Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The anteroposterior (AP) diameter of the neonatal chest is almost as great as its transverse diameter, giving the chest a cylindrical configuration. The degree of rotation is best assessed by comparing the length of the anterior ribs visible on both sides. As newborn chest radiographs are taken in the AP projection, the normal cardiothoracic ratio can be as large as 0.6.
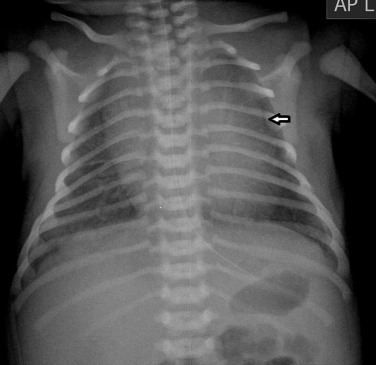
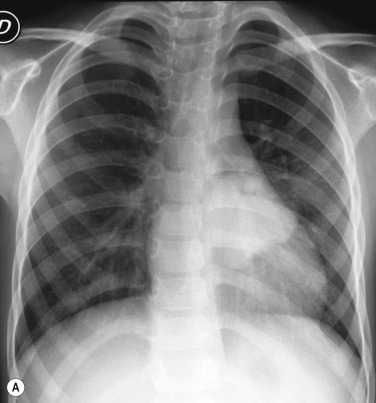
The thymic size is variable and may alter with the degree of lung inflation. It may blend with the cardiac silhouette, may have an undulating boarder due to indentation by the adjacent ribs ( Fig. 70.1 ) or may exhibit the classic ‘sail sign’ more commonly seen on the right side. Thymic tissue may involute rapidly with prenatal or postnatal stress: for example, in severe illnesses such as hyaline membrane disease (HMD), infections, or following corticosteroid treatment.
There are some well-recognised artefacts on a newborn chest radiograph. The hole in the incubator top may be confused with a pneumatocele; and skin folds may overlie the chest wall mimicking a pneumothorax (although these can usually be seen to extend beyond the lateral edge of the lung).
The normal lung development is well described by Agrons et al. During the embryonic phase of gestation (from 26 days to 6 weeks) the lung bud develops from the primitive foregut and divides to form the early tracheobronchial tree. During the pseudoglandular phase (6–16 weeks) there is airway development to the level of the terminal bronchioles, with a deficient number of alveolar saccules. Multiple alveolar ducts develop from the respiratory bronchioles during the canicular or acinar phase (16–28 weeks). These ducts are lined by type II alveolar cells that can produce surfactant, and which differentiate into thin type I alveolar epithelial cells. At the end of this phase, primitive alveoli form. Progressive thinning of the pulmonary interstitium allows gas exchange via approximation of the proliferating capillaries with the type I cells.
During the saccular phase (28–34 weeks) there is an increase in the number of terminal sacs, further thinning of the interstitium, continuing proliferation of the capillary bed and early development of the true alveoli.
The alveolar phase extends from approximately 36 weeks’ gestation until 18 months of age. While most alveoli have formed by 5 to 6 months of age, late alveolarisation continues well into early adulthood.
Idiopathic respiratory distress syndrome (IRDS) or HMD mainly affects the premature infant less than 36 weeks’ gestational age. The primary problem in HMD is a deficiency of the lipoprotein pulmonary surfactant in association with structural immaturity of the lungs. The lipoproteins are produced in the type II pneumocytes, are concentrated in the cell lamellar bodies and then transported to the cell surface and expressed onto the alveolar luminal surface. These lipoproteins then combine with surface surfactant proteins (A, B, C, D), which are also produced by the type II pneumocytes to form tubular myelin. This is the principal contributor at the alveolar air–fluid interface, which lowers alveolar surface tension and prevents acinar collapse on expiration. Without this, there is alveolar collapse and, as a result, poor gas exchange, hypoxia, hypercarbia and acidosis. The alveolar ducts and terminal bronchioles are distended and lined by hyaline membranes that contain fibrin, cellular debris and fluid, thought to arise from a combination of ischaemia, barotrauma and the increased oxygen concentrations used in assisted ventilation. Hyaline membrane formation can also occur in other neonatal chest conditions requiring ventilation.
Clinically these premature infants are usually symptomatic within minutes of birth with grunting, retractions, cyanosis and tachypnoea. Chest radiographic findings may be present shortly after birth but occasionally the maximum features may not be present until 6–24 hours of life.
