Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aortic diseases, including aortic aneurysms, are the 17th leading cause of death in the United States. Descending thoracic aortic aneurysms (TAAs) and thoracoabdominal aortic aneurysms (TAAAs) occur with an estimated incidence of 5.9 cases per 100,000 person-years. A study by Clouse and others suggests that the incidence is increasing. During the last 20 years, the mortality for nonruptured and ruptured TAAs and TAAAs in the US population aged 55 and older ranges from only 0.1 to 2.2 and 0.1 to 2.6 per 100,000, respectively, and increases with age. TAA repair has itself traditionally been associated with high morbidity and mortality. Although aortic aneurysms may affect the ascending aorta and aortic arch, the focus of this chapter is on descending TAAs and TAAAs, which can occur from the left subclavian artery to the aortic bifurcation.
Thoracic aortic pathology is very complex and may be related to a variety of histopathologic conditions. Most TAAs and TAAAs are secondary to either medial degeneration or aortic dissection. Medial degeneration is characterized by disruption and loss of elastic fibers and increased deposition of proteoglycans. Many other thoracic aortic pathologies, including the acute aortic syndromes (aortic dissection, intramural hematoma, and penetrating aortic ulcers) and various forms of vasculitis (giant cell arteritis, Takayasu arteritis, and Behçet disease), can induce TAAAs. Although isolated abdominal aortic aneurysms were once called atherosclerotic, TAAs associated with frank atherosclerosis are much rarer. Other histopathologies associated with the thoracic aorta include coarctations, various brachiocephalic anomalies (e.g., aberrant right subclavian arteries), and tumors. Disastrous complications of TAA include pulmonary and enteric fistulas. This chapter provides an overview of thoracic aortic disease, including guidelines for the diagnosis and management of patients with thoracic aortic disease and the contemporary management of TAAs and TAAAs.
TAAAs are localized dilatations in the thoracic and abdominal aorta secondary to weakening and subsequent expansion of the aortic wall. When all aneurysms of the thoracic aorta are considered, those of the ascending aorta are the most common (40%), followed by those of the descending thoracic aorta (35%), aortic arch (15%), and thoracoabdominal aorta (10%). A TAAA by definition is an aortic dilatation at least 1.5 times its normal value. It is critically important to define these anatomic aortic sizes ( Table 78.1 ) to help identify pathologic aortic growth, because TAAA diameter is the strongest predictor of rupture, with a reported mean aortic diameter of ruptured TAAAs of 6.1 cm.
| Aortic Segment | Mean Aortic Diameter (cm) | Mean Aortic Length (cm) | Percentage of Aneurysms |
|---|---|---|---|
| Ascending | 3 | 5 | 40 |
| Aortic arch | 2.5–3.5 | 4 | 15 |
| Descending thoracic | 2.0–2.3 | NR | 35 |
| Thoracoabdominal | 1.7–2.6 a | NR | 10 |
The normal thoracic aortic is divided into four parts: the aortic root, the ascending aorta, the aortic arch, and the descending thoracic aorta. The normal thoracic aortic wall is composed of three layers, similar to all blood vessels, which include the intima, media, and adventitia. The aorta normally enlarges as it progresses from the aortic root to the terminal aorta. In addition, gender, age, and body surface area influence aortic diameter ( Table 78.2 ). After adjusting for age and body surface area, mean aortic size is significantly smaller, usually 2 to 3 mm, in women than in men. Body surface area is reported to be a better predictor of aortic diameter than height or weight. The annual increase in TAAA cross-sectional diameter is highly variable, with reported increases of 1.9 to 3.4 mm/year. , Importantly, the growth rates of TAAAs are not predictable or linear. However, there is consensus that TAAAs, similar to AAAs, have growth rates that accelerate as they enlarge. , For example, TAAAs larger than 5 cm expand at a rate of 0.30 to 0.79 cm/year, whereas TAAAs less than 5 cm experience growth rates of 0.17 to 0.22 cm/year. , Additionally, the distal aorta manifests more rapid growth than the more proximal segments. Dapunt et al. have documented that patients with ruptured TAAAs experienced growth rates of 0.7 cm/year, while Zafar and colleagues demonstrated a sharp increase in risk of aneurysm rupture at aortic diameters greater than 6 cm.
