Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The male reproductive system consists of two essential elements: the gonads (in this case the testes) and the complex array of glands and ducts that constitute the sex accessory organs ( Fig. 54-1 A, B ).
The testes are responsible for the production of gametes, the haploid cells— spermatozoa, plural of spermatozoon—necessary for sexual reproduction and for the synthesis and secretion of hormones, including the principal male sex hormone, testosterone. These hormones are necessary for functional conditioning of the sex organs, the male secondary sexual characteristics, feedback control of gonadotropin secretion, and modulation of sexual behavior.
The testes (see Fig. 54-1 C ) are composed mainly of seminiferous tubules (see Fig. 54-1 D, E ) and interstitial cells of Leydig, located in the spaces between the tubules. A seminiferous tubule is an epithelium made up of Sertoli cells (see Fig. 54-1 E ) and is also the site of spermatogenesis —the production of the haploid spermatozoa from the diploid germ cells. The seminiferous epithelium rests on a basement membrane, itself supported by a thin lamina propria externa.
The male sex accessory organs include the paired epididymides, the vas deferens, the seminal vesicles, the ejaculatory ducts, the prostate, the bulbourethral glands (Cowper's glands), the urethra, and the penis. The primary role of the male sex accessory glands and ducts is to store and transport spermatozoa to the exterior, and thus enable spermatozoa to reach and fertilize female gametes.
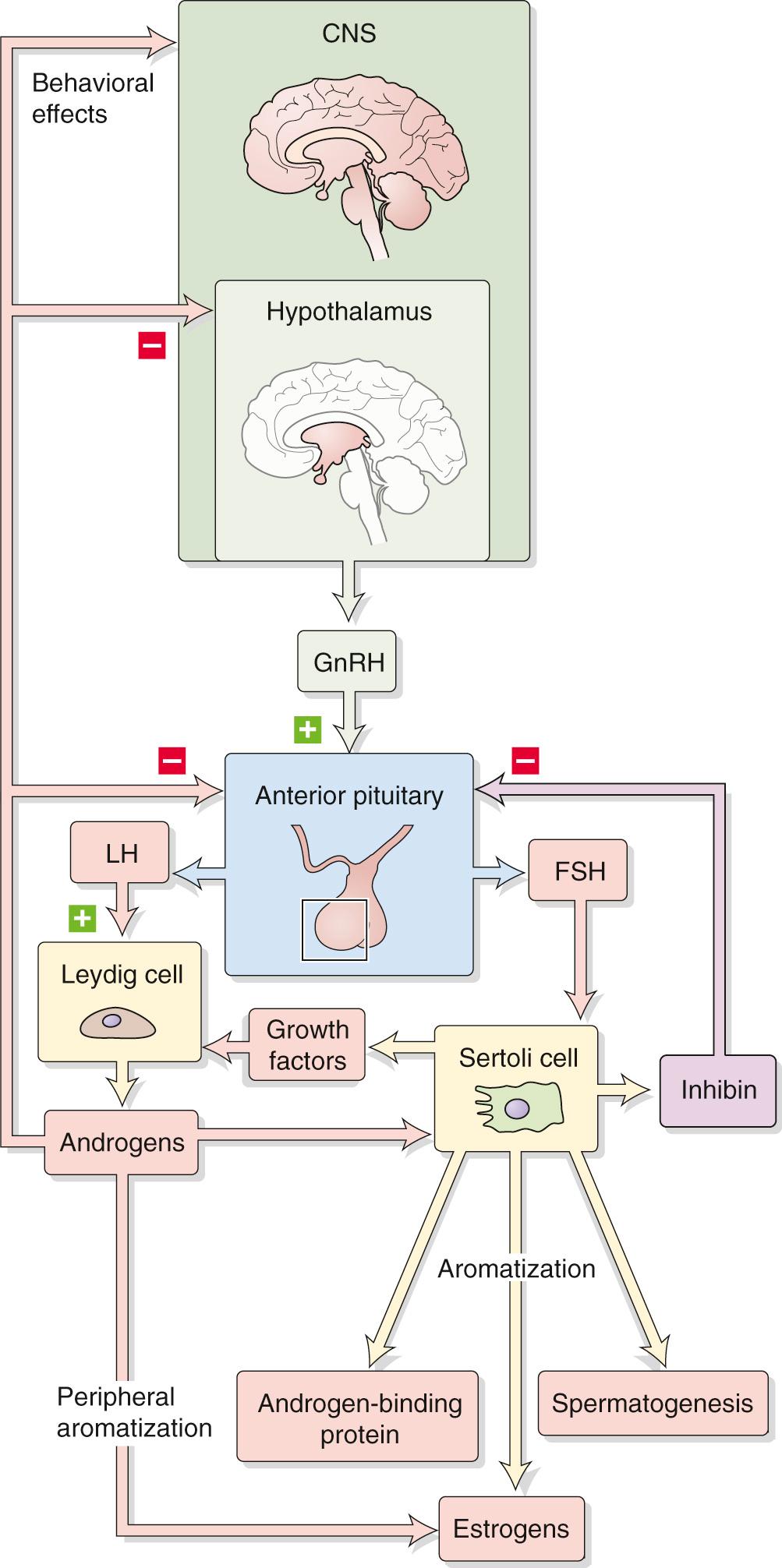
The hypothalamic-pituitary-gonadal axis ( Fig. 54-2 ) controls two primary functions: (1) production of gametes (spermatogenesis in males and oogenesis in females), and (2) gonadal sex steroid biosynthesis (testosterone in males and estradiol and progesterone in females). In both sexes, the hypothalamus produces gonadotropin-releasing hormone (GnRH), which stimulates the gonadotrophs in the anterior pituitary to secrete the two gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Although the names of these hormones reflect their function in the female reproductive system (see pp. 1111–1112 ) they play similar roles in controlling gonadal function in both sexes. The hypothalamic-pituitary axis is therefore the central regulator of male and female reproductive systems. In the male, LH and FSH control, respectively, the Leydig and Sertoli cells of the testes.
