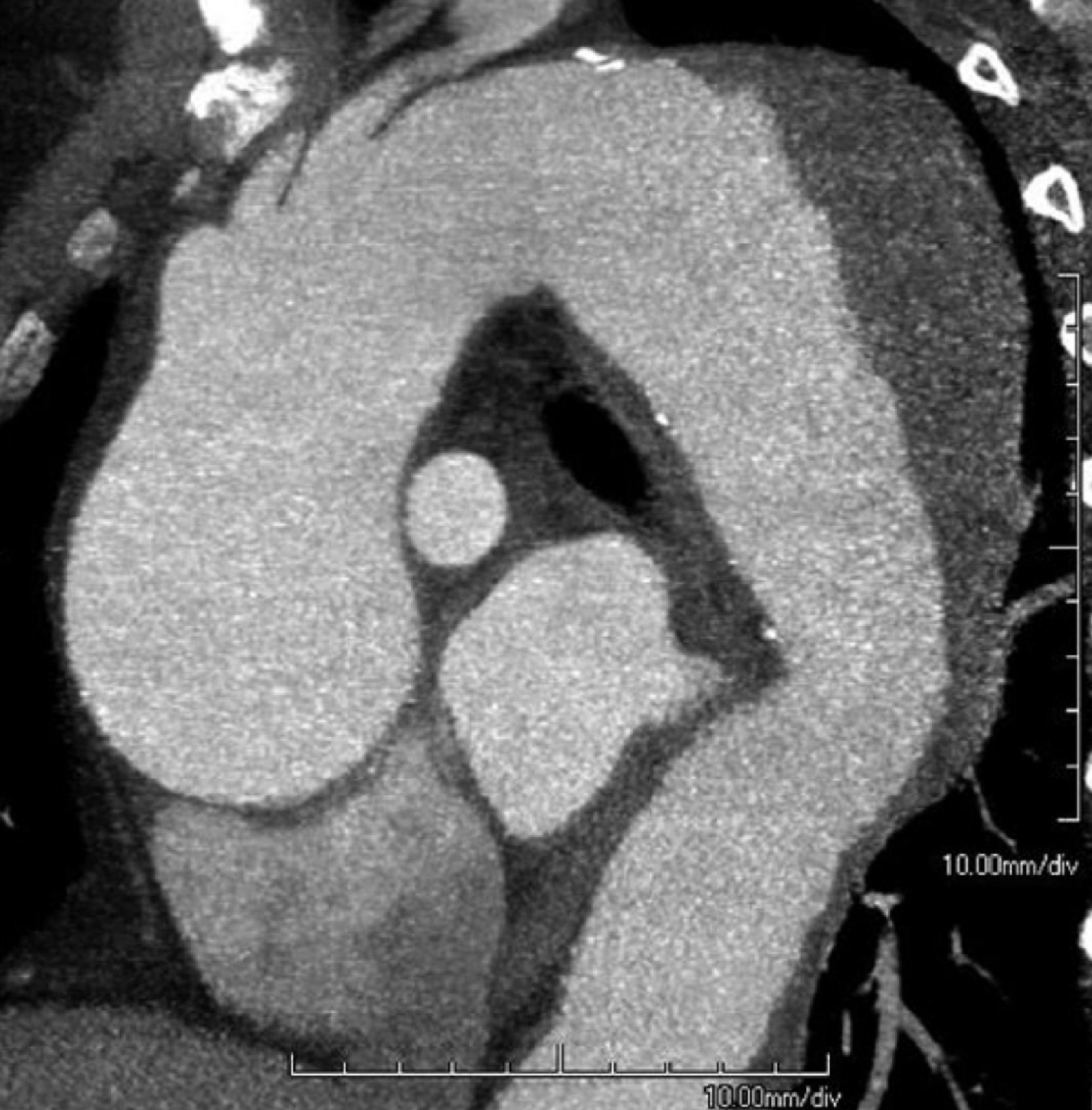
The following Case Presentation and Case Management sections will demonstrate the preoperative, intraoperative, and postoperative care of a young man with Marfan syndrome who is found to have a thoracic aortic aneurysm. We will use this case to underscore some of the important aspects in the care of patients with thoracic aortic aneurysms and discuss some of the most up-to-date and practical literature on the topic.