| Aortic Segment and Gender | Range of Reported Mean (cm) | Modality of Assessment |
|---|---|---|
| Mid-descending (female) | 2.45–2.64 | CT |
| Mid-descending (male) | 2.39–2.98 | CT |
| Diaphragmatic (female) | 2.40–2.44 | CT |
| Diaphragmatic (male) | 2.43–2.68 | CT |
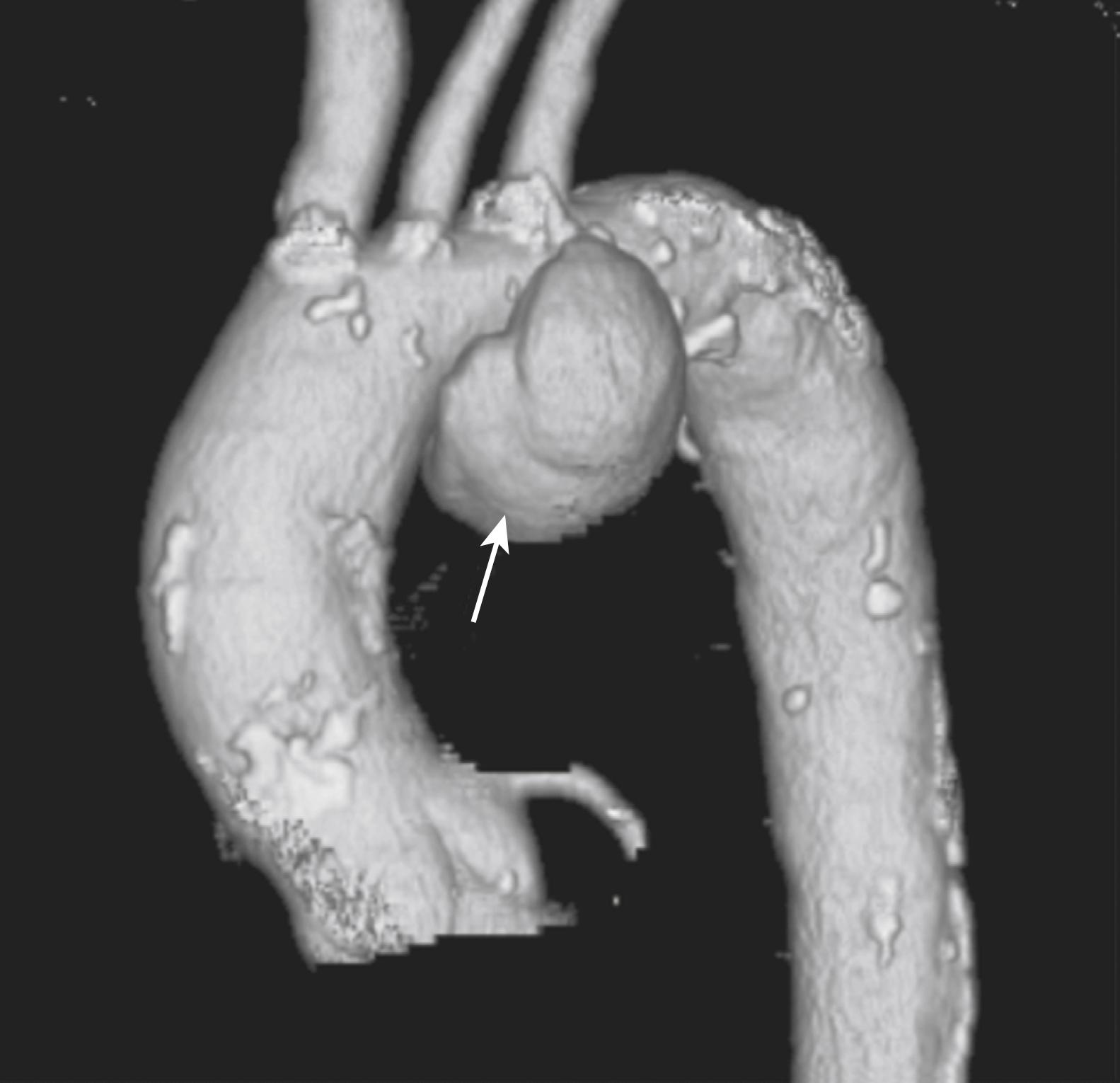
The terms saccular and fusiform should also be defined for TAAAs, because the morphology of an aneurysm will often dictate treatment options, as well as point to specific causes (i.e., mycotic aneurysms are often saccular and at high risk for rupture). Most TAAAs are fusiform aneurysms, which involve a chronic uniform dilatation involving the whole circumference of the aorta. In contrast, saccular aneurysms often represent an eccentric dilatation of the aorta ( Fig. 78.1 ) and most would concur that a lower threshold is an acceptable indication for repair compared to fusiform aneurysms.
TAAAs are primarily a disease of the elderly. The average age of patients with TAAAs is 65 years, with a male:female ratio of 1.7:1 2 ; in contrast, in patients with AAAs whose mean age is 75 years, the male:female ratio is 6:1. TAAAs clearly have a genetic component, since more than 20% of patients will have a first-degree relative affected by aneurysm disease.
Many risk factors that are common in patients with AAAs, including hypertension, smoking, and atherosclerosis in other arteries, are also common in patients with TAAAs. , Systemic hypertension – in particular elevated diastolic blood pressure greater than 100 mm Hg – has been associated with aortic growth and rupture. , Although TAAAs are most often described as degenerative, up to 20% are the sequelae of chronic aortic dissection (see Ch. 83 , Aortic Dissection: Epidemiology, Pathophysiology, Clinical Presentation, and Medical and Surgical Management). In one series of 200 patients, 28% required aortic replacement with a mean follow-up of 28 months; the only factor predictive of growth was an initial aortic diameter >3.5 cm, while false lumen thrombosis was protective against growth.
Multiple genetic syndromes, including, most commonly, Marfan syndrome, have been associated with TAAs and dissections. The vascular manifestations of Marfan syndrome invariably involve aortic root dilation, progressive root and ascending aortic aneurysmal degeneration, and potentially aortic dissection of variable extent. The extent of dissection predicts the need of future intervention on the distal aorta, but there is no consensus on appropriate management of the aortic arch. Reoperation is common in Marfan syndrome, occurring in 20% of patients at a mean of 5 years from the index operation, with most of these requiring multiple reoperations (see Ch. 141 , Arterial Disease in Patients with Connective Tissue Disorders).
