Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Many complex brain systems are organized in a way that allows their function to be readily deduced. For example, although the connections of the somatosensory pathways with the brainstem, thalamus, and cortex are complex, each component plays a straightforward role. The processing of somatosensory information is generally well understood. In contrast, some systems are interconnected in such a way that a given function may be carried out by several components acting in cooperation, and a given component may participate in several functions. The limbic system is a case in point. This system comprises structures that receive inputs from diverse areas of the neuraxis and participate in complicated and interrelated behaviors such as memory, learning, and social interactions. Thus lesions involving the limbic system generally result in a wide range of deficits.
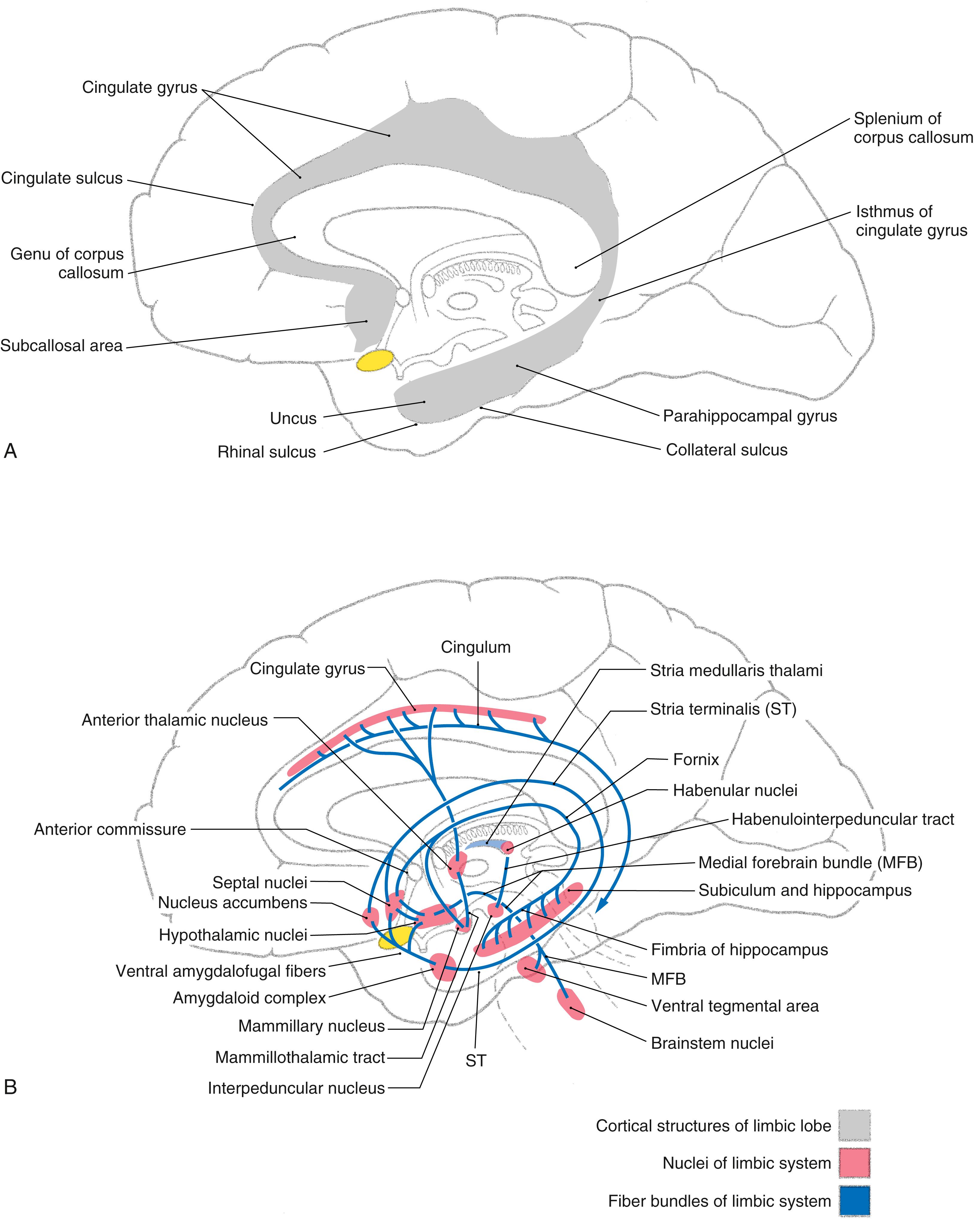
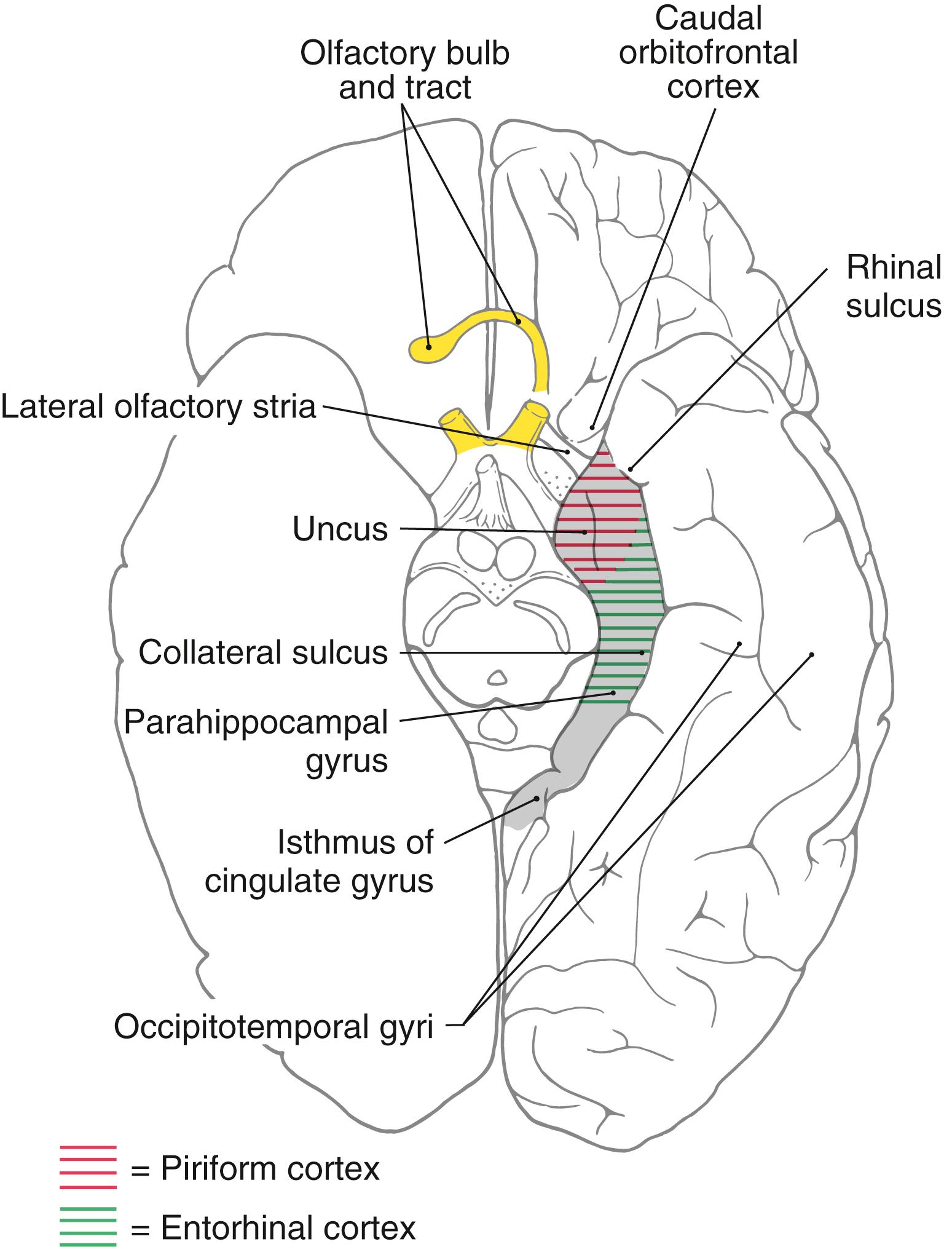
The concept of a limbic system actually encompasses two levels of structural and functional organization. The first level consists of the cortical structures on the most medial edge (the limbus) of the hemisphere; these collectively form the limbic lobe ( Fig. 31.1 A ). Beginning just anterior to the lamina terminalis and proceeding caudally, these are the subcallosal area, containing the parolfactory and paraterminal gyri; the cingulate gyrus; the isthmus of the cingulate gyrus; the parahippocampal gyrus; and the uncus ( Fig. 31.2 ). The limbic lobe also includes the hippocampal formation.
