Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The lungs are ingeniously constructed to carry out their cardinal function, the exchange of gases between inspired air and blood. Developmentally, the respiratory system is an outgrowth from the ventral wall of the foregut. The midline trachea develops two lateral outpocketings, the lung buds, which eventually divide into branches called lobar bronchi, three on the right and two on the left, thus giving rise to three lobes on the right and two on the left. The lobar bronchi allow air to pass in and out of the lung. They have firm cartilaginous walls that provide mechanical support and are lined by columnar ciliated epithelium with abundant subepithelial glands that produce mucus, which impedes the entry of microbes. The right mainstem bronchus is more vertical and directly in line with the trachea. Consequently, aspirated foreign materials such as vomitus, blood, and foreign bodies tend to enter the right lung more often than the left.
The right and left lobar bronchi branch to give rise to progressively smaller airways that are accompanied by a dual arterial supply from the pulmonary and bronchial arteries. Distally, the bronchi give way to bronchioles, which are distinguished by the lack of cartilage and the presence of submucosal glands within their walls. Further branching of bronchioles leads to the terminal bronchioles, which are less than 2 mm in diameter. Beyond the terminal bronchiole is the acinus, a roughly spherical structure with a diameter of about 7 mm. An acinus is composed of a respiratory bronchiole (which gives off several alveoli from its sides), alveolar ducts, and alveolar sacs, the blind ends of the respiratory passages, whose walls are formed entirely of alveoli, the site of gas exchange. A cluster of three to five terminal bronchioles, each with its appended acinus, is referred to as the pulmonary lobule .
Except for the true vocal cords, which are covered by stratified squamous epithelium, the entire respiratory tree, including the larynx, trachea, and bronchioles, is normally lined mainly by tall, columnar, pseudostratified, ciliated epithelial cells and a smaller population of non-ciliated cells called club cells that secrete a number of protective substances into the airway. There also are scattered cells called ionocytes that express high levels of the cystic fibrosis transmembrane conductance regulator (CFTR) and appear to modulate the ion content and viscosity of bronchial secretions. In addition, the bronchial mucosa contains neuroendocrine cells with neurosecretory-type granules that can release a variety of factors including serotonin, calcitonin, and gastrin-releasing peptide (bombesin). Numerous mucus-secreting goblet cells and submucosal glands also are dispersed throughout the walls of the trachea and bronchi (but not the bronchioles).
The microscopic structure of the alveolar walls (or alveolar septa) consists of the following ( Fig. 15.1 ):
An intertwining network of anastomosing capillaries lined with endothelial cells.
Basement membrane and surrounding interstitium, which separates the endothelial cells from the alveolar lining epithelial cells. In thin portions of the alveolar septum the basement membranes of epithelium and endothelium are fused, whereas in thicker portions they are separated by an interstitial space (pulmonary interstitium) containing fine elastic fibers, small bundles of collagen, a few fibroblast-like interstitial cells, smooth muscle cells, mast cells, and rare lymphocytes and monocytes.
Alveolar epithelium, a continuous layer of two cell types: flat, plate-like type I pneumocytes, covering 95% of the alveolar surface, and rounded type II pneumocytes . Type II cells synthesize surfactant (which forms a very thin layer over the alveolar cell membranes) and are involved in the repair of alveolar damage through their ability to proliferate and give rise to type I cells.
The alveolar walls are perforated by numerous pores of Kohn, which permit the passage of bacteria and exudate between adjacent alveoli (see Fig. 15.35B ). Scattered resident alveolar macrophages also are present, either loosely attached to epithelial cells or lying free within the alveolar spaces.
Developmental anomalies of the lung are rare. The most common of these include the following:
Pulmonary hypoplasia is a defect in the development of both lungs (one may be more affected than the other) that results in decreased lung size. It is caused by abnormalities that compress the lung or impede lung expansion in utero, such as congenital diaphragmatic hernia and oligohydramnios. Severe hypoplasia is fatal in the early neonatal period.
Foregut cysts arise from abnormal detachments of primitive foregut and are most often located in the hilum or middle mediastinum. Depending on the wall structure, these cysts are classified as bronchogenic (most common), esophageal, or enteric. A bronchogenic cyst is rarely connected to the tracheobronchial tree. It is lined by ciliated pseudostratified columnar epithelium and has a wall containing bronchial glands, cartilage, and smooth muscle. They may come to attention due to symptoms resulting from compression of nearby structures or superimposed infection or may be incidental findings.
Pulmonary sequestration is a discrete area of lung tissue that (1) is not connected to the airways and (2) has an abnormal blood supply arising from the aorta or its branches. Extralobar sequestration is external to the lung and most commonly presents in infants as a mass lesion. It may be associated with other congenital anomalies. Intralobar sequestration occurs within the lung. It usually presents in older children, often due to recurrent localized infections or bronchiectasis.
Other, less common congenital abnormalities include tracheal and bronchial anomalies (atresia, stenosis, tracheoesophageal fistula), vascular anomalies, congenital pulmonary airway malformation, and congenital lobar overinflation (emphysema).
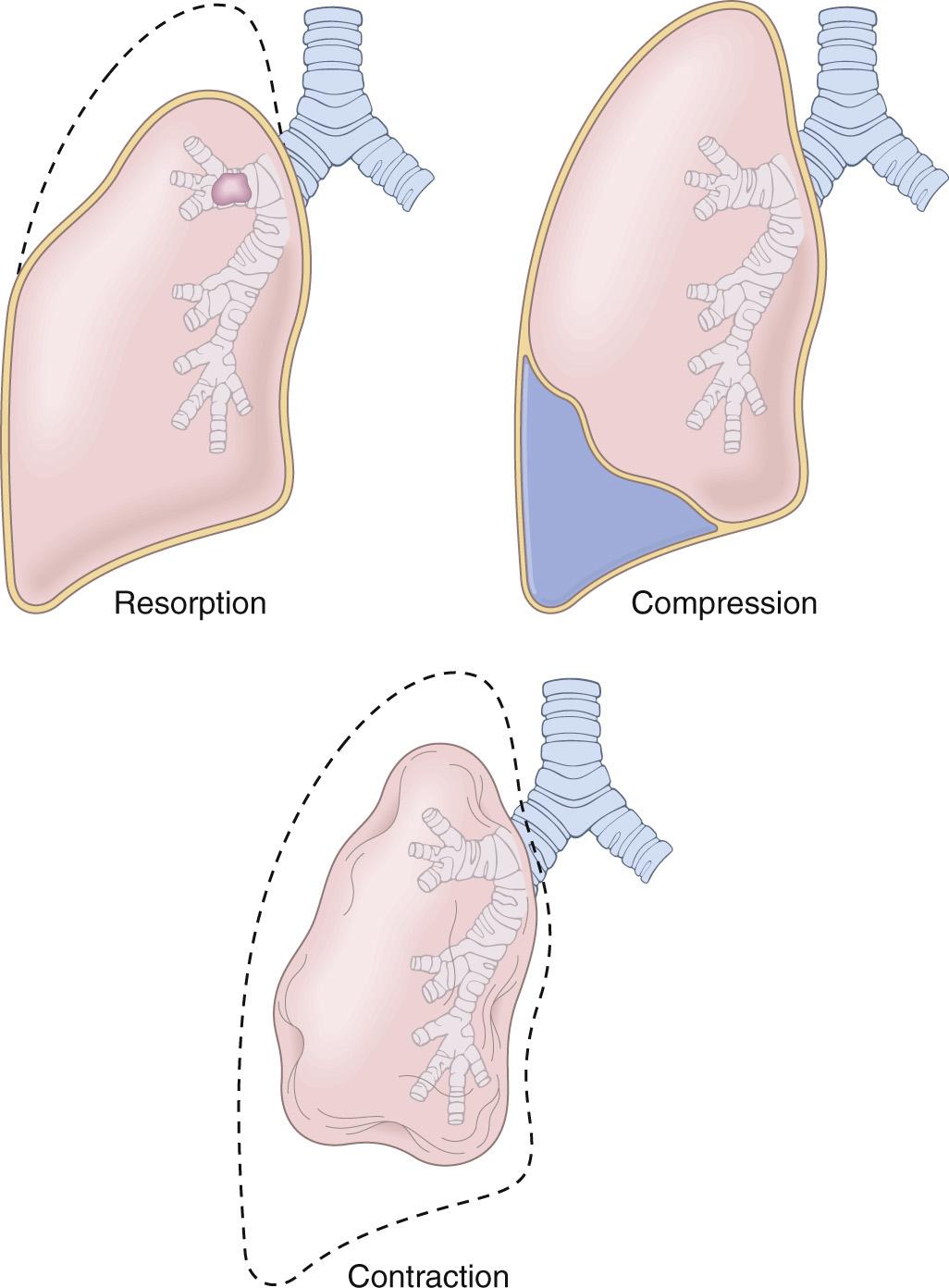
Atelectasis refers either to incomplete expansion of the lungs (neonatal atelectasis) or to the collapse of previously inflated lung, and results in areas of poorly aerated pulmonary parenchyma. The main types of acquired atelectasis, which is encountered principally in adults, are the following ( Fig. 15.2 ).
Resorption atelectasis stems from obstruction of an airway. Over time, air is resorbed from distal alveoli, which collapse. Since lung volume is diminished, the mediastinum shifts toward the atelectatic lung. Airway obstruction is most often caused by excessive secretions (e.g., mucus plugs) or exudates within smaller bronchi, as may occur in bronchial asthma, chronic bronchitis, bronchiectasis, and postoperative states. Aspiration of foreign bodies and intrabronchial tumors may also lead to airway obstruction and atelectasis.
Compression atelectasis results whenever significant volumes of fluid (transudate, exudate, or blood), tumor, or air (pneumothorax) accumulate within the pleural cavity. With compression atelectasis, the mediastinum shifts away from the affected lung.
Contraction atelectasis occurs when focal or generalized pulmonary or pleural fibrosis prevents full lung expansion.
Significant atelectasis reduces oxygenation and predisposes to infection. Except in cases caused by fibrosis, atelectasis is a reversible disorder.
Pulmonary edema (excessive interstitial fluid in the alveoli) can result from hemodynamic disturbances (cardiogenic pulmonary edema) or increased capillary permeability due to microvascular injury (non-cardiogenic pulmonary edema) ( Table 15.1 ). A general consideration of edema is given in Chapter 4 , and pulmonary congestion and edema are described briefly in the context of congestive heart failure ( Chapter 12 ). Whatever the clinical setting, pulmonary congestion and edema produce heavy, wet lungs. Therapy and outcome depend on the underlying etiology.
| Hemodynamic Edema |
| Increased hydrostatic pressure (increased pulmonary venous pressure) |
|
| Decreased oncotic pressure (less common) |
|
| Lymphatic obstruction (rare) |
| Edema Due to Alveolar Wall Injury (Microvascular or Epithelial Injury) |
| Direct injury |
|
| Indirect injury |
|
| Edema of Undetermined Origin |
|
Hemodynamic pulmonary edema is caused by increased hydrostatic pressure and occurs most commonly in left-sided congestive heart failure. Edema accumulates initially in the basal regions of the lower lobes because hydrostatic pressure is greatest in these sites (dependent edema). Histologically, the alveolar capillaries are engorged, and there is an intra-alveolar transudate that appears as finely granular pale pink material. Alveolar microhemorrhages and hemosiderin-laden macrophages (“heart failure” cells) may be present. In long-standing pulmonary congestion (e.g., as seen in mitral stenosis), hemosiderin-laden macrophages are abundant, and fibrosis and thickening of the alveolar walls cause the soggy lungs to become firm and brown (brown induration) . These changes not only impair respiratory function but also predispose to infection.
Non-cardiogenic pulmonary edema is caused by injury of the alveolar septa. Primary injury to the vascular endothelium or damage to alveolar epithelial cells (with secondary microvascular injury) produces an inflammatory exudate that leaks into the interstitial space and, in more severe cases, into the alveoli. Injury-related alveolar edema is an important feature of a serious and often fatal condition, acute respiratory distress syndrome (discussed below).
Acute lung injury (ALI) is characterized by the abrupt onset of hypoxemia and bilateral pulmonary edema in the absence of cardiac failure (non-cardiogenic pulmonary edema). Acute respiratory distress syndrome (ARDS) is a manifestation of severe ALI. Both ARDS and ALI are associated with inflammation-associated increases in pulmonary vascular permeability, edema, and epithelial cell death. The histologic manifestation of these diseases is diffuse alveolar damage .
ALI is a well-recognized complication of diverse conditions including both pulmonary and systemic disorders ( Table 15.2 ). In many cases, several predisposing conditions are present (e.g., shock, oxygen therapy, and sepsis). In other uncommon instances, ALI appears acutely in the absence of known triggers and follows a rapidly progressive clinical course, a condition known as acute interstitial pneumonia.
| Infection |
| Physical/Injury |
|
| Inhaled Irritants |
|
| Chemical Injury |
|
| Hematologic Conditions |
|
| Pancreatitis |
| Uremia |
| Cardiopulmonary Bypass |
| Hypersensitivity Reactions |
|
a More than 50% of cases of acute respiratory distress syndrome are associated with these four conditions.
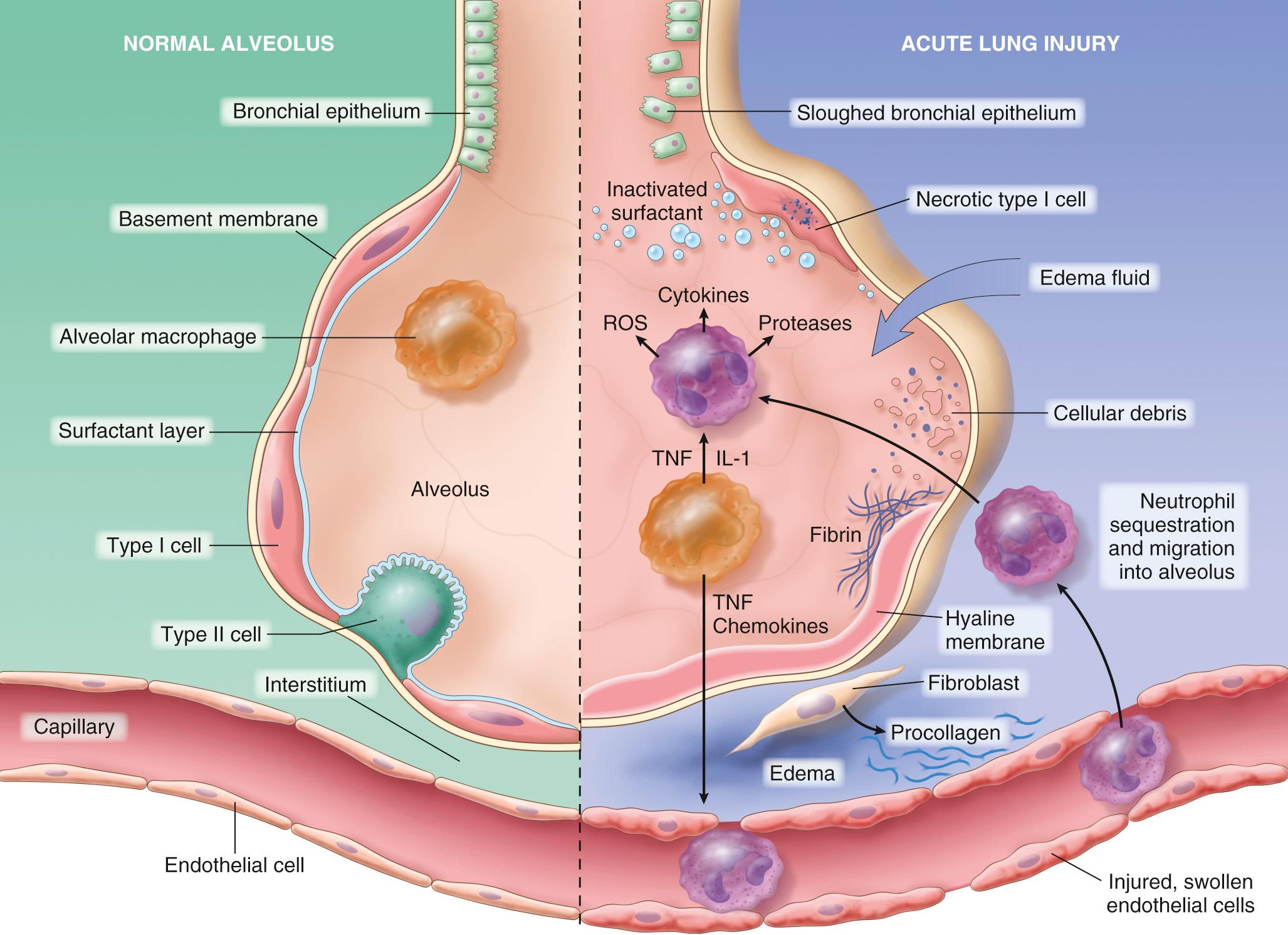
ALI/ARDS is initiated by injury of pneumocytes and pulmonary endothelium, setting in motion a vicious cycle of increasing inflammation and pulmonary damage ( Fig. 15.3 ).
