Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Heart failure has become an increasingly important cause of morbidity and mortality in the United States and in other industrialized nations. Despite improvement in heart failure treatment, its prevalence continues to rise, in large part as a result of the aging of the population and the improved survival of heart failure patients. In 2010, the most recent year for which statistics are available, the estimated prevalence of heart failure in the U.S. population over the age of 20 years was 5.7 million. In the same year, heart failure was listed as the primary diagnosis in over 1 million hospital discharges, a number that has remained stable since 2000. The economic impact is staggering, with a yearly cost of caring for these patients estimated at more than $32 billion, more than half of which is accounted for by direct hospital costs. Despite improvements in therapies, one in five patients will die within 1 year of the initial diagnosis with heart failure, and in the United States alone, heart failure is listed as the principal cause for almost 56,000 deaths annually and as a contributing cause in more than 274,000 deaths.
From a surgical standpoint, heart failure has a significant impact on perioperative morbidity and mortality. Preoperative heart failure is a strong predictor of adverse outcome in patients undergoing noncardiac surgery, and a history of heart failure or depressed left ventricular systolic function is an independent predictor of mortality after coronary artery bypass surgery. A thorough understanding of the pathophysiology and treatment of heart failure is therefore essential for those physicians who manage patients in the perioperative period. Effective preoperative management of heart failure may prevent perioperative decompensation, and rapid recognition and treatment of postoperative heart failure are essential to prevent progressive pulmonary congestion and organ hypoperfusion. For the well-compensated patient, an understanding of the medical management of chronic heart failure ensures continuation of appropriate therapy both perioperatively and at the time of hospital discharge.
This chapter focuses primarily on the various pharmacologic modalities available for the treatment of heart failure, including their mechanisms of action, benefits as shown in clinical trials, and suggested usefulness in the management of patients with acute and chronic heart failure. The use of many of these pharmacotherapies is based on our current understanding of the pathophysiologic mechanisms underlying heart failure. Therefore, a brief mechanistic overview is provided in an effort to place these therapies in a pathophysiologic context.
Our understanding of the pathophysiology of heart failure has evolved dramatically during the past 2 decades. The traditional hemodynamic model, although still applicable in the setting of acutely decompensated heart failure, is less relevant in the setting of chronic heart failure, for which the concepts of progressive ventricular remodeling and neurohormonal activation have come to the forefront. These processes are briefly discussed here as they relate to the pharmacologic treatment of heart failure.
The term heart failure does not refer to a single entity; rather, it denotes a syndrome that is characterized by signs or symptoms of intravascular volume overload or manifestations of inadequate tissue perfusion. It is the end result of a variety of cardiac injuries and the ensuing pathologic remodeling that impair the heart's ability to fill with or to eject blood; it may originate from a wide range of disorders of the myocardium, pericardium, endocardium, or intracardiac valves ( Table 58-1 ). Heart failure can be categorized pathophysiologically in several ways.
Left-sided heart failure is characterized by signs and symptoms of pulmonary congestion (dyspnea, orthopnea, pulmonary rales, pleural effusions). Right-sided heart failure is characterized by peripheral congestion (elevated jugular venous pressure, peripheral edema, hepatic congestion).
Systolic heart failure refers to that occurring in the setting of left ventricular systolic dysfunction (i.e., reduced ejection fraction). Diastolic heart failure refers to that resulting from impaired left ventricular diastolic filling despite normal left ventricular systolic function. These two abnormalities often coexist, although one usually predominates.
Acute heart failure denotes the sudden development of heart failure in the absence of preexisting cardiac dysfunction or the sudden decompensation in a patient with previously stable cardiac disease. This results from an abrupt alteration in cardiac structure or function (e.g., after an acute myocardial infarction or after valvular rupture) and is generally associated with clinical instability. Chronic heart failure results from a more indolent process of myocardial dysfunction and may be associated with less clinical severity because of the development of compensatory mechanisms (see later).
Low-output heart failure is that resulting from a reduction in cardiac output (caused by either systolic or diastolic dysfunction) and is usually characterized by venous congestion and increased arterial resistance (i.e., vasoconstriction). High-output heart failure occurs in the setting of increased cardiac output (e.g., thyrotoxicosis, anemia, beriberi, Paget disease, arteriovenous fistulas) and is characterized by venous congestion and normal or reduced arterial resistance.
Backward heart failure refers to the hypothesis that the manifestations of heart failure are primarily the result of an accumulation of fluid (and pressure) behind the failing ventricle. Forward heart failure proposes that heart failure results from a primary reduction in cardiac output with resultant organ hypoperfusion, sodium and water retention, and subsequent venous congestion. This is not as useful a distinction, because both mechanisms probably operate in most patients with heart failure.
| Myocardial |
| Ischemia or infarction |
| Viral myocarditis |
| Idiopathic cardiomyopathy |
| Hypertrophic cardiomyopathy |
| Hypertension |
| Toxins (alcohol, cocaine, chemotherapeutic agents) |
| Infiltrative diseases (amyloidosis, hemochromatosis) |
| Infectious (Lyme disease, Chagas disease) |
| Peripartum cardiomyopathy |
| Thyroid dysfunction |
| Metabolic abnormalities (thiamine or selenium deficiency) |
| Valvular |
| Aortic stenosis |
| Aortic regurgitation |
| Mitral stenosis |
| Mitral regurgitation |
| Arrhythmic |
| Tachycardia-mediated cardiomyopathy |
| Pericardial |
| Constrictive pericarditis |
Heart failure may be classified symptomatically on the basis of its clinical severity ( Table 58-2 ). Despite the varied causes and classifications of heart failure, the manifestations all reflect intravascular volume overload, inadequate tissue perfusion, or their combination. A thorough explanation of all these forms of heart failure is beyond the scope of this chapter. The following discussion, therefore, focuses primarily on left ventricular systolic failure in both the acute and chronic setting.