A 17.5-year-old male presented to the emergency department of a local hospital because of the sudden onset of sharp, substernal chest pain that he reported as 7 out of 10 in intensity. He stated that the pain did not radiate. The patient did not have any previous discomfort or history of similar symptomatology and was in his normal state of health and not active when the pain began. A basic examination revealed mild pectus excavatum and nearsightedness, which were consistent with a potential diagnosis of Marfan syndrome. The patient had a CT scan, which revealed a 7.7-cm ascending thoracic aortic aneurysm. Following this discovery, the patient was urgently transferred to the Aortic Aneurysm Surveillance Program at the Mount Sinai Medical Center in New York City.
At the Aortic Aneurysm Surveillance Program, further medical history revealed that the patient did not take any medications and had no known allergies. He had no other known past medical history, and he saw a pediatrician regularly. He stated that he was a starting center on his high school’s basketball team, and he had tried out for several prominent college-level basketball programs. The patient was an only child and lived with his parents. The patient’s pain subsided while at the Aortic Surveillance Clinic, and he stated he felt normal.
On examination, the patient’s vital signs were notable for tachycardia (115 beats per minute) and hypertension (145/85 mm Hg measured on both arms). His other vital signs were unremarkable: his respiratory rate was 14 breaths per minute, oxygen saturation (SpO2) 100%, and he was afebrile. The patient had a height of 80.5 inches and a slender build, weighing 92 kg. The patient’s arms and legs were long and seemingly disproportionate to his torso. He was also noted to have a high-arched palate and slight pectus excavatum on his chest exam, which he stated had been noted by previous providers. His cardiac examination was notable for a soft, high-pitched 1-2/6 diastolic decrescendo murmur most prominent at Erb’s point (the left third intercostal space), consistent with aortic regurgitation.
An electrocardiogram showed normal sinus rhythm. The patient then underwent an echocardiogram, which revealed a thoracic aortic root aneurysm with a sinus of Valsalva diameter of 7.7 cm and an aortic root of 7.6 cm. There was evidence of moderate aortic regurgitation but no evidence of aortic dissection. The left ventricle showed mild dilation with a normal ejection fraction. A trace of tricuspid regurgitation with pulmonary insufficiency was evident. Laboratory values, including a coagulation panel, were within normal limits.
The cardiology and surgical teams decided that urgent cardiac surgery was in the patient’s best interest.
The patient was sent to the Preoperative Evaluation Clinic, and the operation was scheduled for the next day. Additional tests were performed to ensure the patient was in an optimized state of health before his thoracic aortic aneurysm resection. Cardiac catheterization revealed normal coronary arteries, and magnetic resonance imaging confirmed the dimensions of the aorta. The patient was informed of the risks and benefits of the operation, and informed consent was obtained. The patient and his relatives were informed about the course of the surgical treatment, including aspects of mechanical ventilation, the length of the intensive care unit stay, as well as the rehabilitation and discharge process.
On the day of surgery, a brief review of his medical status was performed, and his vital signs were checked. Vancomycin and cefazolin were administered 90 minutes before incision. Aminocaproic acid was used as the antifibrinolytic of choice. The availability of type-matched blood and fresh frozen plasma was confirmed with the blood bank. A rapid infusion system was made available in the operating room.
The patient was brought into the operating room, and anesthesia was induced with midazolam, fentanyl, etomidate, and vecuronium, with desflurane used for anesthetic maintenance. Standard anesthesia management for open heart surgery was used with OLV. Using a double lumen tube, the right lung was ventilated while the left lung was collapsed for the thoracic aortic aneurysm resection. In addition to the standard American Society of Anesthesiologists (ASA) monitors, access to the left radial artery was obtained, and near-infrared spectrometry and bispectral index leads were placed on the patient’s forehead. Initial cerebral oximetry readings were 71% and 74% on the right and left hemispheres, respectively. Additional intravenous access was obtained via a 9 Fr multilumen catheter in the right internal jugular vein, as well as a pulmonary artery catheter. A coagulation monitoring system was used.