Gonadotropin-releasing hormone (GnRH), which is synthesized by small-bodied peptidergic neurons in the hypothalamus, stimulates the synthesis, storage, and secretion of gonadotropins by gonadotroph cells in the anterior pituitary. The hypothalamic-pituitary-portal system (see p. 978 ) describes the route by which GnRH and other releasing hormones emanating from the hypothalamus reach the anterior pituitary gland. The neurons that synthesize, store, and release GnRH are dispersed throughout the hypothalamus but are principally located in the arcuate nucleus and preoptic area. During embryonic development, GnRH neurons originate in the olfactory placode and migrate to the hypothalamus. Studies involving both rats and primates show that sites of GnRH production other than the hypothalamus (e.g., the limbic system) can also participate in the control of sex behavior. Neuronal systems originating from other areas of the brain impinge on the hypothalamic GnRH-releasing neurons and thus form a functional neuronal network that integrates multiple environmental signals (e.g., diurnal light-dark cycles) and physiological signals (e.g., extent of body fat stores, stress) to control GnRH release and, ultimately, the function of the reproductive system.
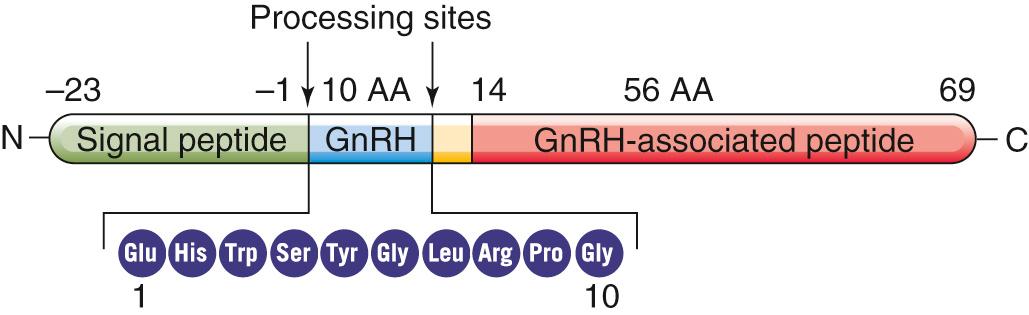
GnRH is a decapeptide hormone encoded by a single gene on chromosome 8. Like many other peptide hormones, GnRH is synthesized as a prohormone—69 amino acids long in this case. Cleavage of the prohormone yields the decapeptide GnRH (residues 1 to 10), a 56–amino-acid peptide (residues 14 to 69) referred to as GnRH-associated peptide (GAP), and three amino acids that link the two ( Fig. 54-3 ). Via the secretory pathway (see p. 34 ), the neuron transports both GnRH and GAP down the axon for secretion into the extracellular space. The role of GAP is unknown.
GnRH neurons project axons directly to a small swelling on the inferior boundary of the hypothalamus, known as the median eminence, which lies above the pituitary stalk. The axons terminate near portal vessels that carry blood to the anterior pituitary (see p. 978 ). Consequently, GnRH secreted at the axon terminals in response to neuron activation enters the portal vasculature and is transported directly to gonadotrophs in the anterior pituitary.
GnRH stimulates the release of both FSH and LH from the gonadotroph cells in the anterior pituitary by interacting with high-affinity membrane receptors on the gonadotroph cell surface (see Fig. 55-5 ). The GnRH receptor (GnRHR) is a G protein–coupled receptor (GPCR; see pp. 51–52 ) linked to Gα q , which activates phospholipase C (PLC; see p. 58 ). PLC acts on membrane phosphatidylinositol 4,5-bisphosphates (PIP 2 ) to liberate inositol 1,4,5-trisphosphate (IP 3 ) and diacylglycerol (DAG). IP 3 stimulates the release of Ca 2+ from internal stores, which triggers exocytosis LH and FSH. DAG stimulates protein kinase C, which indirectly increases expression of genes encoding LH and FSH. The net effect is an increase in the synthesis and release of LH and FSH from the gonadotrophs. Because secretion of GnRH into the portal system is pulsatile, secretion of LH and FSH by the gonadotrophs is also episodic. The frequency of pulsatile LH discharge in men is ~8 to 14 pulses over a 24-hour period. FSH pulses are not as prominent as LH pulses, both because of their lower amplitude and because of the longer half-life of FSH in the circulation.
Upon binding GnRH, the GnRH receptor is internalized and partially degraded in the lysosomes. However, some GnRH receptors are shuttled back to the cell surface, and de novo receptor synthesis continues from GnRH receptor gene expression. Return of the GnRH receptor to the cell membrane is referred to as receptor replenishment. A consequence of receptor internalization, however, is that the responsiveness of gonadotrophs to GnRH can be decreased by prolonged exposure to GnRH. Thus, although pulsatile GnRH discharge elicits a corresponding pulsatile release of LH and FSH, continuous administration of GnRH—or intermittent administration of high doses of GnRH analogs—suppresses the release of gonadotropins. This effect occurs because continuous (rather than pulsatile) exposure to GnRH causes a decrease in the number of GnRH receptors on the surface of the gonadotroph (i.e., receptor internalization exceeds replenishment). The induced desensitization to GnRH can be used therapeutically to control the reproductive function. A clinical application of this principle is chemical castration in prostatic cancer. Here, the administration of long-acting GnRH analogs desensitize the gonadotrophs to GnRH, which leads to low LH and FSH levels and thereby reduces testosterone production (see Box 55-2 ).
Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are members of the same family of hormones as human chorionic gonadotropin (hCG; see p. 1139 ) and thyroid-stimulating hormone (TSH; see p. 1010 ). These glycoprotein hormones are composed of two polypeptide chains designated α and β, both of which are required for full biological activity. The α subunits of LH and FSH, as well as the α subunits of hCG and TSH, are identical. In humans, the common α subunit has 92 amino acids and a molecular weight of ~20 kDa. The β subunits differ among these four hormones and thus confer specific functional and immunological characteristics to the intact molecules.
Each of the unique β subunits of FSH and LH is 115 amino acids in length. The β subunits of LH and hCG are identical except that the β subunit of hCG has an additional 24 amino acids and additional glycosylation sites at the C terminus. ![]() N54-1 The biological activities of LH and hCG are very similar. Indeed, in most clinical uses (e.g., in an attempt to initiate spermatogenesis in oligospermic men), hCG is substituted for LH because hCG is much more readily available.