In 1878, Broca (1824–1880) noted that the limbic lobe, which is present in all mammals, represents a relatively large part of the cerebral cortex in phylogenetically lower forms, and he postulated that it might be related to olfaction. Because of this latter point, the term rhinencephalon (“smell-brain”) was later coined and used interchangeably with limbic lobe. However, it is now known that the limbic lobe has little, if any, olfactory function in humans. Thus the term “rhinencephalon,” although historically interesting, is antiquated and has largely disappeared from use.
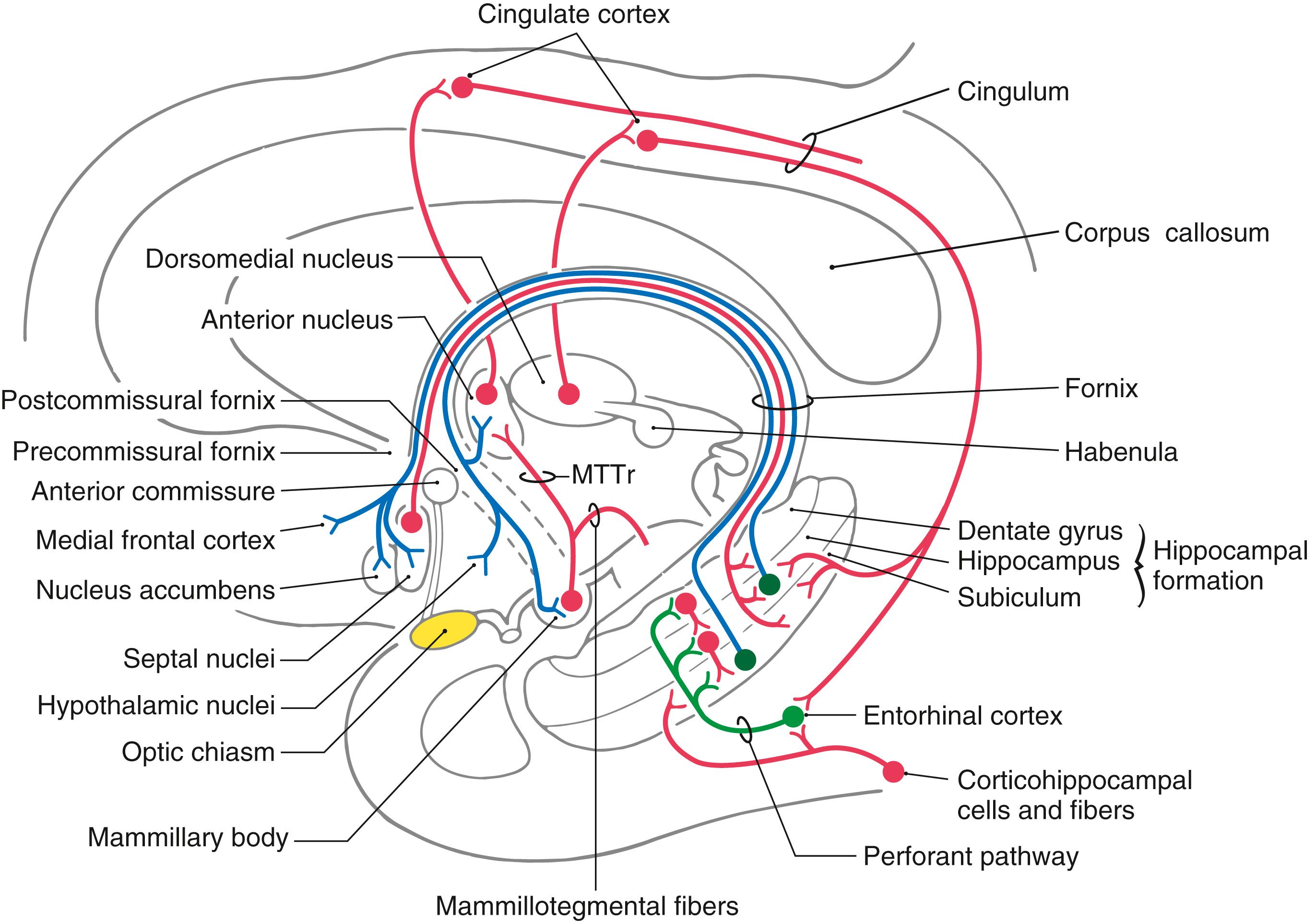
The second level includes structures of the limbic lobe plus a variety of subcortical nuclei and tracts that collectively form the limbic system ( Fig. 31.1 B ). The subcortical nuclei of the limbic system include, among others, the septal nuclei and nucleus accumbens ( nucleus accumbens septi ); various nuclei of the hypothalamus, especially those associated with the mammillary body; the nuclei of the amygdaloid complex and adjacent substantia innominata; and parts of the dorsal thalamus, particularly the anterior and dorsomedial nuclei. Additional structures connected with the limbic system include the habenular nuclei, ventral tegmental area, and periaqueductal gray. Furthermore, the prefrontal cortex is considered by some investigators to be an important component of the limbic system, primarily because of its potential influence on various other cortical and subcortical parts of the limbic system. Cortical targets of the prefrontal cortex include the cingulate gyrus, whereas the hypothalamus, dorsal thalamus, amygdaloid complex, and nuclei of the midbrain represent subcortical targets.