| New York Heart Association Classification | |
| Class I | Symptoms only with greater than usual activity |
| Class II | Asymptomatic at rest but with symptoms during normal activities |
| Class III | Asymptomatic at rest but with symptoms during minimal exertion |
| Class IV | Symptoms at rest |
| American College of Cardiology/American Heart Association Classification | |
| Stage A | Patients with structurally normal hearts, asymptomatic, but at risk for the development of heart failure resulting from the presence of risk factors (e.g., hypertension, coronary artery disease, diabetes) |
| Stage B | Patients with structurally abnormal hearts (e.g., left ventricular systolic dysfunction, left ventricular hypertrophy, valvular dysfunction, prior myocardial infarction) but without symptoms of heart failure |
| Stage C | Patients with structurally abnormal hearts and current or prior symptoms of heart failure |
| Stage D | Patients with end-stage heart failure symptoms not responsive to standard therapy |
The response of the cardiovascular system to the onset of myocardial dysfunction and the pathophysiologic mechanisms underlying the subsequent progression to heart failure depends in large part on the acuity of the dysfunction. An acute cardiac insult results in a series of hemodynamic alterations that account for the clinical manifestations of left ventricular failure. This occurs irrespective of whether the initial insult depresses myocardial contractility (systolic dysfunction) or impairs ventricular filling (diastolic dysfunction). The cascade begins with a rise in left ventricular end-diastolic pressure. This elevated pressure is transmitted to the left atrium and subsequently to the pulmonary venous and capillary system. The increased intravascular pressure results in transudation of fluid into the pulmonary interstitium where it interferes with gas exchange, resulting in hypoxemia and dyspnea. In addition, there is often an associated reduction in cardiac output resulting in inadequate delivery of blood to the arterial system with resultant organ hypoperfusion.
The heart's response to these hemodynamic alterations is the activation of several compensatory mechanisms ( Table 58-3 ). A rapid, generalized activation of the adrenergic system occurs and is associated with a withdrawal of parasympathetic tone. Direct sympathetic stimulation of the heart and β-adrenergically induced release of epinephrine and norepinephrine from the adrenal glands result in tachycardia and an increase in myocardial contractility, both of which serve to augment cardiac output. Catecholamine-induced peripheral arterial vasoconstriction redirects the available cardiac output away from relatively nonessential organs (i.e., skin, skeletal muscle, gut, kidney) and helps maintain sufficient blood pressure to ensure adequate perfusion of more vital organs (i.e., heart and brain). Furthermore, β-adrenergic stimulation of the juxtaglomerular apparatus in the kidneys results in the release of renin and activation of the renin-angiotensin system. The angiotensin II thus produced is a potent vasoconstrictor and acts in concert with the direct α-adrenergic stimulation of the vasculature to maintain blood pressure. The reduced renal blood flow from depressed cardiac output and redistribution of blood volume results in activation of renal baroreceptors. This further stimulates renin release and augments sympathetic activation, thereby contributing to vasoconstriction.
| Compensatory Response | Stimuli | Beneficial Effects | Adverse Effects | Potential Pharmacologic Interventions |
|---|---|---|---|---|
| Renin-angiotensin system activation | ↓CO/BP ↓Renal blood flow ↑β-Adrenergic activity |
Maintain vital organ perfusion through vasoconstriction and sodium retention | ↑Afterload → worsened LV function Adverse LV remodeling (apoptosis, myocyte hypertrophy) |
ACE inhibitors ARBs |
| Adrenergic activation | ↓CO/BP | ↑CO through ↑ in heart rate and contractility ↑BP |
↑Ischemia ↑Afterload → worsened LV function ↑LVEDP → pulmonary congestion Adverse LV remodeling (apoptosis, myocyte hypertrophy) |
β-Adrenergic blocking agents |
| Renal salt and water retention | ↑Antidiuretic hormone ↑Norepinephrine ↑Angiotensin II ↑Aldosterone ↓Renal blood flow |
↑Preload → ↑stroke volume and CO | Pulmonary and systemic congestion Adverse LV remodeling |
Diuretics Aldosterone inhibitors ACE inhibitors, ARBs β-Adrenergic blocking agents |
| ↑Natriuretic peptide secretion | Volume expansion (atrial stretch) | Diuresis Natriuresis Partial inhibition of renin-angiotensin system and norepinephrine |
None known | Natriuretic peptides |
In addition to producing these hemodynamic alterations, acute heart failure is characterized by marked sodium and water retention. This occurs through a variety of mechanisms. Angiotensin II directly promotes the reabsorption of sodium in the proximal nephron and indirectly promotes the reabsorption of sodium from the distal nephron, this latter effect being mediated through angiotensin II–induced release of aldosterone from the adrenal cortex. Furthermore, angiotensin II and norepinephrine stimulate hypothalamic release of arginine vasopressin, resulting in further vasoconstriction and free water reabsorption. These changes produce an expansion of intravascular volume and augmentation of venous return, thereby increasing ventricular end-diastolic volume (preload) . The increased preload results in an increase in stroke volume by the Frank-Starling mechanism ( Fig. 58-1 ) and thereby helps support the cardiac output.