Endothelial activation is an important early event. In some instances, endothelial activation is secondary to pneumocyte injury, which is sensed by resident alveolar macrophages. In response, these immune sentinels secrete mediators such as tumor necrosis factor (TNF) that act on the neighboring endothelium. Alternatively, circulating inflammatory mediators may activate pulmonary endothelium directly in the setting of severe tissue injury or sepsis. Some of these mediators injure endothelial cells, while others (notably cytokines) induce endothelial cells to express increased levels of adhesion molecules, procoagulant proteins, and chemokines.
Adhesion and extravasation of neutrophils. Neutrophils adhere to the activated endothelium and migrate into the interstitium and the alveoli, where they degranulate and release inflammatory mediators including proteases, reactive oxygen species, and cytokines. Experimental evidence suggest that neutrophil extracellular traps (NETs) are released and also contribute directly to lung damage. These injuries and associated proinflammatory factors set in motion a vicious cycle of inflammation and endothelial damage that lies at the heart of ALI/ARDS.
Accumulation of intra-alveolar fluid and formation of hyaline membranes. Endothelial activation and injury make pulmonary capillaries leaky, allowing interstitial and intra-alveolar edema fluid to form. Damage and necrosis of type II alveolar pneumocytes lead to surfactant abnormalities, further compromising alveolar gas exchange. Ultimately the inspissated protein-rich edema fluid and debris from dead alveolar epithelial cells organize into hyaline membranes, a characteristic feature of ALI/ARDS.
Resolution of injury is impeded in ALI/ARDS due to epithelial necrosis and inflammatory damage that impairs the ability of remaining cells to assist with edema resorption. Eventually, however, if the inflammatory stimulus lessens, macrophages remove intra-alveolar debris and release fibrogenic cytokines such as transforming growth factor β (TGF-β) and platelet-derived growth factor. These factors stimulate fibroblast growth and collagen deposition, leading to fibrosis of alveolar walls. Residual type II pneumocytes proliferate to replace type I pneumocytes, reconstituting the alveolar lining. Endothelial restoration occurs through proliferation of uninjured capillary endothelium.
The lesions in ARDS are not evenly distributed, and as a result there are typically areas that are stiff and poorly aerated and regions that have nearly normal levels of compliance and ventilation. Because poorly aerated regions continue to be perfused, there is a mismatch of ventilation and perfusion, a phenomenon that exacerbates the hypoxemia and cyanosis.
Epidemiologic studies have shown that ALI/ARDS is more common and has a worse prognosis in chronic alcoholics and in smokers. Genome-wide association studies have identified a number of genetic variants that increase the risk of ARDS, some of which map to genes linked to inflammation and coagulation.
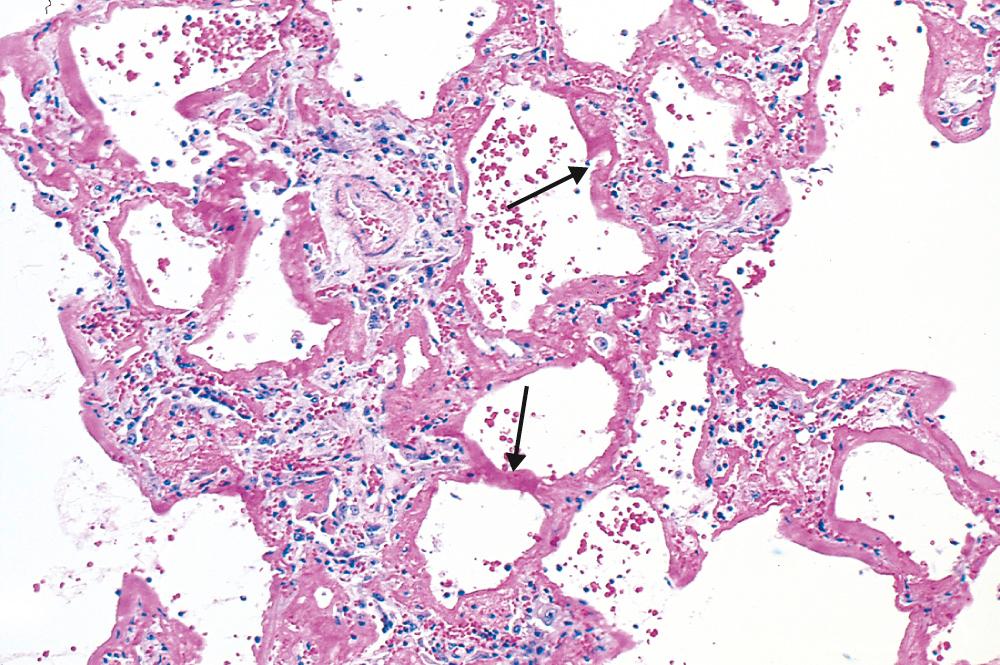
In the acute exudative stage, the lungs are heavy, firm, red, and boggy. They exhibit congestion, interstitial and intra-alveolar edema, inflammation, fibrin deposition, and diffuse alveolar damage. The alveolar walls become lined with waxy hyaline membranes ( Fig. 15.4 ) that are morphologically similar to those seen in hyaline membrane disease of neonates ( Chapter 10 ). Alveolar hyaline membranes consist of fibrin-rich edema fluid mixed with the remnants of necrotic epithelial cells. In the proliferative or organizing stage, type II pneumocytes proliferate, and granulation tissue forms in the alveolar walls and spaces. In most cases the granulation tissue resolves, leaving minimal functional impairment. Sometimes, however, fibrotic thickening (scarring) of the alveolar septa ensues (late fibrotic stage).
Profound dyspnea and tachypnea herald ALI/ARDS, followed by increasing respiratory failure, hypoxemia, cyanosis, and the appearance of diffuse bilateral infiltrates on radiographic examination. Hypoxemia may be refractory to oxygen therapy due to ventilation-perfusion mismatch, and respiratory acidosis can develop. Early in the course, the lungs become stiff due to loss of functional surfactant, leading to the need for intubation and high ventilatory pressures to maintain adequate gas exchange.
There are no proven specific treatments for ARDS, which is common in acutely ill patients and continues to take a high toll, even in patients receiving state-of-the-art supportive care. In a 2016 study of intensive care units in 50 countries, the incidence of ARDS was 10.4%, and mortality rates were 35% for mild, 40% for moderate, and 46% for severe ARDS. The majority of deaths are attributable to sepsis, multiorgan failure, or severe lung injury. Most survivors recover pulmonary function, but in a minority of patients, the lung damage results in interstitial fibrosis and chronic pulmonary disease.
ARDS is a clinical syndrome of progressive respiratory insufficiency caused by diffuse alveolar damage in the setting of sepsis, severe trauma, or diffuse pulmonary infection.
Damage to endothelial and alveolar epithelial cells and secondary inflammation are the key initiating events and the basis of lung damage.
The characteristic histologic finding is hyaline membranes lining alveolar walls, accompanied by edema, scattered neutrophils and macrophages, and epithelial necrosis.
Obstructive lung diseases are characterized by an increase in resistance to airflow due to diffuse airway disease, which may affect any level of the respiratory tract. These are contrasted with restrictive diseases, which are characterized by reduced expansion of lung parenchyma and decreased total lung capacity. The clinical distinction between these diseases is based primarily on pulmonary function tests. In individuals with diffuse obstructive disorders, pulmonary function tests show decreased maximal airflow rates during forced expiration, usually expressed as forced expiratory volume at 1 second (FEV 1 ) over forced ventilatory capacity (FVC). An FEV 1 /FVC ratio of less than 0.7 generally indicates obstructive disease. Expiratory airflow obstruction may be caused by a variety of conditions ( Table 15.3 ), each with characteristic pathologic changes and different mechanisms of airflow obstruction. As discussed later, however, the divisions between these entities are not “clean,” and many patients have diseases with overlapping features. By contrast, restrictive diseases are associated with proportionate decreases in both total lung capacity and FEV 1 , such that the FEV 1 /FVC ratio remains normal. Restrictive defects occur in two broad kinds of conditions: (1) chest wall disorders (e.g., severe obesity, pleural diseases, kyphoscoliosis, and neuromuscular diseases such as poliomyelitis) and (2) chronic interstitial and infiltrative diseases, such as pneumoconioses and interstitial fibrosis.
| Clinical Term | Anatomic Site | Major Pathologic Changes | Etiology | Signs/Symptoms |
|---|---|---|---|---|
| Chronic bronchitis | Bronchus | Mucous gland hyperplasia, hypersecretion | Tobacco smoke, air pollutants | Cough, sputum production |
| Bronchiectasis | Bronchus | Airway dilation and scarring | Persistent or severe infections | Cough, purulent sputum, fever |
| Asthma | Bronchus | Smooth muscle hyperplasia, excess mucus, inflammation | Immunologic or undefined causes | Episodic wheezing, cough, dyspnea |
| Emphysema | Acinus | Airspace enlargement; wall destruction | Tobacco smoke | Dyspnea |
| Small airways disease, bronchiolitis a | Bronchiole | Inflammatory scarring/obliteration | Tobacco smoke, air pollutants, miscellaneous | Cough, dyspnea |
a Can be seen with any form of obstructive lung disease or as an isolated finding.
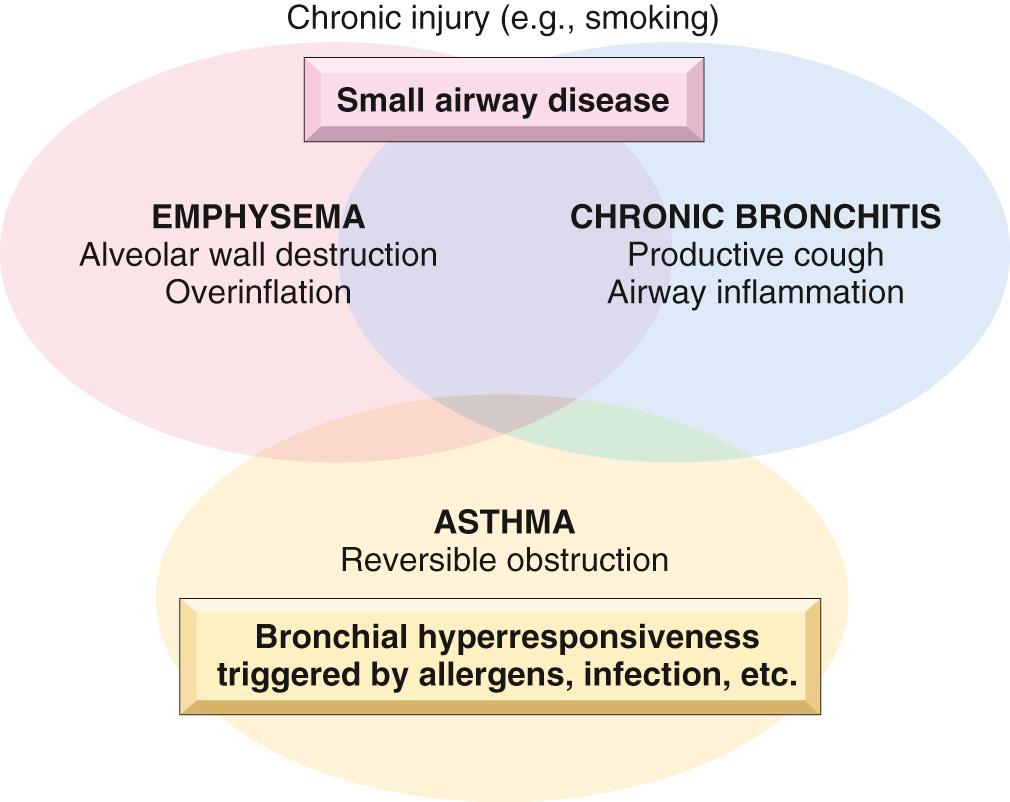
Common obstructive lung diseases include chronic obstructive pulmonary disease (COPD), asthma, and bronchiectasis ( Table 15.3 ). COPD has two major clinicopathologic manifestations, emphysema and chronic bronchitis, which are often found together in the same patient, almost certainly because they share the same major etiologic factor—cigarette smoking. While asthma is distinguished from chronic bronchitis and emphysema by the presence of reversible bronchospasm, some patients with otherwise typical asthma also develop an irreversible component ( Fig. 15.5 ). Conversely, some patients with otherwise typical COPD have a reversible component. Clinicians commonly label such patients as having COPD/asthma.
COPD, a major public health problem, is defined by the World Health Organization (WHO) as “a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities caused by exposure to noxious particles or gases.” It is currently the fourth leading cause of death in the world and is projected to rank third by 2020 due to increases in cigarette smoking in countries such as China. There is a strong association between heavy cigarette smoking and COPD. Overall, 35% to 50% of heavy smokers develop COPD; conversely about 80% of COPD is attributable to smoking. Women and African Americans who smoke heavily are more susceptible than other groups. Additional risk factors include poor lung development early in life, exposure to environmental and occupational pollutants, airway hyperresponsiveness, and certain genetic polymorphisms.
Recognizing that emphysema and chronic bronchitis often occur together in patients with COPD, it is still useful to discuss these patterns of lung injury and associated functional abnormalities individually to highlight the pathophysiologic basis of different causes of airflow obstruction. We will finish our discussion by returning to the clinical features of COPD.
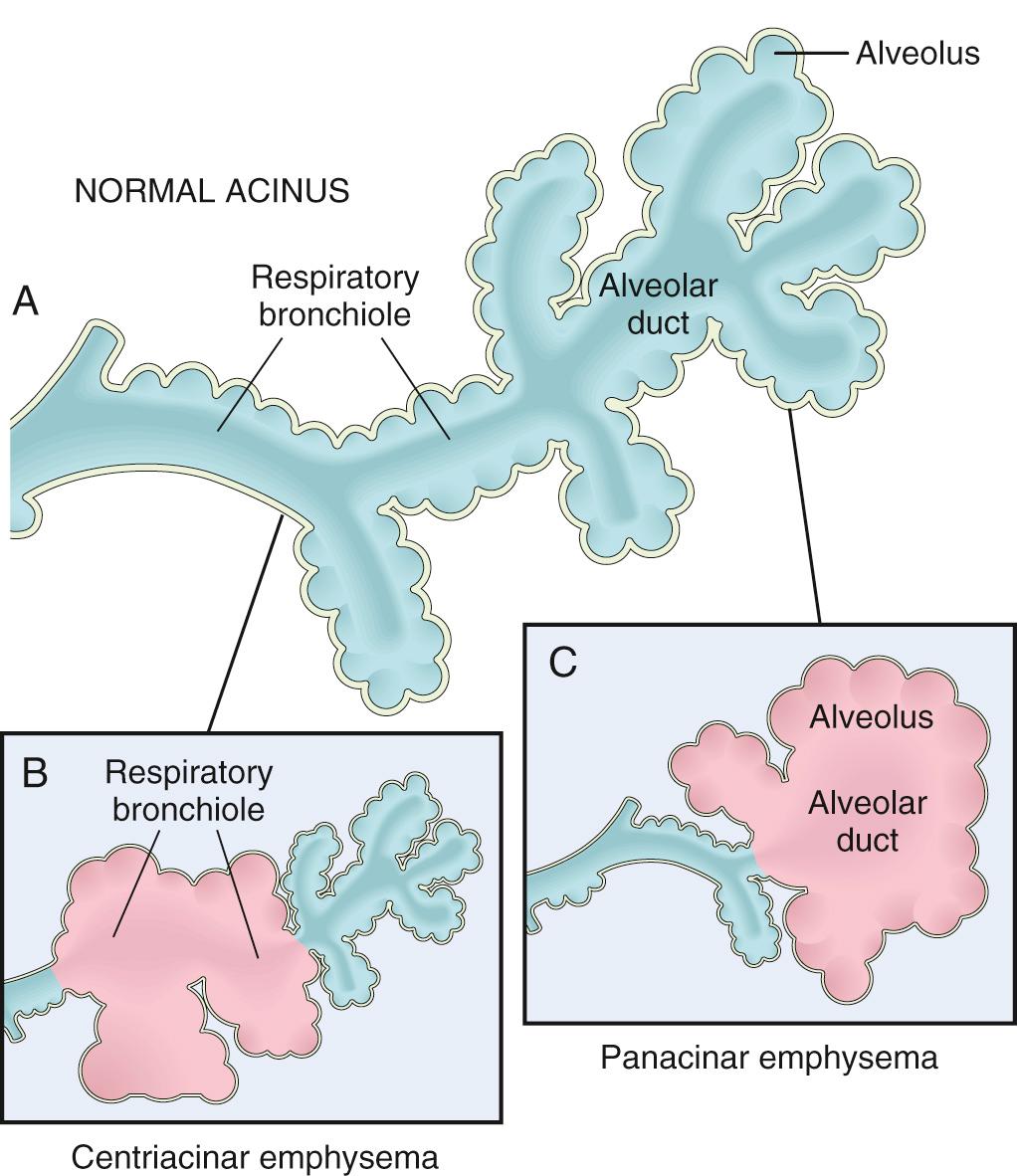
Emphysema is defined by irreversible enlargement of the airspaces distal to the terminal bronchiole, accompanied by destruction of their walls. Subtle but functionally important small airway fibrosis (distinct from chronic bronchitis) is also present and is a significant contributor to airflow obstruction. Emphysema is classified according to its anatomic distribution within the lobule. Recall that the lobule is a cluster of acini, the terminal respiratory units. Based on the segments of the respiratory units that are involved, emphysema is subdivided into four major types: (1) centriacinar, (2) panacinar, (3) paraseptal, and (4) irregular. Of these, only the first two cause clinically significant airflow obstruction ( Fig. 15.6 ).