Familial aggregation studies have shown that between 11% and 19% of patients referred for TAA or dissection repair have a first-degree relative with a TAA or dissection. , , Patients with a strong family history of TAA tend to present at a younger age than those without a positive history, yet at an older age than those with connective tissue disease. Familial thoracic aortic aneurysm (TAAD) appears to be predominately inherited in an autosomal dominant fashion with variable expression. Mapping studies have shown that there is significant genetic heterogeneity in TAAD patients. To date, 37 TAAD-associated genes have been identified, aggregately explaining roughly 30% of nonsyndromic TAAD. , Even among patients with Marfan syndrome, in whom mutations in fibrillin-1 ( FBN1 ) are the culprit genetic defects, the pathogenesis is more complicated than previously thought. The genetic implications of TAAs and dissections are beyond the scope of this chapter and are the subject of many contemporary reviews.
Since most patients with TAAAs are asymptomatic, treatment is focused at preventing rupture. Natural history studies of TAAAs are rarer than those of isolated infrarenal AAAs, likely related to their much less frequent occurrence. In addition, studies on TAAAs often include patients with both acute and chronic aortic dissection, which serves to complicate natural history analyses. Initial studies from the 1970s by Pressler and McNamara documented that approximately 40% of patients who did not undergo surgical repair died of TAAA rupture, whereas 32% died of other cardiovascular diseases. Mean survival was less than 3 years. During the extended period of observation, more than 90% of patients sustained aortic rupture, with 68% of ruptures occurring more than 1 month after the diagnosis. ,
The 5-year survival rate for patients with 6.0-cm TAAAs is 54%, with a risk for rupture of 3.7%–5.9%/year and a risk for death of 12%/year. The risks of rupture and death increase to >9%/year and >18%/year, respectively, for patients with TAAA >6.5 cm. Median survival in patients with untreated TAAAs, at only 3.3 years, is poor. In a natural history study by Crawford and DeNatale of patients who were not candidates for surgery, the survival rate was just 24% at 2 years, with over half the deaths related to aneurysm rupture. Chronic obstructive pulmonary disease (COPD) was noted in 80% of the subgroup with rupture. Similar studies in patients with small infrarenal AAAs have confirmed COPD as a significant risk factor for rupture. Cambria and others followed a series of 57 patients with TAAAs who were not considered operative candidates. In addition to COPD, the authors found an association ( P = 0.06) between rupture and chronic renal failure. In a study by Griepp and colleagues, 165 patients with TAAAs were monitored after being assigned nonoperative status. Twenty percent of patients eventually suffered rupture. Important risk factors for rupture included older age, COPD, uncharacteristic continued pain, and aortic diameter. Patients with aortic dissection ruptured at smaller aortic diameters than did those with degenerative aneurysms.
Maximal aortic dimension has emerged as the most reliable predictor of future aortic dissection or rupture. Those with aortic diameters less than 50 mm experienced estimated annual event rates (rupture or dissection) of less than 1%, as compared with 2.7% to 8.1% and 37.5% to 62.5% among those maximal diameters of 50 to 60 and greater than 60 mm, respectively. Dapunt and colleagues documented that TAAAs greater than 8 cm have an 80% risk for rupture within 1 year of diagnosis. However, the size at which TAAAs rupture is unpredictable. Similar to AAAs, it appears that aneurysm growth rates play a role. The average expansion rate of a TAAA is approximately 0.10 to 0.42 cm/year. , Coady et al. suggested that expansion by more than 1 cm/year signals impending rupture. Juvonen et al. examined 114 patients with TAAAs in detail. Multivariate analysis suggested that increasing age, pain (even atypical), COPD, descending thoracic aortic diameter, and abdominal aortic diameter were predictive of rupture ( Table 78.3 ).