A transesophageal echocardiogram (TEE) probe was inserted. The preoperative TEE examination revealed a 2.6-cm aortic valve annulus with 1+ aortic regurgitation, with no aortic stenosis and mildly distorted aortic valve leaflets because of aortic dilation. The ascending aorta was measured as 7.6 cm in diameter, and the sinus of Valsalva was 7.7 in diameter. There was also mild mitral and tricuspid regurgitation. The left ventricular ejection fraction (LVEF) was 60%.
Surgery started with the exposure of the right axillary artery and right femoral vein. Following heparinization, a median sternotomy was performed under precautionary CPB to protect the patient from the possibility of bleeding problems because of the large thoracic aneurysm and deep pectus excavatum. The initiation of total CPB and cooling began and proceeded uneventfully. The TEE indicated sufficient blood flow into the aortic arch. The esophageal temperature was brought down to 15°C in preparation for circulatory arrest, and the jugular bulb oxygen saturation reached 97%. At this time, perfusion was stopped, and CPB was initiated. Following the induction of hypothermia and the cessation of perfusion before a short period of circulatory arrest, the valve-sparing aortic root replacement (the David procedure) was conducted uneventfully.
Rewarming was subsequently initiated, and the patient was noted to have appropriate urinary output. His hemoglobin and hematocrit levels were maintained with intraoperative cell salvage and one unit of packed red blood cells. Following careful hemostasis, the patient was gradually rewarmed, and he was weaned off CPB. Heparin was neutralized with protamine sulfate. The total bypass time was 350 minutes, and cross-clamp time was 20 minutes. The lowest temperature before deep hypothermic circulatory arrest was 15°C.
The patient was taken to the cardiothoracic intensive care unit for close monitoring. He was extubated on postoperative day one. There was increased chest tube output and a drop in hematocrit on postoperative day two, and the patient was transfused three units of packed red blood cells and one pool of platelets. The patient remained hemodynamically stable afterwards and was started on a low-dose beta blocker. The patient’s neurologic and hemodynamic status was closely monitored, and he was discharged on postoperative day four.
Following discharge, the patient had additional testing to further investigate his Marfan status. He underwent genetic testing, which confirmed a diagnosis of Marfan syndrome, as well as ophthalmologic evaluation. A CT angiogram revealed a normal post-David-procedure aorta, without any evidence of dissection or thrombotic changes. The patient was asymptomatic in good health, and ultimately elected to attend his school of choice on a basketball scholarship.
The management of patients with thoracic aortic aneurysms must involve detailed and clear communication between cardiologists, surgeons, and anesthesiologists. In concert, these physicians should develop a strategy for the preoperative, intraoperative, and postoperative complications that patients may face. In addition to every organ system being evaluated, and the administration of proper perioperative antibacterial prophylaxis, physicians should account for what may be a lengthy procedure using hypothermia and prosthetic materials to reconstruct the aorta. In nonemergent situations, preoperative evaluation before elective open thoracic aortic surgery is of paramount importance, and one must take the time to consider nearly every organ system in any assessment. In both emergent and elective surgical procedures, careful anesthetic management is required for the prevention of intraoperative hypertension and aortic rupture. The anesthetic approach to the patient with a thoracic aortic aneurysm depends on the urgency of surgical intervention. One of the most difficult decisions facing patients and physicians is whether to perform surgery when a thoracic aortic aneurysm is diagnosed. There are several issues to keep in mind, as stated by Dr. John Elefteriades: “Thoracic aortic aneurysm, while lethal, is indolent. Mortality usually does not occur until after years of growth. The aneurysmal ascending thoracic aorta grows slowly: about 0.1 cm per year. Over a patient’s lifetime, ‘hinge points’ at which the likelihood of rupture or dissection skyrockets are seen at 5.5 cm for the ascending aorta. Intervening at 5 cm diameter for the ascending thoracic aortic aneurysm prevents most adverse events. Symptomatic aneurysms require resection regardless of size. The yearly rate of rupture, dissection, or death is 14.1% for a patient with a thoracic aorta of 6 cm diameter.” Elective surgery for nonurgent cases may provide the best results. In cases in which surgery is not recommended, patients are followed over time and evaluated periodically until the potential benefits of surgery outweigh risks. ,
Thoracic aortic aneurysms can be either acquired or inherited. Oftentimes, thoracic aneurysms of the ascending aorta are secondary to associated conditions, such as Marfan syndrome, cystic medial degeneration, the expansion of an aortic dissection, trauma, poststenotic dilatation, or dilatation following previous aortic manipulation. Such thoracic aneurysms are usually seen along with a bicuspid aortic valve, and hypertension is commonly present. Historically, advanced syphilis was associated with ascending thoracic aortic aneurysms, although this is significantly less common today. Furthermore, various familial thoracic aortic aneurysm syndromes have been described.