Centriacinar (centrilobular) emphysema. Centriacinar emphysema is the most common form, constituting more than 95% of clinically significant cases. It occurs predominantly in heavy smokers with COPD. In this type of emphysema the central or proximal parts of the acini, formed by respiratory bronchioles, are affected, whereas distal alveoli are spared ( Figs. 15.6B and 15.7A ). Thus, both emphysematous and normal airspaces exist within the same acinus and lobule. The lesions are more common and usually more pronounced in the upper lobes, particularly in the apical segments. In severe centriacinar emphysema, the distal acinus may also be involved, making differentiation from panacinar emphysema difficult.
Panacinar (panlobular) emphysema. Panacinar emphysema is associated with α1-antitrypsin deficiency ( Chapter 18 ) and is exacerbated by smoking. In this type the acini are uniformly enlarged from the level of the respiratory bronchiole to the terminal blind alveoli ( Figs. 15.6C and 15.7B ). In contrast to centriacinar emphysema, panacinar emphysema tends to occur more commonly in the lower zones and in the anterior margins of the lung, and it is usually most severe at the bases.
Distal acinar (paraseptal) emphysema. Distal acinar emphysema probably underlies many cases of spontaneous pneumothorax in young adults. In this type the proximal portion of the acinus is normal, and the distal part is predominantly involved. The emphysema is more striking adjacent to the pleura, along the lobular connective tissue septa, and at the margins of the lobules. It occurs adjacent to areas of fibrosis, scarring, or atelectasis and is usually more severe in the upper half of the lungs. The characteristic finding is multiple enlarged airspaces, ranging from less than 0.5 cm to more than 2.0 cm in diameter, which sometimes form cyst-like structures.
Airspace enlargement with fibrosis (irregular emphysema). Irregular emphysema, so named because the acinus is irregularly involved, is almost invariably associated with scarring. In most instances it occurs in small foci and is clinically insignificant.
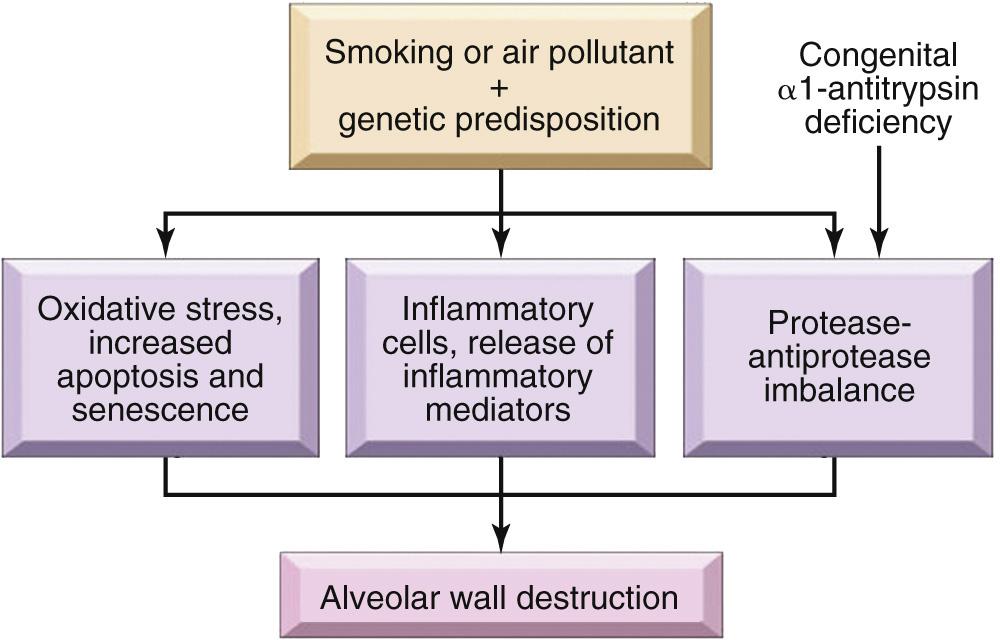
Clinically significant emphysema is largely confined to smokers and to patients with α 1 -antitrypsin deficiency, highlighting the importance of these two etiologic factors. Mechanisms relating to these factors that contribute to the development of emphysema include the following ( Fig. 15.8 ):
Toxic injury and inflammation. Inhaled cigarette smoke and other noxious particles damage respiratory epithelium and cause inflammation, which results in variable degrees of parenchymal destruction. A wide variety of inflammatory mediators (including leukotriene B 4 , interleukin [IL]-8, TNF, and others) are increased in the affected parts of the lung. These mediators are released by resident epithelial cells and macrophages and variously attract inflammatory cells from the circulation (chemotactic factors), amplify the inflammatory process (proinflammatory cytokines), and induce structural changes (growth factors). Chronic inflammation also leads to the accumulation of T and B cells in affected parts of the lung, but the role of adaptive immunity in emphysema is currently uncertain.
Protease-antiprotease imbalance. Several proteases are released from the inflammatory cells and epithelial cells that break down connective tissue components. In patients who develop emphysema, there is a relative deficiency of protective antiproteases, which in some instances has a genetic basis (discussed later).
Oxidative stress. Substances in tobacco smoke, alveolar damage, and inflammatory cells all produce oxidants, which may beget tissue damage, endothelial dysfunction, and inflammation. The role of oxidants is supported by studies of mice in which the NRF2 gene is inactivated. NRF2 is a transcription factor that serves as a sensor for oxidants in many cell types including alveolar epithelial cells. Intracellular oxidants activate NRF2, which upregulates the expression of genes that protect cells from oxidant damage. Mice without NRF2 are significantly more sensitive to tobacco smoke than normal mice, and genetic variants in NRF2, NRF2 regulators, and NRF2 target genes are all associated with smoking-related lung disease in humans.
Infection. Although infection is not thought to play an initiating role in the tissue destruction, bacterial and/or viral infections may acutely exacerbate existing disease.
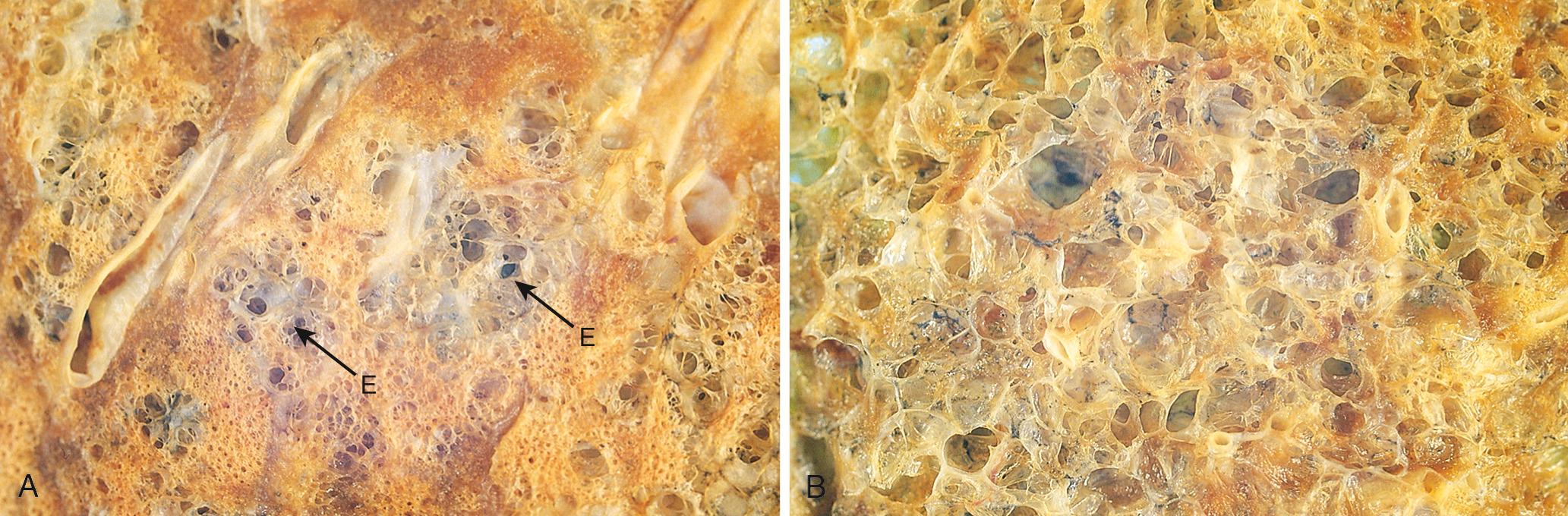
The idea that proteases are important is based in part on the observation that patients with a genetic deficiency of the antiprotease α 1 -antitrypsin have a markedly enhanced tendency to develop emphysema that is compounded by smoking. About 1% of all patients with emphysema have this defect. α 1 -Antitrypsin, normally present in serum, tissue fluids, and macrophages, is a major inhibitor of proteases (particularly elastase) secreted by neutrophils during inflammation. It is encoded by the proteinase inhibitor (Pi) locus on chromosome 14. The Pi locus is polymorphic, and approximately 0.012% of the U.S. population is homozygous for the Z allele, a genotype associated with very low serum levels of α 1 -antitrypsin. More than 80% of ZZ individuals develop symptomatic panacinar emphysema, which occurs at an earlier age and is of greater severity if the individual smokes. It is postulated that any injury (e.g., that induced by smoking) that increases the activation and influx of neutrophils into the lung leads to local release of proteases, which in the absence of α 1 -antitrypsin activity result in excessive digestion of elastic tissue and, with time, emphysema.
Several other genetic variants have also been linked to risk of emphysema. Among these are variants of the nicotinic acetylcholine receptor that are hypothesized to influence the addictiveness of tobacco smoke and thus the behavior of smokers. Not surprisingly, the same variants are also linked to lung cancer risk, emphasizing the importance of smoking in both of these diseases.
A number of factors contribute to airway obstruction in emphysema. Small airways are normally held open by the elastic recoil of the lung parenchyma. The loss of elastic tissue in the walls of alveoli that surround respiratory bronchioles reduces radial traction, leading to collapse of respiratory bronchioles during expiration and functional airflow obstruction in the absence of mechanical obstruction. In addition, even young smokers often have changes related to small airway inflammation that also contribute to airway narrowing and obstruction (described later).
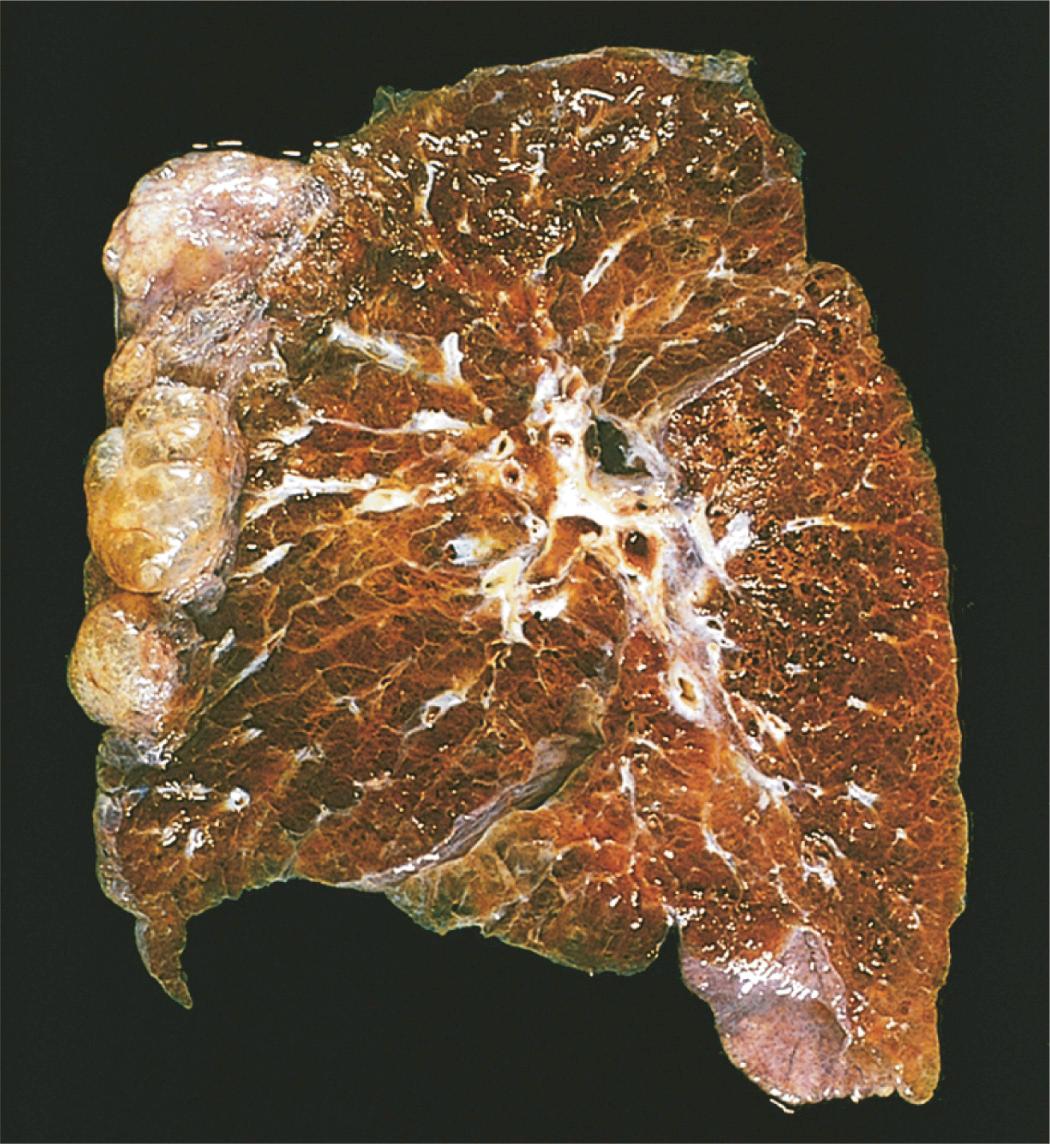
Advanced emphysema produces voluminous lungs, often overlapping the heart anteriorly. Generally, in patients with smoking-related disease, the upper two-thirds of the lungs are more severely affected. Large alveoli can easily be seen on the cut surface of fixed lungs (see Fig. 15.7 ). Apical blebs or bullae characteristic of irregular emphysema may appear in patients with advanced disease.
Microscopically, abnormally large alveoli are separated by thin septa with focal centriacinar fibrosis. There is loss of attachments between alveoli and the outer wall of small airways. The pores of Kohn are so large that septa appear to be floating or protrude blindly into alveolar spaces with a club-shaped end. As alveolar walls are destroyed, there is a decrease in the capillary bed area. With advanced disease, there are even larger abnormal airspaces and possibly blebs or bullae, which often deform and compress the respiratory bronchioles and vasculature of the lung. Inflammatory changes in small airways are often superimposed (described next under chronic bronchitis), as are vascular changes related to pulmonary hypertension stemming from local hypoxemia and loss of capillary beds.
Chronic bronchitis is defined clinically as persistent cough with sputum production for at least 3 months in at least 2 consecutive years in the absence of any other identifiable cause. Longstanding chronic bronchitis is associated with progressive lung dysfunction, which may be so severe as to lead to hypoxemia, pulmonary hypertension, and cor pulmonale.
The primary or initiating factor in the genesis of chronic bronchitis is exposure to noxious or irritating inhaled substances such as tobacco smoke (90% of those affected are smokers) and dust from grain, cotton, and silica. Several factors contribute to its pathogenesis.
Mucus hypersecretion. The earliest feature of chronic bronchitis is hypersecretion of mucus in the large airways, associated with enlargement of the submucosal glands in the trachea and bronchi. The basis for mucus hypersecretion is incompletely understood, but it appears to involve inflammatory mediators such as histamine and IL-13. With time, there is also a marked increase in goblet cells in small airways—small bronchi and bronchioles—leading to excessive mucus production that contributes to airway obstruction. It is thought that both the enlargement of submucosal glands and the increase in numbers of goblet cells are protective reactions against tobacco smoke or other pollutants (e.g., sulfur dioxide and nitrogen dioxide).
Acquired cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. There is substantial evidence that smoking leads to acquired CFTR dysfunction, which in turn causes the secretion of abnormal, dehydrated mucus that exacerbates the severity of chronic bronchitis.
Inflammation. Inhalants that induce chronic bronchitis cause cellular damage, eliciting both acute and chronic inflammatory responses involving neutrophils, lymphocytes, and macrophages. Long-standing inflammation and accompanying fibrosis involving small airways (small bronchi and bronchioles, less than 2 to 3 mm in diameter) can also lead to chronic airway obstruction.
Infection. Infection does not initiate chronic bronchitis, but is probably significant in maintaining it and may be critical in producing acute exacerbations.
Cigarette smoke predisposes to chronic bronchitis in several ways. Not only does it damage airway-lining cells, leading to chronic inflammation, but it also interferes with the ciliary action of the respiratory epithelium, preventing the clearance of mucus and increasing the risk of infection.