Before the commencement of treatment, the typical radiographic features include under-aeration of the lungs, fine granular opacification, which is diffuse and symmetrical, and air bronchograms ( Fig. 70.2 ) due to collapsed alveoli interspersed with distended bronchioles and alveolar ducts. When there is less distension, the granularity is replaced by more generalised opacification or even complete white-out of the lungs ( Fig. 70.3 ). Atelectasis is the main cause of this opacification, but particularly in the very premature infant, oedema, haemorrhage and, occasionally, superimposed infection contribute.
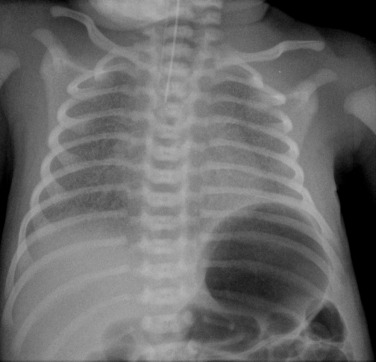
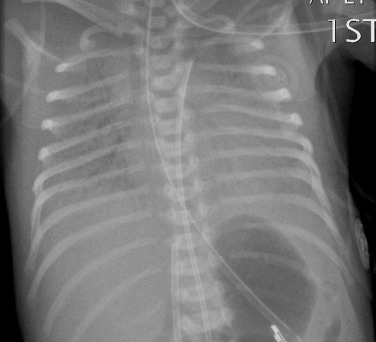
Very premature infants, less than 26 weeks’ gestation, may have clear lungs or mild pulmonary haziness initially. Their lungs are structurally and biochemically immature and require prolonged ventilatory support. Prenatal corticosteroid administration during the 2 days before delivery significantly reduces the incidence of IRDS in premature infants.
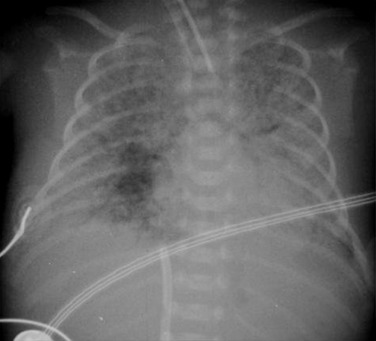
The clinical use of artificial surfactant, given as a liquid bolus through the endotracheal (ET) tube, has been a major therapeutic advance. It may not be evenly distributed throughout the lungs, leading to areas of atelectasis interspersed with areas of good aeration, and may produce radiographic findings similar to neonatal pneumonia or pulmonary interstitial emphysema (PIE) ( Fig. 70.4 ). Correlation with the clinical picture is, therefore, very important.
In general, infants greater than 27 weeks’ gestation respond best to surfactant therapy. In the very premature infant, less than 27 weeks’ gestation, the lungs become clear following surfactant administration, but they are still immature with fewer alveoli than normal. This results in inadequate gas exchange, leads to prolonged ventilation, hazy lung opacification and, occasionally, a picture resembling bronchopulmonary dysplasia (BPD) (a clinical label attached at 28 days of supplemental oxygen requirement from birth) ( Fig. 70.5 ).
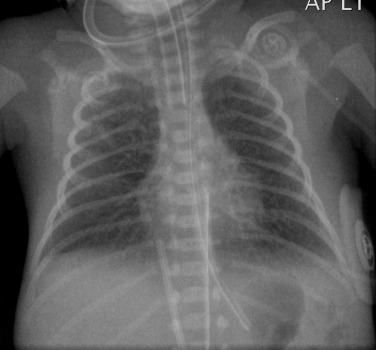
A patent ductus arteriosus (PDA) is frequent in the premature infant and contributes to the disease. The rigid lungs caused by IRDS and the associated hypoxia and hypercarbia may lead to right-to-left shunting through the ductus. With surfactant therapy and improved oxygenation there is reduced pulmonary resistance and as a result there may be left-to-right shunting. Initial treatment, if required, is with ibuprofen, which inhibits prostaglandin production. If unsuccessful, surgical or endovascular occlusion will be required.
The chest radiograph may demonstrate sudden cardiac enlargement, left atrial enlargement causing elevation of the left main bronchus and varying degrees of pulmonary oedema ( Fig. 70.6 ). There is an increasing use of prophylactic continuous positive airway pressure (CPAP) ventilation in infants suspected of developing IRDS, which helps reduce the incidence of complications in these infants. High-frequency oscillatory ventilation is also used to reduce the incidence of barotrauma, particularly in the very premature infant. In these infants the radiographs do not differ significantly from those infants receiving conventional ventilation. The chest radiograph is used to assess the degree of lung inflation with the dome of the diaphragm ideally project at the level of the 8th to 10th posterior ribs if the mean airway pressure is appropriately adjusted.