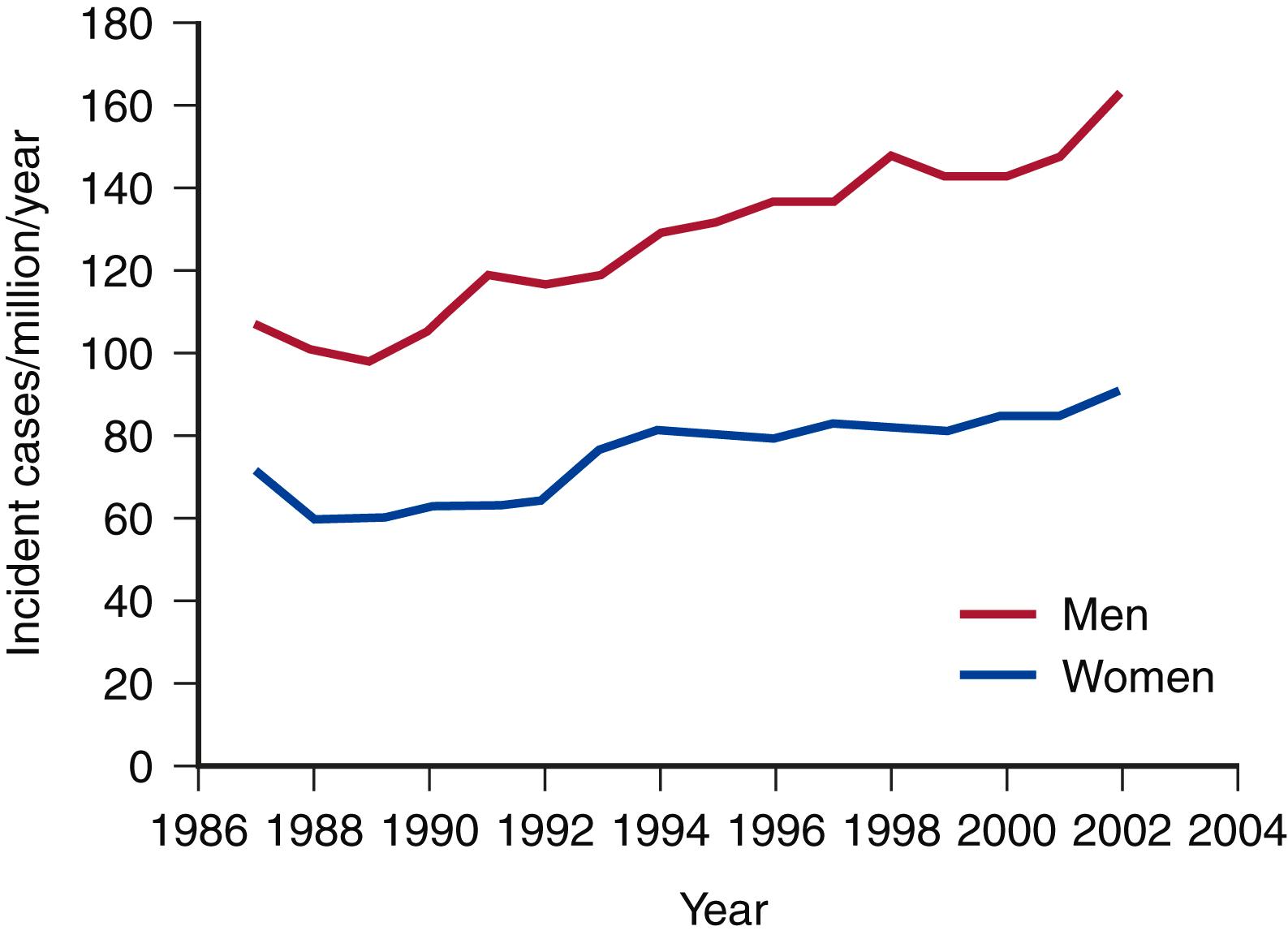
Clouse and coauthors have documented that the incidence of TAAAs in the United States is 10.4 cases per 100,000. Olsson examined the prevalence of TAAA in a large contemporary population. All subjects with thoracic aortic dissections or aneurysms from 1987 to 2002 were identified in the Swedish National Healthcare Registry. Of 14,229 individuals with thoracic aortic disease, the diagnosis was made in 11,039 (78%) before death. The incidence of thoracic aortic disease rose by 52% in men and 28% in women to reach 16.3 and 9.1 per 100,000 per year, respectively. The authors concluded that the prevalence and incidence of thoracic aortic disease were higher than previously reported and were increasing ( Fig. 78.2 ). The increasing prevalence of TAAAs has been attributed to a number of factors, including improved imaging techniques, an aging population, and increased patient and physician awareness.
The development of a TAAA is a multifactorial event that involves a complex interaction of genetic factors, cellular imbalance, and altered hemodynamic factors. The increased incidence of TAAAs in patients with Marfan syndrome and other connective tissue disorders inherited in a Mendelian manner, as well as the familial inheritance of nonsyndromic TAADs typically inherited in an autosomal dominant fashion with marked variability in the age of onset and decreased penetrance, suggests a varying genetic role along different segments of the aorta. , , As alluded to previously, even in Marfan syndrome, the mechanisms behind aortic aneurysm degeneration are more complex than previously appreciated. More recent studies have suggested that genetic variation in extracellular matrix (ECM) actin and myosin may contribute to the development of TAAAs. , Wang and coauthors examined genetic signatures in the peripheral blood of patients with TAAAs ( n = 58) and control patients ( n = 36). This study documented a number of gene families that were different between the two groups, including those involved in the cell cycle, DNA metabolism, glycolysis, interferon-γ signaling, and transcription factors. The authors concluded that analysis of peripheral blood to determine expression signatures may help to identify patients with TAAAs with high accuracy though, at this point, finding clinically relevant biomarkers to aid in identifying patients with nonsyndromic TAAA has proven elusive. ,
Given the myriad factors involved, TAAA formation, therefore, is a complicated, dynamic process involving both extracellular and cellular processes, similar to other aneurysms. Once initiated through a combination of the aforementioned factors, inflammation and pathologic remodeling of the ECM occur. A large body of evidence suggests that ECM degradation by matrix metalloproteinases (MMPs) exceeds matrix production and repair during aneurysm formation. It is important to emphasize that there are significant differences in the composition of the aortic wall as one progresses from the ascending aorta to the iliac bifurcation. Andreotti et al. documented that the ascending aorta has a greater concentration of elastin and is therefore more compliant than the descending aorta. This alteration in elastin concentration leads to a progressive decrease in the elastin–collagen ratio as the aorta progresses from the ascending aorta to the abdominal aorta. The media also becomes thinner as one progresses from proximal to distal along the aorta.