Grossly, there is hyperemia, swelling, and edema of the mucous membranes, frequently accompanied by excessive mucinous or mucopurulent secretions. Sometimes, heavy casts of secretions and pus fill the bronchi and bronchioles. The characteristic microscopic features are chronic inflammation of the airways (predominantly lymphocytes and macrophages); thickening of the bronchiolar wall due to smooth muscle hypertrophy, deposition of extracellular matrix in the muscle layer, and peribronchial fibrosis; goblet cell hyperplasia; and enlargement of the mucus-secreting glands of the trachea and bronchi. Of these, the most striking change is an increase in the size of the mucous glands. This increase can be assessed by the ratio of the thickness of the mucous gland layer to the thickness of the wall between the epithelium and the cartilage (Reid index) . The Reid index (normally 0.4) is increased in chronic bronchitis, usually in proportion to the severity and duration of the disease. The mucus plugging, inflammation, and fibrosis may lead to marked narrowing of bronchioles, and in the most severe cases, there may be obliteration of lumen due to fibrosis (bronchiolitis obliterans) . The bronchial epithelium may also exhibit squamous metaplasia and dysplasia due to the irritating and mutagenic effects of substances in tobacco smoke.
Most affected patients have a smoking history of 40 pack-years or greater. COPD often presents insidiously with slowly increasing dyspnea on exertion and chronic cough with sputum production, slight at first but increasing over time. Other patients present with exacerbations caused by superimposed infection that can lead to confusion with other disorders, such as asthma (due to wheezing). The most important diagnostic test is spirometry, which typically shows an FEV 1 /FVC ratio of less than 0.7.
Once COPD appears, symptoms often wax and wane over time and are generally worse in the morning. The clinical picture varies according to the severity of the disease and the relative contributions of emphysematous and bronchitic changes ( Table 15.4 ). At one extreme end of the spectrum lie “pink puffers,” patients in whom emphysema dominates. Classically, the patient is barrel-chested and dyspneic, with obviously prolonged expiration, sits forward in a hunched-over position, and breathes through pursed lips. Cough is often slight, overdistention of the lungs is severe, diffusion capacity is low, and blood gas values are relatively normal at rest. Weight loss is common and can be so severe as to suggest an occult cancer. At the other end lie patients with pure chronic bronchitis, ingloriously referred to as “blue bloaters.” Their cardinal symptom is a persistent cough productive of sputum, coupled with hypercapnia, hypoxemia, and mild cyanosis. Most patients are somewhere in the middle, with signs and symptoms stemming from both bronchitic and emphysematous changes.
| Bronchitis | Emphysema | |
|---|---|---|
| Age, years | 40–45 | 50–75 |
| Dyspnea | Mild; late | Severe; early |
| Cough | Early; copious sputum | Late; scanty sputum |
| Infections | Common | Occasional |
| Respiratory insufficiency | Early, periodic | End-stage |
| Cor pulmonale | Common | Uncommon, end-stage |
| Airway resistance | Increased | Normal or slightly increased |
| Elastic recoil | Normal | Low |
| Chest radiograph | Prominent vessels; large heart size | Hyperinflation; normal heart size |
| Appearance | Blue bloater | Pink puffer |
Treatment options include smoking cessation, oxygen therapy, long-acting bronchodilators with inhaled corticosteroids, antibiotics, physical therapy, bullectomy, and, in selected patients, lung volume reduction surgery and lung transplantation. Even with intervention, however, COPD often progresses and frequently proves fatal. Long-standing COPD, particularly in patients with a bronchitic component, commonly leads to pulmonary hypertension, cor pulmonale, and death due to heart failure. Death may also result from acute respiratory failure due to acute infections superimposed on COPD. In patients with emphysematous changes, subpleural blebs may rupture, leading to fatal pneumothorax. The best hope for a major change in this dire picture is more effective programs aimed at prevention of smoking and other environmental exposures.
Most common in long-standing tobacco smokers (typically >40 pack-years); air pollutants also contribute.
Underlying pulmonary pathology usually includes both chronic bronchitis and emphysema.
Often fatal due to development of heart failure or of respiratory failure due to superimposed infection.
In COPD, usually follows a centroacinar distribution characterized by permanent enlargement of airspaces distal to terminal bronchioles.
Particularly severe in patients with α 1 -antitrypsin deficiency, in which a panacinar pattern of emphysematous change may be seen.
Tissue destruction is caused by elastases and oxidants released from inflammatory cells, particularly neutrophils, which are responding to cellular injury caused by tobacco smoke and pollutants.
Defined as persistent productive cough for at least 3 consecutive months in at least 2 consecutive years.
Dominant pathologic features are mucus hypersecretion due to enlargement of mucus-secreting glands and chronic inflammation associated with bronchiolar wall fibrosis.
In addition to emphysema occurring in the setting of COPD, several other conditions may be associated with lung overinflation or focal emphysematous change and are mentioned here in brief.
Compensatory hyperinflation. This term is used to designate dilation of alveoli in response to loss of lung substance elsewhere, for example, following surgical removal of a lung or lobe with cancer.
Obstructive overinflation. In this condition the lung expands because air is trapped within it. A common cause is subtotal obstruction of an airway by a tumor or foreign object. In infants, it may be caused by congenital lobar overinflation , which most often results from hypoplasia of bronchial cartilage. Overinflation occurs either (1) because of an obstruction that acts as ball valve, allowing air to enter on inspiration while preventing its exodus on expiration, or (2) because collaterals bring in air behind the obstruction. These collaterals consist of the pores of Kohn and other direct accessory bronchioloalveolar connections (the canals of Lambert ). Obstructive overinflation can be life-threatening if the affected portion distends sufficiently to compress the adjacent uninvolved lung.
Bullous emphysema. This is a descriptive term for large subpleural blebs or bullae (spaces greater than 1 cm in diameter in the distended state) that can occur in any form of emphysema ( Fig. 15.9 ), often near the apex. Rupture of the bullae may give rise to pneumothorax.
Interstitial emphysema. Entrance of air into the connective tissue stroma of the lung, mediastinum, or subcutaneous tissue produces interstitial emphysema. In most instances, it is caused by alveolar tears that occur in patients with pulmonary emphysema due to transient increases in intra-alveolar pressure, for example, during coughing. Less commonly, chest wounds or fractured ribs that puncture the lung provide the portal for entrance of air into surrounding soft tissues.
Asthma is a heterogeneous disease, usually characterized by chronic airway inflammation and variable expiratory airflow obstruction that produces symptoms such as wheezing, shortness of breath, chest tightness, and cough, which vary over time and in intensity. Symptomatic episodes are most likely to occur at night or in the early morning and are produced by bronchoconstriction that is at least partly reversible, either spontaneously or with treatment. Rarely, an unremitting attack called acute severe asthma (formerly known as status asthmaticus ) may prove fatal; usually, such patients have a long history of asthma. Between the attacks, patients may be virtually asymptomatic. Of note, there has been a significant increase in the incidence of asthma in the Western world over the past 40 to 50 years, a trend that has now started to abate. However, the prevalence of asthma continues to increase in lower income countries and in some ethnic groups in which its prevalence was previously low.
Asthma has several distinct clinical phenotypes, each with different underlying pathogenic mechanisms. It may be categorized as atopic (evidence of allergen sensitization and immune activation, often in a patient with allergic rhinitis or eczema) or nonatopic (no evidence of allergen sensitization), of which several subtypes exist. In all types, episodes of bronchospasm may have diverse triggers, such as respiratory infections (especially viral infections), irritants (e.g., smoke, fumes), cold air, stress, and exercise. One biologically meaningful and clinically useful way to classify asthma is based on its triggers. We will first briefly describe the various major subtypes of asthma, and then will delve more deeply into its pathogenesis.
This type of asthma is a classic example of an IgE-mediated (type I) hypersensitivity reaction (discussed in Chapter 6 ). The disease usually begins in childhood and is triggered by environmental allergens, such as dusts, pollens, cockroach or animal dander, and foods, which most frequently act in synergy with other proinflammatory environmental cofactors, most notably respiratory viral infections. A positive family history of asthma is common, and a skin test with the offending antigen in these patients results in an immediate wheal-and-flare reaction. Atopic asthma may also be diagnosed based on high total serum IgE levels or evidence of allergen sensitization by serum radioallergosorbent tests (RASTs), which can detect the presence of IgE antibodies that are specific for individual allergens.
Individuals with non-atopic asthma do not have evidence of allergen sensitization, and skin test results are usually negative. A positive family history of asthma is less common in these patients. Respiratory infections due to viruses (e.g., rhinovirus, parainfluenza virus, and respiratory syncytial virus) are common triggers in non-atopic asthma. Inhaled air pollutants such as tobacco smoke, sulfur dioxide, ozone, and nitrogen dioxide may also contribute to the chronic airway inflammation and hyperreactivity in some cases. As already mentioned, in some instances attacks may be triggered by seemingly innocuous events, such as exposure to cold and even exercise.
Several pharmacologic agents provoke asthma. Aspirin-sensitive asthma is an uncommon type, occurring in individuals with recurrent rhinitis and nasal polyps. These individuals are exquisitely sensitive to small doses of aspirin as well as other non-steroidal anti-inflammatory medications, and they experience not only asthmatic attacks but also urticaria. Aspirin and related drugs trigger asthma in these patients by inhibiting the cyclooxygenase pathway of arachidonic acid metabolism, leading to a rapid decrease in prostaglandin E 2 . Normally prostaglandin E 2 inhibits enzymes that generate proinflammatory mediators such as leukotrienes B 4 , C 4 , D 4 , and E 4 , which are believed to have central roles in aspirin-induced asthma.
This form of asthma may be triggered by fumes (epoxy resins, plastics), organic and chemical dusts (wood, cotton, platinum), gases (toluene), or other chemicals (formaldehyde, penicillin products). Only minute quantities of chemicals are required to induce the attack, which usually occurs after repeated exposure. The underlying mechanisms vary according to stimulus and include type I reactions, direct liberation of bronchoconstrictor substances, and hypersensitivity responses of unknown origin.
Atopic asthma, the most common form of the disease, is caused by a Th2-mediated IgE response to environmental allergens in genetically predisposed individuals. Airway inflammation is central to the disease pathophysiology and causes airway dysfunction partly through the release of potent inflammatory mediators and partly through remodeling of the airway wall. As the disease becomes more severe, there is increased local secretion of growth factors, which induce mucous gland enlargement, smooth muscle proliferation, angiogenesis, and fibrosis. Varying combinations of these processes help explain the different asthma subtypes, their response to treatment, and their natural history over a person's lifetime.
The contributions of the immune response, genetics, and environment are discussed separately below, although they are closely intertwined.
A fundamental abnormality in asthma is an exaggerated Th2 response to normally harmless environmental antigens ( Fig. 15.10 ). Th2 cells secrete cytokines that promote inflammation and stimulate B cells to produce IgE and other antibodies. These cytokines include IL-4, which stimulates the production of IgE; IL-5, which activates locally recruited eosinophils; and IL-13, which stimulates mucus secretion from bronchial submucosal glands and also promotes IgE production by B cells. The T cells and epithelial cells secrete chemokines that recruit more T cells and eosinophils, thus exacerbating the reaction. As in other allergic reactions ( Chapter 6 ), IgE binds to the Fc receptors on submucosal mast cells, and repeat exposure to the allergen triggers the mast cells to release granule contents and produce cytokines and other mediators, which collectively induce the early-phase (immediate hypersensitivity) reaction and the late-phase reaction.
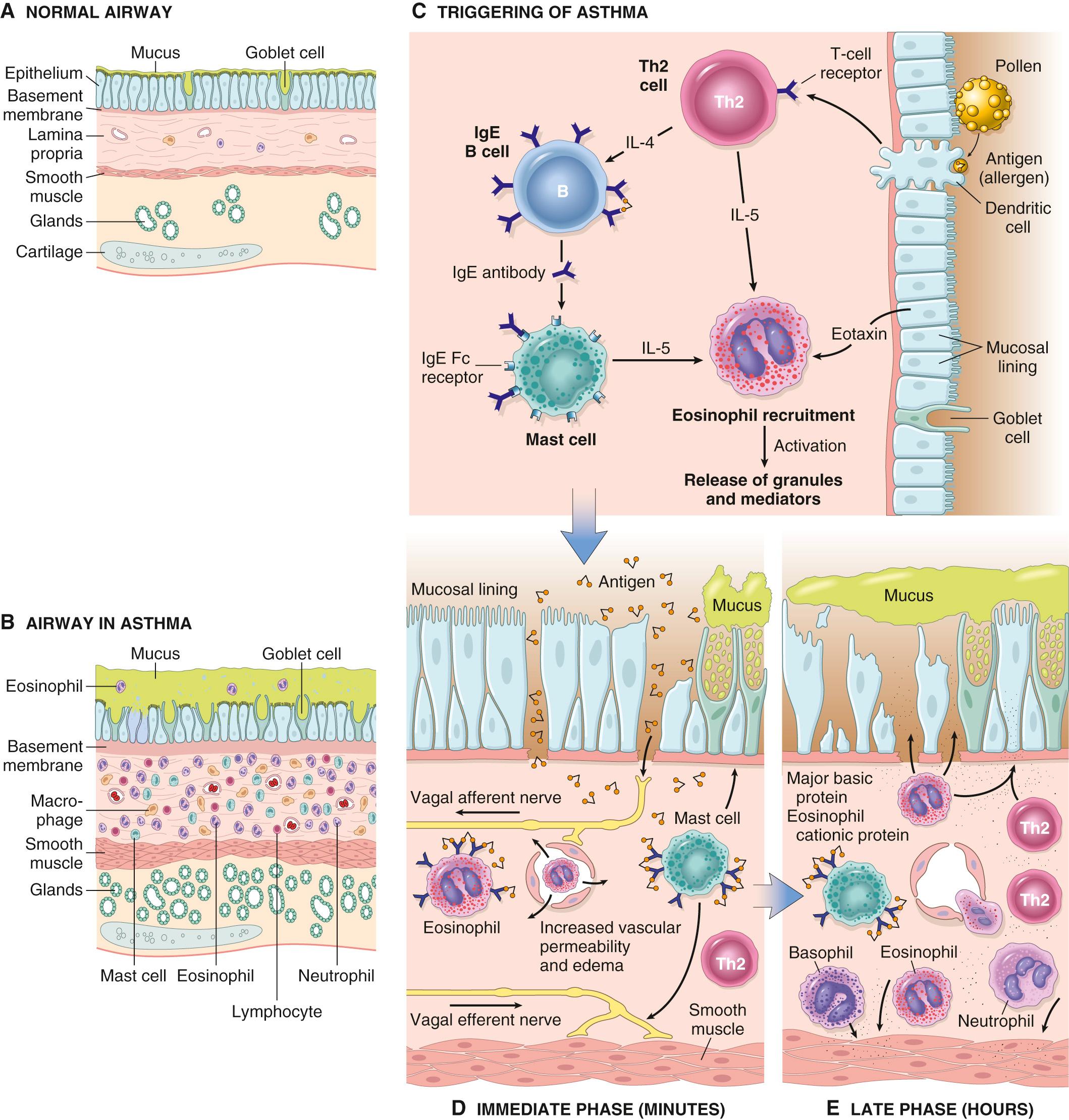
The early-phase reaction is dominated by bronchoconstriction, increased mucus production, variable degrees of vasodilation, and increased vascular permeability. Bronchoconstriction is triggered by direct stimulation of subepithelial vagal (parasympathetic) receptors through both central and local reflexes triggered by mediators produced by mast cells and other cells in the reaction. The late-phase reaction is dominated by recruitment of leukocytes, notably eosinophils, neutrophils, and more T cells. Although Th2 cells are the dominant T-cell type involved in the disease, other T cells that contribute to the inflammation include Th17 (IL-17 producing) cells, which recruit neutrophils.
Many mediators produced by leukocytes and epithelial cells have been implicated in the asthmatic response. The long list of “suspects” in acute asthma can be ranked based on the clinical efficacy of pharmacologic intervention with antagonists of specific mediators.
Mediators whose role in bronchospasm is clearly supported by efficacy of pharmacologic intervention are (1) leukotrienes C 4 , D 4 , and E 4 , which cause prolonged bronchoconstriction as well as increased vascular permeability and increased mucus secretion; (2) acetylcholine, released from intrapulmonary parasympathetic nerves, which can cause airway smooth muscle constriction by directly stimulating muscarinic receptors; (3) IL-5, antagonists of which are effective in treating severe forms of asthma that are associated with peripheral blood eosinophilia; and (4) galectin-10 (GAL10), which is released from eosinophils and forms crystals known as Charcot-Leyden crystals . Long recognized as a feature of asthma, recent studies have shown that these crystals are strong inducers of inflammation and mucus production.
A second group of agents are present at the “scene of the crime” but seem to have relatively minor contributions on the basis of lack of efficacy of potent antagonists or synthesis inhibitors. These include (1) histamine, a potent bronchoconstrictor; (2) prostaglandin D 2 , which elicits bronchoconstriction and vasodilation; and (3) platelet-activating factor, which causes aggregation of platelets and release of serotonin from their granules. These mediators might yet prove important in certain types of chronic or non-allergic asthma.