Aneurysms of the ascending thoracic aorta may extend into the aortic arch. Repair can occur via the head vessels being reinserted individually to a main graft, reimplanted as a unit, or anastomosed to a composite graft, as seen in Figure 39.4 . , In these procedures, hypothermic circulatory arrest is used as the mainstay of cerebral protection. Subsequently, a composite graft can be used to perform selective cerebral perfusion as the arch is reconstructed. Adjunctive therapies for cerebral protection includes the use of steroids to prevent edema, barbiturates, and retrograde cerebral perfusion, and various techniques have also been described in preparation for cerebral circulatory arrest, such as electroencephalography (EEG), jugular bulb saturation monitoring, and cerebral oximetry.
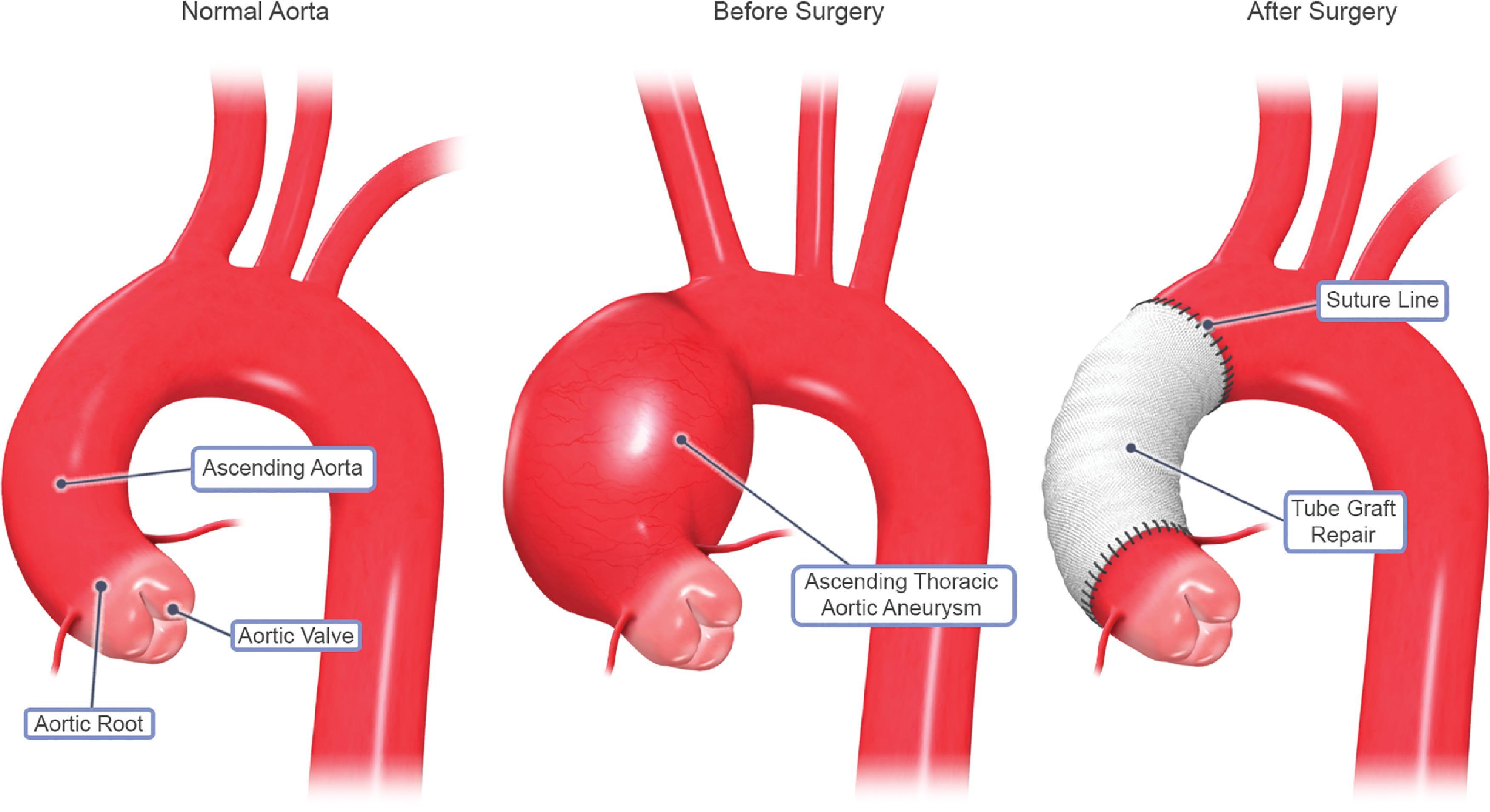
Many patients undergoing thoracic aortic aneurysm resections have medical comorbidities, such as hypertension, coronary artery disease, or atherosclerosis. Others may also have congestive heart failure or ischemic heart disease. Coronary artery ischemia may result from distortions in the anatomy and physiology of the heart, resulting in decreased oxygen supply to the entire organ. Thoracic aortic aneurysms may also distort the aortic valve, leading to valve incompetence and heart failure. Before thoracic aortic aneurysm resection, several tests should be performed. For example, an electrocardiogram can demonstrate ischemia or prior myocardial events, in addition to left ventricular hypertrophy and other electrical abnormalities, suggesting some form of cardiac impairment. The thoracic aneurysm itself should be assessed via echocardiography which can accurately reveal the dimensions of the thoracic aneurysm, as well as valve function and anatomy. Angiography can be used to further visualize the thoracic aneurysm, especially in patients with coronary artery disease.