N54-1 The biological activities of LH and hCG are very similar. Indeed, in most clinical uses (e.g., in an attempt to initiate spermatogenesis in oligospermic men), hCG is substituted for LH because hCG is much more readily available. ![]() N54-2
N54-2
hCG is secreted by the placenta, and some reports have described that small amounts of this substance are made in the testes, pituitary gland, and other nonplacental tissues.
hCG appears in the urine of pregnant women about 12 to 14 days after conception—the basis for pregnancy tests. In former times, hCG was extracted from the urine of pregnant women.
The disappearance of exogenous LH from the circulation is independent of gonadal function and follows a dual exponential time course. The half-life of the fast component is 40 minutes and that of the slow component is 120 minutes. Because of its increased glycosylation, hCG has an even longer half-life. FSH has a slower turnover rate; its disappearance from the blood is described by two exponentials with half-lives of about 4 hours and 3 days, respectively.
Differential secretion of FSH and LH is affected by several other hormonal mediators, including sex steroids, inhibins, and activins (see pp. 1113–1115 ). Thus, depending on the specific hormonal milieu produced by different physiological circumstances, the gonadotroph produces the α and β subunits of FSH and LH at different rates.
The specific gonadotropin and the relative proportions of each gonadotropin released from the anterior pituitary depend on the developmental age. The pituitary gland of the male fetus contains functional gonadotrophs by the end of the first trimester of gestation. Thereafter, gonadotropin secretion rises rapidly and then plateaus. Gonadotropin secretion begins to decline in utero during late fetal life and increases again during the early postnatal period.
Male primates release LH in response to GnRH administration at 1 to 3 months of age, a finding indicative of functional competence of the anterior pituitary gland. Also during this time, a short-lived postnatal surge of LH and testosterone secretion occurs in males. Although the cause of this short-lived surge of gonadotropins remains to be understood, it is clearly independent of sex steroids. The sensitivity of the gonadotrophs to stimulation subsequently diminishes, and the system remains quiescent until just before puberty.
Release of FSH is greater than that of LH during the prepubertal period, a pattern that is reversed after puberty. GnRH preferentially triggers LH release in men. This preferential release of LH may reflect maturation of the testes, which secrete inhibins, a specific inhibitor of FSH secretion at the level of the anterior pituitary gland. Increased sensitivity of the pituitary to increasing gonadal steroid production may also be responsible for the diminished secretion of FSH.
LH derives its name from effects observed in the female, that is, from the ability to stimulate ovulation and the formation and maintenance of the corpus luteum (see p. 1116 ). The comparable substance in the male was originally referred to as interstitial cell–stimulating hormone (ICSH). Subsequently, investigators realized that LH and ICSH are the same substance, and the common name became LH.
LH stimulates the synthesis of testosterone by the testes. Testosterone production decreases in males after hypophysectomy. Conversely, LH (or hCG) treatment of men increases testosterone levels, but only if the testes are intact and functional. The interstitial Leydig cells are the principal targets for LH and the primary source of testosterone production in the male. The plasma membranes of Leydig cells have a high-affinity LH receptor, a GPCR coupled to Gα s ( Fig. 54-4 ). Binding of LH to this receptor activates membrane-bound adenylyl cyclase (see p. 53 ), which catalyzes the formation of cAMP, which in turn activates protein kinase A (PKA). Activated PKA modulates gene transcription (see Fig. 4-13 ) and increases the synthesis of enzymes and other proteins necessary for the biosynthesis of testosterone (see pp. 1097–1100 ).
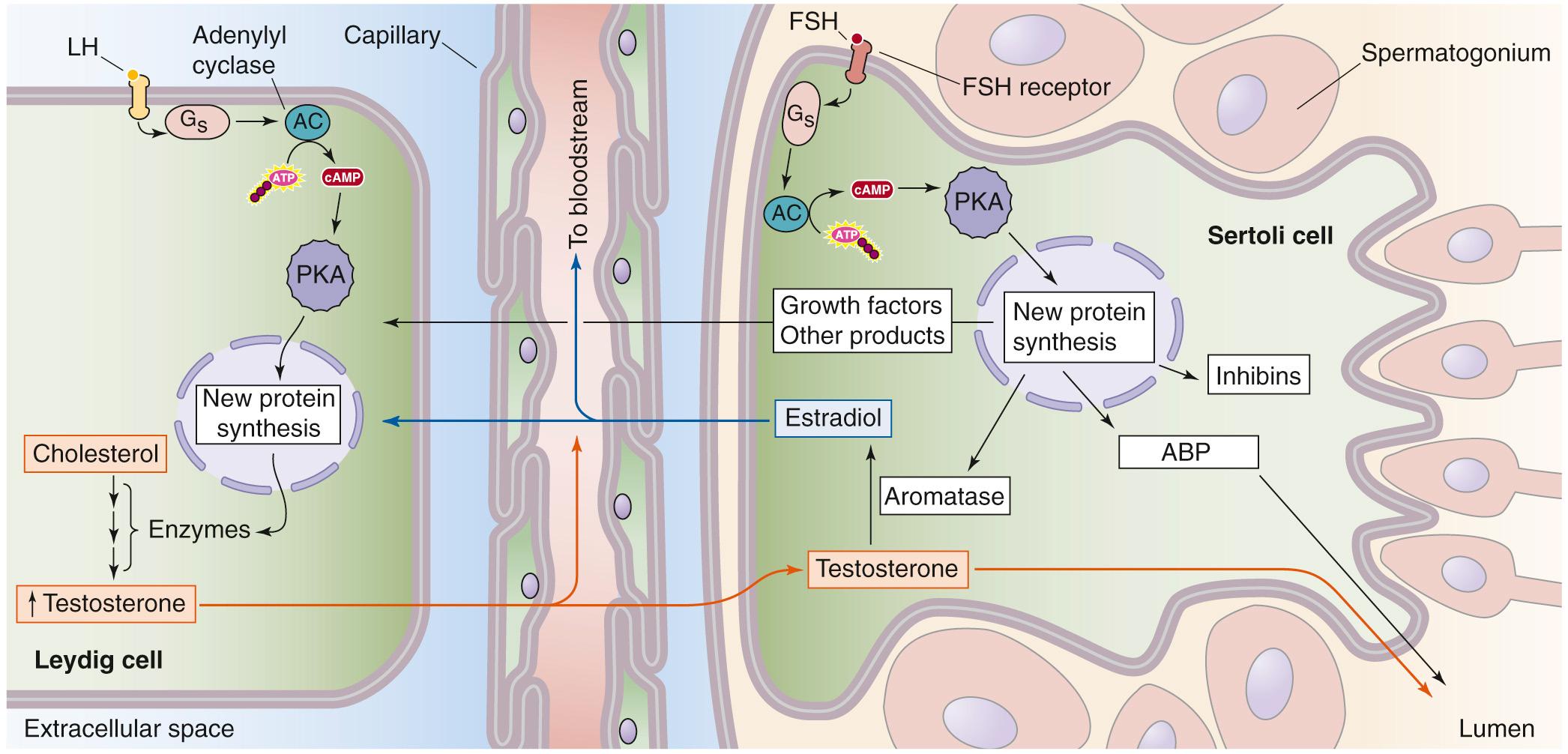
The Sertoli cells are the primary testicular site of FSH action (see Fig. 54-4 ). FSH also regulates Leydig cell physiology via effects on Sertoli cells. The signaling events after FSH binding are similar to those described above for LH on the Leydig cell. Thus, binding of FSH to a GPCR activates Gα s , causing stimulation of adenylyl cyclase, an increase in [cAMP] i , stimulation of PKA, transcription of specific genes, and increased protein synthesis. These proteins are important for synthesis and action of steroid hormones, including the following:
Androgen-binding protein (ABP), which is secreted into the luminal space of the seminiferous tubule, near the developing sperm cells. ABP helps to keep local testosterone levels high.