Finally, a large third group comprises “suspects” for whom specific antagonists or inhibitors are not available or have been insufficiently studied as yet. These include IL-4, IL-13, TNF, chemokines (e.g., eotaxin, also known as CCL11), neuropeptides, nitric oxide, bradykinin, and endothelins.
It is thus clear that multiple mediators contribute to the acute asthmatic response. Moreover, the composition of this “mediator soup” likely varies among individuals or different types of asthma. The appreciation of the importance of inflammatory cells and mediators in asthma has led to greater emphasis on anti-inflammatory drugs, such as corticosteroids, in its treatment.
Susceptibility to atopic asthma is multigenic and often associated with increased incidence of other allergic disorders, such as allergic rhinitis (hay fever) and eczema. Genetic polymorphisms linked to asthma and other allergic disorders were described in Chapter 6 . Suffice it to say here that many of these are likely to influence immune responses and the subsequent inflammatory reaction. Some of the stronger or more interesting genetic variants associated with asthma include the following:
A susceptibility locus for asthma located on chromosome 5q, near the gene cluster encoding the cytokines IL-3, IL-4, IL-5, IL-9, and IL-13 and the IL-4 receptor. Among the genes in this cluster, polymorphisms in the IL13 gene have the strongest and most consistent associations with asthma or allergic disease, while IL-4 receptor gene variants are associated with atopy, elevated total serum IgE, and asthma.
Particular class II HLA alleles linked to production of IgE antibodies against some antigens, such as ragweed pollen.
Variants associated with the genes encoding IL-33, a member of the IL-1 family of cytokines, and its receptor, ST2, which induce the production of Th2 cytokines.
Variants associated with the gene encoding thymic stromal lymphopoietin (TSLP), a cytokine produced by epithelium that may have a role in initiating allergic reactions.
Asthma is a disease of industrialized societies where the majority of people live in cities. Two ideas, neither wholly satisfying, have been proposed to explain this association. First, industrialized environments contain many airborne pollutants that can serve as allergens to initiate the Th2 response. Second, city life tends to limit the exposure of very young children to certain antigens, particularly microbial antigens, and exposure to such antigens may protect children from asthma and atopy. The idea that microbial exposure during early life reduces the later incidence of allergic (and some autoimmune) diseases has been popularized as the hygiene hypothesis. Although the underlying mechanisms of this protective effect are unclear, it has spurred trials of probiotics and intentional early exposure of children to putative allergens to decrease their risk of later developing allergies.
Infections do not cause asthma by themselves, but may be important co-factors. Young children with aeroallergen sensitization who develop lower respiratory tract viral infections (rhinovirus type C, respiratory syncytial virus) have a 10- to 30-fold increased risk of developing persistent and/or severe asthma. Both viral and bacterial infections (identified by cultures and non-culture tools) are associated with acute exacerbations of the disease.
Over time, repeated bouts of allergen exposure and immune reactions result in structural changes in the bronchial wall, referred to as airway remodeling . These changes, described later in greater detail, include hypertrophy and hyperplasia of bronchial smooth muscle, epithelial injury, increased airway vascularity, subepithelial mucous gland enlargement, and subepithelial fibrosis.
A small subset of patients with asthma, many of whom have severe disease that is refractory to glucocorticoids, has inflammatory infiltrates that are enriched for neutrophils rather than eosinophils. This form of the disease may be driven by a Th17 T-cell response to chronic bacterial colonization of the lung.
In patients dying of acute severe asthma (status asthmaticus), the lungs are overinflated and contain small areas of atelectasis. The most striking gross finding is occlusion of bronchi and bronchioles by thick, tenacious mucus plugs, which often contain shed epithelium. A characteristic finding in sputum or bronchoalveolar lavage specimens of patients with atopic asthma is Curschmann spirals, which may result from extrusion of mucus plugs from subepithelial mucous gland ducts or bronchioles. Also present are numerous eosinophils and Charcot-Leyden crystals composed of the eosinophil-derived protein galectin-10. The other characteristic histologic findings of asthma, collectively called airway remodeling ( Figs. 15.10B and 15.11 ), include:
Thickening of airway wall
Sub–basement membrane fibrosis (due to deposition of type I and III collagens)
Increased vascularity
Increase in the size of the submucosal glands and number of airway goblet cells
Hypertrophy and/or hyperplasia of the bronchial wall muscle with increased extracellular matrix
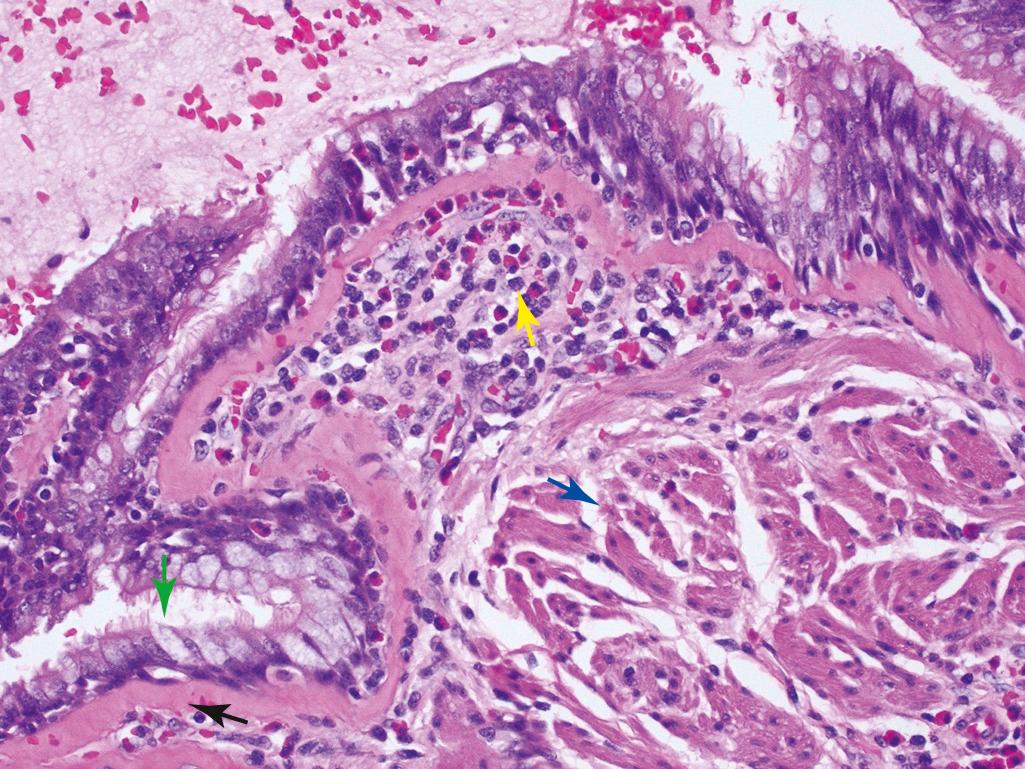
While acute airflow obstruction is primarily attributed to muscular bronchoconstriction, acute edema, and mucus plugging, airway remodeling may contribute to chronic irreversible airway obstruction.
A classic acute asthmatic attack lasts up to several hours. In some patients, however, the cardinal symptoms of chest tightness, dyspnea, wheezing, and coughing (with or without sputum production) are present at a low level constantly. In its most severe form, acute severe asthma, the paroxysm persists for days or even weeks, sometimes causing airflow obstruction that is so extreme that marked cyanosis or even death ensues.
The diagnosis is based on demonstration of an increase in airflow obstruction (from baseline levels); difficulty with exhalation (prolonged expiration, wheeze); and (in those with atopic asthma) identification of eosinophilia in the peripheral blood and eosinophils, Curschmann spirals, and Charcot-Leyden crystals in the sputum. In the usual case with intervals of freedom from respiratory difficulty, the disease is more discouraging and disabling than lethal, and most individuals are able to maintain a productive life.
Therapy is based on the severity of the disease. The centerpieces of standard therapy are bronchodilators, glucocorticoids, and leukotriene antagonists. For severe and difficult-to-treat asthma in adolescents and adults, novel biologic therapies targeting inflammatory mediators such as IL-5 blocking antibodies, which is effective in severe asthma associated with Th2 immune responses and peripheral blood eosinophilia, are now available. Up to 50% of childhood asthma remits in adolescence only to return in adulthood in a significant number of patients. In other cases there is a variable decline in baseline lung function over time.
Asthma is characterized by reversible bronchoconstriction caused by airway hyperresponsiveness to a variety of stimuli.
Atopic asthma is caused by a Th2 and IgE-mediated immunologic reaction to environmental allergens and is characterized by acute-phase (immediate) and late-phase reactions. The Th2 cytokines IL-4, IL-5, and IL-13 are important mediators.
Triggers for non-atopic asthma are less clear but include viral infections and inhaled air pollutants, which can also trigger atopic asthma.
Eosinophils are key inflammatory cells in atopic asthma; other inflammatory cells implicated in its pathogenesis include mast cells, neutrophils, and T lymphocytes.
Airway remodeling (sub–basement membrane fibrosis, hypertrophy of bronchial glands, and smooth muscle hyperplasia) adds an irreversible component to the obstructive disease.
Bronchiectasis is a disorder in which destruction of smooth muscle and elastic tissue by inflammation stemming from persistent or severe infections leads to permanent dilation of bronchi and bronchioles. Because of better control of lung infections, bronchiectasis is now uncommon, but may still develop in association with the following:
Congenital or hereditary conditions that predispose to chronic infections, including cystic fibrosis, intralobar sequestration of the lung, immunodeficiency states, primary ciliary dyskinesia, and Kartagener syndrome.
Severe necrotizing pneumonia caused by bacteria, viruses, or fungi; this may be a single severe episode or recurrent infections.
Bronchial obstruction, due to tumor, foreign body, or mucus impaction; in each instance the bronchiectasis is localized to the obstructed lung segment.
Immune disorders, including rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, and the posttransplant setting (chronic rejection after lung transplant and chronic graft-versus-host disease after hematopoietic stem cell transplantation).
Up to 50% of cases are idiopathic, lacking the aforementioned associations, in which there appears to be dysfunctional host immunity to infectious agents leading to chronic inflammation.
Obstruction and infection are the major conditions associated with bronchiectasis. The infections that lead to bronchiectasis are usually the result of a defect in airway clearance. Sometimes this defect stems from airway obstruction, leading to distal pooling of secretions.
Both mechanisms are readily apparent in a severe form of bronchiectasis that is associated with cystic fibrosis ( Chapter 10 ). In cystic fibrosis the primary defect in ion transport results in thick viscous secretions that perturb mucociliary clearance and lead to airway obstruction. This sets the stage for chronic bacterial infections, which cause widespread damage to airway walls. With destruction of supporting smooth muscle and elastic tissue, the bronchi become markedly dilated, while smaller bronchioles are progressively obliterated as a result of fibrosis (bronchiolitis obliterans).
Primary ciliary dyskinesia is an autosomal recessive disease with a frequency of 1 in 10,000 to 20,000 births. The disease-causing mutations result in ciliary dysfunction due to defects in ciliary motor proteins (e.g., mutations involving dynein), again preventing mucociliary clearance, setting the stage for recurrent infections that lead to bronchiectasis. Ciliary function also is necessary during embryogenesis to ensure proper rotation of the developing organs in the chest and abdomen; in its absence, their location becomes a matter of chance. As a result, approximately half of patients with primary ciliary dyskinesia have Kartagener syndrome, marked by situs inversus or a partial lateralizing abnormality associated with bronchiectasis and sinusitis. Males with this condition also tend to be infertile as a result of sperm dysmotility.
Allergic bronchopulmonary aspergillosis occurs in patients with asthma or cystic fibrosis and frequently leads to the development of bronchiectasis. It is a hyperimmune response to the fungus Aspergillus fumigatus. Sensitization to Aspergillus leads to activation of Th2 helper T cells, which release cytokines that recruit eosinophils and other leukocytes. Characteristically, there are high serum IgE levels, serum antibodies to Aspergillus, intense airway inflammation with eosinophils, and formation of mucus plugs, which play a primary role in the development of bronchiectasis.
Bronchiectasis usually affects the lower lobes bilaterally, particularly air passages that are vertical, and is most severe in the more distal bronchi and bronchioles. When tumor or aspiration of foreign bodies leads to bronchiectasis, the involvement is localized. The airways are dilated, sometimes up to four times normal size. Characteristically, the bronchi and bronchioles are so dilated that they can be followed almost to the pleural surfaces. By contrast, in the normal lung, the bronchioles cannot be followed by eye beyond a point 2 to 3 cm from the pleural surfaces. On the cut surface of the lung, the dilated bronchi appear cystic and are filled with mucopurulent secretions ( Fig. 15.12 ).
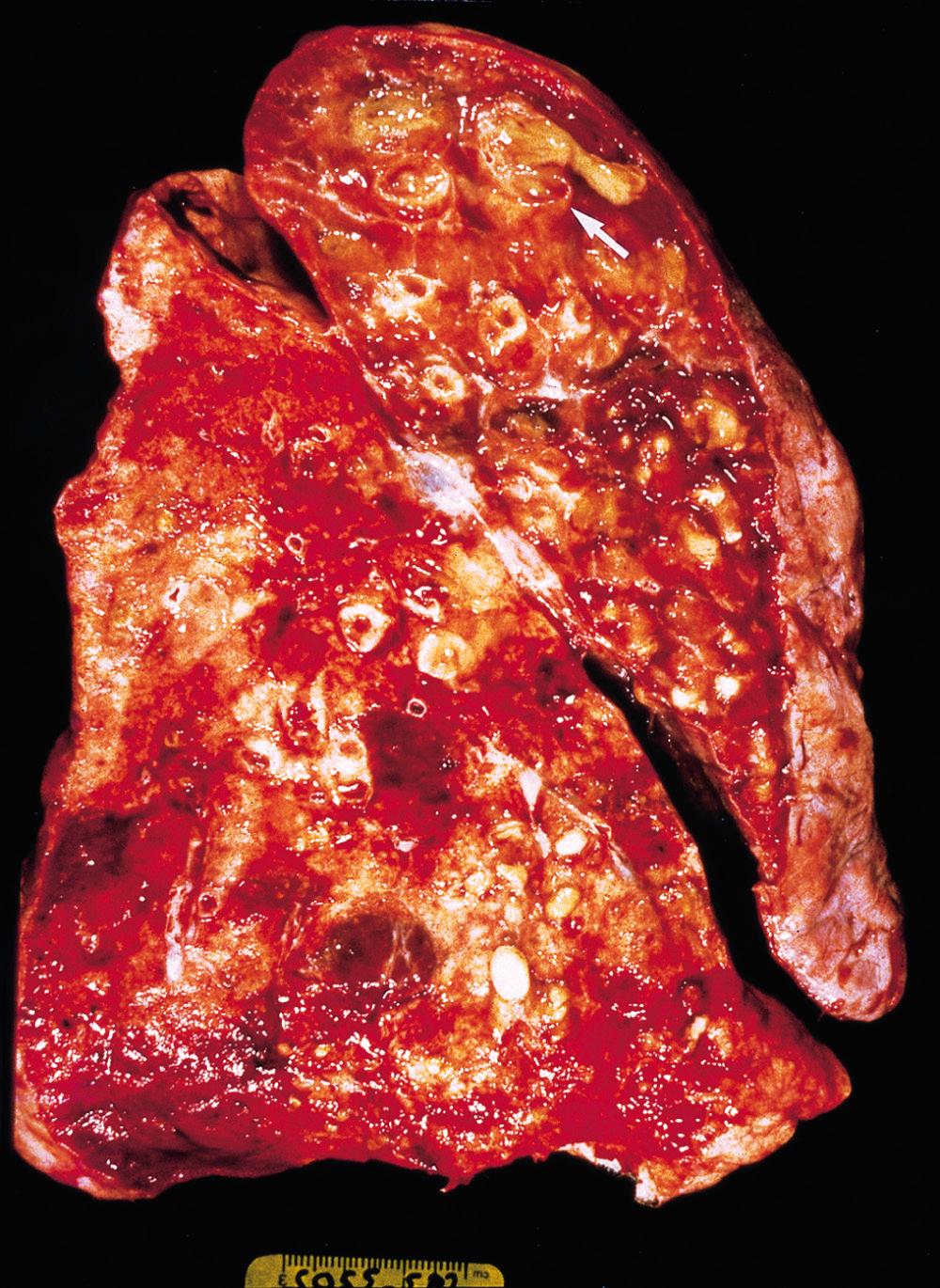
The histologic findings vary with the activity and chronicity of the disease. In the full-blown, active case there is an intense acute and chronic inflammatory exudation within the walls of the bronchi and bronchioles, associated with desquamation of the lining epithelium and extensive ulceration. There also may be squamous metaplasia of the remaining epithelium in response to chronic inflammation, further diminishing mucociliary clearance. In some instances, necrosis destroys the bronchial or bronchiolar walls and forms a lung abscess. Fibrosis of the bronchial and bronchiolar walls and peribronchiolar fibrosis develop in more chronic cases, leading to varying degrees of subtotal or total obliteration of bronchiolar lumens.