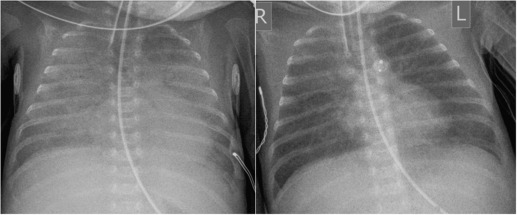
The use of positive pressure ventilation in the newborn is the most common cause of pneumothorax, pneumomediastinum, PIE ( Fig. 70.7 ) and pneumopericardium ( Fig. 70.8 ). These complications have become much less common in infants who have been treated with surfactant and high-frequency ventilation. Areas of atelectasis can occur in surfactant deficiency and are frequently due to poor clearance of secretions ( Fig. 70.9 ). Premature infants are at an increased risk of infective pneumonia, which may coexist with IRDS. Pulmonary haemorrhage resulting in airspace opacification may also be a superimposed problem, and is usually due to severe hypoxia and capillary damage ( Fig. 70.10 ). BPD or chronic lung disease of prematurity is a significant long-term complication of IRDS. Because of the many advances in neonatal care, its incidence and severity have reduced significantly in infants born at 28 weeks’ gestation or older. The unchanged overall incidence is due to the increased survival of the infants of extreme prematurity as they require more prolonged ventilation. Air leaks, PDA and infection are contributing factors as they also prolong ventilation. A higher incidence of BPD has been demonstrated in infants with previous culture-proven Ureaplasma urealyticum pneumonitis.
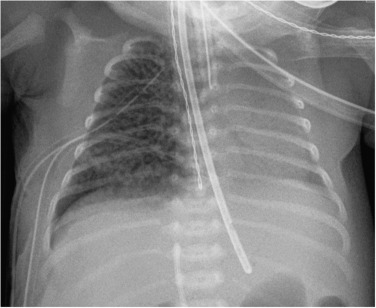
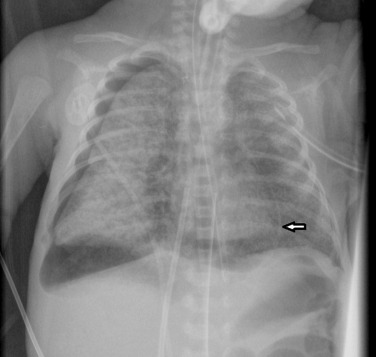
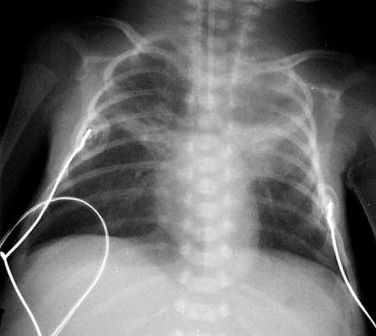
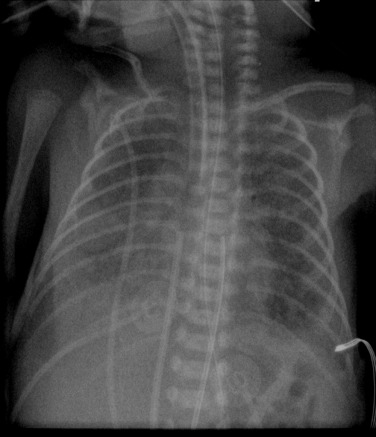
The four classic stages of BPD described by Northway are now very rarely seen. Nowadays the most common radiographic appearance is diffuse interstitial shadowing with mild-to-moderate hyperinflation of gradual onset ( Fig. 70.11 ). The so-called ‘new’ type of BPD was described by Jobe in 1999 in immature infants with minimal lung disease at birth, and who become symptomatic during the first week of life. This entity seems inseparable from the condition described previously as Wilson–Mikity syndrome . In this era of exogenous surfactant administration and improved neonatal intensive care, it could be argued that the appearance of ‘classic’ BPD should trigger a hunt for genetic causes of surfactant dysfunction or other lung growth disorders. Pulmonary vein stenosis is increasingly recognised in association with BPD in premature low birth-weight infants and can result in pulmonary hypertension . Electrocardiogram (ECG)-gated CT angiography is gaining a significant and important role in the diagnosis of pulmonary vein stenosis and may aid with planning of subsequent clinical management.