P-450 aromatase ( P-450 arom ; see p. 1117 and Table 50-2 ), a key steroidogenic enzyme that converts testosterone, which diffuses from the Leydig cells to the Sertoli cells, into estradiol.
Growth factors and other products that support sperm cells and spermatogenesis. These substances significantly increase the number of spermatogonia, spermatocytes, and spermatids in the testis. The stimulatory effect of FSH on spermatogenesis is not a direct action of FSH on the spermatogonia; instead, stimulation of spermatogenesis occurs via the action of FSH on Sertoli cells. FSH may also increase the fertility potential of sperm; it appears that this effect of FSH results from stimulation of motility, rather than from an increase in the absolute number of sperm.
Inhibins, which exert negative feedback on the hypothalamic-pituitary-testicular axis to inhibit FSH secretion (see below). Inhibins are members of the transforming growth factor-β (TGF-β) superfamily, which also includes the activins and antimüllerian hormone (see p. 1080 ). Inhibins are glycoprotein heterodimers consisting of one α and one β subunit that are covalently linked. The granulosa cells in the ovary and the Sertoli cells in the testis are the primary sources of inhibins. We discuss the biology of inhibins and activins in more detail on pages 1113–1115 . Inhibins are secreted into the seminiferous tubule fluid and into the interstitial fluid of the testicle. In addition to exerting an endocrine effect on the axis, inhibins also have paracrine effects, acting as growth factors on Leydig cells.
Leydig cells and Sertoli cells engage in crosstalk (see Fig. 54-4 ). For example, the Leydig cells make testosterone, which acts on Sertoli cells. In the rat, β endorphin produced by fetal Leydig cells binds to opiate receptors in Sertoli cells and inhibits their proliferation. Synthesis of β endorphins could represent a local feedback mechanism by which Leydig cells constrain the number of Sertoli cells. Conversely, Sertoli cells affect Leydig cells. For example, Sertoli cells convert testosterone—manufactured by Leydig cells—to estradiol, which decreases the capacity of Leydig cells to produce testosterone in response to LH. In addition, FSH acting on Sertoli cells produces growth factors that may increase the number of LH receptors on Leydig cells during development and thus result in an increase in steroidogenesis (i.e., an increase in testosterone production).
What, then, is required for optimal spermatogenesis to occur? It appears that two testicular cell types (Leydig cells and Sertoli cells) are required, as well as two gonadotropins (LH and FSH) and one androgen (testosterone). First, LH and Leydig cells are required to produce testosterone. Thus, LH, or rather its substitute hCG, is used therapeutically to initiate spermatogenesis in azoospermic or oligospermic men. Second, FSH and Sertoli cells are important for the nursing of developing sperm cells and for the production of inhibin and growth factors, which affect the Leydig cells. Thus, FSH plays a primary role in regulating development of the appropriate number of the Leydig cells so that adequate testosterone levels are available for spermatogenesis and the development of secondary sex characteristics.
During early puberty in boys, both FSH and LH levels increase while, simultaneously, the Leydig cells proliferate and plasma levels of testosterone increase ( Fig. 54-5 ). ![]() N54-3
N54-3
As noted in the upper panel of eFigure 54-1 , both FSH and LH levels increase during early puberty in boys, while simultaneously the Leydig cells proliferate. As a result, the Leydig cells increase their production of testosterone, and plasma levels of this steroid hormone increase, as shown in the lower panel of eFigure 54-1 .