Haemophilus influenzae is found in approximately half and Pseudomonas aeruginosa in 12% to 30% of sputum cultures from patients with bronchiectasis, with four other types of bacteria (including non-tuberculous mycobacteria) making up most of the remaining cases in patients from different geographic locations. This observation suggests that the inflamed, mucoid, sometimes anaerobic, bronchiectatic microenvironment favors colonization by a relatively few microbial species. In allergic bronchopulmonary aspergillosis, fungal hyphae can be seen on special stains within the mucoinflammatory contents of the dilated segmental bronchi. In late stages the fungus may infiltrate the bronchial wall.
Bronchiectasis causes severe, persistent cough; expectoration of foul smelling, sometimes bloody sputum; dyspnea and orthopnea in severe cases; and, on occasion, hemoptysis, which may be massive. Symptoms are often episodic and are precipitated by upper respiratory tract infections or the introduction of new pathogenic agents. Paroxysms of cough are particularly frequent when the patient rises in the morning, as the change in position causes collections of pus and secretions to drain into the bronchi. Obstructive respiratory insufficiency can lead to marked dyspnea and cyanosis. However, current treatments with better antibiotics and physical therapy have improved outcomes considerably, and life expectancy has almost doubled. Hence, cor pulmonale, brain abscesses, and amyloidosis are less frequent complications of bronchiectasis currently than in the past.
Restrictive lung disorders fall into two general categories: (1) chronic interstitial and infiltrative diseases, such as pneumoconioses and interstitial fibrosis of unknown etiology, and (2) chest wall disorders (e.g., neuromuscular diseases such as poliomyelitis, severe obesity, pleural diseases, and kyphoscoliosis), which are not discussed here.
Chronic interstitial pulmonary diseases are a heterogeneous group of disorders characterized predominantly by inflammation and fibrosis of the lung interstitium associated with pulmonary function studies indicative of restrictive lung disease. Diffuse restrictive diseases are categorized based on histology and clinical features ( Table 15.5 ). Many of the entities are of unknown cause and pathogenesis, and some have an intra-alveolar as well as an interstitial component. Patients have dyspnea, tachypnea, end-inspiratory crackles, and eventual cyanosis, without wheezing or other evidence of airway obstruction. The classic functional abnormalities are reductions in diffusion capacity, lung volume, and lung compliance. Chest radiographs show bilateral lesions that take the form of small nodules, irregular lines, or ground-glass shadows, all corresponding to areas of interstitial fibrosis. Although the entities can often be distinguished in their early stages, advanced forms are hard to differentiate because all result in diffuse scarring of the lung, often referred to as end-stage lung or honeycomb lung . Eventually, secondary pulmonary hypertension and right-sided heart failure (cor pulmonale) may result.
| Fibrosing |
|
| Granulomatous |
|
| Eosinophilic |
| Smoking-Related |
|
| Other |
|
Idiopathic pulmonary fibrosis (IPF) refers to a clinicopathologic syndrome marked by progressive interstitial pulmonary fibrosis and respiratory failure. In Europe the term cryptogenic fibrosing alveolitis is more popular. IPF has characteristic radiologic, pathologic, and clinical features. The histologic pattern of fibrosis is referred to as usual interstitial pneumonia (UIP), which can often be diagnosed based on its characteristic appearance on computed tomography scans. The UIP pattern can also be seen in other diseases, notably connective tissue diseases, chronic hypersensitivity pneumonia, and asbestosis; these must be distinguished from IPF based on other clinical, laboratory, and histologic features.
While the cause of IPF remains unknown, it appears that it arises in genetically predisposed individuals who are prone to aberrant repair of recurrent alveolar epithelial cell injuries caused by environmental exposures ( Fig. 15.13 ). The implicated factors are as follows:
Environmental factors. Most important among these is cigarette smoking, which increases the risk of IPF several-fold. IPF incidence is also increased in individuals who are exposed to air pollution, microaspiration, metal fumes, and wood dust, or who work in certain occupations, including farming, hairdressing, and stone polishing. It is hypothesized that exposure to environmental irritants or toxins in each of these contexts causes recurrent alveolar epithelial cell damage.
Genetic factors. The vast majority of individuals who smoke or who have other environmental exposures linked to IPF do not develop the disorder, indicating that additional factors are required for its development. Mutations in the TERT, TERC, PARN, and RTEL1 genes, all of which are involved with the maintenance of telomeres, are associated with increased risk of IPF. You will recall that maintenance of telomeres (the ends of chromosomes) is necessary to prevent cellular senescence. Up to 15% of familial IPF is associated with inherited defects in genes that maintain telomeres, while up to 25% of sporadic IPF cases are associated with abnormal telomere shortening in peripheral blood lymphocytes, a finding that also points to a problem with telomere maintenance. Other, rare familial forms of IPF are associated with mutations in genes encoding components of surfactant; these mutations create folding defects in the affected proteins, leading to activation of the unfolded protein response in type II pneumocytes. This in turn appears to make pneumocytes more sensitive to environmental insults, leading to cellular dysfunction and injury. Finally, roughly one-third of IPF cases are associated with a single-nucleotide polymorphism in the promoter of the MUC5B gene that greatly increases the secretion of MUC5B, a member of the mucin family. This may in turn alter mucociliary clearance, but precisely how this alteration relates to IPF risk is uncertain.
Age. IPF is a disease of older individuals, rarely appearing before the age of 50 years. Whether this association stems from aging-related telomere shortening or from other acquired changes associated with aging is unknown.
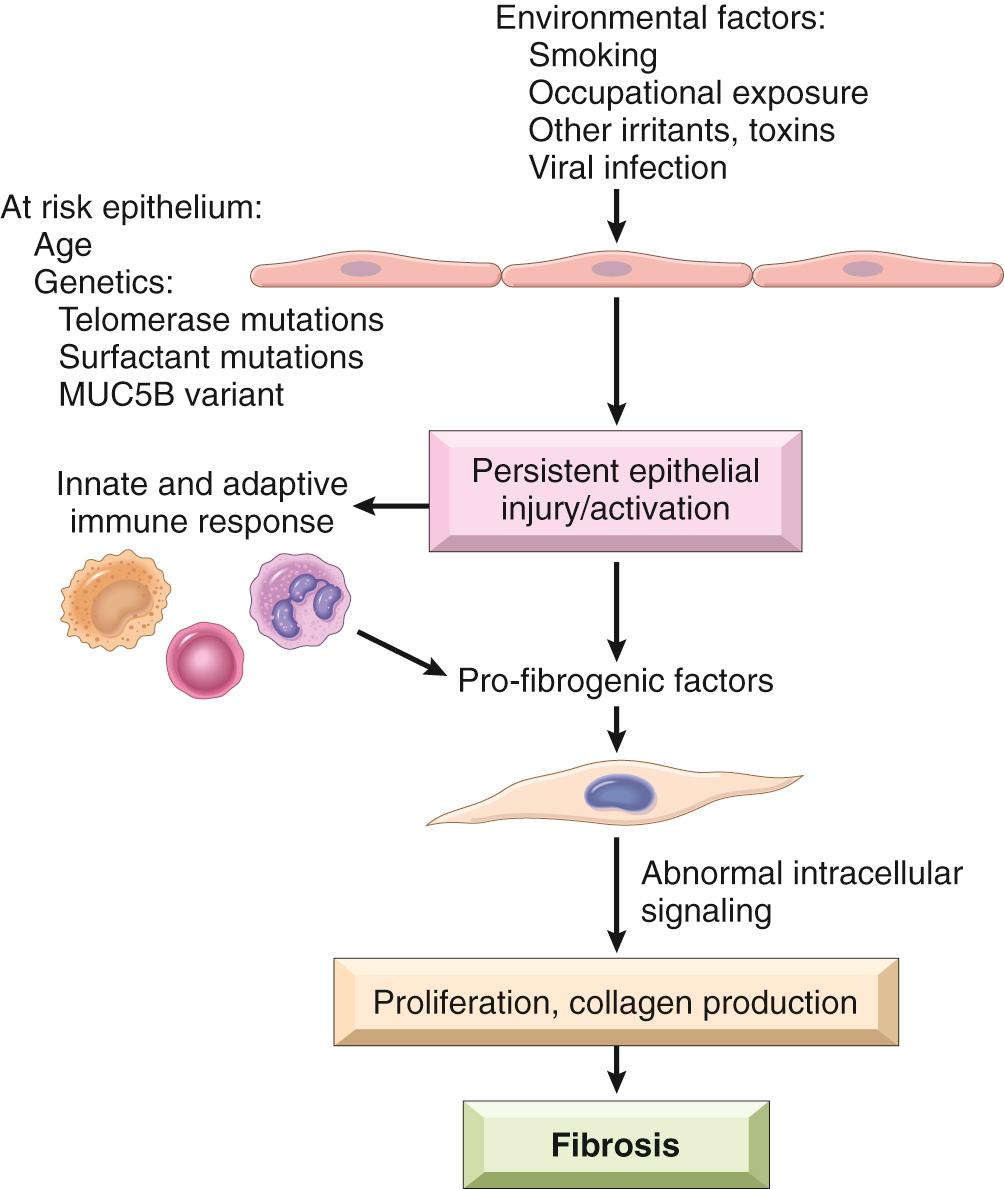
It is easy to imagine how some of the factors cited herein might combine to exacerbate alveolar epithelial cell damage and senescence, which seems to be the initiating event in IPF, but it must be admitted that the pathogenesis of IPF is complex and poorly understood. For example, it is unknown precisely how alveolar epithelial cell damage translates into interstitial fibrosis. One model holds that the injured epithelial cells are the source of profibrogenic factors such as TGF-β, whereas a second, non–mutually exclusive model proposes that innate and adaptive immune cells produce such factors as part of the host response to epithelial cell damage. Other work has described abnormalities in the fibroblasts themselves that involve changes in intracellular signaling and features reminiscent of epithelial mesenchymal transition ( Chapter 7 ), but a causal link between these alterations and fibrosis has not been established.
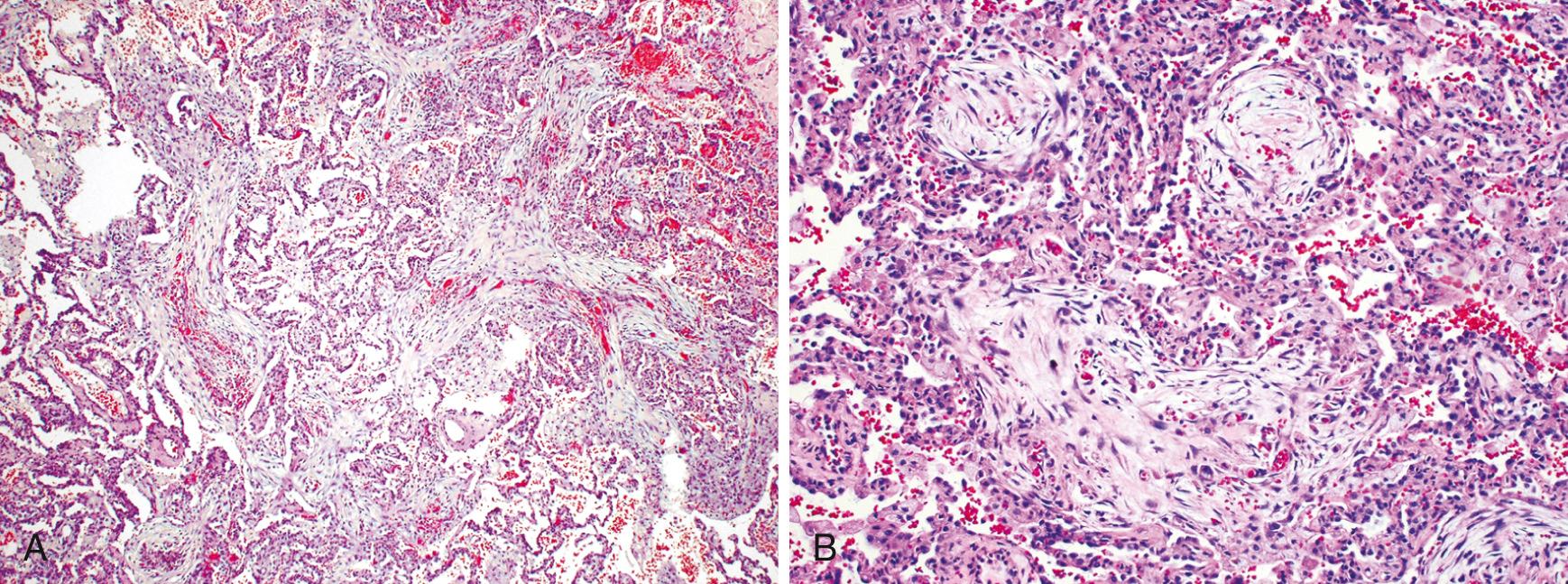
Grossly, the pleural surfaces of the lung are cobblestoned as a result of the retraction of scars along the interlobular septa. The cut surface shows firm, rubbery white areas of fibrosis, which occurs preferentially in the lower lobes, the subpleural regions, and along the interlobular septa. Microscopically, the hallmark is patchy interstitial fibrosis, which varies in intensity ( Fig. 15.14 ) and age. The earliest lesions contain an exuberant proliferation of fibroblasts (fibroblastic foci) . With time these areas become more fibrotic and less cellular. Quite typical is the coexistence of both early and late lesions ( Fig. 15.15 ). The dense fibrosis causes the destruction of alveolar architecture and the formation of cystic spaces lined by hyperplastic type II pneumocytes or bronchiolar epithelium (honeycomb fibrosis) . With adequate sampling, these diagnostic histologic changes (i.e., areas of dense fibrosis and fibroblastic foci) can be identified even in advanced IPF. There is mild to moderate inflammation within the fibrotic areas, consisting of mostly lymphocytes admixed with a few plasma cells, neutrophils, eosinophils, and mast cells. Foci of squamous metaplasia and smooth muscle hyperplasia may be present, along with pulmonary arterial hypertensive changes (intimal fibrosis and medial thickening). In acute exacerbations, DAD may be superimposed on these chronic changes.
IPF begins insidiously with gradually increasing dyspnea on exertion and dry cough. Most patients are 55 to 75 years old at presentation. Hypoxemia, cyanosis, and clubbing occur late in the course. The course in individual patients is unpredictable. Usually there is slowly progressive respiratory failure, but some patients have acute exacerbations and follow a rapid downhill clinical course. The median survival is about 3.8 years after diagnosis. Lung transplantation is the only definitive therapy; however, two drugs, a tyrosine kinase inhibitor and a TGF-β antagonist, have both been shown to slow disease progression and represent the first effective (albeit modestly so) targeted therapies for IPF.
Despite its “nonspecific” name, this entity is important to recognize, since these patients have a much better prognosis than patients with UIP. Nonspecific interstitial pneumonia is most often associated with connective tissue disease but may also be idiopathic.
On the basis of its histology, nonspecific interstitial pneumonia is divided into cellular and fibrosing patterns. The cellular pattern consists primarily of mild to moderate chronic interstitial inflammation, containing lymphocytes and a few plasma cells, in a uniform or patchy distribution. The fibrosing pattern consists of diffuse or patchy interstitial fibrotic lesions of roughly the same stage of development, an important distinction from UIP. Fibroblastic foci, honeycombing, hyaline membranes, and granulomas are absent.
Patients present with dyspnea and cough of several months’ duration. They are more likely to be female nonsmokers in their sixth decade of life. On imaging, the lesions have the appearance of bilateral, symmetric, predominantly lower lobe reticular opacities. Patients having the cellular pattern are somewhat younger than those with the fibrosing pattern and have a better prognosis.