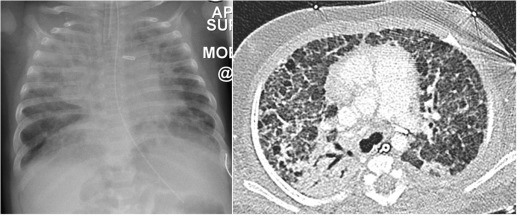
This condition is also referred to as retained fetal lung fluid or wet-lung syndrome. Normally fluid is cleared from the lungs at, or shortly after, birth by the pulmonary lymphatics and capillaries. In transient tachypnoea of the newborn (TTN) the normal physiological clearance is delayed. The incidence is greater in infants delivered by caesarean section, in hypoproteinaemia, hyponatraemia and maternal fluid overload. There is also an increased incidence in small, hypotonic and sedated infants who have had a precipitous delivery.
Typically the infants have mild-to-moderate respiratory distress without cyanosis in the first couple of hours. The process resolves rapidly with almost complete resolution in 48 hours. Treatment consists of supportive oxygen and maintenance of body temperature. Radiographically, the most common appearances are mild overinflation, prominent blood vessels, perihilar interstitial shadowing and fluid in the transverse fissure with occasional small pleural effusions ( Fig. 70.12 ). There may be mild associated cardiomegaly. The appearances may be asymmetrical with right-sided predominance, a phenomenon which remains unexplained. When severe, the features may simulate meconium aspiration syndrome and congenital neonatal pneumonia.
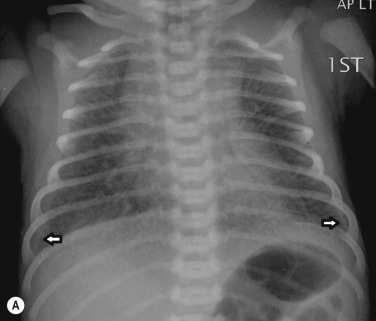
Meconium aspiration syndrome is diagnosed when an infant born through meconium-stained amniotic fluid displays symptoms that cannot otherwise be explained and is confirmed by the presence of meconium below the level of the vocal cords. It is thought that fetal hypoxia causes fetal intestinal hyperperistalsis and passage of meconium, which is then aspirated by the fetus. It is most common in infants who are post-mature.
The radiographic features may, in part, be due to inhalation of the meconium itself – a thick viscous substance that may migrate to the distal airways, causing complete or partial obstruction, the latter leading to a ‘ball-valve’ effect. This may lead to alternating areas of segmental atelectasis and overinflation. Meconium may also cause a chemical pneumonitis ( Fig. 70.13 ), alterations in the pulmonary vasculature and resultant pulmonary arterial hypertension. Air leaks are common and small associated pleural effusions may be seen.
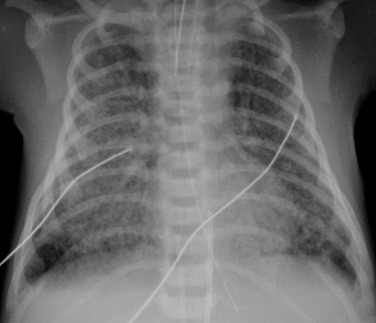
Approximately 30% of affected infants will require mechanical ventilation. The mortality rate has been improved by the use of inhaled nitric oxide, to treat severe pulmonary hypertension, and also by extracorporeal membrane oxygenation (ECMO), used in those infants where the conventional treatments have failed. ECMO can be used either with the veno-arterial (VA) method (VA-ECMO), where one catheter is placed via the internal jugular vein and one via the common carotid artery, or the veno-venous (VV) method, most often via a double lumen catheter placed via the internal jugular vein ( Fig. 70.14 ). The ECMO circuit allows gaseous exchange to maintain physiological levels of oxygen saturation whilst VA-ECMO allows the circulation to bypass the lungs when pulmonary vascular resistance is particularly high.
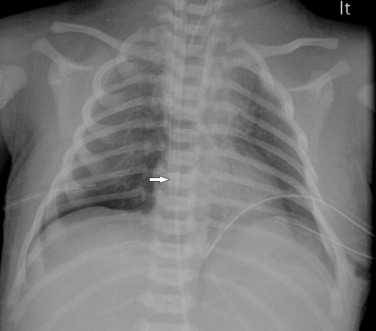
Neonatal infections acquired transplacentally, such as TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes), are rare and seldom develop pulmonary abnormalities. Infections acquired perinatally can occur via ascending infection from the vagina, transvaginally during birth or as a hospital-acquired infection in the neonatal period. Prolonged rupture of membranes before delivery is a major risk factor. It is thought that most cases of neonatal pneumonia occur during birth, when the infant may swallow and/or aspirate infected amniotic fluid or vaginal tract secretions. Group B streptococcus is the most common organism identified. The radiological features are non-specific.