The main efferent fiber bundles of the limbic system are the fornix (primarily efferents of the hippocampus and subiculum), the stria terminalis and ventral amygdalofugal pathway (both are mainly efferents of the amygdaloid complex), and the mammillothalamic tract (efferents of the medial mammillary nucleus; Fig. 31.1 B ). A few additional nuclei and smaller tracts will be introduced as connections and functions of the limbic system.
The human cerebral cortex can be divided into several areas on the basis of the number of cell layers present. Most of the cerebral cortex (more than 90%) has six cell layers and is called the neocortex ( isocortex ). Examples of neocortex include the primary sensory, motor, and association cortices. The cortical regions that have fewer than six layers are structurally and functionally associated with the limbic system or with olfaction and are classified as allocortex. Those structures that comprise three to five cellular layers are called the paleocortex ( periallocortex ) and are represented by the cortex of the parahippocampal gyrus (the entorhinal cortex ), the uncus (the piriform cortex) , and the cortex overlying the termination of the lateral olfactory stria (lateral olfactory gyrus) ( Fig. 31.2 ). The lateral olfactory stria is directly rostromedial to the piriform cortex. Structures having only three cellular layers are classified as archicortex ( allocortex ) and are represented by the dentate gyrus and hippocampus.
The separation between neocortex and allocortex is never sharp but instead consists of transitional areas called periallocortex, where one cortical region blends into the next. Such areas are represented by caudal parts of the orbitofrontal cortex, the temporal pole, parts of the insula, and portions of the parahippocampal and cingulate gyri. These important areas funnel input from association areas of the neocortex into the allocortex.
In the late 1930s, two pivotal observations were made that formed the basis for the concept of a limbic system. First, largely on the basis of the morphology of the brain, a circuit for the elaboration of emotion was proposed. This pathway is now called the Papez circuit in recognition of the anatomist James Papez (1883–1958), who initially described its components. This model, although surprisingly simple, has proved to be quite important in understanding limbic function. The circuit suggested that emotion, mediated through the hypothalamus, is controlled and modulated by fibers from the fornix. Specifically, the cortical control of emotional activity can be viewed as a pathway originating from the cingulate gyrus (see Fig. 31.5 ). The Papez circuit consists of the following structures and cell groups. The cingulate gyrus projects, via the cingulum, to the hippocampal formation (mainly the subiculum and the Ammon horn) as well as to the entorhinal cortex. The hippocampus then projects, via the alveus and the various parts of the fornix, to the mammillary nuclei by coursing through the postcommissural fornix. In turn, the medial mammillary nucleus projects to the anterior nucleus of the thalamus by way of the mammillothalamic tract, and the anterior nucleus projects to the cortex of the cingulate gyrus. This was the first time a specific anatomic substrate was proposed for a phenomenon as complex as emotion.
The second pivotal observation was that bilateral removal of large parts of the temporal lobe in monkeys resulted in a constellation of dysfunctions that came to be known as the Klüver-Bucy syndrome, in recognition of Heinrich Klüver (1897–1979) and Paul Bucy (1904–1992). Whereas deficits in memory and behavioral changes were initially described in animal experiments, numerous cases of humans with bilateral temporal lobe injuries further elucidated the importance of the limbic system in the formation of new or recent memories.