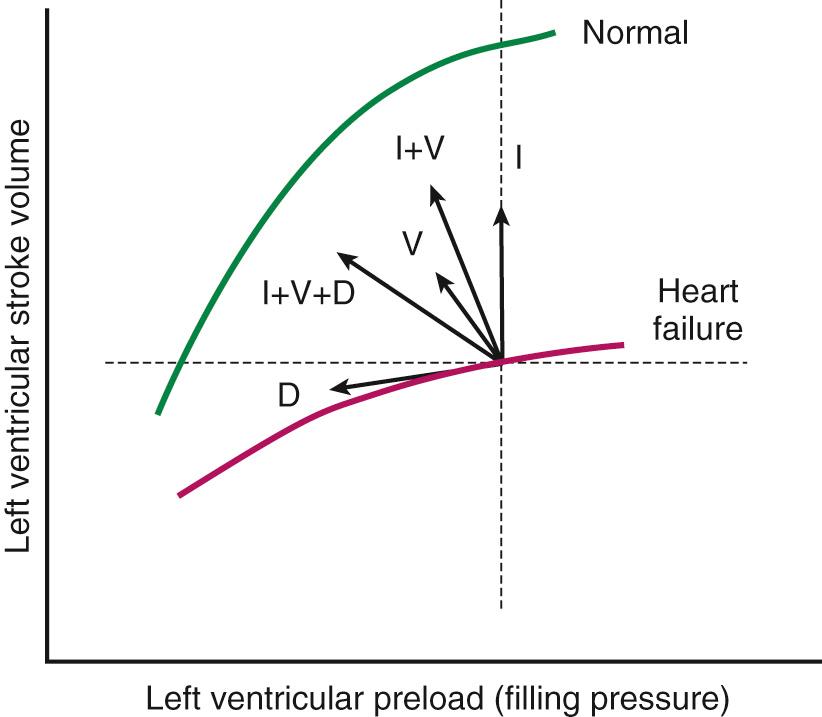
In combination, the aforementioned mechanisms serve a compensatory role in acute heart failure, helping to maintain cardiac output and blood pressure to allow adequate perfusion of vital organs. These compensatory mechanisms may initially be adequate to allow clinical stability, and the patient may subsequently go through a stage of asymptomatic ventricular dysfunction, maintained in part by chronic stimulation of the adrenergic and renin-angiotensin systems. As heart failure progresses, the hemodynamic overload induces changes in the shape and size of the ventricle, a process known as ventricular remodeling . The specific changes that occur depend in part on the hemodynamic stressors facing the ventricle. In predominantly pressure-overloaded conditions (e.g., hypertension, aortic stenosis), there is a rise in systolic wall stress that results in left ventricular hypertrophy. If this hypertrophy is insufficient to normalize the wall stress, dilation occurs. Under conditions of volume overload (e.g., aortic or mitral insufficiency), there is a rise in diastolic wall stress that induces ventricular dilation. This dilation in turn results in increased systolic wall stress (by the Laplace relationship) and subsequent hypertrophy. These hypertrophic changes help maintain systolic wall stress within a normal range and help preserve ventricular contractile function. However, with continued hemodynamic overload, there is progressive ventricular dilation, eventuating in the development of a dilated, spherical heart. This altered ventricular morphology produces less efficient ventricular contraction, may induce mitral regurgitation caused by annular dilation and malcoaptation of the valve leaflets, and is associated with an adverse prognosis.
The stimuli that induce ventricular remodeling are varied and the mechanisms underlying the remodeling process are complex ( Table 58-4 ). It appears that increased wall stress (resulting from ventricular dilation and increased afterload) and neurohormones (i.e., β-adrenergic and renin-angiotensin systems), vasoactive peptides (e.g., endothelin), and cytokines (e.g., tumor necrosis factor α) mediate remodeling. These factors may have direct effects on the cardiac myocytes or may act indirectly through stimulation of second-messenger systems and thereby induce a variety of changes in myocyte structure and function. On a cellular level, myocyte hypertrophy results from the replication of sarcomeres either in parallel (producing ventricular hypertrophy) or in series (producing ventricular dilation). Alterations in the expression of various contractile proteins occur with reexpression of fetal genes and reduced expression of adult contractile genes, resulting in abnormalities of calcium handling and excitation-contraction coupling. Chronic stimulation of the sympathetic system is accompanied by a reduction in the density of β-adrenergic receptors in the myocardium and an uncoupling of the receptors from their intracellular mediators. This results in a blunted response of the failing myocardium to either endogenous (e.g., exercise) or exogenous (e.g., dopamine or dobutamine) adrenergic stimulation.
| Stimulants of Ventricular Remodeling | Molecular and Cellular Events That Mediate Ventricular Remodeling |
|---|---|
|
|
In addition to these alterations of the contractile apparatus within the myocytes, there is a progressive reduction in the number of myocytes, in part the result of apoptosis induced by the various stimuli of remodeling. Furthermore, changes occur in the extracellular matrix related to fibroblast proliferation, interstitial fibrosis, and increased expression of degradative enzymes such as matrix metalloproteinases. The last factor results in the loss of mechanical coupling of myocytes and may contribute to the remodeling process by facilitating “myocyte slippage” and, thereby, ventricular dilation.
As the remodeling process develops, the neurohormonal effects of the β-adrenergic and renin-angiotensin systems on the peripheral vasculature and renal salt and water handling continue. The intense vasoconstriction, while maintaining flow to vital organs, contributes to hypoperfusion of the kidneys and progressive renal dysfunction. The augmented preload and cardiac output resulting from sodium and water retention help maintain circulating blood volume and tissue perfusion. However, the associated increase in end-diastolic pressure and increased ventricular wall stress contribute to progressive ventricular remodeling and result in pulmonary and systemic venous hypertension and precipitation of congestive symptoms. Thus, these initially compensatory changes become deleterious in the chronic setting. The inhibition of these processes offers not only a mechanism for the treatment of heart failure but also the potential to reverse the adverse remodeling seen in the chronic state (see Table 58-3 ).