Cardiovascular complications in patients with Marfan syndrome are recognized as the leading cause of morbidity and mortality. The risk of an acute aortic event in a patient with Marfan syndrome increases steadily with age, and such events result in death in up to 50% of undiagnosed patients by age 40 years. Additional cardiovascular pathologies are seen in Marfan syndrome, such as mitral valve prolapse with regurgitation, pulmonary artery dilatation, and aortic valve regurgitation. About two in three patients with Marfan syndrome have an associated cardiothoracic abnormality other than aortic root dilatation, and physicians must be aware of these comorbidities. In children with early-onset and severe Marfan syndrome, these pathologies are particularly alarming, and mitral valve insufficiency can lead to congestive heart failure and death.
Proper perioperative fluid management is essential to the prevention of kidney injury during aortic resection surgery. Anesthesiologists should implement renal-protective strategies, such as the use of diuretics like mannitol or furosemide, as well as the possible use of low-dose dopamine to enhance renal perfusion. The use of CPB can result in some kidney dysfunction. Patients with thoracic aortic aneurysms are susceptible to kidney dysfunction. Those at highest risk for renal failure include patients with preoperative renal dysfunction, those with aneurysms extending distally to include renal artery involvement, and those with a history of congestive heart failure. In addition, transient elevations of kidney enzymes can be seen with the administration of contrast dyes. Relevant to our patient who had an ascending thoracic aortic aneurysm, it has been demonstrated that up to 10% of ascending thoracic aortic aneurysm resections can result in kidney failure. All patients should have preoperative creatinine and blood urea nitrogen measurements to attain a baseline before thoracic aortic aneurysm repair.