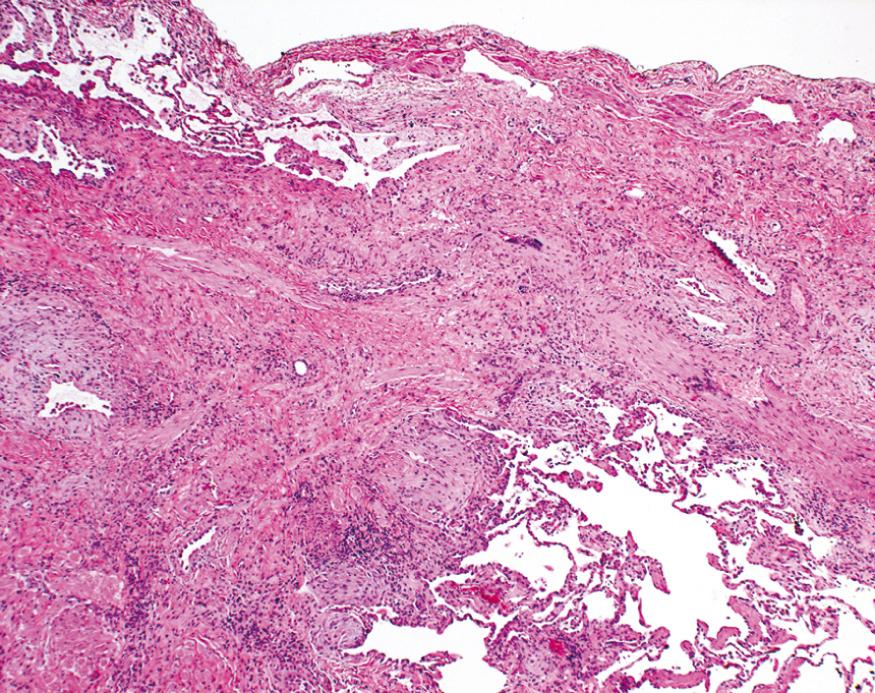
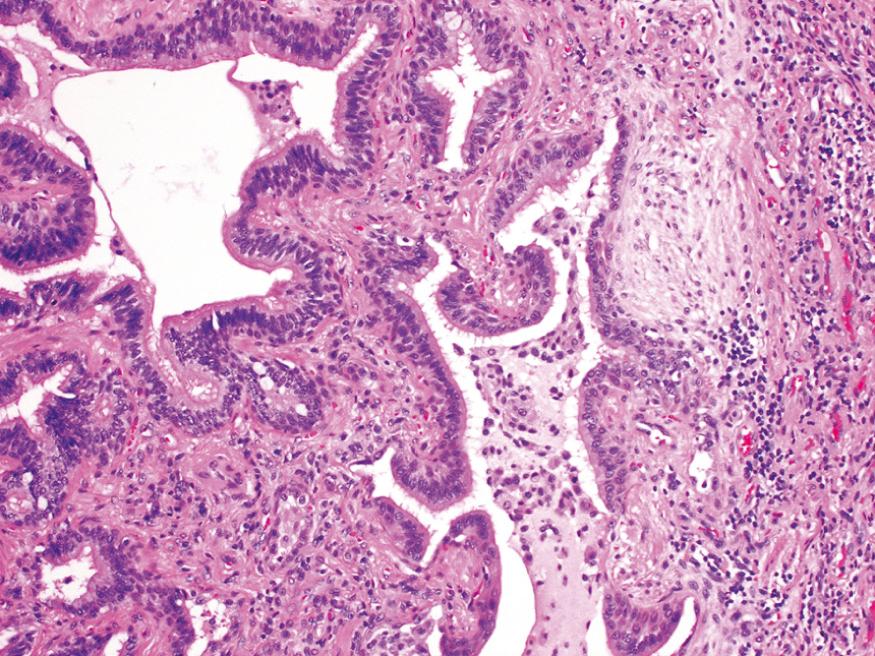
Cryptogenic organizing pneumonia is most often seen as a response to infection or inflammatory injury of the lungs. It has been associated with viral and bacterial pneumonias, inhaled toxins, drugs, connective tissue disease, and graft-versus-host disease in hematopoietic stem cell transplant recipients. Patients present with cough and dyspnea and have patchy subpleural or peribronchial areas of airspace consolidation radiographically. Histologically, it is characterized by the presence of polypoid plugs of loose organizing connective tissue (Masson bodies) within alveolar ducts, alveoli, and often bronchioles ( Fig. 15.16 ). The connective tissue is all of the same age, and the underlying lung architecture is normal. There is no interstitial fibrosis or honeycomb lung. Some patients recover spontaneously, but most need treatment with oral steroids for 6 months or longer for complete recovery. The long-term prognosis is dependent on the underlying disorder.
Many autoimmune diseases (also referred to as connective tissue diseases because of their frequent association with arthritis) can involve the lung at some point in their course. Those that are well recognized for producing pulmonary disease include systemic lupus erythematosus, rheumatoid arthritis, progressive systemic sclerosis (scleroderma), and dermatomyositis-polymyositis. Pulmonary involvement can take different histologic patterns; nonspecific interstitial pneumonia, usual interstitial pneumonia, organizing pneumonia, and bronchiolitis are the most common.
Rheumatoid arthritis is associated with pulmonary involvement in 30% to 40% of patients as (1) chronic pleuritis, with or without effusion; (2) diffuse interstitial pneumonitis and fibrosis; (3) intrapulmonary rheumatoid nodules; (4) follicular bronchiolitis; or (5) pulmonary hypertension. When lung disease occurs in the setting of rheumatoid arthritis and a pneumoconiosis (described next), it is referred to as Caplan syndrome .
Systemic sclerosis (scleroderma) is associated with diffuse interstitial fibrosis (nonspecific interstitial pattern more common than usual interstitial pattern) and pleural involvement.
Lupus erythematosus may cause patchy, transient parenchymal infiltrates or occasionally severe lupus pneumonitis, as well as pleuritis and pleural effusions.
Pulmonary involvement in these diseases has a variable prognosis that is determined by the extent and histologic pattern of involvement.
Diffuse interstitial fibrosis of the lung gives rise to restrictive lung diseases characterized by reduced lung compliance and reduced FVC. The ratio of FEV 1 to FVC is normal.
Ididopathic pulmonary fibrosis is prototypic of restrictive lung diseases. It is characterized by patchy interstitial fibrosis, fibroblastic foci, and formation of cystic spaces (honeycomb lung). This histologic pattern is known as usual interstitial fibrosis.
The cause of idiopathic pulmonary fibrosis is unknown, but genetic analyses point to roles for senescence of alveolar epithelium (due to telomere shortening), altered mucin production, and abnormal signaling in alveolar fibroblasts. Injury to alveolar epithelial cells sets in motion events that lead to increased local production of fibrogenic cytokines, such as TGF-β.
The term pneumoconiosis, originally coined to describe the non-neoplastic lung reaction to inhalation of mineral dusts encountered in the workplace, now also includes disease induced by chemical fumes and vapors. A simplified classification is presented in Table 15.6 . Where implemented, regulations limiting worker exposure have resulted in a marked decrease in dust-associated diseases.
| Agent | Disease | Exposure |
|---|---|---|
| Mineral Dusts | ||
| Coal dust | Anthracosis Macules Progressive massive fibrosis Caplan syndrome |
Coal mining (particularly hard coal) |
| Silica | Silicosis Caplan syndrome |
Metal casting work, sandblasting, hard rock mining, stone cutting, others |
| Asbestos | Asbestosis Pleural plaques Caplan syndrome Mesothelioma Carcinoma of the lung, larynx, stomach, colon |
Mining, milling, manufacturing, and installation and removal of insulation |
| Beryllium | Acute berylliosis Beryllium granulomatosis Lung carcinoma (?) |
Mining, manufacturing |
| Iron oxide | Siderosis | Welding |
| Barium sulfate | Baritosis | Mining |
| Tin oxide | Stannosis | Mining |
| Organic Dusts That Induce Hypersensitivity Pneumonitis | ||
| Moldy hay | Farmer's lung | Farming |
| Bagasse | Bagassosis | Manufacturing wallboard, paper |
| Bird droppings | Bird breeder's lung | Bird handling |
| Organic Dusts That Induce Asthma | ||
| Cotton, flax, hemp | Byssinosis | Textile manufacturing |
| Red cedar dust | Asthma | Lumbering, carpentry |
| Chemical Fumes and Vapors | ||
| Nitrous oxide, sulfur dioxide, ammonia, benzene, insecticides | Bronchitis, asthma Pulmonary edema ARDS Mucosal injury Fulminant poisoning |
Occupational and accidental exposure |
Specific factors that influence the development of dust-borne pneumoconiosis include the following:
Dust retention, which is determined by the dust concentration in ambient air, the duration of exposure, and the effectiveness of clearance mechanisms. Any influence, such as cigarette smoking, that impairs mucociliary clearance significantly increases the accumulation of dust in the lungs.
Particle size. The most dangerous particles are from 1 to 5 µm in diameter because particles of this size can reach the terminal small airways and air sacs and deposit in their linings.
Particle solubility and cytotoxicity, which are influenced by particle size. In general, small particles composed of injurious substances of high solubility are more likely to produce rapid-onset acute lung injury. By contrast, larger particles are more likely to resist dissolution and may persist within the lung parenchyma for years. These tend to evoke fibrosing collagenous pneumoconioses, such as is characteristic of silicosis.
Particle uptake by epithelial cells or egress across epithelial linings, which allows direct interactions with fibroblasts and interstitial macrophages to occur. Some particles may reach the lymph nodes through lymphatic drainage directly or within migrating macrophages and thereby initiate an adaptive immune response to components of the particulates or to self-proteins modified by the particles or both.
Activation of the inflammasome ( Chapter 3 ), which occurs following the phagocytosis of certain particles by macrophages. This innate immune response amplifies the intensity and the duration of the local reaction.
Tobacco smoking, which worsens the effects of all inhaled mineral dusts, but particularly those caused by asbestos.
In general, only a small percentage of exposed people develop occupational respiratory diseases, implying a genetic predisposition to their development. Many of the diseases listed in Table 15.6 are quite uncommon; hence only a select few that cause pulmonary fibrosis are presented next.
Coal workers’ pneumoconiosis is lung disease caused by inhalation of coal particles and other admixed forms of dust. Dust reduction measures in coal mines around the globe have drastically reduced its incidence. The spectrum of lung findings in coal workers is wide, varying from asymptomatic anthracosis, to simple coal workers’ pneumoconiosis with little to no pulmonary dysfunction, to complicated coal workers’ pneumoconiosis, or progressive massive fibrosis, in which lung function is compromised. Contaminating silica in the coal dust favors the development of progressive disease. In most cases, carbon dust itself is the major culprit, and studies have shown that complicated lesions contain much more dust than simple lesions. Coal workers may also develop emphysema and chronic bronchitis independent of smoking.
Carbon deposits are dark black in color and are readily visible grossly and microscopically. Anthracosis is the most innocuous coal-induced pulmonary lesion in coal miners and is also seen to some degree in urban dwellers and tobacco smokers. Inhaled carbon pigment is engulfed by alveolar or interstitial macrophages, which accumulate in the connective tissue adjacent to the lymphatics and in organized lymphoid tissue adjacent to the bronchi or in the lung hilus.
Simple coal workers’ pneumoconiosis is characterized by coal macules (1 to 2 mm in diameter) and somewhat larger coal nodules. Coal macules consist of carbon-laden macrophages; nodules also contain a delicate network of collagen fibers. Although these lesions are scattered throughout the lung, the upper lobes and upper zones of the lower lobes are more heavily involved. They are located primarily adjacent to respiratory bronchioles, the site of initial dust accumulation. In due course dilation of adjacent alveoli occurs, sometimes giving rise to centrilobular emphysema.
Complicated coal workers’ pneumoconiosis (progressive massive fibrosis) occurs on a background of simple disease and generally requires many years to develop. It is characterized by intensely blackened scars 1 cm or larger, sometimes up to 10 cm in greatest diameter. They are usually multiple. Microscopically, the lesions consist of dense collagen and pigment ( Fig. 15.17 ). The center of the lesion is often necrotic, most likely due to local ischemia.
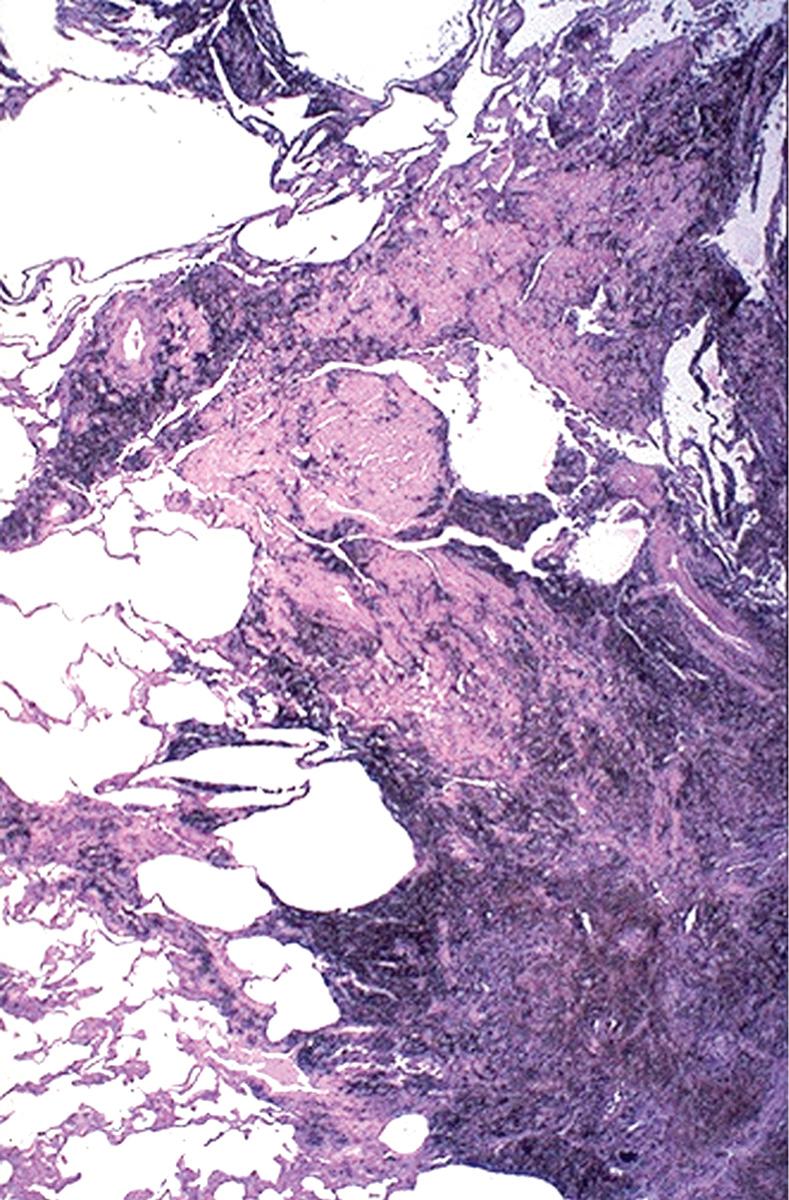
Coal workers’ pneumoconiosis is usually benign, causing little decrement in lung function. Even mild forms of complicated coal workers’ pneumoconiosis do not affect lung function significantly. In a minority of cases (fewer than 10%), progressive massive fibrosis develops, leading to increasing pulmonary dysfunction, pulmonary hypertension, and cor pulmonale. Once progressive massive fibrosis develops, it may continue to worsen even if further exposure to dust is prevented. Unlike silicosis (discussed next), there is no convincing evidence that coal workers' pneumoconiosis increases susceptibility to tuberculosis, nor does it predispose to cancer in the absence of smoking. However, domestic indoor use of “smoky coal” (bituminous) for cooking and heating, a common practice in lower income parts of the world, is associated with an increased risk of lung cancer death, even in those who do not smoke.
Silicosis is a common lung disease caused by inhalation of proinflammatory crystalline silicon dioxide (silica). It usually presents after decades of exposure as slowly progressing, nodular, fibrosing pneumoconiosis. Currently, silicosis is the most prevalent chronic occupational disease in the world. Both dose and race are important in developing silicosis (African Americans are at higher risk than Caucasians). As shown in Table 15.6 , workers in a large number of occupations are at risk, including individuals involved with the repair, rehabilitation, or demolition of concrete structures such as buildings and roads. The disease also occurs in workers producing stressed denim by sandblasting, stone carvers, and jewelers using chalk molds. Occasionally, heavy exposure over months to a few years can result in acute silicosis, a disorder characterized by the accumulation of abundant lipoproteinaceous material within alveoli (identical morphologically to alveolar proteinosis, discussed later).
Phagocytosis of inhaled silica crystals by macrophages activates the inflammasome and stimulates the release of inflammatory mediators, particularly IL-1 and IL-18. This in turn leads to the recruitment of additional inflammatory cells and activates interstitial fibroblasts, leading to collagen deposition. Silica occurs in both crystalline and amorphous forms, but crystalline forms (including quartz, cristobalite, and tridymite) are much more fibrogenic. Of these, quartz is most commonly implicated. The lack of severe responses to silica in coal and hematite miners is thought to be due to coating of silica with other minerals, especially clay components, which render the silica less toxic. Although amorphous silicates are biologically less active than crystalline silica, heavy lung burdens of these minerals may also produce lesions.
Silicosis is characterized grossly in its early stages by tiny, barely palpable, discrete pale to blackened (if coal dust is also present) nodules in the hilar lymph nodes and upper zones of the lungs. As the disease progresses, these nodules coalesce into hard, collagenous scars ( Fig. 15.18 ). Some nodules may undergo central softening and cavitation due to superimposed tuberculosis or to ischemia. Fibrotic lesions may also occur in the hilar lymph nodes and pleura. Sometimes, thin sheets of calcification occur in the lymph nodes and are seen radiographically as eggshell calcification (i.e., calcium surrounding a zone lacking calcification). If the disease continues to progress, expansion and coalescence of lesions may produce progressive massive fibrosis. Histologic examination reveals the hallmark lesion characterized by a central area of whorled collagen fibers with a more peripheral zone of dust-laden macrophages ( Fig. 15.19 ). Examination of the nodules by polarized microscopy reveals weakly birefringent silicate particles.