Most pharmacologic agents used for the treatment of heart failure effect benefit either by interfering with the hemodynamic alterations described earlier or through inhibition of the neurohormonal activation underlying these alterations (see Fig. 58-1 ). These medications fall into several categories: diuretics, vasodilators, inotropic agents, and neurohormonal inhibitors. Diuretics act to reduce preload (leftward shift on the Frank-Starling curve), resulting in decreased filling pressures and improved congestive symptoms. Although this fall in preload may be associated with a reduction in stroke volume, this effect is minimal in patients with elevated filling pressures. Pure venodilators similarly reduce filling pressures and congestive symptoms and have minimal effect on stroke volume. Arterial vasodilators and inotropic agents predominantly augment cardiac output and thereby improve organ perfusion; arterial vasodilators act indirectly through a reduction in vascular resistance, whereas inotropic agents directly increase contractility and stroke volume. Although the increased cardiac output seen with these agents may result in a fall in filling pressures, the effect may be relatively modest. As a result of the differential effects of these agents, many patients with heart failure attain the greatest benefit from combination therapy. Neurohormonal inhibitors may have mixed hemodynamic effects. The blockade of adrenergic tone (beta blockers) and inhibition of the renin-angiotensin system (angiotensin-converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARBs]) results in vasodilation and augmented cardiac output. These agents may also decrease preload and reduce filling pressures through a reduction in neurohormonally mediated renal sodium and water retention.
Diuretics have long played an important role in the symptomatic treatment of heart failure ( Table 58-5 ). The induced diuresis and natriuresis reduce extracellular volume and ventricular filling pressures, thereby ameliorating congestive symptoms. This effect occurs without a significant decrease in cardiac output or systemic blood pressure unless excessive diuresis and intravascular volume depletion occur. Whereas diuretics are beneficial in controlling symptoms and improving exercise capacity in patients with heart failure, with the exception of aldosterone inhibitors (spironolactone and eplerenone) diuretic use has not resulted in a decrease in mortality.
| Class and Examples | Daily Dose Range | Duration of Action | Adverse Effects (by Class) |
|---|---|---|---|
| Loop Diuretics | |||
| Furosemide (Lasix) | 20-480 mg PO 20-300 mg IV |
4-6 hours | Hypokalemia, hyperuricemia, metabolic alkalosis, ototoxicity at high doses |
| Torsemide (Demadex) | 5-200 mg PO | 12 hours | |
| Bumetanide (Bumex) | 0.5-5 mg PO | 4-6 hours | |
| Ethacrynic acid (Edecrin) | 25-100 mg PO | 12 hours | |
| Thiazide Diuretics | |||
| Chlorothiazide (Diuril) | 125-500 mg PO | 6-12 hours | Hypokalemia, hyponatremia, hyperuricemia, hyperglycemia, hyperlipidemia |
| Hydrochlorothiazide (HydroDIURIL) | 12.5-50 mg PO | 12-18 hours | |
| Chlorthalidone (Hygroton) | 25-100 mg PO | 24 hours | |
| Thiazide-like Diuretics | |||
| Metolazone (Zaroxolyn) | 0.5-10 mg PO | 24 hours | Hypokalemia, hypomagnesemia |
| Aldosterone Inhibitors | |||
| Spironolactone (Aldactone) | 25 mg PO | 8-12 hours | Hyperkalemia, nausea, gynecomastia (spironolactone) |
| Eplerenone (Inspra) | 25-50 mg PO | 4-6 hours | |
| Potassium-Sparing Diuretics | |||
| Amiloride (Midamor) | 5-10 mg PO | 24 hours | Hyperkalemia when combined with angiotensin-converting enzyme inhibitor or angiotensin receptor blocker |
| Triamterene (Dyrenium) | 50-100 mg PO | 12 hours | |
Loop diuretics act in the thick ascending limb of the loop of Henle, where they inhibit the Na + -K + -2Cl − transporter, resulting in increased delivery of sodium and water to the distal nephron. They also decrease the tonicity of the medullary interstitium and thereby limit the osmotic reabsorption of free water from the collecting tubules. Currently available loop diuretics include furosemide, bumetanide, torsemide, and ethacrynic acid. Ethacrynic acid carries an increased risk of ototoxicity; therefore, this agent should be reserved for patients who are allergic to or intolerant of other agents.
Furosemide is the loop diuretic most commonly used for the treatment of heart failure. For patients with mild to moderate congestive symptoms, it can be given orally at initial doses of 20 to 40 mg daily. Its bioavailability ranges from 40% to 70%, and gradual dose titration is frequently required. Furosemide has a relatively short half-life. Once renal tubular levels of the drug decline, avid sodium reabsorption occurs throughout the nephron, potentially limiting or preventing effective natriuresis. A twice-daily dosing regimen may therefore be required to produce adequate salt and water loss. In patients with more severe volume retention or decompensated heart failure, intravenous administration of furosemide (20 to 100 mg) may produce a more rapid and effective diuresis. The maximum intravenous dose is 300 mg; however, the risk of ototoxicity increases at such high doses. For patients who require frequent high doses of intravenous furosemide, a continuous infusion may be used and may be associated with less hypotension; however, it does not appear to be any more effective at reducing symptoms or stimulating diuresis than are intermittent intravenous boluses. The infusion is usually started at 5 to 10 mg/hr and titrated as needed to obtain the desired effect. Bumetanide and torsemide have greater bioavailability than does furosemide (≈80%) but have not demonstrated better efficacy and are significantly more expensive.
Thiazide diuretics act in the distal convoluted tubule, where they inhibit the Na + -Cl − cotransporter. Their efficacy is dependent on the delivery of sodium to the distal nephron; therefore, their diuretic effect is limited by sodium reabsorption from more proximal regions, as occurs during intravascular volume depletion or in low-flow states. In addition, they are ineffective when glomerular filtration rates fall below 30 mL/min. Thiazides may be useful as the sole diuretic in treatment of mild congestive symptoms; however, their predominant role in the management of more advanced heart failure is as an adjunct to other diuretic therapy in patients who exhibit diuretic resistance. Although several thiazides are available, the most frequently used are hydrochlorothiazide (12.5 to 50 mg daily) and metolazone (2.5 to 10 mg daily). These agents exhibit synergism with loop diuretics and should be given approximately 30 minutes before administration of furosemide, bumetanide, or torsemide.