A number of recent studies have documented the overexpression and increased activity of various ECM proteinases, specifically the MMPs, in human TAAAs. Sinha et al. documented asymmetric production of MMP9 in the expanding human TAAA wall, which correlated with increased numbers of macrophages. In contrast, MMP2 was documented to be increased in the wall of the TAAA that was preserved, in particular, at the point where smooth muscle cells were more abundant and the wall was preserved. Ikonomidis and associates studied the role of MMPs in a murine model of TAAAs. These authors documented that MMP9 gene deletion attenuated TAA formation despite increases in MMP2 activity. They concluded that interactions between MMP9 and MMP2 are necessary to facilitate TAAA progression. Recent studies by Ikonomidis and others have documented altered membrane type-1 matrix metalloproteinase and tissue inhibitor of metalloproteinase2 (TIMP2 an inhibitor of MMPs) in the murine TAA model. The association between mutations in TIMP1 and 3 and the development of thoracic aneurysms lends additional evidence supporting this concept. Further studies by Johnston, Pope and others have also suggested a critical role for IL-1β and IL-6 in TAA based on animal studies. ,
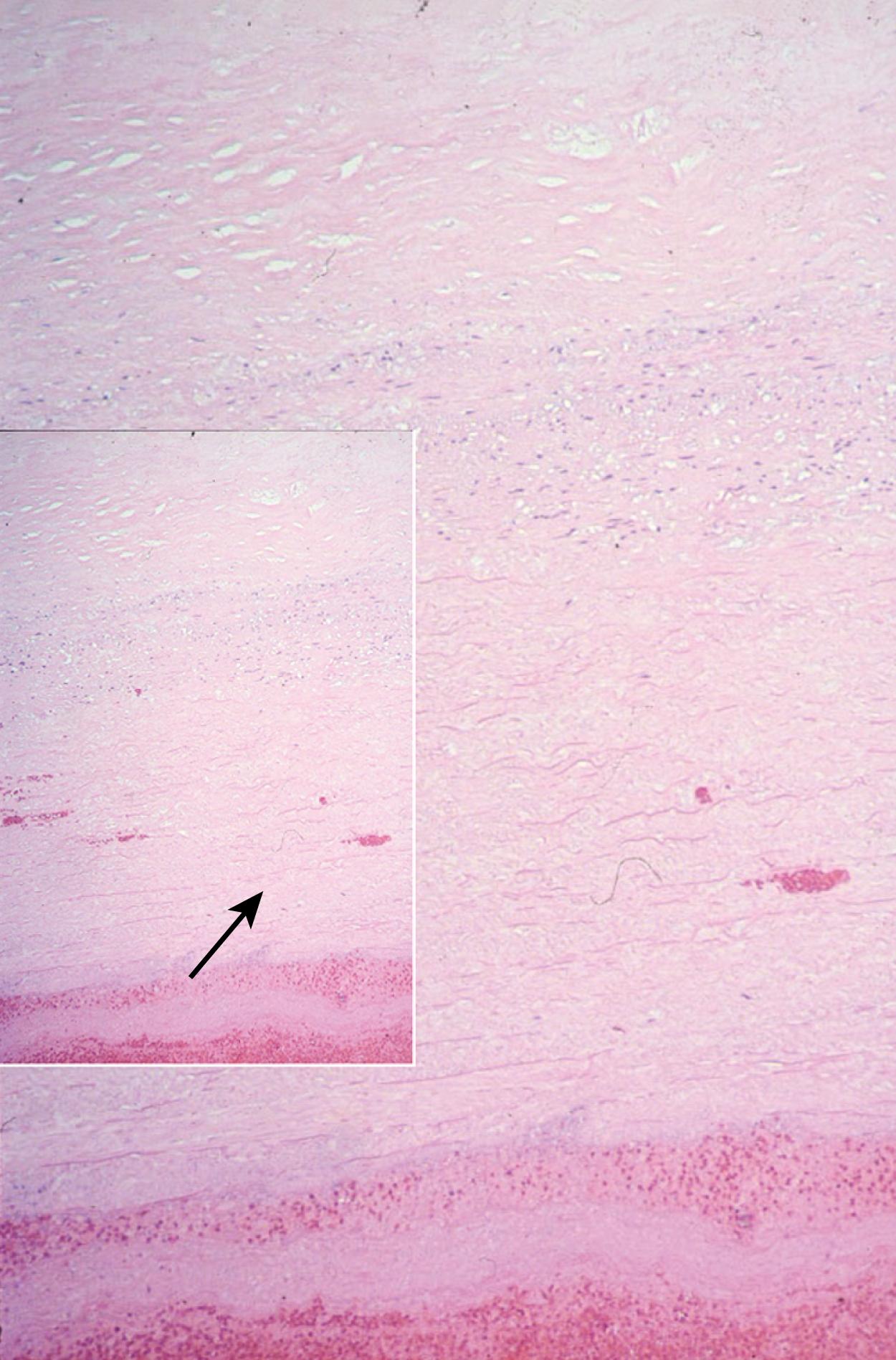
The histology of TAAAs is most closely associated with medial degeneration, formerly known as cystic medial necrosis ( Fig. 78.3 ). Medial degeneration is characterized by fragmentation and loss of elastic fibers, loss of smooth muscle cells, and collections of interstitial collagenous tissue, basophilic ground substance, and proteoglycans. , Although medial degeneration is considered part of the normal aging process, it is accelerated by certain clinical conditions such as hypertension and atherosclerosis. Given the relatively widespread process along the thoracic aorta, medial degeneration is most closely associated with the development of fusiform aneurysms. Genetic abnormalities, such as Marfan syndrome, also accelerate aortic medial degeneration. Although TAAAs were originally described as noninflammatory, recent studies have shown that infiltration of leukocytes contributes to the formation and development of TAAAs. The significant difference in the epidemiology and histology of TAAAs and AAAs suggests distinct causes.