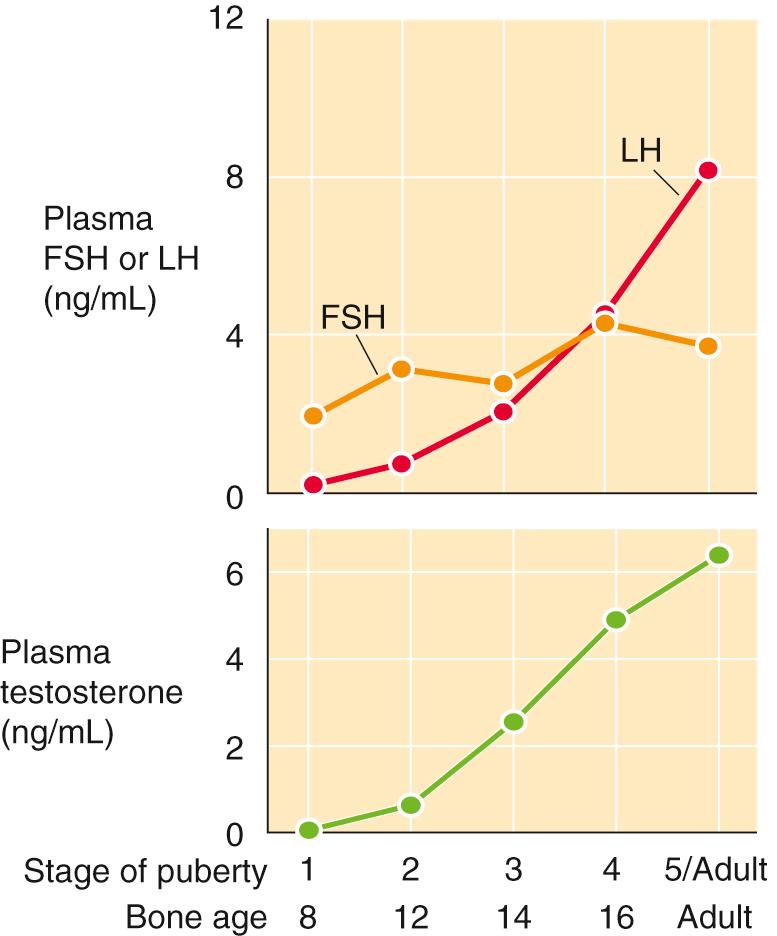
The primary target of FSH in the testis is the Sertoli cell (see Fig. 54-4 ). Via this action on Sertoli cells, FSH indirectly increases the number of Leydig cells, which is a key part of pubertal development. In hypogonadotropic-hypogonadal men (i.e., individuals who have decreased levels of both LH and FSH), treating with exogenous FSH stimulates the Sertoli cells to release factors that induce differentiation and maturation of Leydig cells. Subsequent treatment with hCG (i.e., which acts like LH) acts on these Leydig cells to synthesize testosterone and thereby support spermatogenesis.
During puberty, a related change is that Sertoli cells become relatively less sensitive to FSH, but at the same time they become more dependent on the testosterone that the Leydig cells produce. Thus, there is a continuum in the development of the Sertoli cells: as proliferation and maturation of the Sertoli cells proceeds during puberty, the responsiveness of the Sertoli cell to FSH declines while its responsiveness to testosterone increases. The mechanism for this switch appears to be that FSH stimulates the synthesis of androgen receptors on Sertoli cells.
The hypothalamic-pituitary-testicular axis in postpubertal males not only induces production of testosterone and inhibin by the testes but also receives negative feedback from these substances (see Fig. 54-2 ).
Normal circulating levels of testosterone inhibit the pulsatile release of GnRH by the hypothalamus and thereby reduce the frequency and amplitude of the LH- and FSH-secretory pulses. Testosterone also has negative-feedback action on LH secretion at the level of the pituitary gonadotrophs.
The inhibins also feed back on FSH secretion. Evidence for such negative feedback is that plasma FSH concentrations increase in proportion to the loss of germinal elements in the testis. Thus, FSH specifically stimulates the Sertoli cells to produce inhibin, and inhibin “inhibits” FSH secretion. Inhibin appears to diminish FSH secretion by acting at the level of the anterior pituitary rather than the hypothalamus.
The secretion of LH and FSH is under the additional control of neuropeptides, amino acids such as aspartate, corticotropin-releasing hormone (CRH), and endogenous opioids.
Cholesterol is the obligate precursor for androgens and other testicular steroids. The Leydig cell can synthesize cholesterol de novo from acetyl coenzyme A or can take it up as low-density lipoproteins from the extracellular fluid by receptor-mediated endocytosis (see p. 42 ). The two sources appear to be equally important in humans.
Preceding the metabolism of cholesterol is the translocation of this precursor to the mitochondrial inner membrane, which requires two proteins. The first is sterol-carrier protein 2 (SCP-2), a 13.5-kDa protein that translocates cholesterol from the plasma or organellar membranes to other organellar membranes, including the outer mitochondrial membrane. The second protein is the steroidogenic acute regulatory protein (StAR), which belongs to a large family of proteins involved in lipid trafficking and metabolism. The 37-kDa pro-StAR protein—the precursor to StAR—ferries cholesterol from the endoplasmic reticulum to the outer mitochondrial membrane. The 30-kDa mature StAR protein resides in the mitochondrial intermembrane space and extracts cholesterol from the mitochondrial outer membrane and ferries it across to the mitochondrial inner membrane.
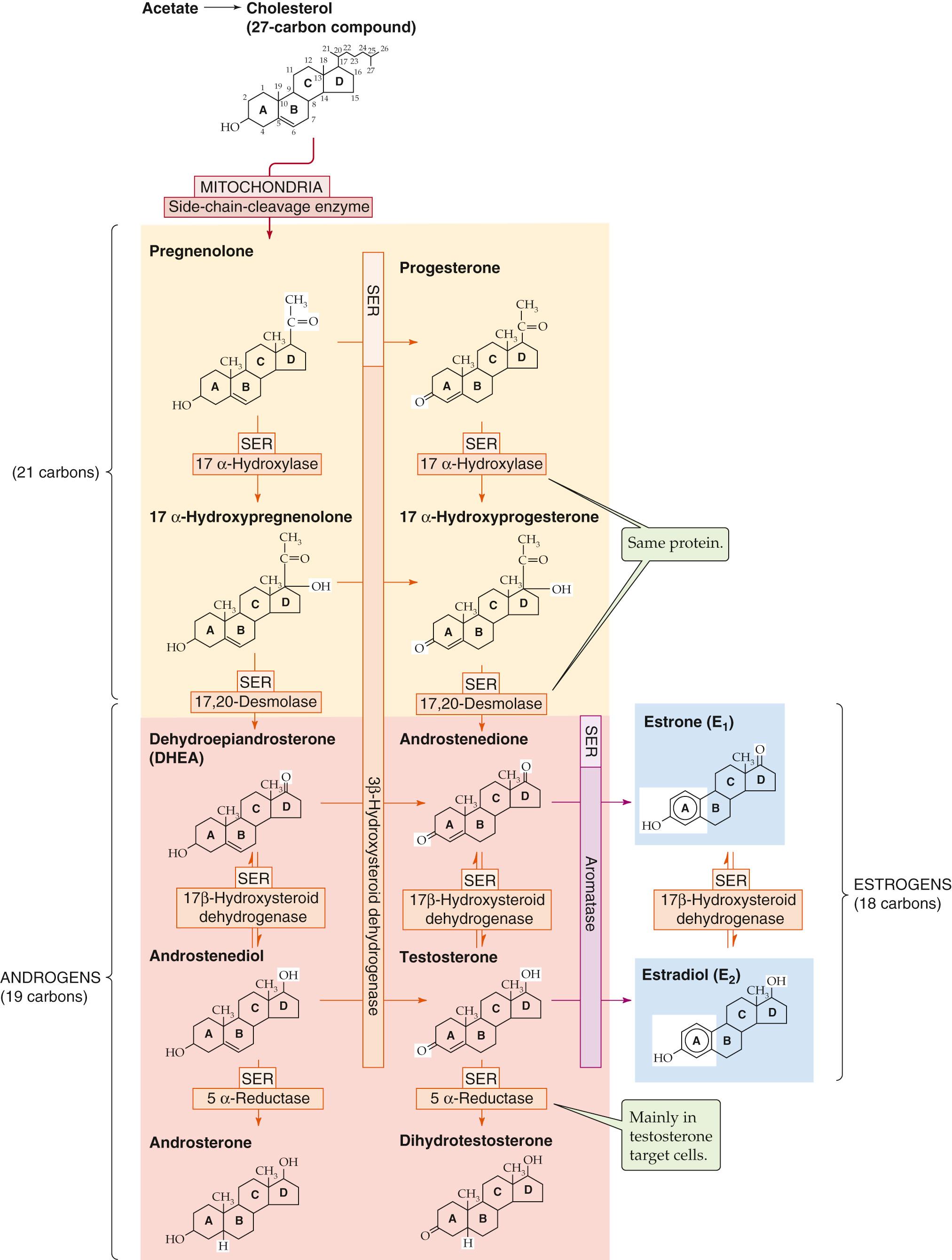
The Leydig cell uses a series of five enzymes to convert cholesterol to testosterone. Three of these enzymes are P-450 enzymes (see Table 50-2 ). ![]() N54-4 As summarized in Figure 54-6 , because 3β-hydroxysteroid dehydrogenase (3β-HSD) can oxidize the A ring of four intermediates, testosterone synthesis from cholesterol can take four pathways. The following is the “preferred” pathway:
N54-4 As summarized in Figure 54-6 , because 3β-hydroxysteroid dehydrogenase (3β-HSD) can oxidize the A ring of four intermediates, testosterone synthesis from cholesterol can take four pathways. The following is the “preferred” pathway:
The term cytochrome P-450 enzymes refers to a family of several hundred heme-containing enzymes that are located primarily in the SER. See Table 50-2 for some examples of these enzymes that play a role in steroidogenesis. We discuss the roles of these P-450 enzymes for the adrenal gland on page 1021 , for the testis on page 1097 , and for the ovary on page 1117 . On page 64 , we discuss the role of a P-450 enzyme (i.e., epoxygenase) in the metabolism of arachidonic acid (see also Fig. 3-11 ).
These enzymes are mono oxygenases. *
* Enzymes that transfer both oxygen atoms of molecular oxygen to an organic substrate are termed dioxygenases. In contrast, oxidases (e.g., cytochrome oxidase in the electron transport chain of mitochondria) are enzymes that catalyze oxidations in which neither of the atoms of molecular oxygen becomes part of the oxidized product. Instead, the molecular oxygen acts as an electron acceptor to form a molecule such as H 2 O or H 2 O 2 .
That is, they transfer one atom of molecular oxygen to an organic substrate RH to form ROH, whereas the other oxygen atom accepts two protons from the reduced form of the enzyme to form water:
This monooxygenation reaction is also referred to as a hydroxylation reaction because the enzyme hydroxylates RH to form ROH. Note that at the end of the reaction, the P-450 monooxygenase is in its reduced form. Another enzyme—a cytochrome P-450 reductase —recycles the P-450 monooxygenase to its reduced form; in the process, this P-450 reductase becomes oxidized. Finally, the oxidized P-450 reductase recycles to its reduced form by oxidizing the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) to NADP + or the reduced form of nicotinamide adenine dinucleotide (NADH) to NAD + or one of the flavin nucleotides (reduced flavin adenine dinucleotide [FADH 2 ] or reduced flavin mononucleotide [FMNH 2 ]).
The P-450 enzymes are so named because when the reduced forms of the enzymes bind carbon monoxide, they absorb light strongly at 450 nm.
Cholesterol conversion to pregnenolone. The pathway for testosterone synthesis begins in the mitochondrial inner membrane, where the cytochrome P-450 side-chain-cleavage enzyme ( P-450 SCC , also called 20,22-desmolase) ![]() N54-4 removes the long side chain (carbons 22 to 27) from the carbon at position 20 of the cholesterol molecule (27 carbon atoms), yielding pregnenolone (21 carbon atoms). This reaction is the rate-limiting step in the biosynthesis of testosterone, as it is for other steroid hormones. LH stimulates this reaction in the Leydig cell in two ways. First, LH increases the affinity of P-450 SCC for cholesterol. Second, LH has long-term actions of increasing the levels of SCP-2, StAR, and P-450 SCC via PKA-stimulated gene transcription.
N54-4 removes the long side chain (carbons 22 to 27) from the carbon at position 20 of the cholesterol molecule (27 carbon atoms), yielding pregnenolone (21 carbon atoms). This reaction is the rate-limiting step in the biosynthesis of testosterone, as it is for other steroid hormones. LH stimulates this reaction in the Leydig cell in two ways. First, LH increases the affinity of P-450 SCC for cholesterol. Second, LH has long-term actions of increasing the levels of SCP-2, StAR, and P-450 SCC via PKA-stimulated gene transcription.
Pregnenolone conversion to 17α-hydroxypregnenolone. In the smooth endoplasmic reticulum (SER), 17α-hydroxylase (P-450 c17 ) ![]() N54-4 then adds a hydroxyl group at position 17 to form 17α-hydroxypregnenolone. P-450 c17 , a key branch-point enzyme in the steroidogenic pathway, also converts progesterone to 17α-hydroxyprogesterone (see Fig. 54-6 , middle column).