Spironolactone is a competitive inhibitor of aldosterone in the distal convoluted tubule, whereas eplerenone is a selective aldosterone blocker. These agents stimulate a mild natriuresis and potassium reabsorption and may be most effective in patients with advanced heart failure, in whom marked activation of the renin-angiotensin-aldosterone system results in aldosterone levels as high as 20 times normal. In the Randomized Aldactone Evaluation Study (RALES), patients with moderate to severe congestive heart failure (NYHA class III-IV) were treated with spironolactone (25 to 50 mg daily) and had improved symptoms, reduced rates of hospitalization for heart failure, and 30% reduction in mortality. In the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), patients with acute myocardial infarction, left ventricular ejection fraction of 40% or less, and evidence of heart failure were treated with eplerenone (25 to 50 mg daily) and had a 15% reduction in mortality and significantly fewer episodes of heart failure. The mechanism of benefit of these agents is unlikely to be related to a diuretic effect because they are relatively weak diuretics. Rather, it probably reflects inhibition of aldosterone-induced myocardial fibrosis and ventricular remodeling. The major side effect of spironolactone is gynecomastia, which is not seen with eplerenone because of its selective mineralocorticoid blockade. The dose of these agents should not exceed 50 mg daily because of the risk of hyperkalemia, especially in patients with a serum creatinine concentration of 2.5 mg/dL or higher or when they are used in conjunction with an ACE inhibitor or ARB for the treatment of heart failure.
Amiloride and triamterene inhibit the reabsorption of sodium in the distal convoluted tubule and proximal collecting duct, resulting in a mild natriuresis and reduction of the ionic gradient required for potassium secretion into the urine. These agents produce a mild diuresis without the potassium wasting seen with loop diuretics and thiazides and may be effective for the control of mild congestive symptoms. However, when they are given alone, they are not effective in maintaining a negative fluid balance in patients with advanced heart failure. In such patients, these agents may provide benefit as part of a combination diuretic regimen, especially given their potassium-sparing properties.
Patients treated with diuretics require close monitoring of their renal function and serum electrolytes. Loop diuretics and thiazides can lead to profound hypokalemia and hypomagnesemia, especially when they are used in combination; spironolactone may result in hyperkalemia. In contrast to loop diuretics, thiazides do not alter the tonicity of the renal medullary interstitium and may produce significant hyponatremia as a result of the reabsorption of free water from the distal convoluted tubule in the face of a preserved interstitial gradient. In addition to these metabolic effects, thiazides may adversely affect serum lipid levels, and spironolactone may induce gynecomastia.
Nitric oxide is formed by normal endothelial and smooth muscle cells throughout the vasculature and functions in both a paracrine and an autocrine fashion. Its primary mechanism of action involves an induced increase in intracellular cyclic guanosine monophosphate, which results in vascular smooth muscle relaxation. Nitrovasodilators such as sodium nitroprusside and organic nitrates (i.e., nitroglycerin) are metabolized to nitric oxide within the vasculature. They are potent vasodilators and, as such, are useful in the management of heart failure.
Nitroprusside is the sodium salt of nitric oxide and ferricyanide. It is a balanced vasodilator and produces both vasodilation and venodilation in both systemic and pulmonary systems. These effects result in favorable hemodynamic changes, including a decrease in right atrial and pulmonary capillary wedge pressures (i.e., decreased preload), a reduction in pulmonary and systemic vascular resistance (i.e., decreased afterload), and an increase in stroke volume and cardiac output/index ( Fig. 58-2 ). In contrast to other arterial vasodilators, nitroprusside does not cause a significant increase in heart rate, and its use is usually associated with a decrease in myocardial oxygen demand. Nitroprusside is useful for the management of heart failure associated with elevated filling pressures, low cardiac output, and high vascular resistance, as occurs in patients with decompensated systolic failure. It is also ideally suited for the management of heart failure associated with profound hypertension, acute mitral regurgitation, acute aortic insufficiency, or acute ventricular septal defect.
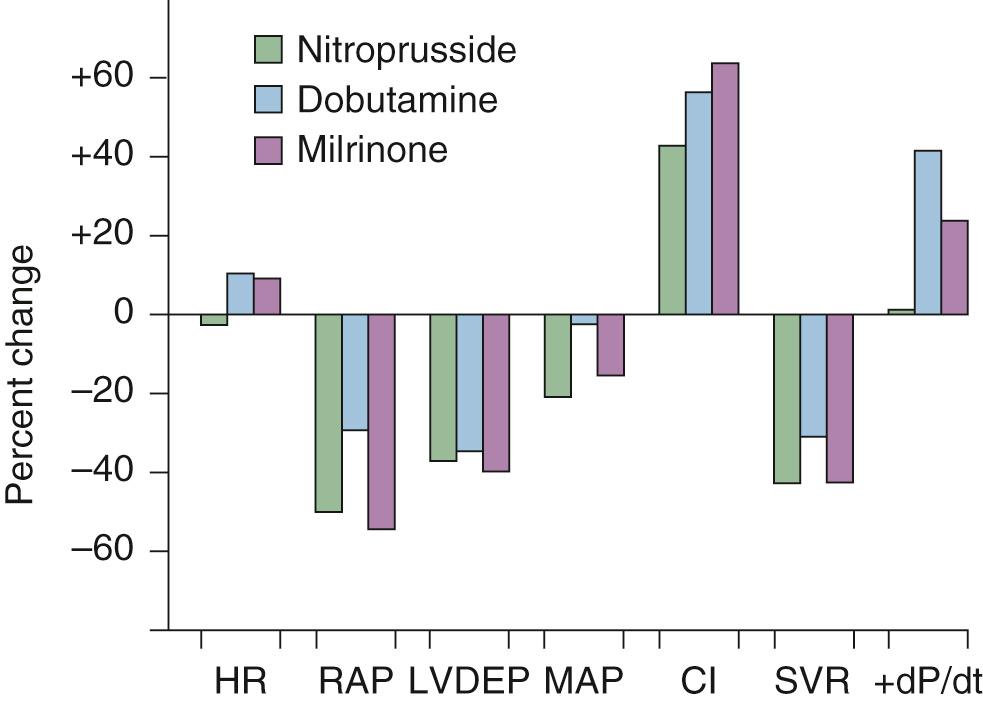
Nitroprusside is administered as a continuous infusion. Its onset of action is rapid (within 30 seconds), and it reaches peak effect within 2 minutes. Similarly, its effects completely resolve within 3 minutes of discontinuation of its infusion. Because of these rapid changes in hemodynamics, it is best administered under the guidance of pulmonary (i.e., Swan-Ganz catheter) and systemic arterial monitoring. The usual starting dose is 0.1 to 0.25 µg/kg/min. The dose may be titrated up by 0.25 µg/kg/min every 5 to 10 minutes until the desired effect is achieved or the maximum dose is reached (10 µg/kg/min). The use of nitroprusside may be limited by the development of hypotension, especially in patients with normal left ventricular systolic function or low filling pressures. Rapid cessation of nitroprusside may result in rebound hypertension, probably reflecting neurohormonal activation. Nitroprusside is metabolized in the vasculature to nitric oxide and cyanide; cyanide is further metabolized in the liver to thiocyanate, which is excreted by the kidneys. Accumulation of these toxic metabolites is more likely to occur when nitroprusside is infused at higher doses or for prolonged periods, especially in the setting of hepatic and renal dysfunction. Cyanide toxicity may be manifested as abdominal pain, confusion, or seizure and is usually preceded by lactic acidosis. Thiocyanate toxicity usually manifests as nausea, confusion, fatigue, psychosis, and, in rare cases, coma. If toxicity is suspected, the infusion should be discontinued and serum levels of the metabolites should be measured. Cyanide toxicity can be treated with sodium nitrite (300 mg) or sodium thiosulfate (12.5 g), but thiocyanate toxicity may require hemodialysis.