Eighty percent of TAAAs are secondary to medial degeneration, with approximately 15% to 20% caused by aortic dissection ( Table 78.4 ) (see Ch. 83 , Aortic Dissection: Epidemiology, Pathophysiology, Clinical Presentation, and Medical and Surgical Management). Patients with TAAAs secondary to aortic dissection are typically younger and have more extensive aortic involvement than those with degenerative aneurysms. The aorta in patients with Marfan syndrome is particularly prone to aortic dissection and subsequent TAAA formation. Both systemic autoimmune disorders, such as Takayasu arteritis, and chronic nonspecific aortitis can destroy the aortic media with progressive aneurysm formation. Aneurysms associated with arteritis are more commonly seen in women than are degenerative aneurysms. Aneurysms of the upper thoracic aorta can also be secondary to congenital aortic coarctations, either in unrepaired coarctations or after repair.
|
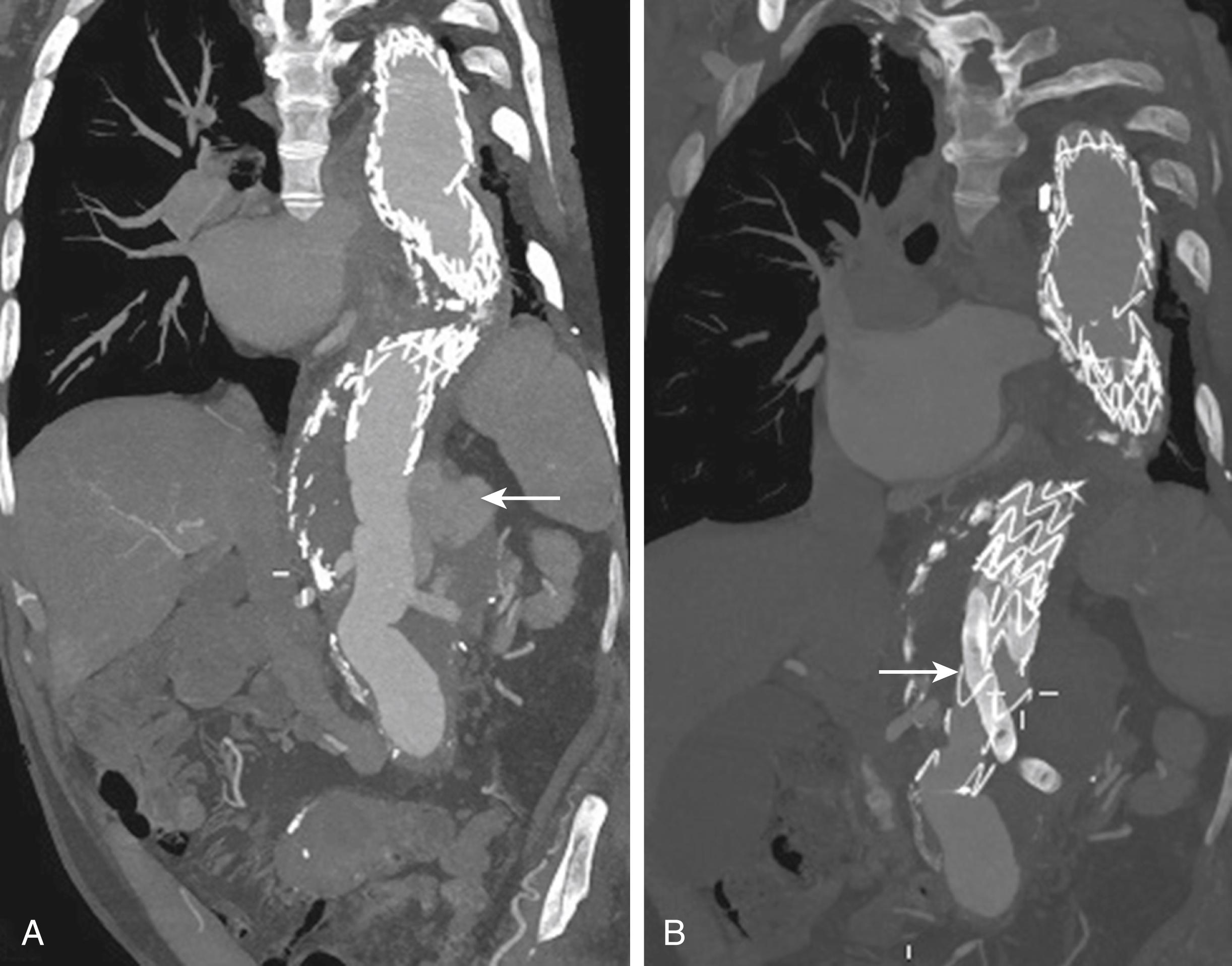