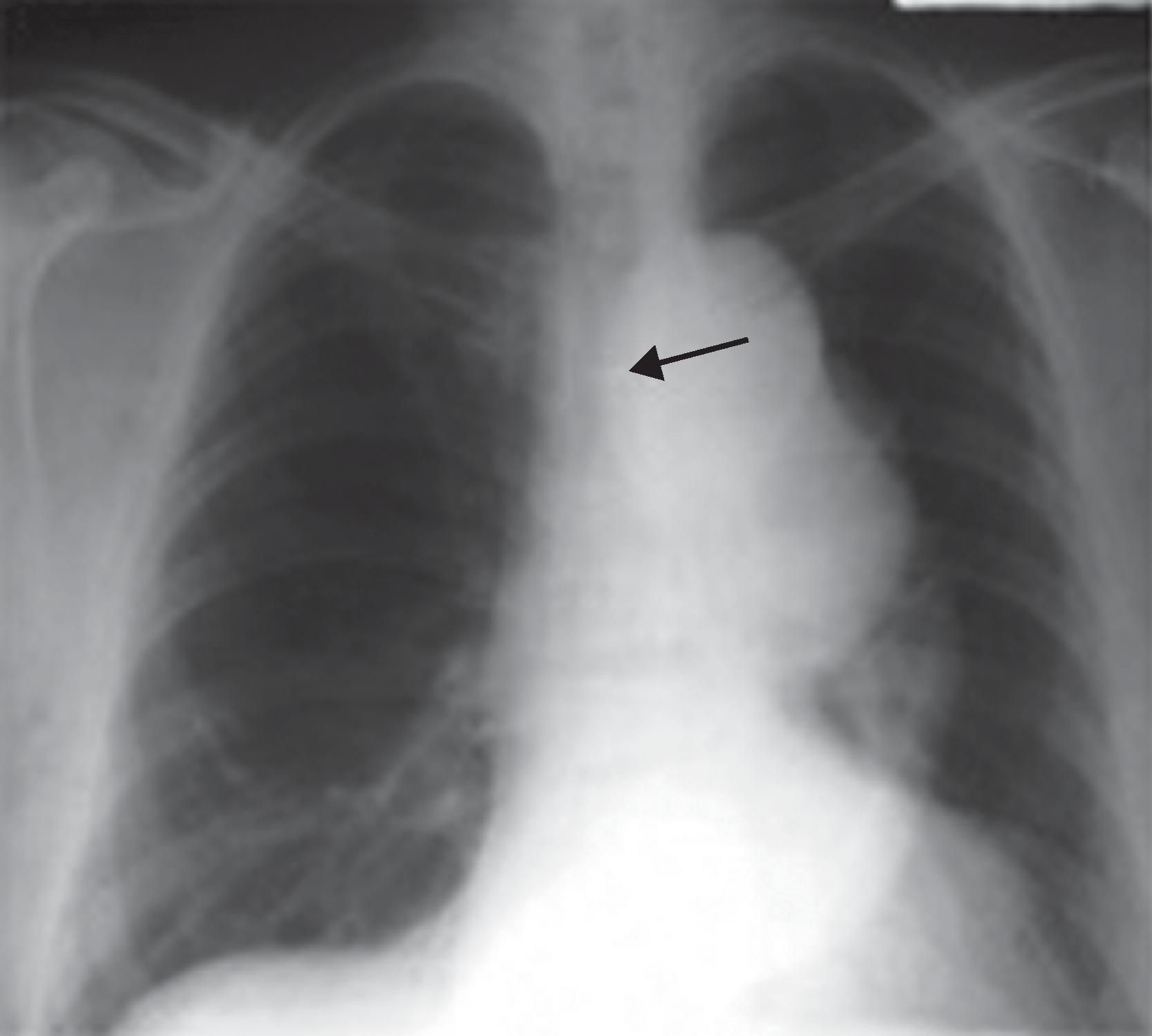
Baseline arterial blood gas analyses and pulmonary function testing should be obtained before thoracic aortic aneurysm surgery because these data can aid in ventilator weaning following the surgical procedure. In addition, the preoperative physical examination is crucial to lung function and potential respiratory disease. For example, stridor or dyspnea may suggest compression on the trachea or bronchi, and overt hemoptysis. An x-ray depicting the compression of an airway by a large thoracic aortic aneurysm can be seen in Fig. 39.5 . Hoarseness on examination may indicate a recurrent laryngeal nerve palsy in patients with ascending thoracic aortic aneurysms. In addition, patients should be aware that postoperative hoarseness may occur if there is damage to the recurrent laryngeal nerve during aortic surgery. Other considerations regarding recurrent laryngeal nerve injury include predisposition to aspiration, and patients should be counseled about the seriousness of this potential but rare complication. Our patient had no hoarseness and his pulmonary examination was otherwise normal, with lungs clear to auscultation bilaterally and no wheezes or rhonchi noted.
Chronic obstructive pulmonary disease is common in patients with thoracic aortic aneurysms, as many have a lengthy smoking history. A history of smoking fundamentally changes anesthetic and ventilatory parameters, and any history of smoking should be clarified with the patient. Our patient (with no smoking history) was successfully weaned without incident from the DLT. However, this process could have been far more tumultuous had the patient been a smoker, and interestingly, the most common complication of ascending and aortic arch repairs is prolonged intubation following surgery.