The most common features seen on the chest radiograph in term infants who present with severe acute symptoms in the first 24–48 hours are coarse bilateral asymmetrical alveolar opacification with or without associated interstitial change ( Fig. 70.15 ). In these infants the radiographic changes may mimic meconium aspiration syndrome or severe transient tachypnoea. The presence of pleural effusions, pulmonary hyperinflation and mild cardiomegaly may not be helpful in differentiating pneumonia from these other conditions.
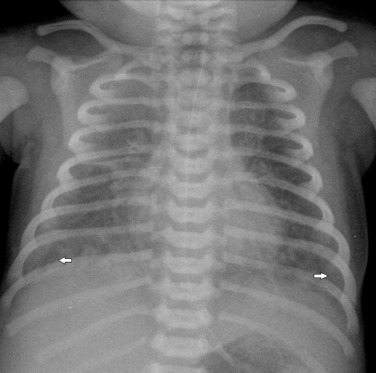
In the premature infant there may be diffuse fine granular opacification, similar to the appearances seen in IRDS, and some infants may have both IRDS and group B streptococcus pneumonia. Chlamydial infection classically presents first with conjunctivitis at 1–2 weeks after birth and the lung infection does not usually become evident until 4–12 weeks of age. Typically the radiograph demonstrates interstitial opacification with some hyperinflation.
The association of Ureaplasma urealyticum with neonatal pneumonia is increasingly recognised. These infants have a mild early course and develop features of BPD at an earlier age than would be expected in a premature infant.
Spontaneous pneumothorax and pneumomediastinum cause respiratory distress in the newborn infant. Many are small and transient and do not require intervention. A pneumothorax may be radiographically subtle in sick infants as supine radiographs are usually performed and free air accumulates over the anterior lung surface, producing a hyperlucent hemithorax, increased sharpness of the mediastinal and/or diaphragmatic contours and a deep costophrenic recess or so-called ‘deep sulcus sign’ (see Figs 70.7 and 70.14 ). A pneumomediastinum usually outlines the thymus ( Fig. 70.16 ) and when there is a pneumopericardium the air surrounds the heart (see Fig. 70.8 ).
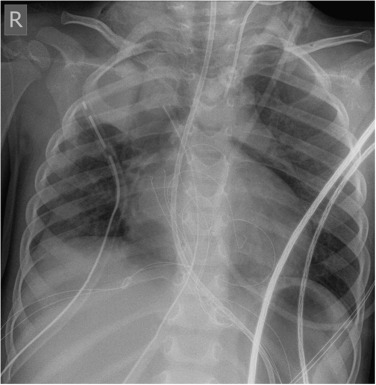
In infants who do not have hydrops, the most common cause of a congenital pleural effusion is chylothorax. The cause is unknown, and late maturation of the thoracic duct has been suggested as an aetiology. The abnormality is usually detected on antenatal ultrasound (US) and, if large, in utero drainage may be performed to prevent pulmonary hypoplasia. Postnatally, the chest radiograph demonstrates the pleural effusions ( Fig. 70.17 ). Aspirated fluid will have a high lymphocyte count but will not have a milky appearance until such time as the infant is fed with fat. Resolution is usually complete, but often after multiple aspirations.
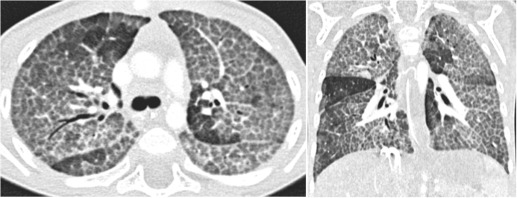
Disorders of surfactant function/metabolism, due to a genetic abnormality in the surfactant protein B (SpB), SpC or ATP-binding cassette transporter protein A3 (ABCA3), can lead to interstitial lung disease. Inherited mutations in the SpB and ABCA3 are autosomal recessive and may present immediately after birth with respiratory symptoms. Mutations in the SpC are often autosomal dominant and may present later in infancy. The chest radiograph may initially show diffuse hazy opacification, with the later development of reticulation, which may be progressive. CT appearances vary according to the underlying histopathological pattern of damage and may demonstrate the ‘crazy paving’ of pulmonary alveolar proteinosis (PAP) or variable combinations of ground-glass opacification, septal thickening and cystic change (consistent with cellular non-specific interstitial pneumonia (NSIP) or chronic pneumonitis of infancy) ( Fig. 70.18 ).