The blood supply to the limbic system originates from several sources. The main vessels serving the limbic system are the anterior and posterior cerebral arteries, the anterior choroidal artery, and branches arising from the circle of Willis.
The subcallosal area and rostral parts of the cingulate gyrus are supplied by branches of the anterior cerebral artery as it loops around the genu of the corpus callosum (see Fig. 8.7 ). Most of the cingulate gyrus and its isthmus receive blood supply via the pericallosal artery, a branch of the anterior cerebral artery. Temporal branches of the posterior cerebral artery (P 3 segment) supply the parahippocampal gyrus. Although the uncus may receive some small branches from the posterior cerebral artery, it is served primarily by uncal arteries, which are branches of the M 1 segment of the middle cerebral artery (see Fig. 8.6 ).
The anterior choroidal artery usually originates from the internal carotid artery and follows the general trajectory of the optic tract. It sends branches into the choroidal fissure of the temporal horn of the lateral ventricle. This vessel serves the choroid plexus of the temporal horn, the hippocampal formation, parts of the amygdaloid complex, and adjacent structures, such as the tail of the caudate nucleus, the stria terminalis, and the sublenticular and retrolenticular limbs of the internal capsule.
Vessels serving hypothalamic nuclei that are functionally associated with the limbic system originate from the circle of Willis. In general, rostral areas of the hypothalamus are served by branches from the anterior communicating artery and anterior cerebral artery; posterior areas are served by branches from the posterior communicating artery and proximal posterior cerebral artery (see Fig. 8.17 ). The anterior nucleus of the thalamus, an important synaptic station in the limbic system, is supplied by thalamoperforating arteries that arise from the P 1 segment of the posterior cerebral artery.
The hippocampal formation is composed of the subiculum, hippocampus (also called the hippocampus proper or horn of Ammon), and dentate gyrus (see Fig. 31.4 ), all of which constitute the allocortex of Brodmann. The subiculum is laterally continuous with the cortex of the parahippocampal gyrus and an area of the periallocortex. Medially, the edge of the hippocampal formation is formed by the dentate gyrus and the fimbria of the hippocampus.