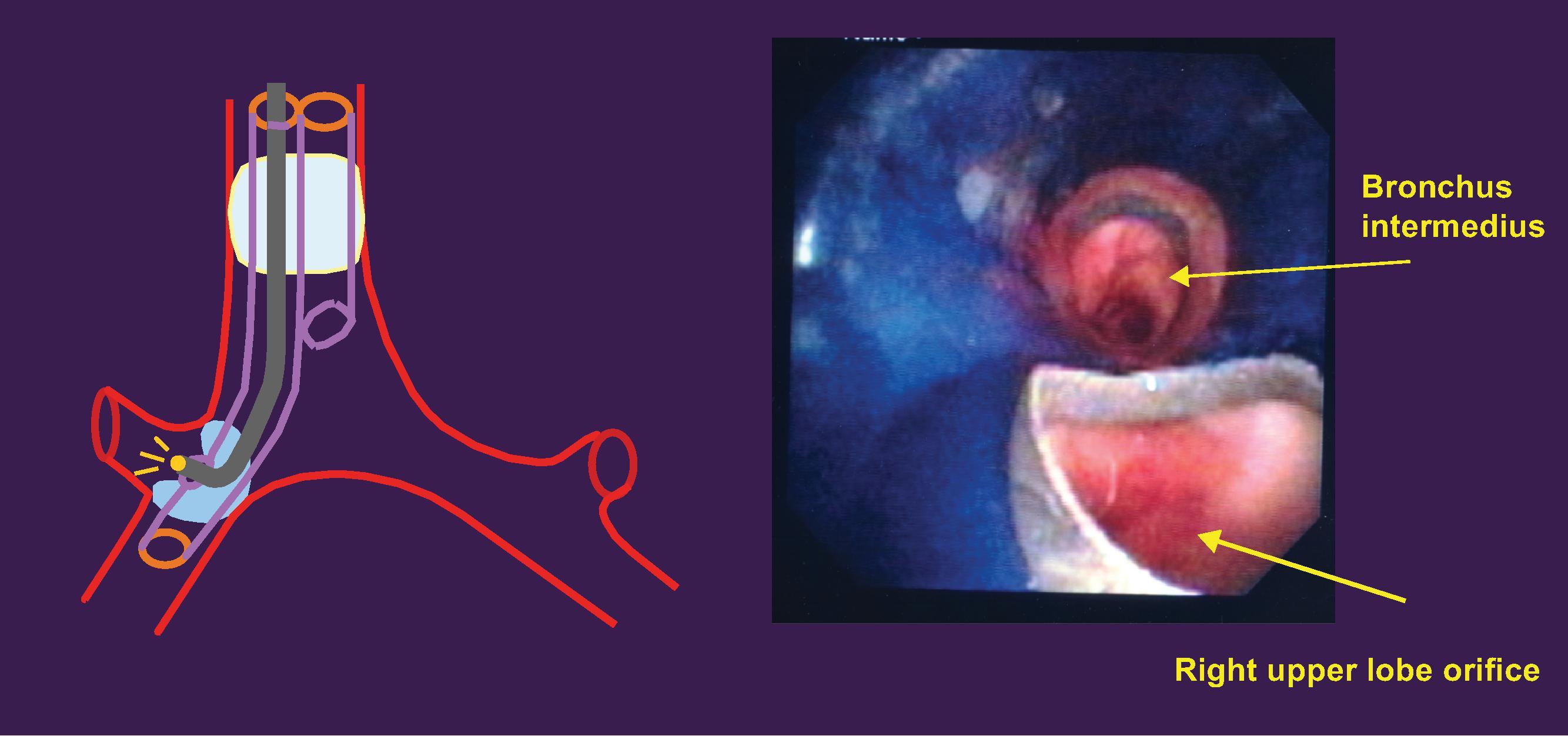
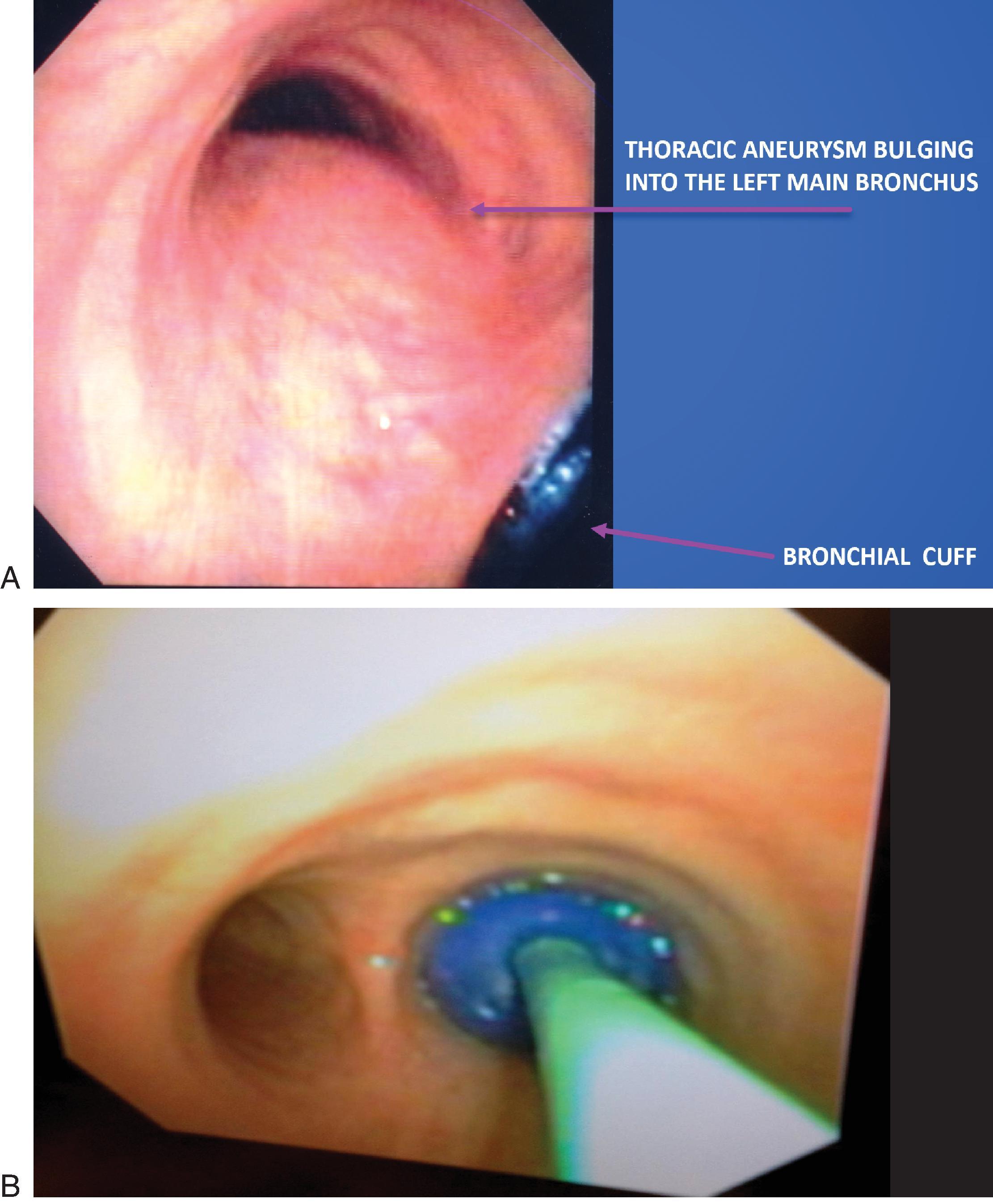
Preoperative chest x-rays are an effective way to identify potential complications of thoracic aortic aneurysms, such as a deviated trachea or left mainstem bronchus, which are both important considerations when placing a left-sided DLT. Left-sided endotracheal tubes are overwhelmingly preferred for this procedure and not only provide a safety benefit, but also grant surgeons more space in the chest cavity to operate. The most significant complication of such endotracheal tubes is blunt trauma to the thoracic aneurysm, which could cause the aneurysm to tear. A tracheal bronchoscopy through a right-sided double-lumen tube in a patient with a thoracic aneurysm can be seen in Figs. 39.6 ; and 39.7A and B.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here