Nitroglycerin, like nitroprusside, is a potent vasodilator; however, it has dose-dependent effects in the arterial and venous systems. At low doses, it is a relatively selective venodilator, resulting in increased venous capacitance and decreased left and right ventricular filling pressures. At higher doses, it is also an arterial dilator and results in a fall in pulmonary and systemic vascular resistance, although less predictably and to a lesser extent than nitroprusside. Intravenous nitroglycerin is an effective agent in the management of acute decompensated heart failure characterized by increased filling pressures and elevated vascular resistance. In addition, nitroglycerin has a significant vasodilative effect on epicardial coronary arteries and may indirectly improve left ventricular function by improving blood flow to ischemic myocardium. It is thus an agent of choice in managing heart failure associated with acute myocardial ischemia or infarction.
Intravenous nitroglycerin is usually initiated at 20 µg/min and titrated up by 10 to 20 µg/min every 5 to 10 minutes until the desired hemodynamic effect is achieved or the maximum dose is reached (400 µg/min). Its effects are immediate and resolve rapidly after discontinuation of the infusion. Nitroglycerin may result in hypotension, especially at high doses and in patients with low filling pressures. Its use is commonly associated with a headache, occasionally requiring down-titration or discontinuance of the infusion. Nitrate tolerance frequently develops but can usually be overcome by increasing the infusion rate.
Hydralazine is a direct vasodilator that causes relaxation of arteriolar smooth muscle by an unknown mechanism. It does not cause venodilation or dilation of epicardial coronary arteries; thus, its hemodynamic effects are primarily limited to a reduction in vascular resistance. Hydralazine is an effective antihypertensive agent, especially when it is used in combination with other agents. When it is administered to patients with congestive heart failure, it is most effective in combination with venodilating agents (e.g., organic nitrates). The combination of hydralazine and oral nitrates, when it is added to a regimen of digoxin and diuretics, has been shown in randomized trials to reduce mortality, to improve left ventricular systolic function, and to reduce symptoms in patients with heart failure. However, the benefit of this regimen on mortality and left ventricular function is less than that of ACE inhibitors. In general, hydralazine is not a first-line agent for the treatment of heart failure. Nonetheless, it should be considered in patients who are intolerant of ACE inhibitors because of allergy or renal insufficiency, and it is the agent of choice for afterload reduction in pregnant patients. In addition, it may offer further relief in patients with heart failure who remain symptomatic despite treatment with ACE inhibitors.
Hydralazine therapy is initiated at a dose of 10 mg four times daily and titrated upward as blood pressure tolerates to a maximum dose of 100 mg four times daily. Organic nitrates are given concurrently (i.e., isosorbide dinitrate, 30 to 120 mg daily). Hydralazine-induced vasodilation is associated with a baroreceptor-mediated increase in sympathetic activity, resulting in a reflex tachycardia, increased ventricular contractility, increased renin activity, and fluid retention. In patients with underlying coronary artery disease, the arteriolar dilation may result in a coronary steal phenomenon and, combined with the tachycardia, may precipitate myocardial ischemia. Coadministration with β-adrenergic blocking agents may prevent this complication; nonetheless, hydralazine should be used with caution in patients with an ischemia cardiomyopathy. Other side effects occur more frequently and include headaches, flushing, palpitations, nausea, and dizziness. A lupus-like syndrome occurs in 5% to 10% of patients and may require discontinuation of the drug.
In general, the use of calcium channel blocking agents for the treatment of heart failure has been disappointing, despite the fact that they are relatively potent vasodilators. Verapamil and diltiazem have negative inotropic effects and may worsen symptoms in patients with systolic heart failure. However, these agents may improve diastolic function because of their rate-slowing effects and induced alterations in calcium homeostasis and thus may be beneficial in the treatment of diastolic heart failure. The first-generation dihydropyridine nifedipine has been associated with an increase in adverse effects, including a trend toward increased mortality in patients with systolic heart failure. This may relate to neurohormonal activation resulting from fluctuations in its hemodynamic effects, especially with short-acting formulations. Newer second-generation dihydropyridines, such as amlodipine and felodipine, appear to be safe in patients with heart failure but have not demonstrated significant benefit with regard to morbidity or mortality. Therefore, although these agents could be considered for the treatment of hypertension or angina in patients with left ventricular systolic dysfunction, calcium channel blocking agents in general should not be used for primary treatment of heart failure.