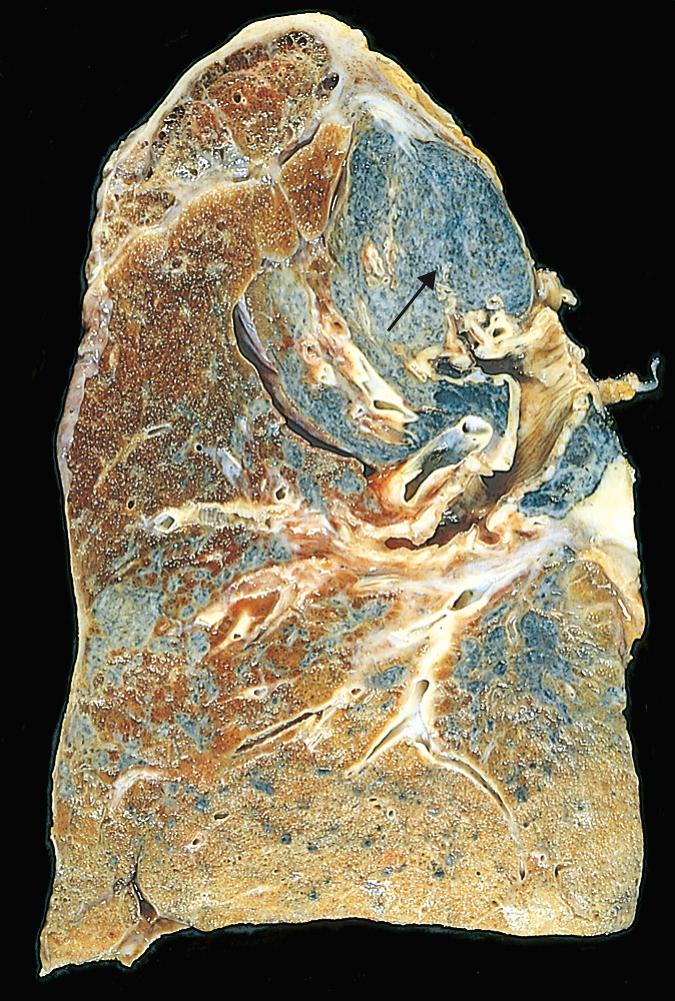
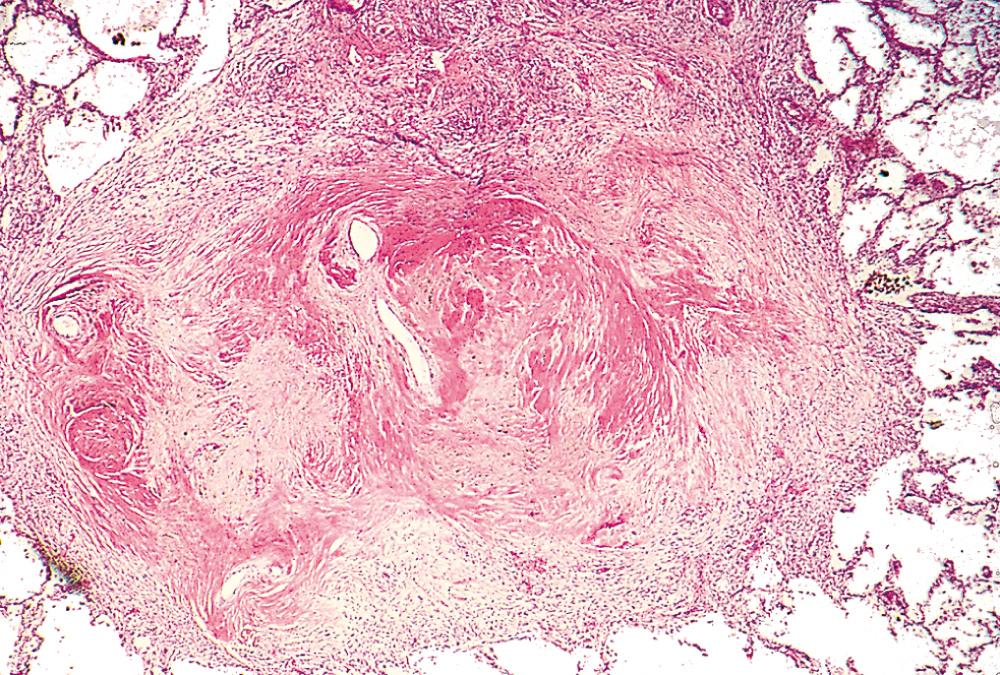
The onset of silicosis may be slow and insidious (10 to 30 years after exposure; this is most common), accelerated (within 10 years of exposure), or rapid (weeks or months after intense exposure to fine dust high in silica; this is rare). Chest radiographs typically show a fine nodularity in the upper zones of the lung. Pulmonary function is either normal or only moderately affected early in the course, and most patients do not develop shortness of breath until progressive massive fibrosis supervenes. The disease may continue to worsen even if the patient is no longer exposed. It is slow to kill, but impaired pulmonary function may severely limit activity.
Silicosis also is associated with an increased susceptibility to tuberculosis and a twofold increased risk of lung cancer. The former may be because crystalline silica inhibits the ability of pulmonary macrophages to kill phagocytosed mycobacteria. The link to cancer is not fully understood, but it is but one of many chronic inflammatory conditions that increase the risk of carcinoma in involved tissues ( Chapter 7 ).
Asbestos is a family of proinflammatory crystalline hydrated silicates that are associated with pulmonary fibrosis and various forms of cancer. Use of asbestos is tightly restricted in many higher income countries; however, there is little, if any, control in lower income parts of the world. Asbestos-related diseases include:
Localized fibrous plaques or, rarely, diffuse pleural fibrosis
Pleural effusions, recurrent
Parenchymal interstitial fibrosis (asbestosis)
Lung carcinoma
Mesothelioma
Laryngeal, ovarian, and perhaps other extrapulmonary neoplasms, including colon carcinoma
Increased risks for systemic autoimmune diseases and cardiovascular disease also have been proposed
The increased incidence of asbestos-related cancers in family members of asbestos workers has alerted the general public to the potential hazards of even low-level exposure to asbestos. However, the necessity of expensive asbestos abatement programs for environments such as schools with low, but measurable, airborne asbestos fiber counts remains a matter of contention.
The disease-causing capabilities of the different forms of asbestos depend on concentration, size, shape, and solubility. Asbestos occurs in two distinct geometric forms, serpentine and amphibole. The serpentine chrysotile form accounts for 90% of the asbestos used in industry. Amphiboles, even though less prevalent, are more pathogenic than chrysotiles, particularly with respect to induction of mesothelioma, a malignant tumor derived from the lining cells of pleural surfaces (described later).
The greater pathogenicity of amphiboles is apparently related to their aerodynamic properties and solubility. Chrysotiles, with their more flexible, curled structure, are likely to become impacted in the upper respiratory passages and removed by the mucociliary elevator. Furthermore, once trapped in the lungs, chrysotiles are gradually leached from the tissues because they are more soluble than amphiboles. In contrast, the straight, stiff amphiboles may align themselves with the airstream and thus be delivered deeper into the lungs, where they can penetrate epithelial cells and reach the interstitium. Both amphiboles and serpentines are fibrogenic, and increasing doses are associated with a higher incidence of asbestos-related diseases.
In contrast to other inorganic dusts, asbestos acts as a tumor initiator and a tumor promoter ( Chapter 7 ). Some of its oncogenic effects are mediated by reactive free radicals generated by asbestos fibers, which preferentially localize in the distal lung, close to the mesothelial cells of the pleura. Toxic chemicals adsorbed onto the asbestos fibers also likely contribute to the oncogenicity of the fibers. For example, the adsorption of carcinogens in tobacco smoke onto asbestos fibers may be the basis for the remarkable synergy between tobacco smoking and the development of lung carcinoma in asbestos workers. Smoking also enhances the effect of asbestos by interfering with the mucociliary clearance of fibers. One study of asbestos workers found a fivefold increase of lung carcinoma with asbestos exposure alone, while asbestos exposure and smoking together led to a 55-fold increase in the risk.
Once phagocytosed by macrophages, asbestos fibers activate the inflammasome and stimulate the release of proinflammatory factors and fibrogenic mediators. The initial injury occurs at bifurcations of small airways and ducts, where asbestos fibers land, penetrate, and are directly toxic to pulmonary parenchymal cells. Macrophages, both alveolar and interstitial, attempt to ingest and clear the fibers. Long-term deposition of fibers and persistent release of mediators (e.g., reactive oxygen species, proteases, cytokines, and growth factors) eventually lead to generalized interstitial pulmonary inflammation and fibrosis.
Asbestosis is marked by diffuse pulmonary interstitial fibrosis, which is distinguished from diffuse interstitial fibrosis resulting from other causes only by the presence of asbestos bodies. Asbestos bodies are golden brown, fusiform or beaded rods with a translucent center that consist of asbestos fibers coated with an iron-containing proteinaceous material ( Fig. 15.20 ). They arise when macrophages phagocytose asbestos fibers; the iron is presumably derived from phagocyte ferritin. Other inorganic particulates may become coated with similar iron-protein complexes and are called ferruginous bodies.
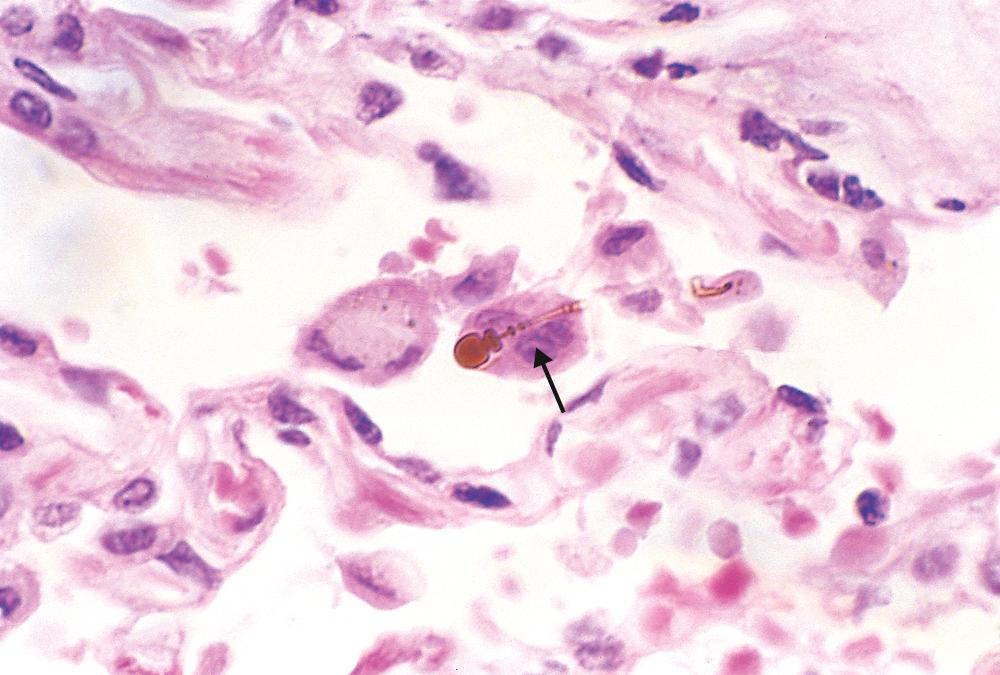
Asbestosis begins as fibrosis around respiratory bronchioles and alveolar ducts and extends to involve adjacent alveolar sacs and alveoli. The fibrosis distorts the architecture, creating enlarged airspaces enclosed by thick fibrous walls; eventually the affected regions become honeycombed. The pattern of fibrosis is histologically similar to that seen in usual interstitial fibrosis, with fibroblastic foci and varying degrees of fibrosis. In contrast to coal workers’ pneumoconiosis and silicosis, asbestosis begins in the lower lobes and subpleurally, with the middle and upper lobes becoming affected as fibrosis progresses. The scarring may trap and narrow pulmonary arteries and arterioles, causing pulmonary hypertension and cor pulmonale.
Pleural plaques, the most common manifestation of asbestos exposure, are well-circumscribed plaques of dense collagen that are often calcified ( Fig. 15.21 ). They develop most frequently on the anterior and posterolateral aspects of the parietal pleura and over the domes of the diaphragm. The size and number of pleural plaques do not correlate with the level of exposure to asbestos or the time since exposure. They also do not contain identifiable asbestos bodies; however, only rarely do they occur in individuals without a history or evidence of asbestos exposure. Uncommonly, asbestos exposure induces pleural effusions, which are usually serous but may be bloody. Rarely, diffuse visceral pleural fibrosis may occur and, in advanced cases, bind the lung to the thoracic wall.
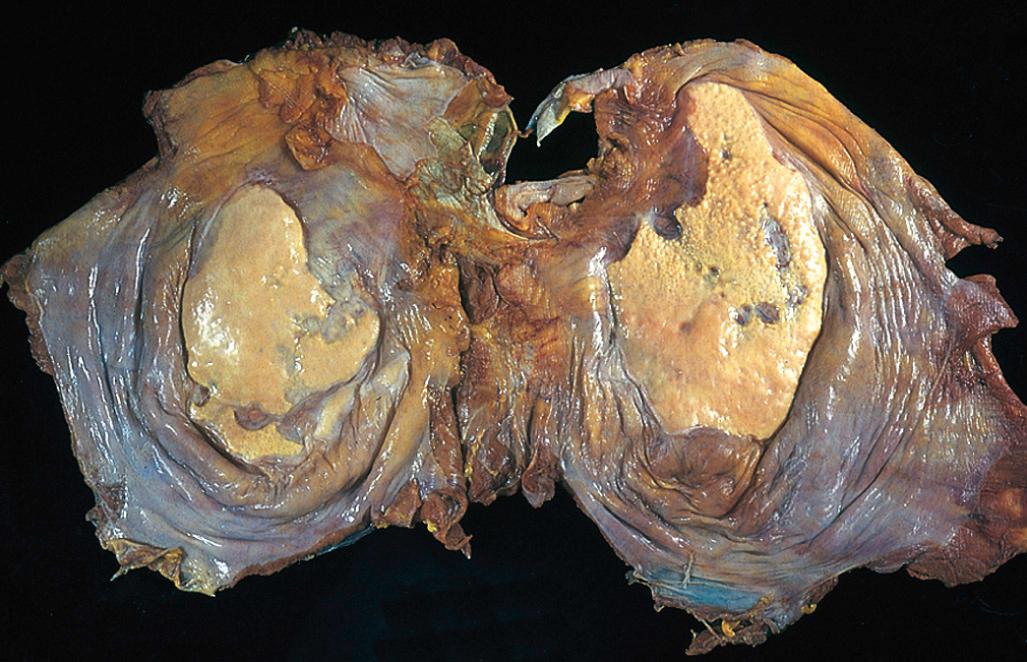
Both lung carcinomas and mesotheliomas (pleural and peritoneal) develop in workers exposed to asbestos (see sections on Carcinomas and Pleural Tumors ).
The clinical findings in asbestosis are very similar to those caused by other diffuse interstitial lung diseases (discussed earlier). They rarely appear fewer than 10 years after first exposure and are more common after 20 to 30 years. Dyspnea is usually the first manifestation; at first, it is provoked by exertion, but later is present even at rest. Cough associated with production of sputum, when present, is likely to be due to smoking rather than asbestosis. Chest x-ray studies reveal irregular linear densities, particularly in both lower lobes. With advancement of the pneumoconiosis, a honeycomb pattern develops. The disease may remain static or progress to respiratory failure, cor pulmonale, and death. Pleural plaques are usually asymptomatic and are detected on radiographs as circumscribed densities. Asbestosis complicated by lung or pleural cancer is associated with a particularly grim prognosis.
Pneumoconioses encompass a group of chronic fibrosing diseases of the lung resulting from exposure to organic and inorganic particulates, most commonly mineral dust.
Pulmonary alveolar macrophages play a central role in the pathogenesis of lung injury by promoting inflammation and producing fibrogenic cytokines.
Coal dust–induced disease varies from asymptomatic anthracosis to simple coal workers’ pneumoconiosis (coal macules or nodules, and centrilobular emphysema), to progressive massive fibrosis, manifested by increasing pulmonary dysfunction, pulmonary hypertension, and cor pulmonale.
Silicosis is the most common pneumoconiosis in the world, and crystalline silica (e.g., quartz) is the usual culprit. The lung disease is progressive even after exposure stops.
The manifestations of silicosis range from asymptomatic silicotic nodules to large areas of dense fibrosis; persons with silicosis also have an increased susceptibility to tuberculosis. There is twofold increased risk of lung cancer.
Asbestos fibers come in two forms; the stiff amphiboles have a greater fibrogenic and carcinogenic potential than the serpentine chrysotiles.
Asbestos exposure is linked with six disease processes: (1) parenchymal interstitial fibrosis (asbestosis); (2) localized pleural plaques (asymptomatic) or rarely diffuse pleural fibrosis; (3) recurrent pleural effusions; (4) lung carcinoma; (5) malignant pleural and peritoneal mesotheliomas; and (6) laryngeal cancer.
Cigarette smoking increases the risk of lung cancer in the setting of asbestos exposure; even family members of workers exposed to asbestos are at increased risk for lung carcinoma and mesothelioma.
An increasing number of prescription drugs have been found to cause a variety of acute and chronic alterations in lung structure and function, interstitial fibrosis, bronchiolitis obliterans, and eosinophilic pneumonia. For example, cytotoxic drugs used in cancer therapy (e.g., bleomycin) cause pulmonary damage and fibrosis as a result of direct toxicity and by stimulating the influx of inflammatory cells into the alveoli. Amiodarone, a drug used to treat cardiac arrhythmias, is preferentially concentrated in the lung and causes significant pneumonitis in 5% to 15% of patients receiving it. Cough induced by angiotensin-converting enzyme inhibitors is very common.
Illicit intravenous drug abuse most often causes lung infections. In addition, particulate matter used to cut drugs may lodge in the lung microvasculature, producing granulomatous inflammation and fibrosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here