Pulmonary interstitial glycogenosis (PIG) may present in the preterm or term infant very soon after birth. It has been reported in isolation but is frequently associated with conditions that affect lung growth, and the diagnosis is made by the pathological examination of lung tissue. The imaging features include ground glass and pulmonary parenchymal cysts, similar to those seen in the disorders of surfactant deficiency.
The tip of an ET tube may vary considerably with head and neck movement and the correct position must therefore be assessed by taking the patient's head position and the tip of the tube into consideration.
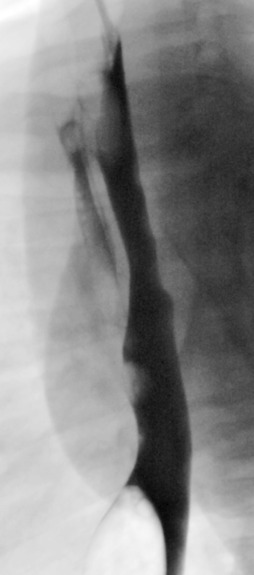
An umbilical arterial line should course inferiorly within the umbilical artery, into the internal and common iliac arteries and then into the aorta. The tip should be positioned to avoid the origins of the major vessels, and are usually positioned between T6 and T9 (high) ( Fig. 70.19 ) or in some institutions inferior to the L3 vertebral body (low).
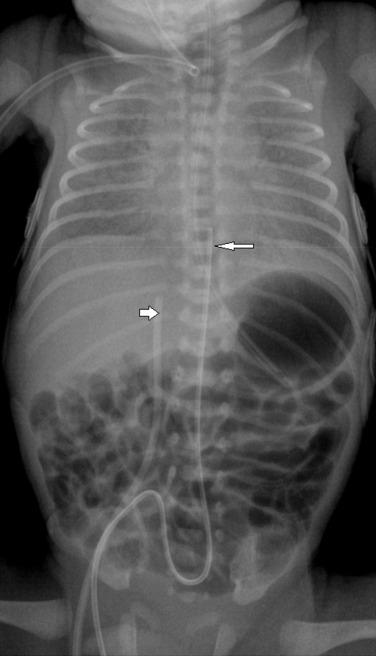
An umbilical venous line should course superiorly toward the liver, enter the left portal vein, traverse the ductus venosus and end within the inferior vena cava (IVC). The ideal tip position is at the junction of the IVC and the right atrium (see Fig. 70.19 ).
The position of the tips of central venous catheters, inserted via the internal jugular veins, and peripherally inserted central catheter (PICC) line tips, inserted through the upper limbs, is usually in the superior vena cava. The tips of lines inserted through the lower limbs is more controversial. US may be helpful in assessing a catheter's position and injection of very small amounts of intravenous water-soluble, low osmolar contrast medium may be useful in checking the position of the tip, patency of the line and assessing for the presence of thrombin sheaths around the tip or line fractures.
Nasogastric tube tip positions should always be reported, in order to avoid misplacement of nasogastric feeds. Nasojejunal tubes often include a weighted tip and care should be taken to identify the position of the exit hole (often a few cm proximal to the line tip) in order to avoid misreporting the appropriateness of the tip position.
ET tube – tip above the carina (consider chin position)
UAC – T6-T9 (high position) or below L3 (low position), not inbetween
UVC – junction between IVC and right atrium
Temperature probe – within the thoracic oesophagus
NG tube – within the stomach
NJ tube – side hole at the duodenojejunal flexure (noting that the side hole is often proximal to a long weighted section of tubing)
Diseases of the respiratory tract occur frequently in children. These will range from the presentation of congenital abnormalities and common infections, through to complex immunodeficiency syndromes and malignancy.
The chest radiograph is the most frequently requested radiological investigation encountered within paediatric practice, and although pathological manifestations may mimic those seen in adults, a thorough knowledge of the variations within paediatric practice is vital to the general radiologist. In this section, we cover some of the unique aspects of chest disease in the older child.
The plain chest radiograph remains the first radiological examination in use for the evaluation of the chest in children. Frontal chest radiographs are widely performed. Lateral views tend only to be performed after review of the frontal radiograph, when there are unanswered clinical questions.