N54-4 then adds a hydroxyl group at position 17 to form 17α-hydroxypregnenolone. P-450 c17 , a key branch-point enzyme in the steroidogenic pathway, also converts progesterone to 17α-hydroxyprogesterone (see Fig. 54-6 , middle column).
17α-hydroxypregnenolone conversion to dehydroepiandrosterone. In the SER, the 17,20-desmolase (a different activity of the same P-450 c17 whose 17α-hydroxylase activity catalyzes the previous step) removes the position-20 side chain from position 17 of 17α-hydroxypregnenolone, producing a 19-carbon steroid called dehydroepiandrosterone (DHEA). This so-called delta-5 pathway on the left of Figure 54-6 is the preferred route in Leydig cells to yield DHEA, the precursor for all sex steroids.
DHEA conversion to androstenediol. In the SER of the Leydig cell, a 17β-hydroxysteroid dehydrogenase (17β-HSD, which is not a P-450 enzyme) converts the ketone at position 17 of DHEA to a hydroxyl group to form androstenediol.
Androstenediol conversion to testosterone. Finally, in the SER, 3β-HSD ( not a P-450 enzyme) oxidizes the hydroxyl group of androstenediol at position 3 of the A ring to a ketone, forming testosterone. ![]() N54-5
N54-5
Pregnenolone is called P5. Progesterone is P4. This is where the terms delta-5 and delta-4 come from.
3β-HSD is a major branch-point enzyme in the steroidogenic pathway (see Fig. 54-6 ). It converts all delta-5 steroids to delta-4 steroids via an isomerase activity and therefore is essential for the production of mineralocorticoids and glucocorticoids. The competition between 17α-hydroxylase/17,20-desmolase (two enzymatic activities mediated in the same protein, also known as P-450 c17 ) and 3β-HSD for pregnenolone and 17α-hydroxypregnenolone is a major determinant of whether a steroidogenic cell will produce mineralocorticoids, glucocorticoids, or sex steroids. In the Leydig cell, 17α-hydroxylase/17,20-desmolase prevails to produce DHEA, which 17β-HSD1 then converts to androstenediol. DHEA can also undergo conversion, via 3β-HSD, to androstenedione, which 17β-HSD1 then converts to testosterone.
In addition, the testis can also use 5α-reductase, which is located in the SER, to convert testosterone to dihydrotestosterone (DHT). However, extratesticular tissue is responsible for most of the production of DHT. The conversion of testosterone to DHT is especially important in certain testosterone target cells (see pp. 1097–1099 ).
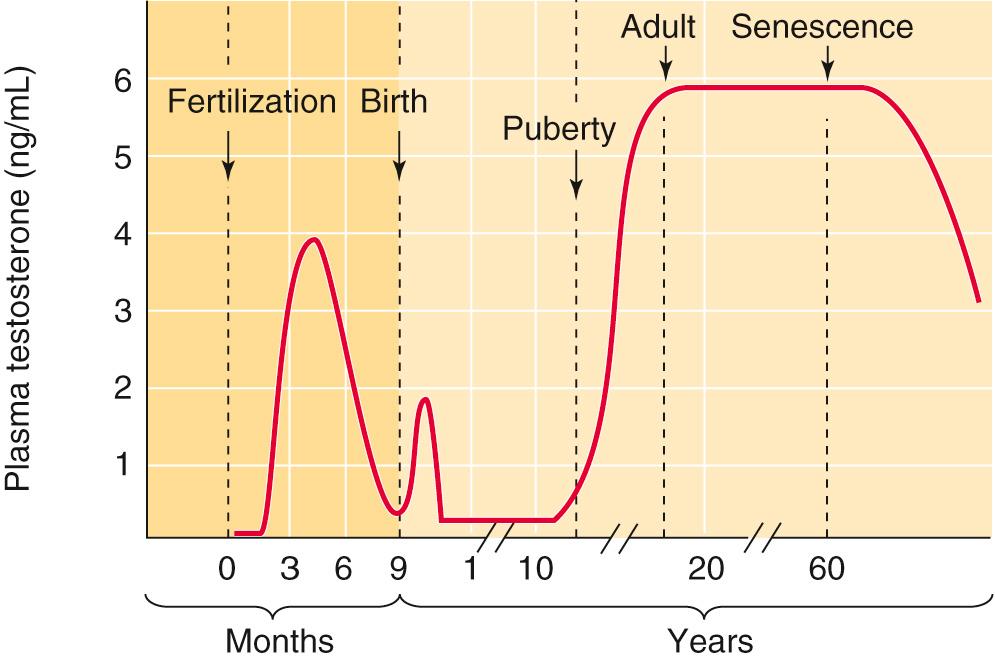
The Leydig cells of the testes make ~95% of the circulating testosterone. Although testosterone is the major secretory product, the testes also secretes pregnenolone, progesterone, 17-hydroxyprogesterone, androstenedione, androsterone, and DHT. The conversion of testosterone to DHT by Leydig cells is minor compared with its production in certain testosterone target cells (see p. 1085 ). Androstenedione is of major importance because it serves as a precursor for extraglandular estrogen formation. In men who are between the ages of 25 and 70 years, the rate of testosterone production remains relatively constant ( Table 54-1 ). Figure 54-5 summarizes the changes in plasma testosterone levels as a function of age in human males. ![]() N54-6
N54-6
| STEROID | BLOOD PRODUCTION RATE—HORMONE DELIVERED TO THE BLOOD (µg/day) | PLASMA CONCENTRATION (µg/L) |
|---|---|---|
| Testosterone | 6500 | 6.5 |
| Androstenedione | 2000–6000 | 1.5 |
| Dihydrotestosterone | 300 | 0.5 |
In the text, we noted that plasma testosterone levels are relatively constant in males between the ages of 25 and 70 years. As for any substance in the blood, the stability of plasma levels of testosterone indicates that the rate of testosterone production is equal to the rate of testosterone removal. However, the stability of plasma testosterone levels says nothing about the individual rates of production and removal.