Developmentally, the hippocampal formation originates dorsally and migrates into its ventral and medial positions in the temporal lobe. During this migration, small remnants of the hippocampal formation remain behind to form the medial and lateral longitudinal striae and their associated gray matter, the indusium griseum ( Fig. 31.3 ). These structures are small in the human brain and extend rostrally along the dorsal aspect of the corpus callosum into the subcallosal area.
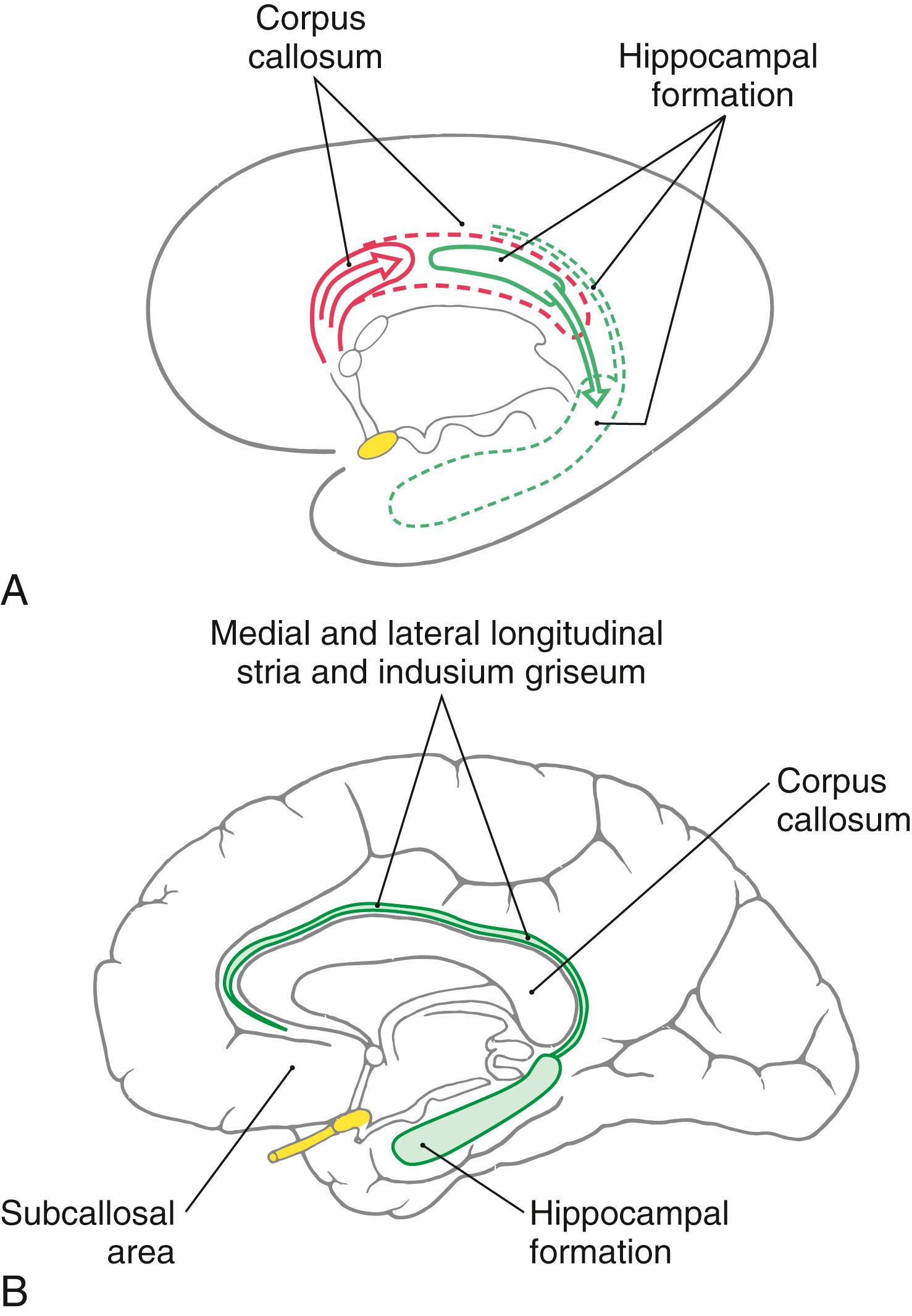
The subiculum of the hippocampal formation is the transitional area between the three-layered hippocampus (allocortex) and the five-layered entorhinal cortex (periallocortex) of the parahippocampal gyrus ( Fig. 31.4 ). This transitional zone, although small, can be divided into a prosubiculum, subiculum proper, presubiculum, and parasubiculum. These areas are essential for the flow of information into the hippocampal formation.
The dentate gyrus and the hippocampus are each composed of three layers ( Fig. 31.4 ). The external layer is called the molecular layer and contains afferent axons and dendrites of cells intrinsic to each structure. The middle layer, called the granule cell layer in the dentate gyrus and the pyramidal layer in the hippocampus, contains the efferent neurons of each structure ( Fig. 31.4 ). These layers are named according to the shape of the cell body of the principal type of neuron found therein. The dendrites of granule and pyramidal cells radiate into the molecular layer. The inner layer, called the polymorphic layer (also called the stratum oriens in the hippocampus), contains the axons of pyramidal and granule cells, a few intrinsic neurons, and many glial elements. In addition, the polymorphic layer of the hippocampus contains the elaborate basal dendrites of some larger pyramidal somata that are located in the pyramidal layer. These are called double pyramidal cells because they have dendrites extending into both molecular and polymorphic layers ( Fig. 31.4 ). The innermost part of the hippocampus borders on the wall of the lateral ventricle and is a layer of myelinated axons arising from cell bodies located in the subiculum and hippocampus. This layer, called the alveus, is continuous with the fimbria of the hippocampus, which in turn becomes the fornix ( Fig. 31.4 ).