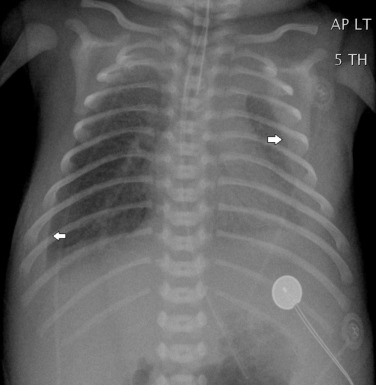
A PA erect radiograph taken in full inspiration is optimal but difficult to obtain in uncooperative children; hence, an AP supine view is usually obtained in infants and small children. An inspiratory plain chest radiograph is considered adequate when the right hemidiaphragm is at the level of the eighth rib posteriorly. Poor inspiration may cause significant misinterpretation of the chest radiograph ( Fig. 70.20 ). Radiographs obtained in expiration frequently show a rightward kink in the trachea, owing to the soft cartilage, relatively long trachea and the presence of a left aortic arch in the majority of children. Other features of an expiratory radiograph include some degree of ground-glass opacification of the lungs and relative enlargement of the heart. Rotation of the patient causes problems with interpretation, including apparent mediastinal shift/distortion of vasculature, the thymus and vessels mimicking a ‘mass’, or lobar consolidation ( Fig. 70.21 ) and relative lucency of one lung compared with the other, simulating oligaemia/hyperinflation.
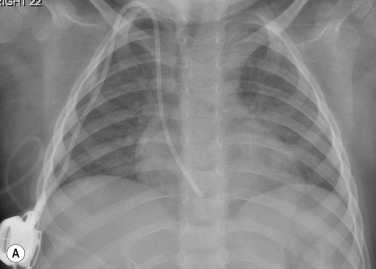
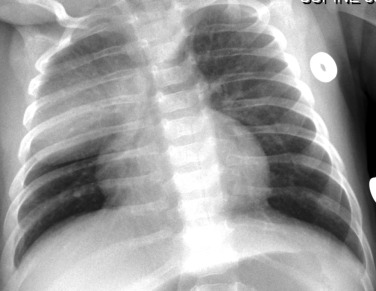
Care should be taken to avoid confusing overlying plaits or braids of hair superimposed over the upper zones of the chest radiograph as intraparenchymal lung disease.
The normal thymus is a frequent cause of physiological widening of the anterior mediastinum occurring during the early years of life.
Normal thymic tissue is soft, malleable and compliant; hence, it often undulates beneath the overlying ribs, giving it a lobulated appearance known as the ‘thymic wave’. The right thymic margin can often have a sharp ‘sail-like’ configuration (see Fig. 70.1 ). The thymus may involute during periods of illness, severe stress or whilst on steroids or other chemotherapy. Subsequent ‘rebound hyperplasia’ of the thymus may then occur following recovery or cessation of therapy, and this should not be confused with the development of mediastinal disease. If chest radiographic differentiation between normal thymus and pathology proves difficult on the radiograph, US can help distinguish intrathymic or adjacent masses within the anterior mediastinum from a normal-appearing isoechoic homogeneous thymus. Most mediastinal disease processes appear heterogeneous, and may cause compression or indeed occlusion of adjacent airway or vasculature, something which should not occur with a normal thymus. In some cases where US is inconclusive, magnetic resonance imaging (MRI) is performed to differentiate a normal thymus from mediastinal disease. On T 2 weighted spin-echo sequences, the normal thymus has an intermediate signal similar to that of the spleen. On gadolinium-enhanced T 1 weighted spin-echo sequences, the thymus should show only minimal enhancement.
When the chest radiograph shows asymmetrical lung volumes, the lung with fewer vessels per unit area is usually the abnormal lung. The lack of, or reduction in, vascular markings is most commonly due to the presence of primary airways disease in children and the resultant homeostatic reflex vasoconstriction ( Table 70.1 ) ( Fig. 70.22 ). The presence of reduced vascularity in the hyperlucent areas resulting from a primary vascular pathological process, such as thromboembolism or pulmonary hypertension, is rare in children, although various complex congenital cardiac disorders can result in pulmonary oligaemia.