It is important to distinguish between the secretion rate of a hormone and the production rate. Secretion refers to the release of the hormone from a specific organ or gland, and may be determined by selectively catheterizing the artery and vein supplying that tissue and ascertaining the arterial-venous difference in the concentration of that substance. For example, the concentration of testosterone is 400 to 500 µg/L in effluent of the spermatic vein; this level is ~75 times higher than the concentration found in the arterial blood. Thus, if we knew the blood flow out of the spermatic vein, then we could compute the rate of secretion of testosterone by the testis.
Production rate refers to the total appearance of the hormone in the circulation as the result of the secretion by all tissues in the body. Thus, the secretion rate for the testes equals the whole-body production rate only when other tissues make no contribution.
In the steady state, the amount of testosterone cleared from the circulation equals the amount produced. Thus,
Here, PR is the whole-body production rate, MCR is the metabolic clearance rate, and [S] is the concentration of the substance in the plasma. MCR is defined in the same way as renal clearances (see Table 33-2 ). That is, MCR is the virtual number of liters per day that are fully cleared of testosterone. This clearance is due to the metabolism of testosterone, which is discussed on pages 1099–1100 . Because the mean metabolic clearance rate for testosterone is ~1000 L/day, and the testosterone concentration is about 6.5 µg/L (range, 3 to 10 µg/L), the production rate must be about 6500 µg/day. Evidence for this high clearance rate is the fact that the plasma half-life of testosterone is only 10 to 20 minutes.
Several tissues besides the testes—including adipose tissue, skin, adrenal cortex, brain, and muscle—produce testosterone and several other androgens. These substances may be synthesized de novo from cholesterol or produced by peripheral conversion of precursors. Moreover, the peripheral organs and tissues may convert sex steroids to less active forms (see Fig. 54-6 ). Notable sites of extragonadal conversion include adipose tissue and the skin. Androstenedione is converted to testosterone in peripheral tissues. In this case, androstenedione is the precursor for the hormone testosterone. Testosterone can be converted to estradiol or DHT or go “backward” by reversible interconversion to androstenedione. Thus, a potent hormone such as testosterone may also serve as a precursor for a weaker hormone (androstenedione), a hormone with different activities (estradiol), or a more potent hormone having similar activities (DHT). This last example may be illustrated by the effects of DHT on hair follicles, sebaceous glands, and the sex accessory organs. In these tissues, the androgenic effects of circulating testosterone are amplified by its conversion by 5α-reductase to DHT, which has a much higher affinity for the androgen receptor ( AR; see p. 1085 ). Some tissues, including the brain, aromatize testosterone to estradiol, and thus the action of this metabolite occurs via the estrogen receptor.
The adrenal cortex (see p. 1021 ) is another source of androgen production in both males and females. Normal human adrenal glands synthesize and secrete the weak androgens DHEA, conjugated DHEA sulfate, and androstenedione. Essentially, all the DHEA in male plasma is of adrenal origin. However, <1% of the total testosterone in plasma is derived from DHEA. As summarized in Table 54-1 , the plasma concentration of androstenedione in males is only ~25% that of testosterone. About 20% of androstenedione is generated by peripheral metabolism of other steroids. Although the adrenal gland contributes significantly to the total androgen milieu in males, it does not appear to have significant effects on stimulation and growth of the male accessory organs ( Box 54-1 ). This occurs primarily as a result of DHT production from circulating testosterone.
For a long time, the abrupt hormonal alterations that signal the dramatic changes of female menopause were believed to have no correlate in men. We now know that men do experience a gradual decline in their serum testosterone levels (see Fig. 54-5 ) ![]() N54-6 and that this decline is closely correlated with many of the changes that accompany aging: decreases in bone formation, muscle mass, growth of facial hair, appetite, and libido. The blood hematocrit also decreases. Testosterone replacement can reverse many of these changes by restoring muscle and bone mass and correcting the anemia.
N54-6 and that this decline is closely correlated with many of the changes that accompany aging: decreases in bone formation, muscle mass, growth of facial hair, appetite, and libido. The blood hematocrit also decreases. Testosterone replacement can reverse many of these changes by restoring muscle and bone mass and correcting the anemia.
Although the levels of both total and free testosterone decline with age, levels of LH are frequently not elevated. This finding is believed to indicate that some degree of hypothalamic-pituitary dysfunction accompanies aging.
Most testosterone in the circulation is bound to specific binding proteins. About 45% of plasma testosterone binds to sex hormone–binding globulin (SHBG) and ~55% binds to serum albumin and corticosteroid-binding globulin (CBG; see p. 1021 ). A small fraction (~2%) of the total circulating testosterone circulates free, or unbound, in plasma. The free form of testosterone enters the cell by passive diffusion and subsequently exerts biological actions or undergoes metabolism by other organs such as the prostate, liver, and intestines (see the next section). The quantity of testosterone entering a cell is determined by the plasma concentration and by the intracellular milieu of enzymes and binding proteins.
Once it diffuses into the cell, testosterone either binds to a high-affinity AR in the nucleus or undergoes conversion to DHT, which also binds to the AR. This receptor functions as a homodimer (AR/AR) and is a member of the family of nuclear receptors (see Table 3-6 ) that includes receptors for glucocorticoids, mineralocorticoids, progesterone, estrogens, vitamin D, thyroid hormone, and retinoic acid. The gene coding for the AR is located on the X chromosome. The androgen-AR complex is a transcription factor that binds to hormone response elements on DNA located in the promoter regions of the target genes. Interaction between the androgen-AR complex and nuclear chromatin causes marked increases in transcription, which ultimately lead to the synthesis of specific proteins. As a result of these synthetic processes, specific cell functions ensue, including growth and development. Thus, presence of the AR in a cell or tissue determines whether that tissue responds to androgens.
Whether the active compound in any tissue is DHT or testosterone depends on the presence or absence in that tissue of the microsomal enzyme 5α-reductase, which converts testosterone to DHT. The biological activity of DHT is 30 to 50 times higher than that of testosterone. Some tissues, including the brain, aromatize testosterone to estradiol, and thus the action of this metabolite occurs via the estrogen receptor.
Some of the effects of androgens may be nongenomic. For example, androgens may stimulate hepatic microsomal protein synthesis by a mechanism independent of binding to the AR. Other evidence indicates that the action of androgens on the prostate gland may occur via the adenylyl cyclase/PKA system (see pp. 56–57 ) and could result in gene activation under some circumstances.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here