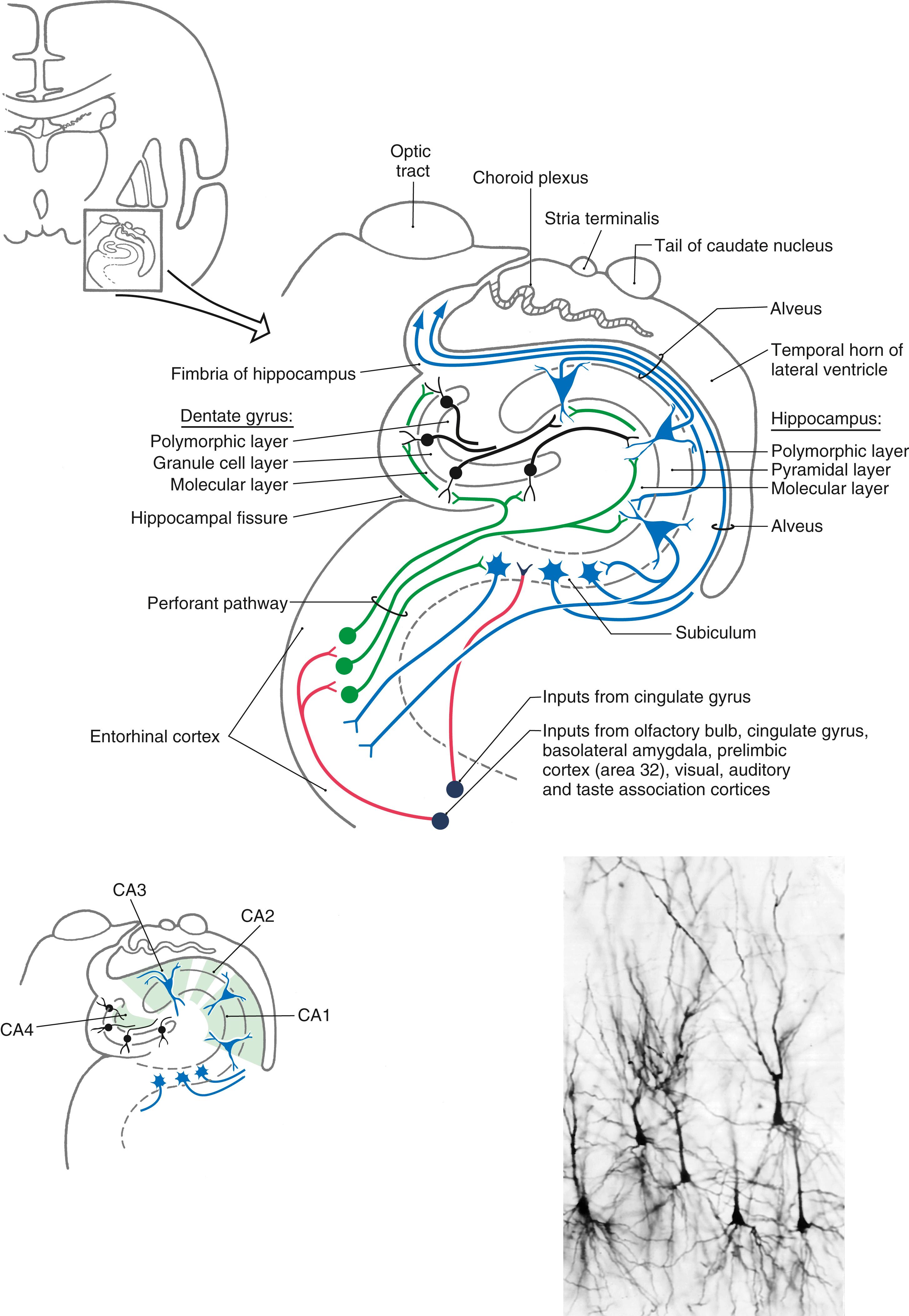
The hippocampus can be divided into four regions on the basis of a variety of cytoarchitectural criteria ( Fig. 31.4 ). These areas are designated CA1 to CA4, where CA stands for cornu ammonis (horn of Ammon). Area CA1 (a parvocellular region that can be separated into two cell layers in humans) is located at the subiculum-hippocampal interface. Area CA2 (a mixed zone) and area CA3 (a magnocellular zone) are located within the hippocampus. Area CA4 is located at the junction of the hippocampus with the dentate gyrus but within the hilus of the dentate gyrus.
The major input to the hippocampal formation is from cells of the entorhinal cortex via a diffuse projection called the perforant pathway ( Figs. 31.4 and 31.5 ). Most fibers of the perforant pathway terminate in the molecular layer of the dentate gyrus, although a few terminate in the subiculum and hippocampus. Granule cells in the dentate gyrus project into the molecular layer of the CA3 region of the hippocampus. CA3 neurons project into CA1 of the hippocampus, which in turn provides an input to the subiculum. In addition, the subiculum also receives a modest projection from the amygdaloid complex. Although the fornix is mainly an efferent path from the hippocampus, it also conveys cholinergic septohippocampal projections to the hippocampal formation and entorhinal cortex.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here