TAAAs may also form secondary to infection. Although saccular aneurysms have been described as mycotic, these aneurysms are most often bacterial and occur because of hematogenous spread of emboli laden with bacteria. Infected TAAAs usually arise as a result of seeding of atherosclerotic plaque in the aorta, the development of a focal inflammatory process in the aortic wall, and ultimately the formation of a false aneurysm. Infected TAAAs are challenging, with the goal of therapy to debulk the infection and restore arterial continuity. For years this has involved in situ repair with its attendant lifetime risk of reinfection. Recent reports of treatment of infected TAAAs with endovascular prostheses are beginning to accumulate. , The endovascular approach can be utilized as either definitive management or as a bridge to stabilize an ill patient to eventual open operation. An endovascular approach, if durable, may definitively circumvent the challenges posed by open aortic reconstruction in an infected field ( Fig. 78.4 ). In-hospital mortality for this approach in recently published series ranges from 9%–17% with secondary conversion to subsequent open repair in 5%–40% of initial survivors. In one of the largest series published to date including 123 patients with mycotic aortic aneurysms – not all in the thoracoabdominal aorta – 1, 5- and 10-year survival following endovascular repair was 75%, 55% and 41%, respectively.
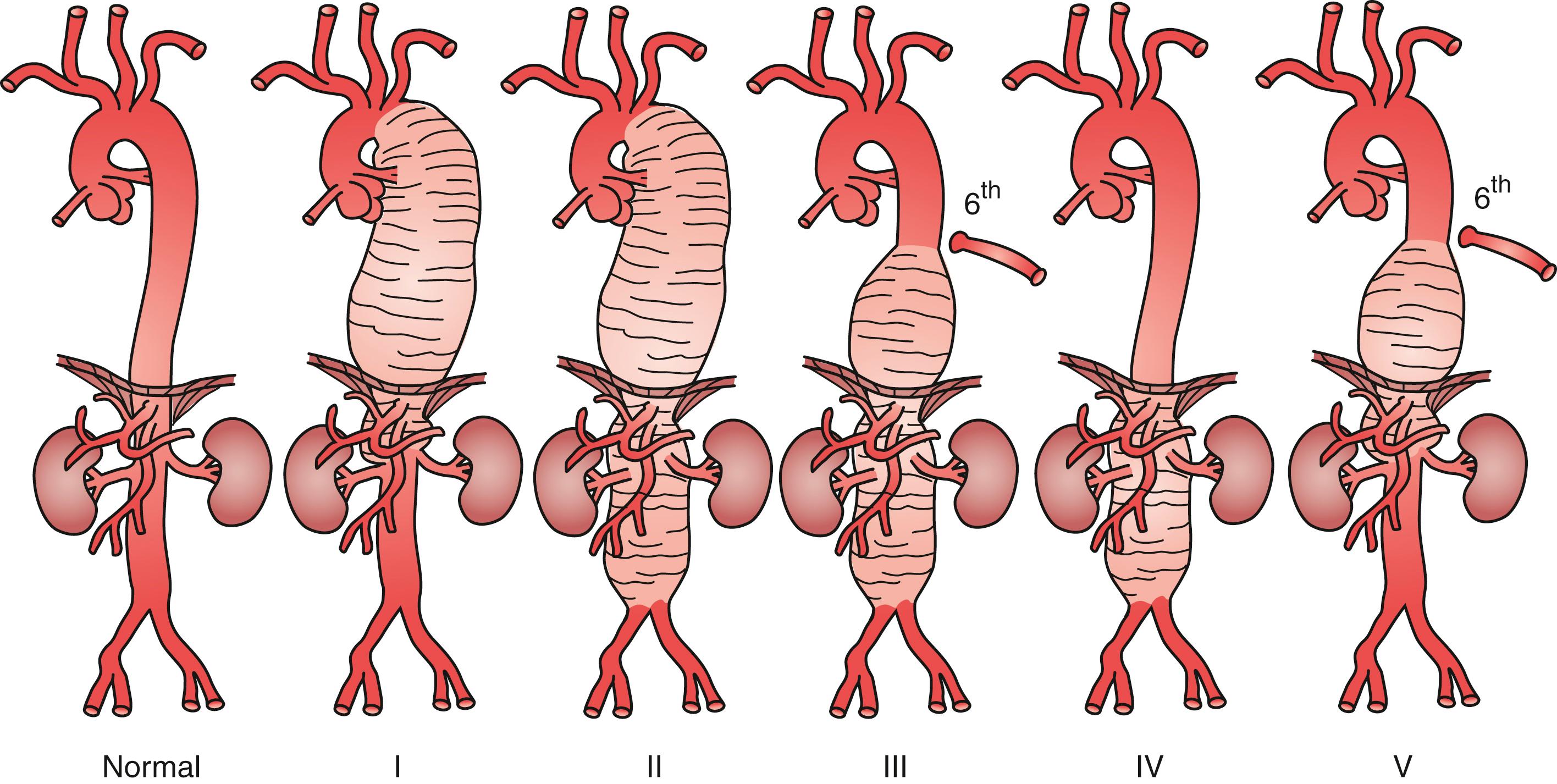
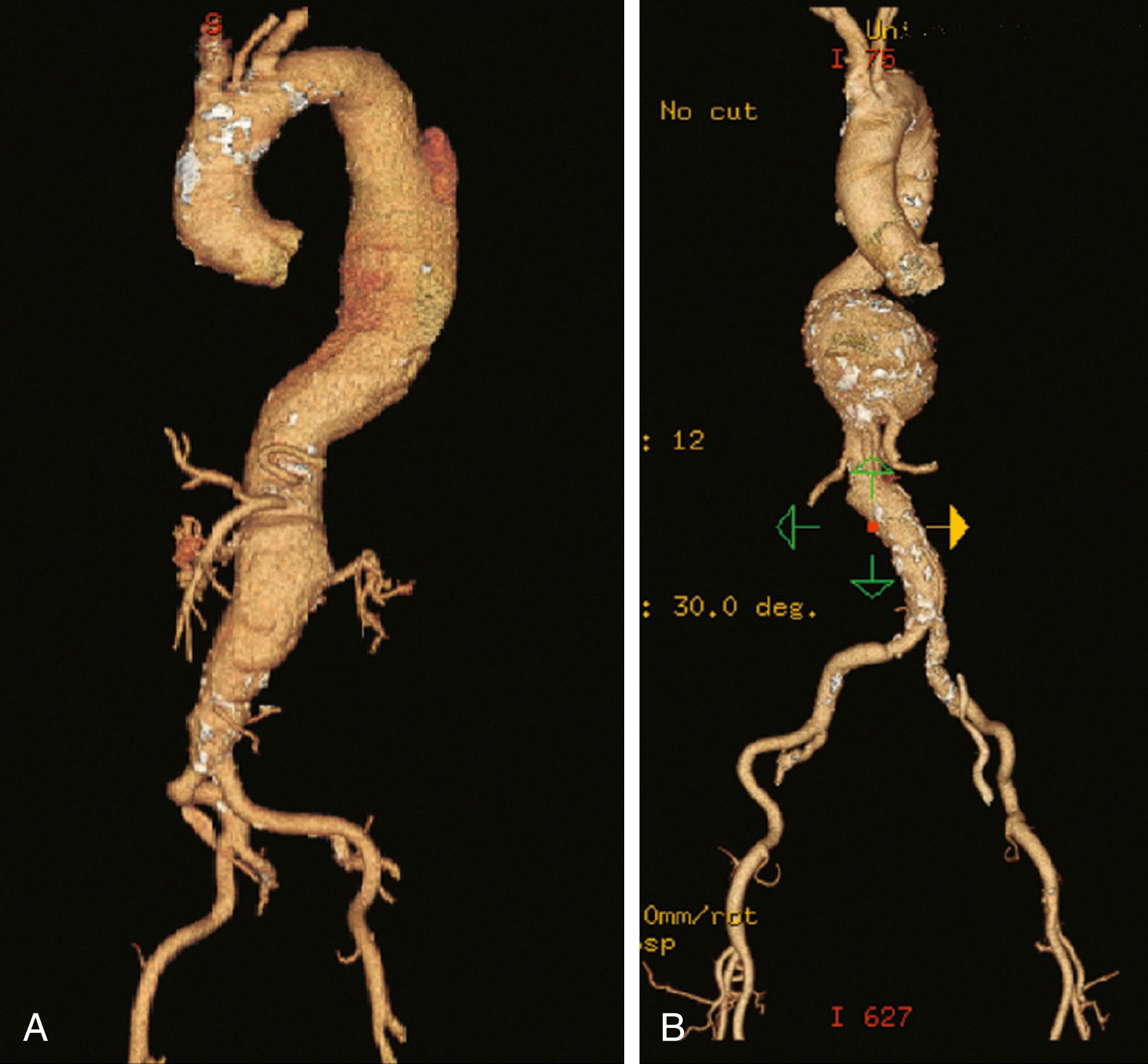
Evaluation of a patient with a TAAA, as well as the technical performance of either open or endovascular repair, depends upon the Crawford classification ( Fig. 78.5 ). Classification of TAAAs also has important therapeutic implications for the operation to be performed as well as the risk for specific complications. Type I TAAAs account for approximately 25% of all TAAAs. They involve the entire descending thoracic aorta and extend only to the upper abdominal aorta. Type II TAAAs (approximately 30% of TAAAs) involve the entire descending thoracic aorta and most of or the entire abdominal aorta ( Fig. 78.6A ). Type III TAAAs (<25%) involve variable lengths of the descending thoracic aorta and extend into the abdominal aorta ( Fig. 78.6B ). Type IV TAAAs (<25%) are limited to the abdominal aorta, including the visceral and renal arteries.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here