| Respiratory Causes With Contralateral Mediastinal Shift | Respiratory Causes Without Mediastinal Shift | Other Causes |
|---|---|---|
| Foreign body aspiration—may be normal on inspiratory image, fluoroscopy can help | Unilateral hypoplastic lung Congenital venolobar syndrome/scimitar syndrome Constrictive bronchiolitis—formerly known as Swyer-James-Macleod syndrome |
Congenital thoracic anomalies (pulmonary agenesis/aplasia, Poland syndrome) |
| Mucous plugging—particularly asthma, cystic fibrosis and ventilated patients Congenital lobar overinflation External mass compression—mediastinal mass compressing a bronchus |
Congenital cardiac disease with asymmetric pulmonary vascularity (anomalous pulmonary venous return, major aortopulmonary collateral arteries, etc.) | |
| Post-cardiac surgery—e.g., Blalock–Taussig shunt | ||
| Bronchomalacia | ||
| Endobronchial lesion—e.g., bronchial carcinoid |
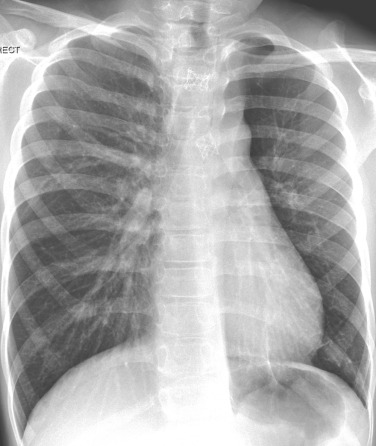
A widespread increase in/prominence/enlargement of pulmonary parenchymal vessels could indicate the presence of a left-to-right shunt at either intracardiac or great vessel level. Cardiac failure as a primary cause of pleural effusion in children is not common. In children, fluid overload tends to cause peribronchovascular oedema with loss of clarity of vascular structures (the x-ray equivalent of ‘ground-glass’), perihilar infiltrates and upper lobe venous diversion.
Respiratory infections in children are the most frequent disorders encountered by paediatricians. Chest radiography is the primary imaging technique used to evaluate acute lung disease. However, chest CT has an important role in evaluating immunocompromised patients and both the acute and chronic complications of respiratory tract infection, such as pulmonary abscesses, complex empyema, potential underlying lesions (e.g. congenital thoracic malformations) and bronchiectasis. A frontal radiograph is usually adequate to confirm or exclude pulmonary infection/pneumonia.
Within all age groups, viral infection is more common than bacterial. Viral infection usually affects the respiratory mucosa and airways, causing bronchial and bronchiolar oedema. This results in hyperinflation (due to air trapping as a result of partial bronchial obstruction as a result of peribronchial thickening), segmental and subsegmental atelectasis and small patches of consolidation, frequently occurring in a perihilar location ( Fig. 70.23 ).
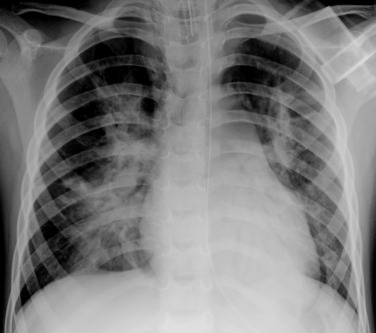
Bacterial pneumonia, in general, causes inflammation within the acini, resulting in oedema and intra-alveolar exudate. This causes consolidation within the air spaces and results in the presence of ‘air bronchograms’ seen on radiographs. The typical distribution is lobar or segmental, and associated pleural (parapneumonic) effusions are not uncommon ( Fig. 70.24 ). Although these patterns have traditionally been associated with viral and bacterial pathogens, studies indicate that prediction of causative pathogen using radiographic patterns is notoriously inaccurate. In addition, viral and bacterial infection may be present simultaneously.
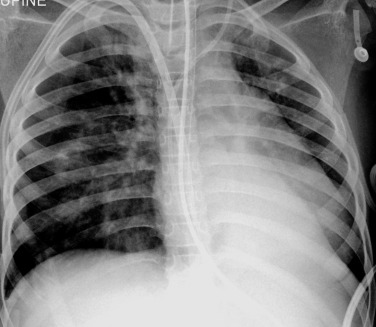
Round pneumonia occurs frequently in young children, usually under 8 years of age, due to the presence of immature collateral ventilation pathways between the small airways ( Fig. 70.25 ). Streptococcus pneumoniae is the causative pathogen in >90% of normal hosts. Radiographs show a rounded opacity with poorly defined margins (unlike primary or metastatic chest tumours, which are usually very well circumscribed). Follow-up chest radiography to ensure resolution of pneumonia is not routinely necessary, in an otherwise previously healthy child. Follow-up should be reserved for children who fail to improve clinically with persistent or recurrent symptoms, or have an underlying condition such as immunodeficiency. Causes of recurrent/persistent pneumonia include infection within a pre-existing lung abnormality, bronchial obstruction, aspiration associated with gastro-oesophageal reflux and, rarely, an H-type congenital trachea-oesophageal fistula ( Fig. 70.26 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here