ACE inhibitors have a variety of beneficial effects on the pathophysiologic mechanism of heart failure. Hemodynamically, ACE inhibitors are potent vasodilators and reduce both preload and afterload. The subsequent fall in intracardiac pressures and reduction in wall stress result in a decrease in myocardial oxygen demand, potentially reducing ischemia, and a decrease in the activity of the sympathetic nervous system, thereby reducing electrical instability. In addition, the ACE inhibitor–induced reduction in angiotensin as well as the subsequent reduction in adrenal aldosterone release may have direct effects on the extent of fibrosis and collagen deposition that characterize myocardial remodeling in heart failure. The effects of ACE inhibitors are primarily mediated by inhibition of the enzyme responsible for the conversion of angiotensin I to angiotensin II, thereby decreasing production of angiotensin II. However, some of the benefit of ACE inhibitors may result from their effects on the kinin system; ACE inhibitors decrease the degradation of kinins (e.g., bradykinin) and thereby enhance their vasodilative effects and potentiate kinin-mediated synthesis of vasodilative prostaglandins.
ACE inhibitors have been extensively studied in a wide variety of patients with heart failure and have almost universally demonstrated benefit in hemodynamics, symptoms, exercise capacity, hospitalization, and mortality. In the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), patients with severe (NYHA class IV) systolic heart failure who were already treated with digoxin and diuretics had a 40% reduction in mortality at 6 months when treated with enalapril. Patients with less-severe heart failure (NYHA class II-III and an ejection fraction ≤ 35%) were studied in the treatment arm of the SOLVD (Studies Of Left Ventricular Dysfunction) trial. In this trial, patients who were treated with enalapril had a 16% reduction in mortality and a 26% reduction in the risk of death or hospitalization for worsening heart failure. In the Acute Infarction Ramipril Efficacy study, patients with symptomatic heart failure, a recent myocardial infarction, and ejection fractions below 40% demonstrated a 27% decrease in mortality after 30 days of treatment with ramipril. Furthermore, several studies have demonstrated that asymptomatic patients with depressed ejection fractions (<35% to 40%) have reduced morbidity and mortality when treated with ACE inhibitors, although the magnitude of this benefit is less than that seen in patients with overt heart failure. Thus, the results of clinical trials with ACE inhibitors reveal a consistent benefit in patients with symptomatic heart failure or asymptomatic left ventricular dysfunction ( Fig. 58-3 ).
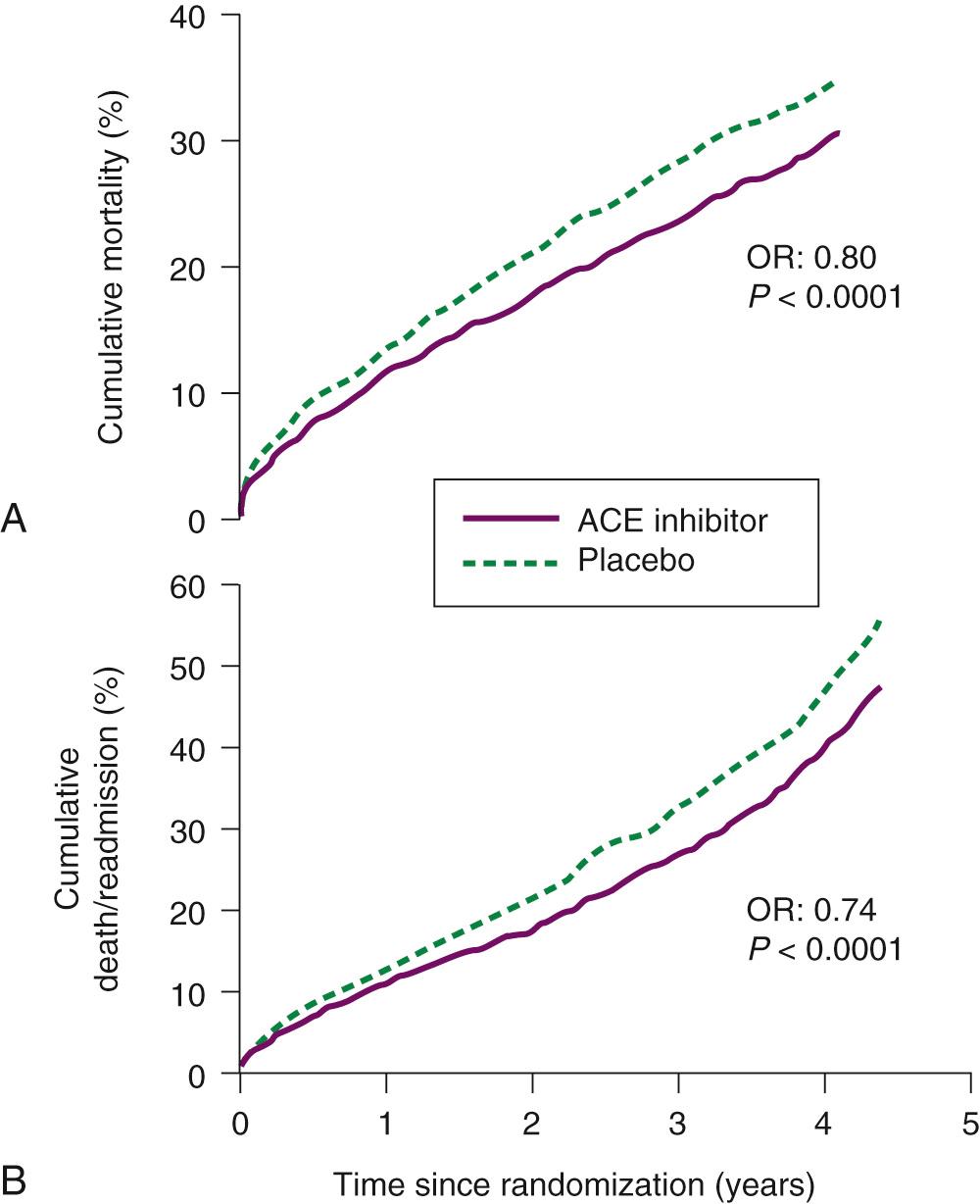
The various ACE inhibitors that are currently available ( Table 58-6 ) differ in plasma half-life, dosing regimen, and ability to inhibit ACE at the tissue level. However, available data suggest that the beneficial effects of these agents are a class effect and not dependent on individual pharmacologic characteristics. Nonetheless, in selecting an ACE inhibitor for the treatment of heart failure, preference should be given to those agents that have demonstrated efficacy in large-scale trials (enalapril, captopril, lisinopril, and ramipril). ACE inhibitors should be initiated at low doses, especially in patients with hypotension before treatment. If initial doses are tolerated hemodynamically, the dose should be gradually titrated upward over several days to several weeks. In general, these agents should be titrated upward to goal doses as determined by clinical trials or to the highest dose that can be tolerated. Although lower doses may offer mortality benefit similar to that of higher doses, higher doses are associated with augmented symptom control. In patients with decompensated heart failure who cannot receive oral agents, intravenous enalaprilat can be used. Enalaprilat is the active form of the oral ACE inhibitor enalapril. When it is given intravenously, it is a balanced vasodilator resulting in a reduction in left and right ventricular filling pressures and vascular resistance.
| Class and Examples | Starting Dose | Target or Maximum Dose | Adverse Effects (by Class) |
|---|---|---|---|
| Nitrovasodilators | |||
| Isosorbide mononitrate (Imdur) | 30 mg qd | 120 mg qd | Headache; nitrate tolerance with continuous use |
| Isosorbide dinitrate | 10 mg tid | 30 mg tid | |
| Direct-Acting Vasodilators | |||
| Hydralazine (Apresoline) | 10 mg qid | 100 mg qid | Reflex tachycardia, lupus-like syndrome |
| Angiotensin-Converting Enzyme Inhibitors | |||
| Captopril (Capoten) | 6.25 mg tid | 50 mg tid | Hypotension, cough, rash, angioedema, hyperkalemia Renal dysfunction (especially in patients with bilateral renal artery stenosis) |
| Enalapril (Vasotec) | 2.5 mg bid | 20 mg bid | |
| Lisinopril (Zestril, Prinivil) | 2.5 mg qd | 40 mg qd | |
| Ramipril (Altace) | 2.5 mg qd | 10 mg qd | |
| Quinapril (Accupril) | 10 mg bid | 40 mg qd | |
| Fosinopril (Monopril) | 10 mg qd | 40 mg qd | |
| Trandolapril (Mavik) | 0.5 mg qd | 8 mg qd | |
| Angiotensin Receptor Blockers | |||
| Losartan (Cozaar) | 50 mg qd | 100 mg qd | Hyperkalemia Renal dysfunction (especially in patients with bilateral renal artery stenosis) |
| Candesartan (Atacand) | 4 mg qd | 32 mg qd | |
| Valsartan (Diovan) | 80 mg qd | 320 mg qd | |
| β-Adrenergic Blocking Agents | |||
| Carvedilol (Coreg) | 3.125 mg bid | 25 mg bid | Bradycardia, hypotension, bronchospasm May worsen heart failure during initiation and titration |
| Metoprolol (Toprol XL) | 25 mg qd | 200 mg qd | |
| Bisoprolol (Zebeta) | 1.25 mg qd | 10 mg qd | |
| Inotropic Agents | |||
| Digoxin (Lanoxin) | 0.125 mg qd | Serum digoxin level: 0.5-0.8 ng/mL | Nausea, bradycardia, heart block, ventricular tachyarrhythmias |
| Calcium Channel Blockers * | |||
| Amlodipine (Norvasc) | 2.5 mg qd | 10 mg qd | Amlodipine: pedal edema Verapamil and diltiazem: bradycardia, worsened systolic heart failure |
| Verapamil (Calan, Verelan) | 120 mg qd | 480 mg qd | |
| Diltiazem (Cardizem, Dilacor) | 120 mg qd | 540 mg qd | |
* Calcium channel blockers should not be routinely used for the treatment of systolic heart failure. Although amlodipine is safe in this setting, verapamil and diltiazem may worsen systolic heart failure, and their use is limited to the treatment of diastolic heart failure.
Use of ACE inhibitors may be limited by the development of side effects. Hypotension is the most common adverse effect, occurs most frequently during the initiation of therapy, and is more common in patients who are volume depleted. It can usually be managed by reducing diuretic dosing and titrating the ACE inhibitor slowly. Moderate hypotension (systolic blood pressure >85 mm Hg) can frequently be tolerated as long as organ hypoperfusion is not present. ACE inhibitors produce vasodilation of renal efferent arterioles and thereby reduce glomerular filtration rate. Worsening renal function can be seen in 5% to 30% of patients treated with these agents; the risks are significantly higher in patients with more severe heart failure and in those with bilateral renal artery stenosis. Hyperkalemia may also occur, even in the absence of declining renal function. At least 5% to 10% of patients treated with ACE inhibitors develop a dry, nonproductive cough. This probably results from the inhibition of bradykinin metabolism and resolves with cessation of the drug. Less than 1% of patients develop angioedema when they are treated with ACE inhibitors. This can be life-threatening and precludes further use of the drug. Both the efficacy and side effect profile of ACE inhibitors are affected by volume status, and careful monitoring of volume and appropriate diuretic dosing are important. Volume overload will blunt the therapeutic effects of ACE inhibitors, and whereas dietary sodium restriction may enhance the response to ACE inhibitors, volume depletion will exaggerate their hypotensive effects.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here